072ff93b9a211723b03210eb4a7ff9e1.ppt
- Количество слайдов: 76

The International Consortium for Evidence-Based Perfusion: Moving from Concept to Reality Robert A. Baker 1, 2, Timothy A Dickinson 2, Donald S Likosky 2, Kenneth G Shann 2 1 Flinders Medical Centre, Bedford Park, South Australia. 2 Executive, International Consortium for Evidence Based Perfusion

Potential Conflicts of Interest ØResearch Support § Terumo, National Heart Foundation, Somanetics, Lunar Innovations ØTravel and Conference Support § Terumo, Medtronic, Cellplex, Bayer ØPerfusion Downunder Organisation § Deputy Chair § Chair of the PDU Collaboration

Outline ØWhat is the ICEBP? ØStructure and Progress ØHow and Why to get involved.

What is the ICEBP? Øwww. icebp. org

Mission Statement The International Consortium for Evidence-Based Perfusion (ICEBP) partners and collaborates with perfusion societies, professional medical societies, and interested clinicians, to improve continuously the delivery of care and outcomes for our patients. Vision of the ICEBP To achieve this mission, we will • Evaluate current practice through a dedicated international perfusion registry. Mission Statement • Develop and publish evidence based guidelines, and support their integration The International Consortium for Evidence-Based Perfusion (ICEBP) partners and into clinical practice. collaborates with perfusion societies, professional medical societies, and interested clinicians, to improve continuously the delivery of care and outcomes for our patients. • Identify gaps in the medical literature and empower clinical teams to conduct Vision of the ICEBP research in areas where evidence is lacking. To achieve this mission, we will • Identify gaps between current and evidence-based clinical practice to promote • Evaluate current practice through a dedicated international perfusion registry. the improvement in patient care. • Develop and publish evidence based guidelines, and support their integration into clinical practice. • Identify gaps in the medical literature and empower clinical teams to conduct research in areas where evidence is lacking.

International Consortium for Evidence-Based Perfusion Pediatric Process Improvement Subcommittee Clinically-Based Registry Subcommittee Communication Subcommittee Steering Committee Scientific Sessions Subcommittee Adult Process Improvement Subcommittee Evidence-Based Guideline Writing Subcommittee Educational Subcommittee Research Development Subcommittee

