fe5ecdec85f9b95d53ada16606fc7c33.ppt
- Количество слайдов: 45

The greenhouse effect, global warming and ozone depletion : facts and myths [or the agnostics views of a chemical physicist !] Professor Richard Tuckett (School of Chemistry) r. p. tuckett@bham. ac. uk RSC West Midlands Chem. Net, 12 th December 2006 Specialist details in : Trifluoromethyl sulphur pentafluoride CF 3 SF 5 : the ultimate greenhouse gas, and its contribution to global warming [Adv. Fluorine Science (Elsevier) 1 (2006) chapter 3] [Thanks to Dr Peter Barnes (Warwick University) for technical help]

Myths of atmospheric science ? ‘The greenhouse effect and ozone depletion have the same scientific causes’ (John Selwyn Gummer, 1994 – Today programme) ‘The Greenhouse Effect is all bad news ; detrimental to life on earth’ Facts ? The ozone depletion issue is slowly getting better. The stratosphere may recover within 50 -100 years. The science is well understood. The planet is warming up. The chemistry of the troposphere, where greenhouse warming occurs, is poorly understood. Opinions ? ‘Global warming is not occurring. Even if it is, it is unrelated to man’s activities on earth’ (George Bush, 2001 -2004, 2006 ? ) ‘Global warming is the most serious phenomenon affecting the world’s security and prosperity, more so than terrorism’ (David King, UK Government Chief Scientific Adviser, 2003) Scientists ? ‘Global warming is not due to man’s activities since the Industrial Revolution, but to a ‘natural’ cycle of ice ages with warm periods in between’. Most scientists from chemistry and physics backgrounds disagree. Some geologists / geographers see the situation differently.
