306d285311298dee3599798f8afc816e.ppt
- Количество слайдов: 23
 The Impact of the EU Proposed Research Regulation on the Legal, Ethical, and Social Aspects of Clinical Trials Clinical Research Conference 2012 Clinical Drug Research in Greece Athens, Greece 5 December 2012 Francis P. Crawley Good Clinical Practice Alliance - Europe & Strategic Initiative for Developing Capacity in Ethical Review 1 fpc@gcpalliance. org
The Impact of the EU Proposed Research Regulation on the Legal, Ethical, and Social Aspects of Clinical Trials Clinical Research Conference 2012 Clinical Drug Research in Greece Athens, Greece 5 December 2012 Francis P. Crawley Good Clinical Practice Alliance - Europe & Strategic Initiative for Developing Capacity in Ethical Review 1 fpc@gcpalliance. org
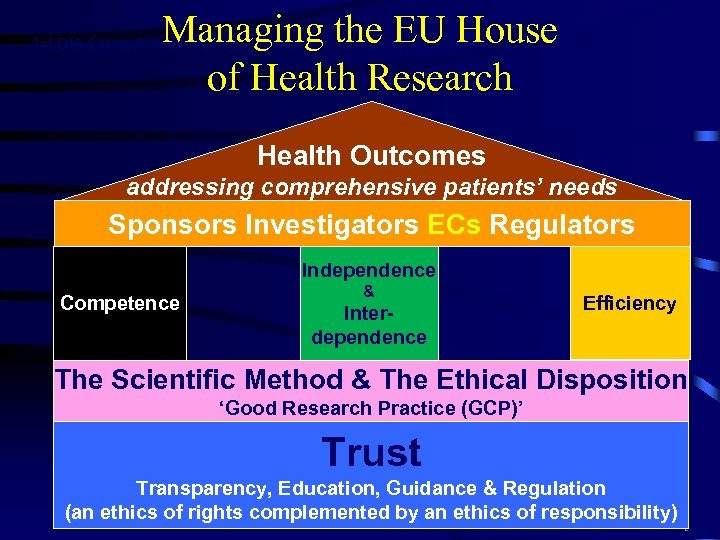 Managing the EU House of Health Research 1 st DIA China Annual Meeting | November 1 -3, Beijing, China Health Outcomes addressing comprehensive patients’ needs Sponsors Investigators ECs Regulators Independence Competence & Interdependence Efficiency The Scientific Method & The Ethical Disposition ‘Good Research Practice (GCP)’ Trust Transparency, Education, Guidance & Regulation (an ethics of rights complemented by an ethics of responsibility) 2
Managing the EU House of Health Research 1 st DIA China Annual Meeting | November 1 -3, Beijing, China Health Outcomes addressing comprehensive patients’ needs Sponsors Investigators ECs Regulators Independence Competence & Interdependence Efficiency The Scientific Method & The Ethical Disposition ‘Good Research Practice (GCP)’ Trust Transparency, Education, Guidance & Regulation (an ethics of rights complemented by an ethics of responsibility) 2
 Risk: The EU Future Focus for the Ethical and Social Aspects of Clinical Trials A past with a future?
Risk: The EU Future Focus for the Ethical and Social Aspects of Clinical Trials A past with a future?