Current practice Evidence-based practice
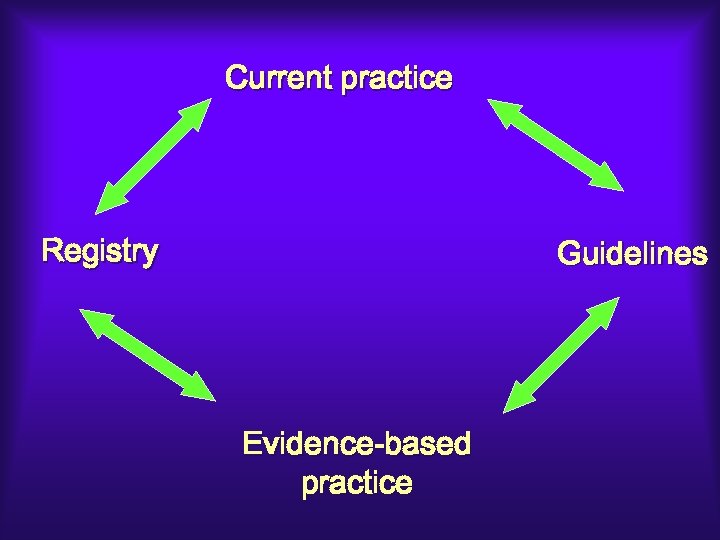
Current practice Registry Guidelines Evidence-based practice
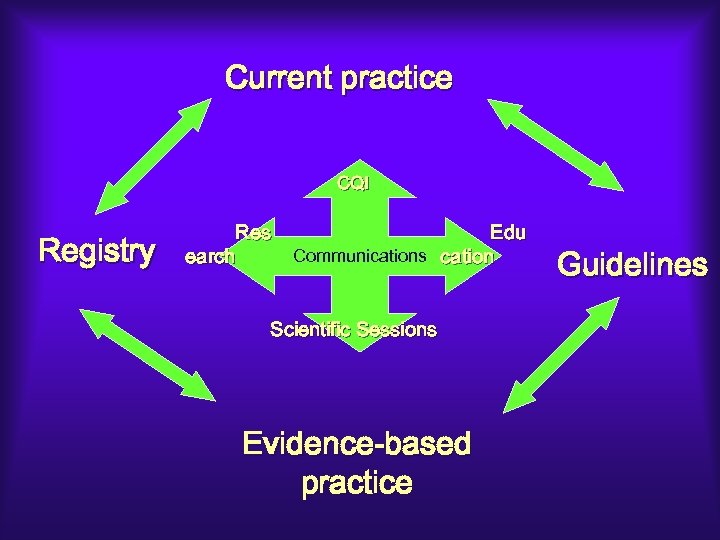
Current practice CQI Registry Res earch Edu Communications cation Scientific Sessions Evidence-based practice Guidelines
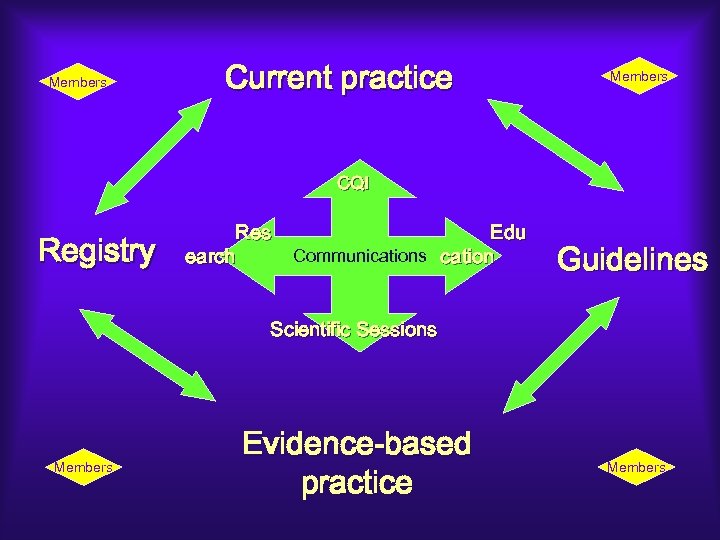
Members Current practice Members CQI Registry Res earch Edu Communications cation Guidelines Scientific Sessions Members Evidence-based practice Members

Communications Subcommittee

Website – www. icebp. org

Website – Committee Pages
Committee Sites
Committee Sites

Website – Meetings / Events
Tools and Tips

Newsletters

www. icebp. org

Scientific Sessions Planning Subcommittee

Scientific Subcommittee ØMission: § Develop and organize an annual scientific meeting focused on meeting the goals of the ICEBP mission statement. The scientific meeting will cover topics related to all other subcommittees. In addition, the subcommittee will be charged with examining areas into which the meeting can grow to allow demonstration of sustained improvement in the care

Scientific Subcommittee ØMission: § Develop and organize an annual scientific meeting focused on meeting the goals of the ICEBP mission statement. The scientific meeting will cover topics related to all other subcommittees. In addition, the subcommittee will be charged with examining areas into which the meeting can grow to allow demonstration of sustained improvement in the care

Scientific Subcommittee ØMission: § Develop and organize an annual scientific meeting focused on meeting the goals of the ICEBP mission statement. The scientific meeting will cover topics related to all other subcommittees. In addition, the subcommittee will be charged with examining areas into which the meeting can grow to allow demonstration of sustained improvement in the care