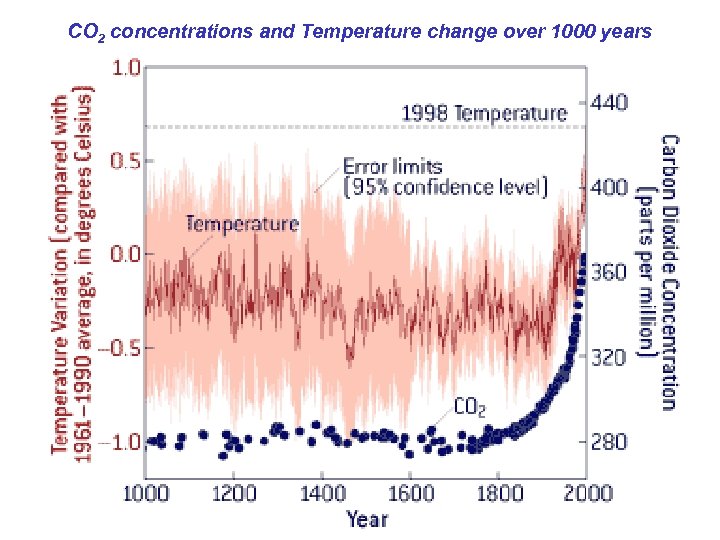
CO 2 concentrations and Temperature change over 1000 years
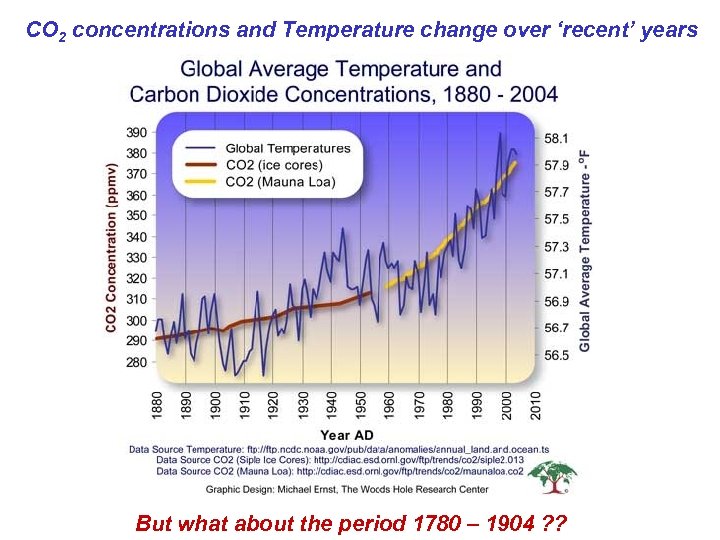
CO 2 concentrations and Temperature change over ‘recent’ years But what about the period 1780 – 1904 ? ?
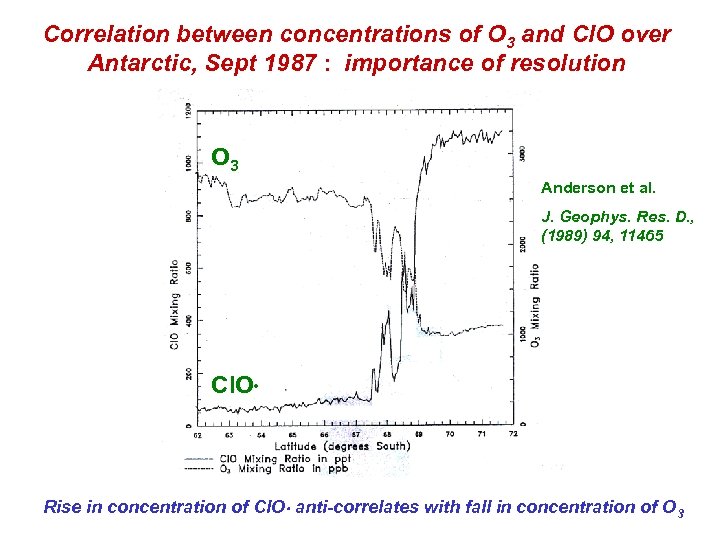
Correlation between concentrations of O 3 and Cl. O over Antarctic, Sept 1987 : importance of resolution O 3 Anderson et al. J. Geophys. Res. D. , (1989) 94, 11465 Cl. O Rise in concentration of Cl. O anti-correlates with fall in concentration of O 3
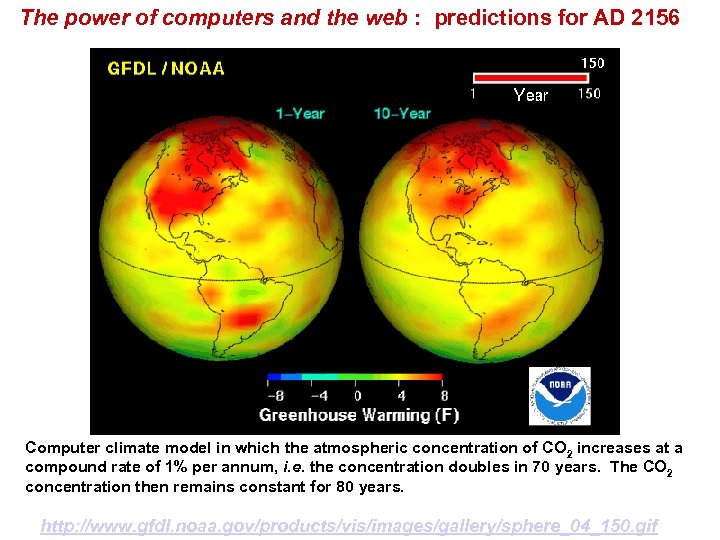
The power of computers and the web : predictions for AD 2156 Computer climate model in which the atmospheric concentration of CO 2 increases at a compound rate of 1% per annum, i. e. the concentration doubles in 70 years. The CO 2 concentration then remains constant for 80 years. http: //www. gfdl. noaa. gov/products/vis/images/gallery/sphere_04_150. gif
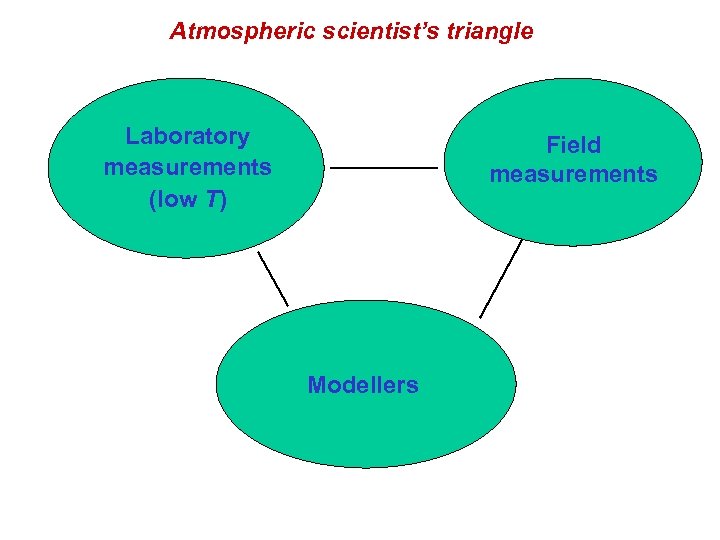
Atmospheric scientist’s triangle Laboratory measurements (low T) Field measurements Modellers
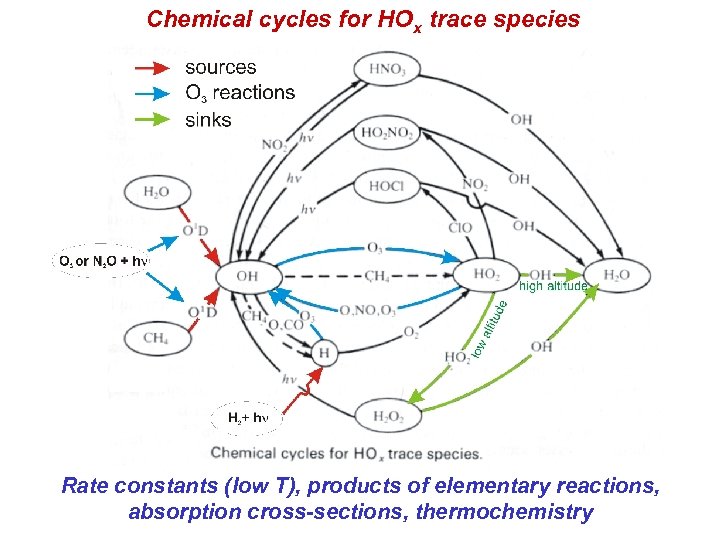
Chemical cycles for HOx trace species Rate constants (low T), products of elementary reactions, absorption cross-sections, thermochemistry