 Risk-proportionality in EU CTs 1. Clinical Trial Methodology 2. Selection of Research Populations, including vulnerable groups : children, elderly, social and psychological profiles 3. Healthy Volunteers 4. The selection of control arms, including placebo or no treatment groups
Risk-proportionality in EU CTs 1. Clinical Trial Methodology 2. Selection of Research Populations, including vulnerable groups : children, elderly, social and psychological profiles 3. Healthy Volunteers 4. The selection of control arms, including placebo or no treatment groups
 The Simplification of the EU Substantial Rules for CTs The regulation of clinical trials addresses two distinct risks: the risk to subject safety and the risk to data reliability [and robustness]. The former can vary widely, depending on a range of factors, in particular: • The extent of knowledge and prior experience with the investigational medicinal product (in particular, whether or not the investigational medicinal product is authorised in the EU); and • The type of intervention (which can range from a simple blood sample to a sophisticated biopsy). [10]
The Simplification of the EU Substantial Rules for CTs The regulation of clinical trials addresses two distinct risks: the risk to subject safety and the risk to data reliability [and robustness]. The former can vary widely, depending on a range of factors, in particular: • The extent of knowledge and prior experience with the investigational medicinal product (in particular, whether or not the investigational medicinal product is authorised in the EU); and • The type of intervention (which can range from a simple blood sample to a sophisticated biopsy). [10]
 Helsinki & the Therapeutic Misconception The history of the Declaration of Helsinki is a history of therapeutic misconception. 1964 I. Basic Principles II. Clinical Research Combined with Professional Care III. Non-therapeutic Clinical Research 6
Helsinki & the Therapeutic Misconception The history of the Declaration of Helsinki is a history of therapeutic misconception. 1964 I. Basic Principles II. Clinical Research Combined with Professional Care III. Non-therapeutic Clinical Research 6
 The Core Principle ‘In medical research involving human subjects, the well-being of the individual research subject must take precedence over all other interests. ’ Declaration of Helsinki 2008, paragraph 8 7
The Core Principle ‘In medical research involving human subjects, the well-being of the individual research subject must take precedence over all other interests. ’ Declaration of Helsinki 2008, paragraph 8 7
 Principles for Those in Research and Experimentation (WMA 1954) 1. Scientific and Moral Aspects of Experimentation The word experimentation applies not only to experimentation itself but also to the experimenter. . Likewise, there must be strict adherence to the general rules of respect of the individual. 8
Principles for Those in Research and Experimentation (WMA 1954) 1. Scientific and Moral Aspects of Experimentation The word experimentation applies not only to experimentation itself but also to the experimenter. . Likewise, there must be strict adherence to the general rules of respect of the individual. 8
 Draft Code on Ethics of Human Experimentation (WMA 1962) paragraph II. 4 Controlled trials in therapeutic and preventive medicine should be conducted according to the general and special ethical rules concerning experiments on the individual. 9
Draft Code on Ethics of Human Experimentation (WMA 1962) paragraph II. 4 Controlled trials in therapeutic and preventive medicine should be conducted according to the general and special ethical rules concerning experiments on the individual. 9
 Declaration of Helsinki 1964 paragraph II. 2 The doctor can combine clinical research with professional care, the objective being the acquisition of new medical knowledge, only to the extent that clinical research is justified by its therapeutic value for the patient. 10
Declaration of Helsinki 1964 paragraph II. 2 The doctor can combine clinical research with professional care, the objective being the acquisition of new medical knowledge, only to the extent that clinical research is justified by its therapeutic value for the patient. 10
 Declaration of Helsinki 1975 -1983 paragraph II. 2 & II. 3 The potential benefits, hazards and discomfort of a new method should be weighed against the advantages of the best current diagnostic and therapeutic methods. (II. 2) In any medical study, every patient - including those of a control group, if any - should be assured of the best proven diagnostic and therapeutic method. (II. 3) 11