Scientific Subcommittee ØMission: § Develop and organize an annual scientific meeting focused on meeting the goals of the ICEBP mission statement. The scientific meeting will cover topics related to all other subcommittees. In addition, the subcommittee will be charged with examining areas into which the meeting can grow to allow demonstration of sustained improvement in the care

Scientific Subcommittee ØMission: § Develop and organize an annual scientific meeting focused on meeting the goals of the ICEBP mission statement. The scientific meeting will cover topics related to all other subcommittees. In addition, the subcommittee will be charged with examining areas into which the meeting can grow to allow demonstration of sustained improvement in the care

Best Practices in Perfusion ØTwo Successful Meetings: 2006 Seattle, Washington (USA) 2007 Montreal, Quebec (CAN)

Planning Committee - 2007 Ø Dwayne Jones, CPC, Ø Donald S. Likosky, Ph. D Ø Ø Ø CCP, RN (CAN) Christos Calaritis, BSc, CPC, CCP (CAN) Gordon R. De. Foe, CCP Timothy A. Dickinson, MS (Chair) (USA) Robert C. Groom, MS, CCP (USA) Deborah Hubble, CCP (USA) Ø Ø Ø (USA) Jeffrey B. Riley, MHPE, CCT (USA) Dirck A. Rilla, LP, CCP (USA) David J. Rosinski, CCP (USA) Kenneth G. Shann, CCP (USA) Alfred H. Stammers, MSA, CCP (USA) Robert Baker, Ph. D, CCP

Program Highlights - 2007 Ø ICEBP guideline subcommittee update Ø Quality improvement skills training Ø The World Society for Pediatric and Congenital Heart Surgery –Dr. Tchervenkov Ø Adult & pediatric registries Ø Public reporting and transparency Ø Credentialing of perfusionists as a best practice Ø Abstracts on key aspects of best
International Attendees - 2007 Ø Australia Ø Pakistan Ø Belgium Ø Saudi Arabia Ø Canada Ø Spain Ø Japan Ø Sweden Ø Germany Ø United Kingdom Ø Netherlands Ø United States Ø New Zealand

Manufacturer Support - 2007 Ø Bayer Pharmaceuticals Ø The Medicines Company Ø Terumo Cardiovascular Ø Maquet-Dynamed Inc. Ø Somanetics Corporations Ø CAS Medical Ø Global Blood Resources Ø Luna Innovations Ø Medtronic, Inc. Ø Quest Medical, Inc. Ø Rocky Mountain Research Ø Sorin Group Ø Spectrum Medical

Best Practices – 2008 ØDate: Early October 2008 ØLocation: Southern USA § San Antonio § Galverston § Dallas

Paediatric Subcommittee

The Opportunity “The professions caring for patients with congenital heart disease have the unique opportunity to create the first comprehensive international database for a medical subspecialty. ” Jacobs JP. International Congenital Heart Disease Nomenclature: Introduction to Mapping and Computer Based Mapping Solutions. Presented at The International Summit on Nomenclature for Congenital Heart Disease at The Third World Congress of Pediatric Cardiology and Cardiac Surgery, Toronto, Canada, May 27, 2001.

Meaningful Multi-institutional Outcomes Analysis Requirements - Accomplishments Ø Ø Ø Common Language = Nomenclature Mechanism of Data Collection (Database - Registry) Mechanism of Evaluating Case Complexity Mechanism to Verify Data Validity and Accuracy Collaboration Between Subspecialties
Multi-Societal Database Committee for Pediatric and Congenital Heart Disease Ø Ø Ø Ø Ø The STS Congenital Database Taskforce The EACTS/ECHSA Congenital Database Committee headed by Bohdan Maruszewski The STS Congenital Database Taskforce Core Users Group headed by Hal Walters The STS Congenital Database Data Verification Subcommittee headed by Dave Clarke The Aristotle Project headed by Francois Lacour Gayet The Multi-Center Panel of Experts for Cardiac Surgical Outcomes headed by Kathy Jenkins The Congenital Cardiac Anesthesia Society (CCAS) Database headed by David Vener The Joint Council of Congenital Heart Disease (JCCHD) headed by Gerard Martin, MD and representing the American Heart Association and the American College of Cardiology The Association of European Pediatric Cardiology Nomenclature Committee headed by Rodney Franklin