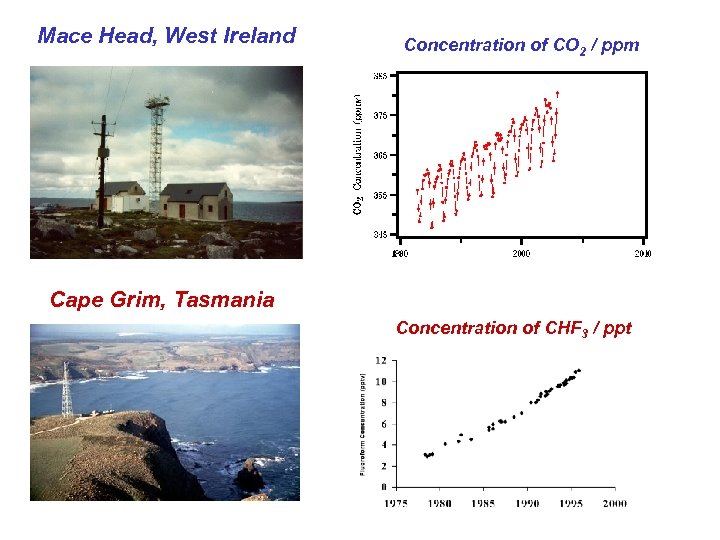
Mace Head, West Ireland Concentration of CO 2 / ppm Cape Grim, Tasmania Concentration of CHF 3 / ppt
TOMS (Total ozone monitoring spectrometer) NASA
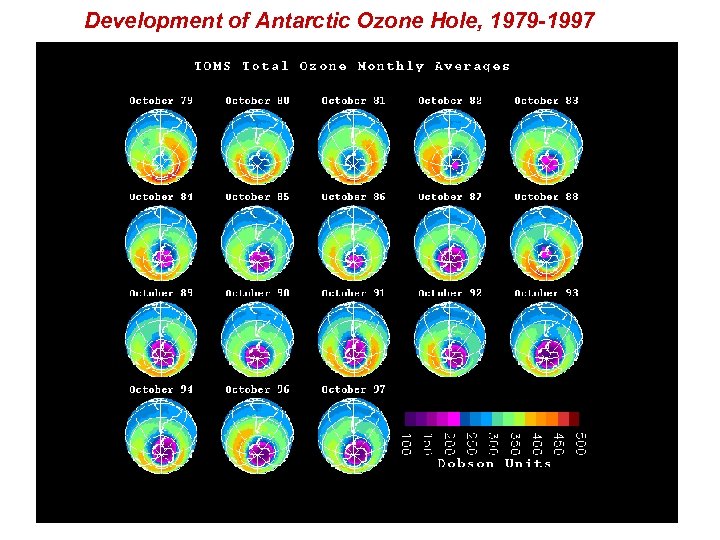
Development of Antarctic Ozone Hole, 1979 -1997
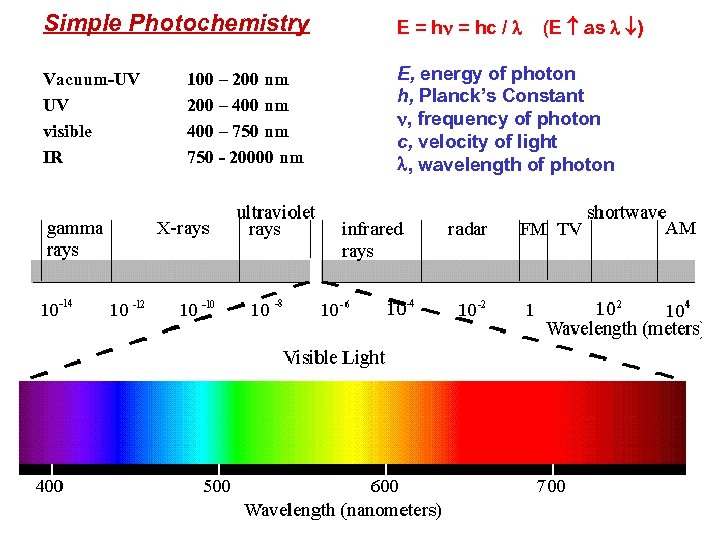
Simple Photochemistry E = hn = hc / l (E as l ) Vacuum-UV UV visible IR E, energy of photon h, Planck’s Constant n, frequency of photon c, velocity of light l, wavelength of photon 100 – 200 nm 200 – 400 nm 400 – 750 nm 750 - 20000 nm
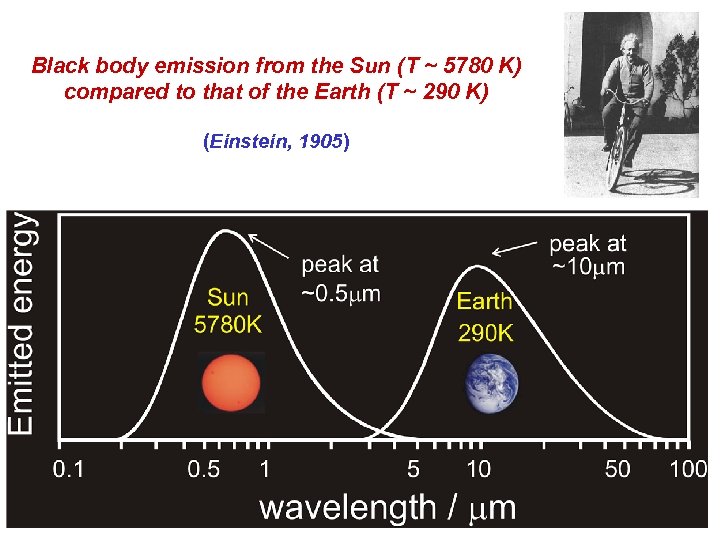
Black body emission from the Sun (T ~ 5780 K) compared to that of the Earth (T ~ 290 K) (Einstein, 1905)
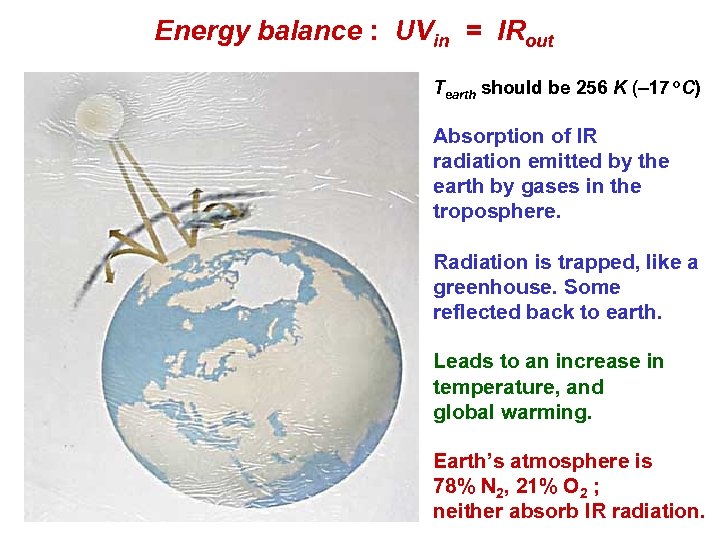
Energy balance : UVin = IRout Tearth should be 256 K ( 17 o. C) Absorption of IR radiation emitted by the earth by gases in the troposphere. Radiation is trapped, like a greenhouse. Some reflected back to earth. Leads to an increase in temperature, and global warming. Earth’s atmosphere is 78% N 2, 21% O 2 ; neither absorb IR radiation.
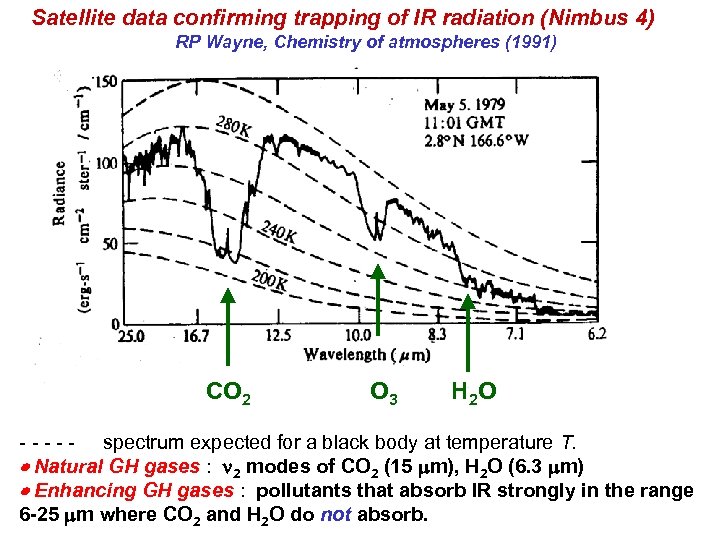
Satellite data confirming trapping of IR radiation (Nimbus 4) RP Wayne, Chemistry of atmospheres (1991) CO 2 O 3 H 2 O - - - spectrum expected for a black body at temperature T. Natural GH gases : n 2 modes of CO 2 (15 mm), H 2 O (6. 3 mm) Enhancing GH gases : pollutants that absorb IR strongly in the range 6 -25 mm where CO 2 and H 2 O do not absorb.