Declaration of Helsinki 1975 -1983 paragraph II. 2 & II. 3 The potential benefits, hazards and discomfort of a new method should be weighed against the advantages of the best current diagnostic and therapeutic methods. (II. 2) In any medical study, every patient - including those of a control group, if any - should be assured of the best proven diagnostic and therapeutic method. (II. 3) 11
 Declaration of Helsinki 1996 Paragraph II. 3 In any medical study, every patient - including those of a control group, if any - should be assured of proven effective prophylactic, diagnostic, and therapeutic methods. This does not exclude the use of inert placebo in studies where no proven diagnostic or therapeutic method exists. 12
Declaration of Helsinki 1996 Paragraph II. 3 In any medical study, every patient - including those of a control group, if any - should be assured of proven effective prophylactic, diagnostic, and therapeutic methods. This does not exclude the use of inert placebo in studies where no proven diagnostic or therapeutic method exists. 12
 Declaration of Helsinki 2000 Paragraph 29 ‘The benefits, risks, burdens and effectiveness of a new method should be tested against those of the best current prophylactic, diagnostic, and therapeutic methods. This does not exclude the use of placebo, or no treatment, in studies where no proven prophylactic, diagnostic or therapeutic method exists. ’ 13
Declaration of Helsinki 2000 Paragraph 29 ‘The benefits, risks, burdens and effectiveness of a new method should be tested against those of the best current prophylactic, diagnostic, and therapeutic methods. This does not exclude the use of placebo, or no treatment, in studies where no proven prophylactic, diagnostic or therapeutic method exists. ’ 13
 Declaration of Helsinki 2002 paragraph 29, note of clarification ‘ The WMA hereby reaffirms its position that extreme care must be taken in making use of a placebo-controlled trial and that in general this methodology should only be used in the absence of existing proven therapy. However, a placebo-controlled trial may be ethically acceptable, even if proven therapy is available, under the following circumstances: 14
Declaration of Helsinki 2002 paragraph 29, note of clarification ‘ The WMA hereby reaffirms its position that extreme care must be taken in making use of a placebo-controlled trial and that in general this methodology should only be used in the absence of existing proven therapy. However, a placebo-controlled trial may be ethically acceptable, even if proven therapy is available, under the following circumstances: 14
 Declaration of Helsinki 2002 paragraph 29, note of clarification (cont) – Where for compelling and scientifically sound methodological reasons its use is necessary to determine the efficacy or safety of a prophylactic, diagnostic or therapeutic method; or – Where a prophylactic, diagnostic or therapeutic method is being investigated for a minor condition and the patients who receive placebo will not be subject to any additional risk of serious or irreversible harm. All other provisions of the Declaration of Helsinki must be adhered to, especially the need for appropriate ethical and scientific review. 15
Declaration of Helsinki 2002 paragraph 29, note of clarification (cont) – Where for compelling and scientifically sound methodological reasons its use is necessary to determine the efficacy or safety of a prophylactic, diagnostic or therapeutic method; or – Where a prophylactic, diagnostic or therapeutic method is being investigated for a minor condition and the patients who receive placebo will not be subject to any additional risk of serious or irreversible harm. All other provisions of the Declaration of Helsinki must be adhered to, especially the need for appropriate ethical and scientific review. 15
 Declaration of Helsinki 2008 paragraph 32 ‘The benefits, risks, burdens and effectiveness of a new intervention must be tested against those of the best current proven intervention, except in the following circumstances: • The use of placebo, or no treatment, is acceptable in studies where no current proven intervention exists; or • Where for compelling and scientifically sound methodological reasons the use of placebo is necessary to determine the efficacy or safety of an intervention and the patients who receive placebo or no treatment will not be subject to any risk of serious or irreversible harm. Extreme care must be taken to avoid abuse of 16 this option. ’
Declaration of Helsinki 2008 paragraph 32 ‘The benefits, risks, burdens and effectiveness of a new intervention must be tested against those of the best current proven intervention, except in the following circumstances: • The use of placebo, or no treatment, is acceptable in studies where no current proven intervention exists; or • Where for compelling and scientifically sound methodological reasons the use of placebo is necessary to determine the efficacy or safety of an intervention and the patients who receive placebo or no treatment will not be subject to any risk of serious or irreversible harm. Extreme care must be taken to avoid abuse of 16 this option. ’