Multi-Societal Database Committee for Pediatric and Congenital Heart Disease Ø Ø Ø Ø Ø The Pediatric Cardiac Intensive Care Society (PCICS) The VPS (Virtual Pediatric Intensive Care Unit Database System) The International Consortium for Evidence Based Perfusion (ICEBP) The International Working Group for Mapping and Coding of Nomenclatures for Paediatric and Congenital Heart Disease (Nomenclature Working Group – NWG) headed by Christo Tchervenkov The World Society for Pediatric and Congenital Heart Disease headed by Christo Tchervenkov Center for Quality Improvement and Patient Safety of Agency for Healthcare Research and Quality (AHRQ) of the United States Department of Health and Human Services Birth Defect Branch of the Centers for Disease Control and Prevention (CDC) The National Association of Children's Hospitals and Related Institutions (NACHRI ) The National Quality Forum (NQF)

Multi-Societal Database Committee for Pediatric and Congenital Heart Disease ØICEBP Pediatric Committee is now a member ØAttend 3 meetings per year ØInvolved with all projects ØImplementation of perfusion specific variables into STS Congenital heart surgery database

Complications Project Ø In congenital heart surgery, mortality in 2006 is 4%. In order to assess better quality of care involving the remaining 96% of patients, we must agree on universally accepted definitions of morbidity. Ø Not all complications are caused by medical error and not all medical error results in complications. Ø Not all complications are medical negligence or medical malpractice. Ø Many subtypes of complications exist.

Complications Project Ø ICEBP Pediatric Committee responsible for CPB, ECLS, and VAD complication list Ø Biweekly conference calls Ø E-mail Ø Multiple revisions Ø Creation of manuscript to be submitted to Cardiology in the Young alongside other organ system complication lists (>1000)

Ongoing Initiatives Ø Collaborative Database initiatives with the: Ø Congenital Cardiac Anesthesia Society (CCAS) Ø Joint Council of Congenital Heart Disease (JCCHD), representing ACC and AHA Ø Pediatric Cardiac Intensive Care Society (PCICS) Ø STS Regional Congenital Database Reports Ø Data Verification Site Visit Project Ø Ongoing collaborative research to Ø validate the Aristotle Basic Complexity Score Ø with the developers of both the Aristotle Basic Complexity Score (ABC Score) and the Risk Adjustment for Congenital Heart Surgery (RACHS-1) methodology with the goal of unifying these two systems.

Registry Subcommittee International Perfusion Registry
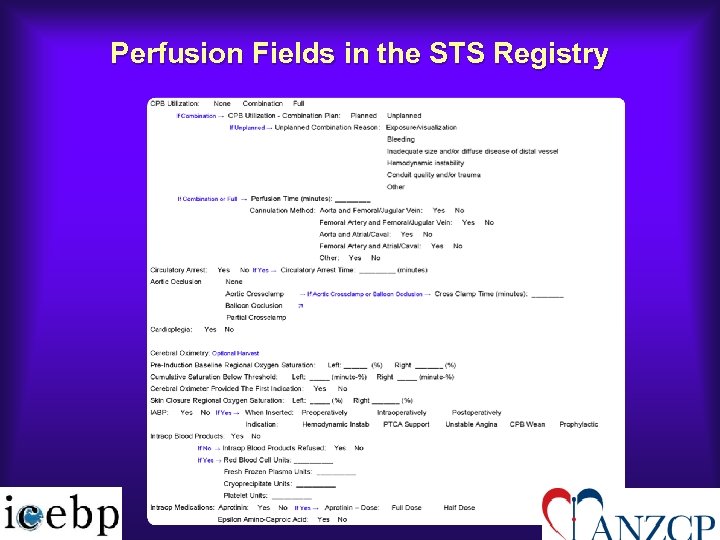
Perfusion Fields in the STS Registry