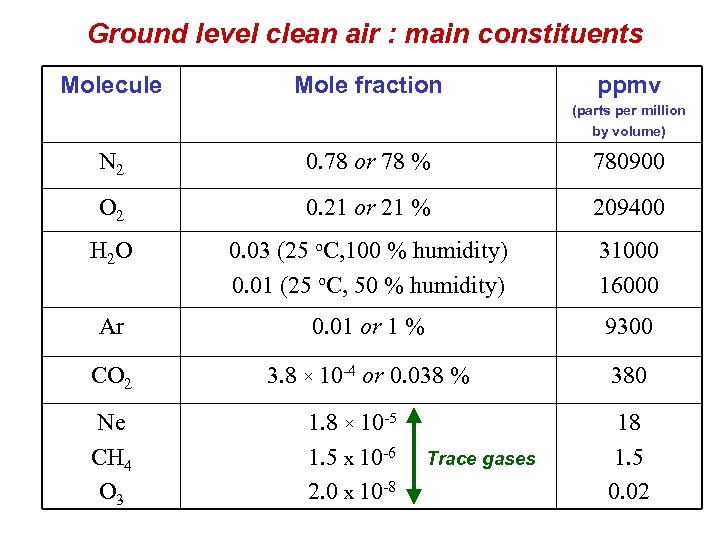
Ground level clean air : main constituents Molecule Mole fraction ppmv (parts per million by volume) N 2 0. 78 or 78 % 780900 O 2 0. 21 or 21 % 209400 H 2 O 0. 03 (25 o. C, 100 % humidity) 0. 01 (25 o. C, 50 % humidity) 31000 16000 Ar 0. 01 or 1 % 9300 CO 2 3. 8 × 10 -4 or 0. 038 % 380 Ne CH 4 O 3 1. 8 × 10 -5 1. 5 x 10 -6 Trace gases 2. 0 x 10 -8 18 1. 5 0. 02
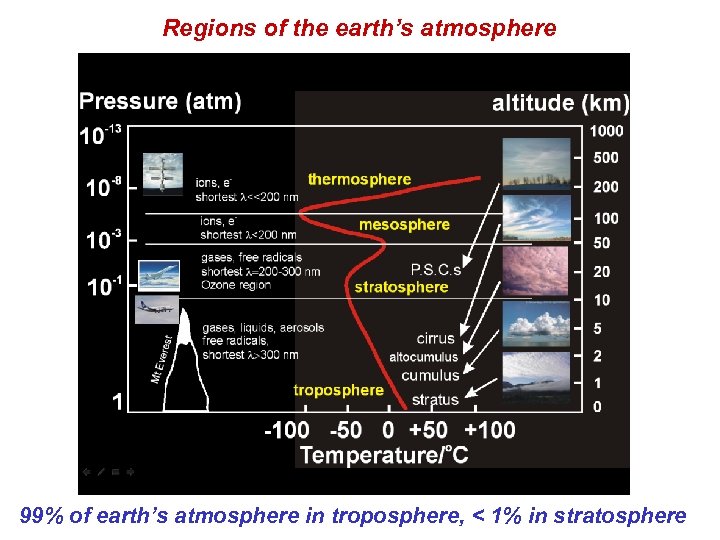
Regions of the earth’s atmosphere 99% of earth’s atmosphere in troposphere, < 1% in stratosphere
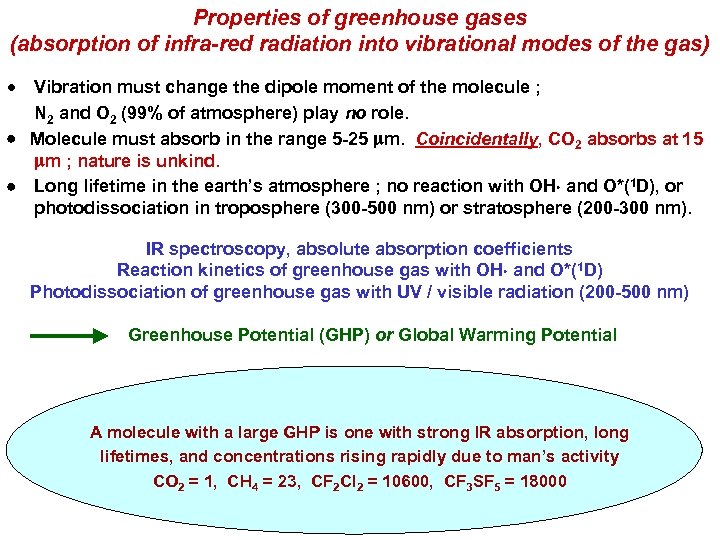
Properties of greenhouse gases (absorption of infra-red radiation into vibrational modes of the gas) Vibration must change the dipole moment of the molecule ; N 2 and O 2 (99% of atmosphere) play no role. Molecule must absorb in the range 5 -25 mm. Coincidentally, CO 2 absorbs at 15 mm ; nature is unkind. Long lifetime in the earth’s atmosphere ; no reaction with OH and O*(1 D), or photodissociation in troposphere (300 -500 nm) or stratosphere (200 -300 nm). IR spectroscopy, absolute absorption coefficients Reaction kinetics of greenhouse gas with OH and O*(1 D) Photodissociation of greenhouse gas with UV / visible radiation (200 -500 nm) Greenhouse Potential (GHP) or Global Warming Potential A molecule with a large GHP is one with strong IR absorption, long lifetimes, and concentrations rising rapidly due to man’s activity CO 2 = 1, CH 4 = 23, CF 2 Cl 2 = 10600, CF 3 SF 5 = 18000