 New FDA Ruling on GCP for Foreign Clinical Studies Not Conducted Under an IND (Federal Register, 28 April 2008) essentially replacing the rule to follow the Declaration of Helsinki (1989) with Good Clinical Practice 17
New FDA Ruling on GCP for Foreign Clinical Studies Not Conducted Under an IND (Federal Register, 28 April 2008) essentially replacing the rule to follow the Declaration of Helsinki (1989) with Good Clinical Practice 17
 Criticizing the FDA Ruling ‘if the FDA jettisons Helsinki. . . it risks sending a message that ethical considerations are expendable when research subjects live half a world away’ 18
Criticizing the FDA Ruling ‘if the FDA jettisons Helsinki. . . it risks sending a message that ethical considerations are expendable when research subjects live half a world away’ 18
 ‘Helsinki discords: FDA, ethics, and international drug trials’ Kimmelman, Weijer, & Meslin, The Lancet 373, 3 Jan 2009: 13 -14. • ‘First, the Declaration of Helsinki has a moral authority that GCP lacks. The Declaration has long been recognised as a leading international ethical standard for research. ’ • ‘Second, the Declaration of Helsinki has a breadth and depth that GCP lacks. ’ • ‘Third, the FDA’s departure from the Declaration of Helsinki could undermine its stated goals of clarity 19 and regulatory harmonisation. ’
‘Helsinki discords: FDA, ethics, and international drug trials’ Kimmelman, Weijer, & Meslin, The Lancet 373, 3 Jan 2009: 13 -14. • ‘First, the Declaration of Helsinki has a moral authority that GCP lacks. The Declaration has long been recognised as a leading international ethical standard for research. ’ • ‘Second, the Declaration of Helsinki has a breadth and depth that GCP lacks. ’ • ‘Third, the FDA’s departure from the Declaration of Helsinki could undermine its stated goals of clarity 19 and regulatory harmonisation. ’
 Workgroup Report on Placebo Control in Clinical Trials Main Results of the Conference in Sao Paulo (1 -3 February 2010) • Report Accepted by the WMA Council on 20 -22 May 2010 • ‘However, there is no urgent need to change the central ethical position of the Declaration of Helsinki in terms of placebo control. The current version of the Declaration comes close to an acceptable middle ground. A careful evolution is worth considering; however a totally new approach is not needed. ’ • ‘Looks that we are already on count down for the next revision. ’ (O. Kloiber to FP Crawley, email, 13 July 2010) 20
Workgroup Report on Placebo Control in Clinical Trials Main Results of the Conference in Sao Paulo (1 -3 February 2010) • Report Accepted by the WMA Council on 20 -22 May 2010 • ‘However, there is no urgent need to change the central ethical position of the Declaration of Helsinki in terms of placebo control. The current version of the Declaration comes close to an acceptable middle ground. A careful evolution is worth considering; however a totally new approach is not needed. ’ • ‘Looks that we are already on count down for the next revision. ’ (O. Kloiber to FP Crawley, email, 13 July 2010) 20
 A Suggestion from the EU on the Declaration of Helsinki 2013 The design and performance of the research should be appropriate to the health needs and expectations of the (potential) research participant(s).
A Suggestion from the EU on the Declaration of Helsinki 2013 The design and performance of the research should be appropriate to the health needs and expectations of the (potential) research participant(s).
 The Future Assessment of EU CT Applications • ‘The assessment of the application for a clinical trial should address in particular the anticipated therapeutic and public health benefits ('relevance') and the risk and inconveniences for the subject. ’ (10) • ‘It should be left to the Member State concerned to determine the appropriate body or bodies to be involved in this assessment. ’ (14)
The Future Assessment of EU CT Applications • ‘The assessment of the application for a clinical trial should address in particular the anticipated therapeutic and public health benefits ('relevance') and the risk and inconveniences for the subject. ’ (10) • ‘It should be left to the Member State concerned to determine the appropriate body or bodies to be involved in this assessment. ’ (14)
 The challenge to future European clinical trials is to address risk in methodological area of clinical trials as well as in the ethical and social aspects. fpc@gcpalliance. org
The challenge to future European clinical trials is to address risk in methodological area of clinical trials as well as in the ethical and social aspects. fpc@gcpalliance. org