What Might Be Some Items on My Data Form? Traditional • Preoperative factors – Age, comorbid conditions • Intraoperative factors – Duration of cardiopulmonary bypass – Prime volume • Outcome Variables – Death x acuity, return to the OR for bleeding

What Might Be Some Items on My Data Form? Traditional • Preoperative factors – Age, comorbid conditions • Intraoperative factors – Duration of cardiopulmonary bypass – Prime volume • Outcome Variables – Death x acuity, return to the OR for bleeding What you Can Vary • Process variables – Use of cell processing – Use and type of arterial line filter – Type of circuit – Prevention of air entrainment – Highest blood glucose level during bypass – Nadir Hct - by gender

Guiding Principles Ø Ø Quality over quantity Definitions, definitions Validation of case count and status Smart registry Ø Ø Center Thumbprint Assist in submissions to other registries Software independent Cross match to STS and PDUCDB
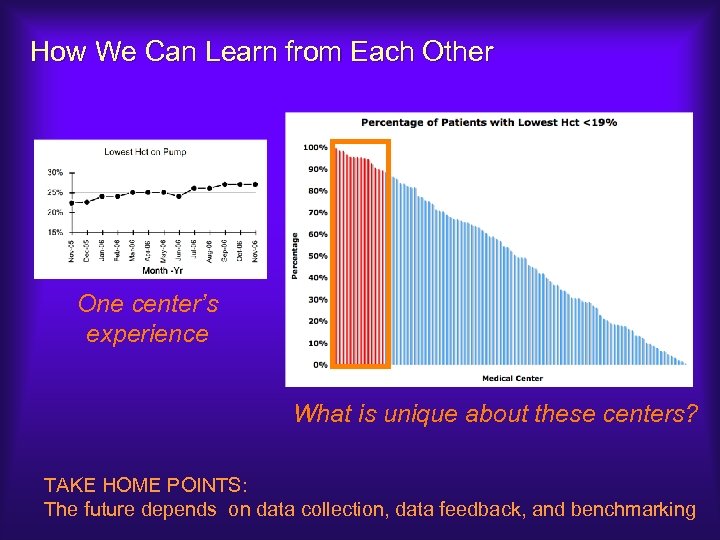
How We Can Learn from Each Other One center’s experience What is unique about these centers? TAKE HOME POINTS: The future depends on data collection, data feedback, and benchmarking
Areas of Focus Ø Patient demographics (to adjust for potential patient-level confounders) Ø Compliance with perfusion guidelines that were published in JTCVS (amend the list as the ICEBP publishes guidelines) Ø Cell processing and filtration Ø Renal Management Ø Factors influence low EF (among patients with normal EF)

Guideline Writing Subcommittee ØThe mission of the guideline writing subcommittee is to develop evidencebased clinical practice guidelines for cardiovascular perfusion. § Methodology used by the American College of Cardiology/American Heart Association (ACC/AHA) § Written and subsequently updated to remain concurrent with the medical literature. § Adoption of these guidelines in practice would be tracked through the clinical registry subcommittee.