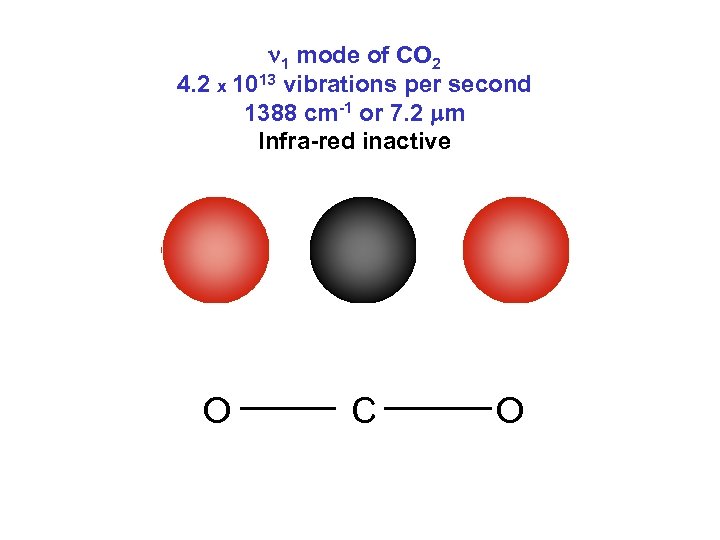
n 1 mode of CO 2 4. 2 x 1013 vibrations per second 1388 cm-1 or 7. 2 mm Infra-red inactive O C O
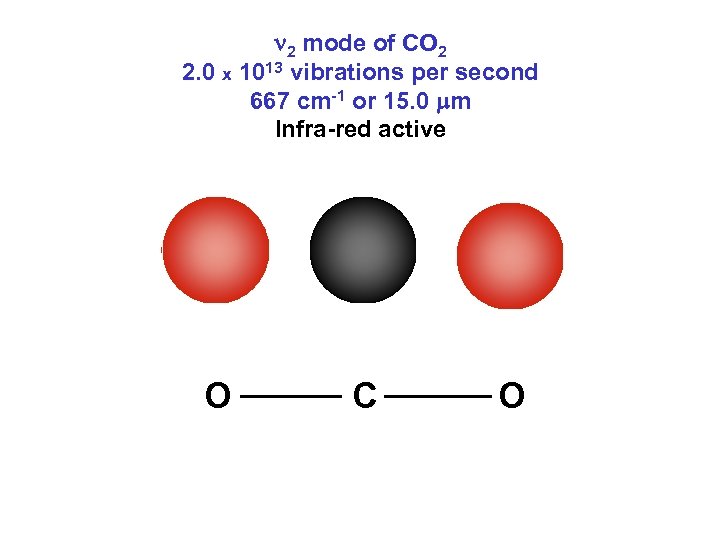
n 2 mode of CO 2 2. 0 x 1013 vibrations per second 667 cm-1 or 15. 0 mm Infra-red active O C O
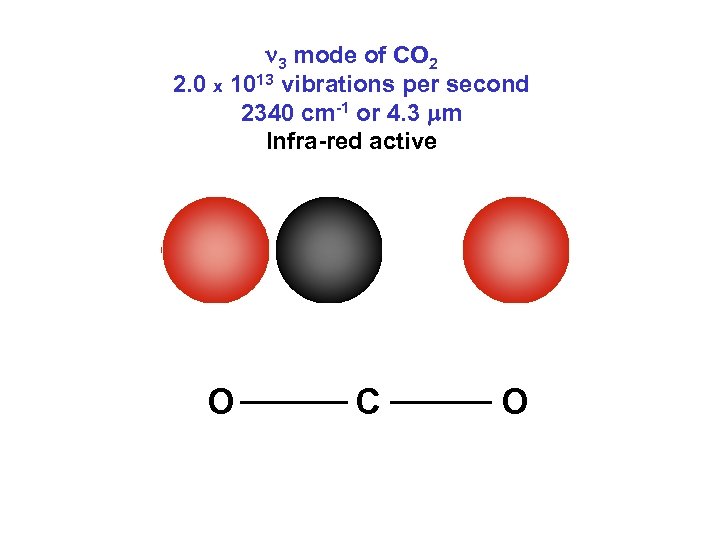
n 3 mode of CO 2 2. 0 x 1013 vibrations per second 2340 cm-1 or 4. 3 mm Infra-red active O C O
![Examples of greenhouse gases, and their importance to global warming [IPCC 2001] _____________________________________ Greenhouse Examples of greenhouse gases, and their importance to global warming [IPCC 2001] _____________________________________ Greenhouse](https://present5.com/presentation/fe5ecdec85f9b95d53ada16606fc7c33/image-22.jpg)
Examples of greenhouse gases, and their importance to global warming [IPCC 2001] _____________________________________ Greenhouse gas CO 2 CH 4 CF 2 Cl 2 CF 3 SF 5 Concentration / ppm 380 1. 75 0. 0003 < 10 6 DConcn / % per year 0. 45 0. 60 ca. 5 ca. 6 Microscopic radiative 1. 68 x 10 5 4. 59 x 10 4 0. 32 forcing / W m-2 ppb-1 0. 60 Total radiative forcing / W m-2 1. 46 7. 2 x 10 5 Lifetime / years 50 -200 12 _____________________________________ 0. 48 0. 16 100 ca. 1000 18000 GHP (100 year projection) 1 23 10600 Contribn to GH effect / % 52 17 ca. 6 « 0. 1 ______________________________________