Guideline Writing Subcommittee ØGuidelines § Involvement of representatives from each of the participating perfusion organizations in the guideline writing subcommittee should reduce any un/anticipated hurdles for the endorsement of any given guideline. § Submitted to the participating perfusion organizations for their review and endorsement

Platelet Preservation: Do perfusion strategies really make a difference? Gordon R. De. Foe, BA, CCP Dartmouth-Hitchcock Medical Center Dartmouth Medical School Lebanon, NH, USA
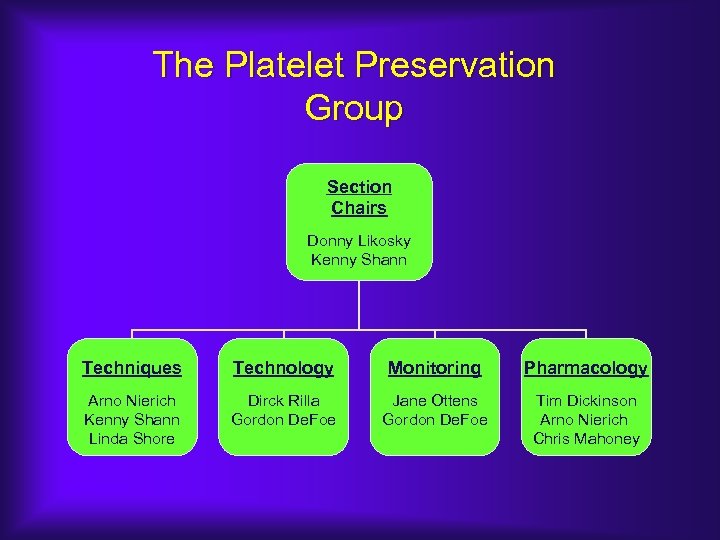
The Platelet Preservation Group Section Chairs Donny Likosky Kenny Shann Techniques Technology Monitoring Pharmacology Arno Nierich Kenny Shann Linda Shore Dirck Rilla Gordon De. Foe Jane Ottens Gordon De. Foe Tim Dickinson Arno Nierich Chris Mahoney

Defining the problem Statement: We believe that platelets are good. Ø Can we physically and qualitatively preserve platelets during cardiopulmonary bypass? § § Surface coatings or treatments Pump types Circuit components Cell salvage devices Ø Are there holes in our knowledge?


What are the steps? Ø Evaluate the peer-reviewed medical literature in a rigorous and consistent fashion Ø Focus expertise on specific topics Ø Develop an informed opinion regarding effectiveness and assign levels of evidence Ø Formulate a “finding” and a written summary for publication

Search methodology Ø NCBI - National Center For Bio. Technology Information - MEDLINE search, ≥ 1996 Ø Search parameters § ((platelet OR platelets OR flow cytometry) AND (cardiac surgery OR ((("cardiopulmonary bypass"[TIAB] NOT Medline[SB]) OR "cardiopulmonary bypass"[Me. SH Terms] OR ("coronary artery bypass"[TIAB] NOT Medline[SB]) OR "coronary artery bypass"[Me. SH Terms]) OR (valve OR valves OR valvular) AND and surgery))) AND (biocompatible coated materials OR coated circuits)

Evaluation of search results Ø An automated Excel spreadsheet was automatically populated by NCBI search Ø NCBI download includes abstract (if available) Ø Reviewers can sift through references based upon abstract, or decide to review entire paper Ø Almost all citations retrievable on-line Ø Fate of all citations is tracked.

Classification of Recommendations Ø Class I - Conditions for which there is evidence, general agreement, or both that a given procedure or treatment is useful and effective Ø Class II – Procedure or treatment should be performed or administered Ø Class IIa – Additional studies with focused objectives are needed Ø Class IIb – Additional studies with broad objectives are needed; additional registry data would be helpful Ø Class III – Procedure or treatment should not be performed or administered because it is

Levels of evidence ØLevel A – Data is derived from multiple randomized clinical trials ØLevel B – Data is derived from a single randomized trial or nonrandomized studies ØLevel C – Consensus opinion of experts

Results of literature search Ø 103 “hits” on MEDLINE ØAll proved to be retrievable on-line Ø 44 citations (inc. 2 review articles) were judged to be relevant to the topic of “technology for platelet preservation on bypass” ØIn all, we evaluated studies on 4, 234 adult patients in 41 distinct trials

“Biocompatible” v “Standard” ØThe clear preponderance of the evidence is that coated circuits better preserve platelet counts and reduce platelet deposition and activation, when used in either the “tip-to-tip” or “all but cannula” mode ØNo clear benefit was observed when only the oxygenator was coated
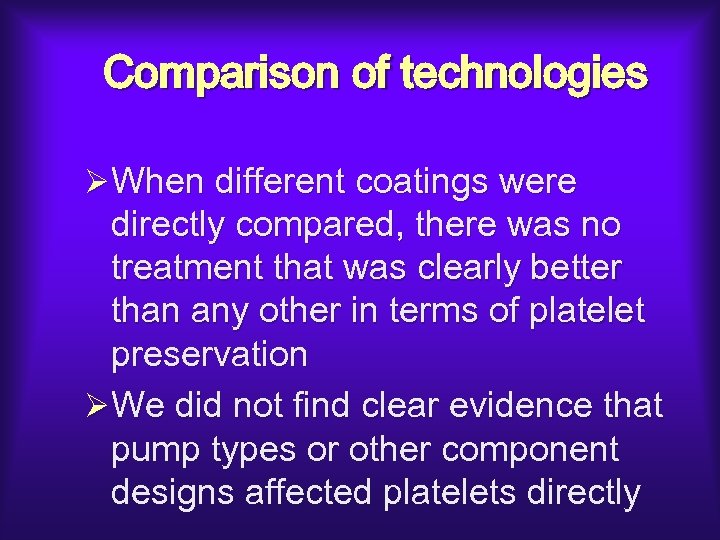
Comparison of technologies ØWhen different coatings were directly compared, there was no treatment that was clearly better than any other in terms of platelet preservation ØWe did not find clear evidence that pump types or other component designs affected platelets directly

Work still to be done ØCochrane meta-analysis will be run ØWrite discussion section for next publication ØThere are holes in our knowledge § § Roller versus centrifugal Closed versus open Role of pump suction versus IRCR Is there a “best coating? ”

Proposed “Finding” When used in either the “tip to tip” or “all but cannula” configuration, biocompatible cardiopulmonary bypass circuits offer superior preservation and protection of platelets during and after cardiac surgery. (Class TBD, Level TBD)