![CF 3 SF 5 : atmospheric background Sturges et al. [Science (2000) 289, 611] CF 3 SF 5 : atmospheric background Sturges et al. [Science (2000) 289, 611]](https://present5.com/presentation/fe5ecdec85f9b95d53ada16606fc7c33/image-23.jpg)
CF 3 SF 5 : atmospheric background Sturges et al. [Science (2000) 289, 611] report observation of CF 3 SF 5 in the Antarctic from ice firn data. Anthropogenic. Highest radiative forcing per molecule of any greenhouse gas. Current concentrations are low (0. 12 pptv), but growing at 6% per annum. Stratospheric profiles suggest it is long-lived. The value of the CF 3 SF 5 bond strength is important to determine if this greenhouse gas can be photolysed in the stratosphere. or is the sink route determined by ionic processes in the mesosphere ? Measure Dr. Ho 0 (CF 3 SF 5 CF 3+ + SF 5+ e ) as a route to determine the C S bond strength. DIE (CF 3 SF 5) = Do 0(CF 3 SF 5) + Adiabatic IE (CF 3)
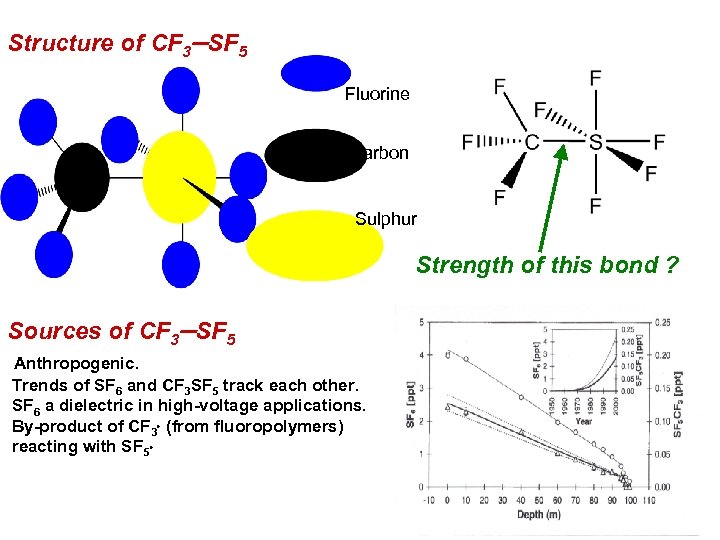
Structure of CF 3 -SF 5 Fluorine Carbon Sulphur Strength of this bond ? Sources of CF 3 -SF 5 Anthropogenic. Trends of SF 6 and CF 3 SF 5 track each other. SF 6 a dielectric in high-voltage applications. By-product of CF 3 (from fluoropolymers) reacting with SF 5