The Inflammatory Response: It’s not all about Pharmacological Intervention Rob Baker Flinders Medical Centre and Flinders University, Bedford Park Adelaide, AUSTRALIA
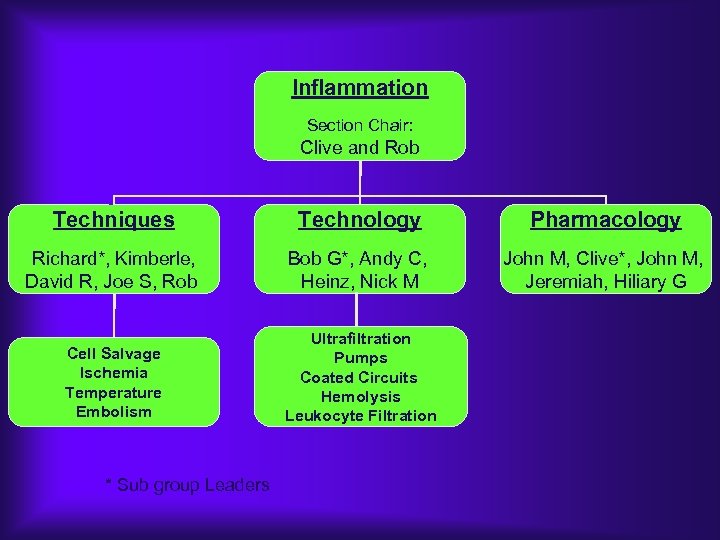
Inflammation Section Chair: Clive and Rob Techniques Technology Pharmacology Richard*, Kimberle, David R, Joe S, Rob Bob G*, Andy C, Heinz, Nick M John M, Clive*, John M, Jeremiah, Hiliary G Cell Salvage Ischemia Temperature Embolism Ultrafiltration Pumps Coated Circuits Hemolysis Leukocyte Filtration * Sub group Leaders
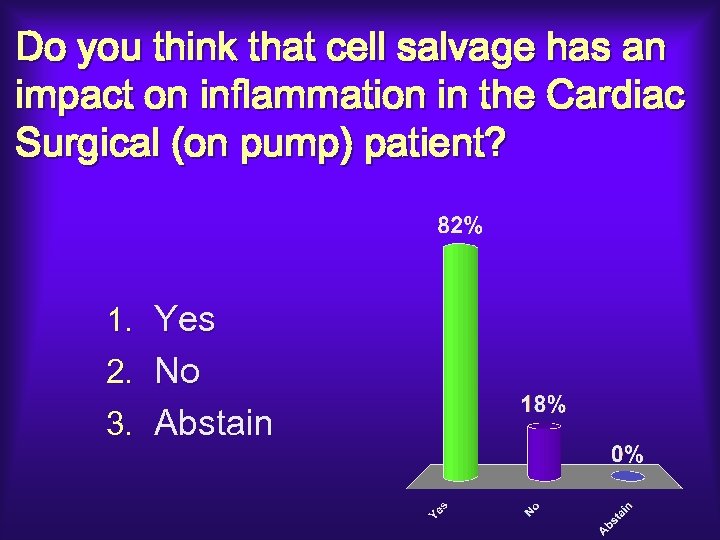
Do you think that cell salvage has an impact on inflammation in the Cardiac Surgical (on pump) patient? 1. Yes 2. No 3. Abstain
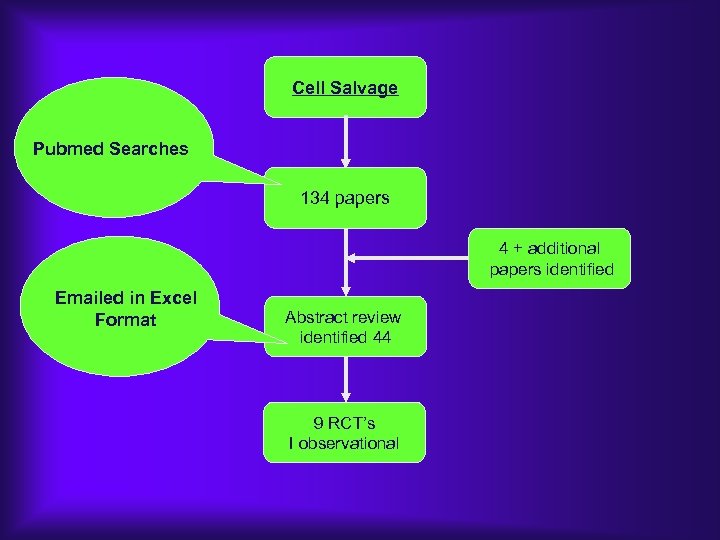
Cell Salvage Pubmed Searches 134 papers 4 + additional papers identified Emailed in Excel Format Abstract review identified 44 9 RCT’s I observational

Recommendation Cell salvage is reasonable for the reduction of inflammatory markers in CS blood prior to its return to the patient. (Class TBD, Level TBD).

Who is currently involved Ø David Rosinski, Richard Newland, Kimberle Mc. Gill; Nicholas Mellas, Andrew Cleland, Bob Groom, Heinz Weitkemper, John Murkin, Jeremiah Brown, John Motley, Hiliary Grocott, Kenneth Shann, Tim Dickinson, Chris Brown Mahoney, Sander Spanjersberg, Arno Nierich, Donny Likosky, Linda Shore Brown, Gordon De. Foe, Dirck Rilla, Jane Ottens, Huong Pham,

072ff93b9a211723b03210eb4a7ff9e1.ppt