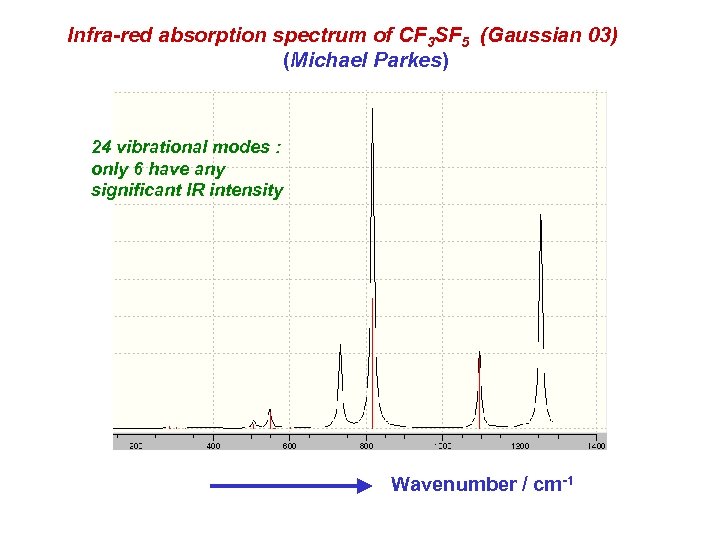
Infra-red absorption spectrum of CF 3 SF 5 (Gaussian 03) (Michael Parkes) 24 vibrational modes : only 6 have any significant IR intensity Wavenumber / cm-1
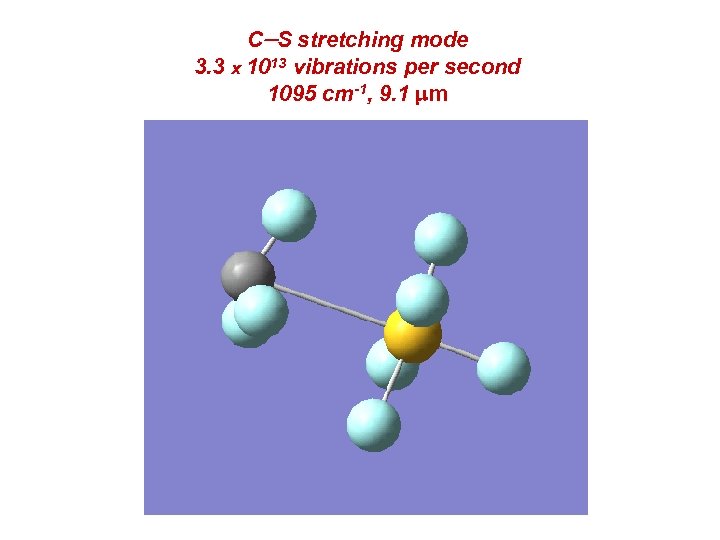
C-S stretching mode 3. 3 x 1013 vibrations per second 1095 cm-1, 9. 1 mm
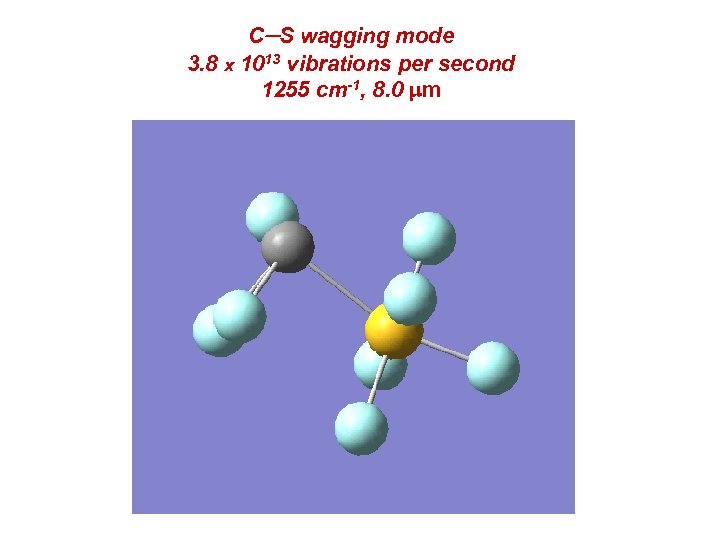
C-S wagging mode 3. 8 x 1013 vibrations per second 1255 cm-1, 8. 0 mm
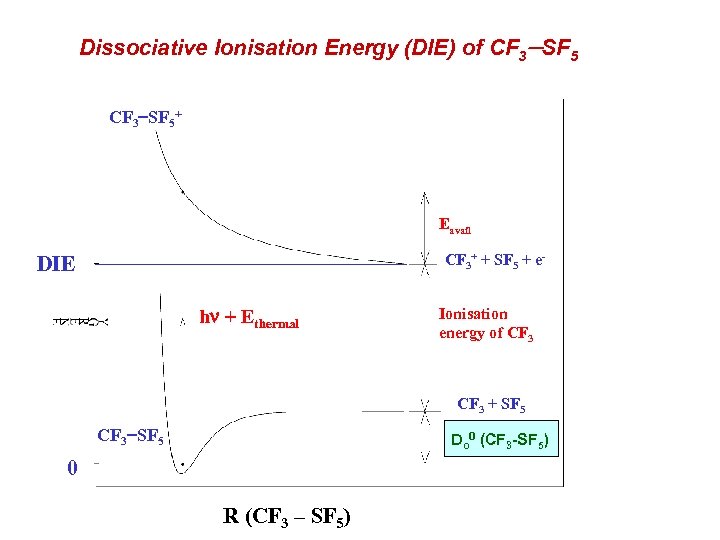
Dissociative Ionisation Energy (DIE) of CF 3 -SF 5 CF 3 SF 5+ Eavail CF 3+ + SF 5 + e- DIE hn + Ethermal Ionisation energy of CF 3 + SF 5 CF 3 SF 5 Do 0 (CF 3 -SF 5) 0 R (CF 3 – SF 5)

Daresbury Synchrotron Radiation Source, Cheshire
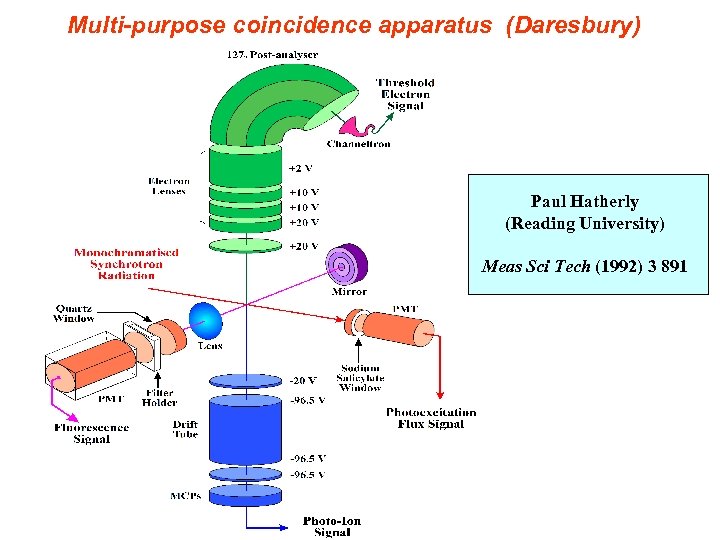
Multi-purpose coincidence apparatus (Daresbury) Paul Hatherly (Reading University) Meas Sci Tech (1992) 3 891
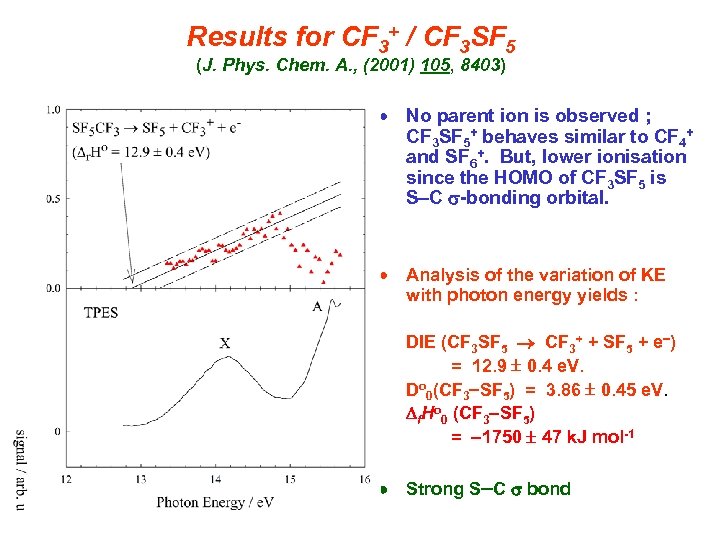
Results for CF 3+ / CF 3 SF 5 (J. Phys. Chem. A. , (2001) 105, 8403) No parent ion is observed ; CF 3 SF 5+ behaves similar to CF 4+ and SF 6+. But, lower ionisation since the HOMO of CF 3 SF 5 is S C s-bonding orbital. Analysis of the variation of KE with photon energy yields : DIE (CF 3 SF 5 CF 3+ + SF 5 + e ) = 12. 9 0. 4 e. V. Do 0(CF 3 SF 5) = 3. 86 0. 45 e. V. Df. Ho 0 (CF 3 SF 5) = 1750 47 k. J mol-1 Strong S C s bond
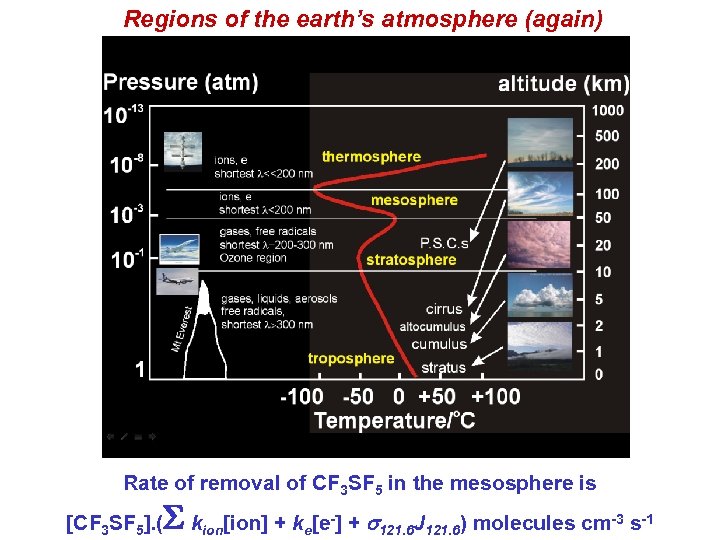
Regions of the earth’s atmosphere (again) Rate of removal of CF 3 SF 5 in the mesosphere is [CF 3 SF 5]. ( S k ion[ion] + ke[e -] + s 121. 6 J 121. 6) molecules cm-3 s-1
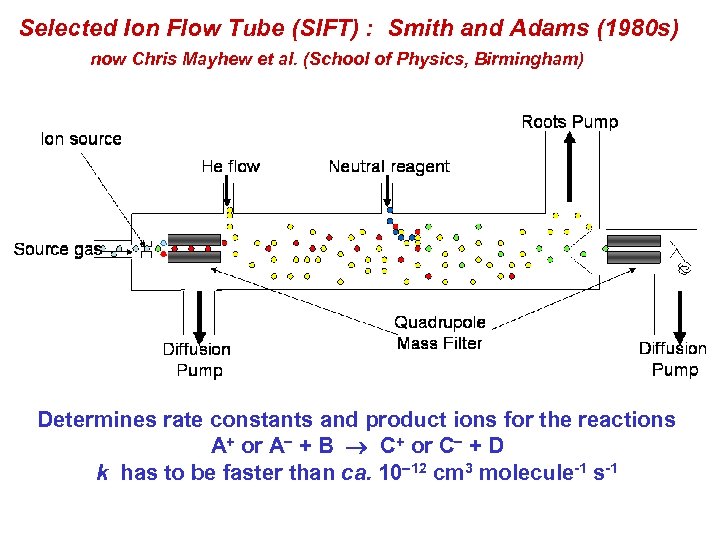
Selected Ion Flow Tube (SIFT) : Smith and Adams (1980 s) now Chris Mayhew et al. (School of Physics, Birmingham) Determines rate constants and product ions for the reactions A+ or A + B C+ or C + D k has to be faster than ca. 10 12 cm 3 molecule-1 s-1
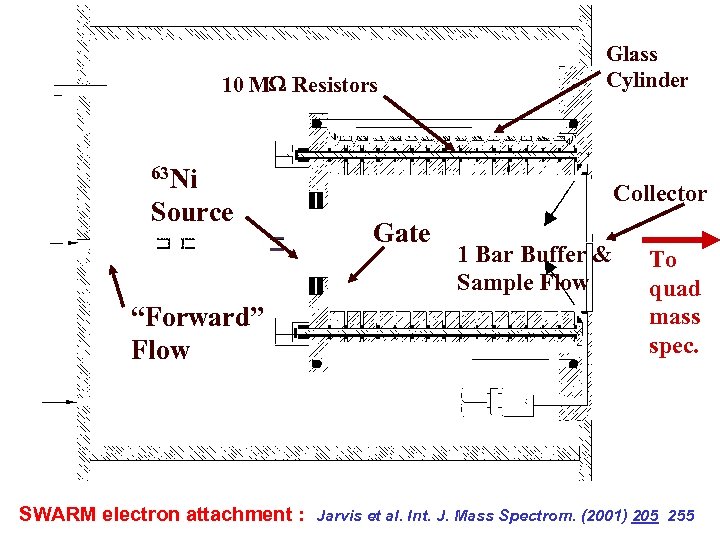
10 M Resistors Glass Cylinder 63 Ni Source “Forward” Flow SWARM electron attachment : Collector Gate 1 Bar Buffer & Sample Flow To quad mass spec. Jarvis et al. Int. J. Mass Spectrom. (2001) 205 255
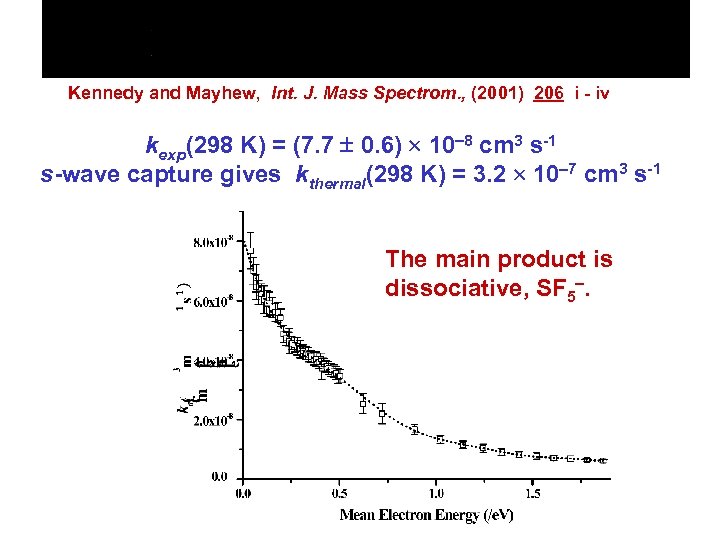
Kennedy and Mayhew, Int. J. Mass Spectrom. , (2001) 206 i - iv kexp(298 K) = (7. 7 0. 6) 10 8 cm 3 s-1 s-wave capture gives kthermal(298 K) = 3. 2 10 7 cm 3 s-1 The main product is dissociative, SF 5.
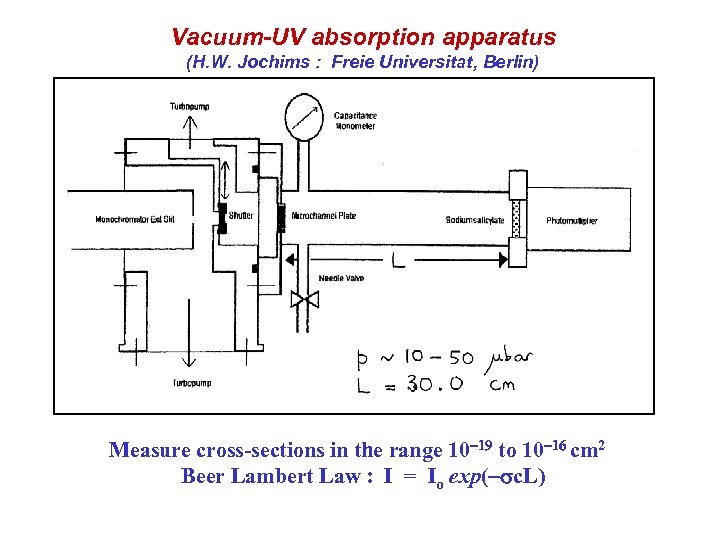
Vacuum-UV absorption apparatus (H. W. Jochims : Freie Universitat, Berlin) Measure cross-sections in the range 10 19 to 10 16 cm 2 Beer Lambert Law : I = Io exp( sc. L)
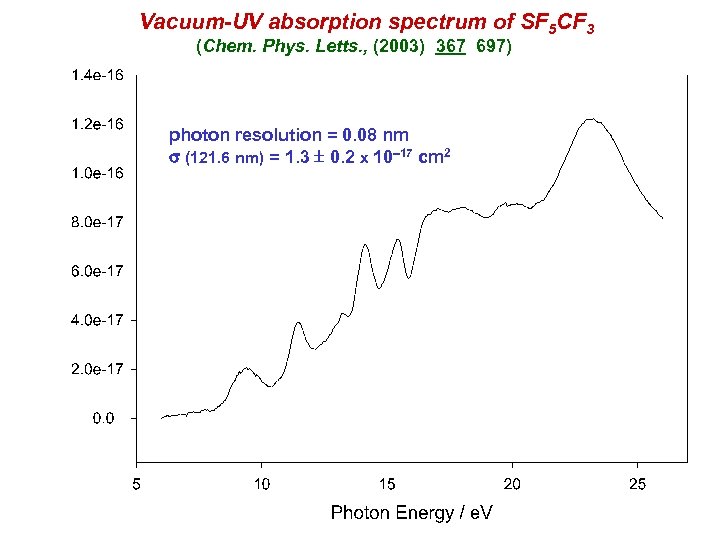
Vacuum-UV absorption spectrum of SF 5 CF 3 (Chem. Phys. Letts. , (2003) 367 697) photon resolution = 0. 08 nm s (121. 6 nm) = 1. 3 0. 2 x 10 17 cm 2
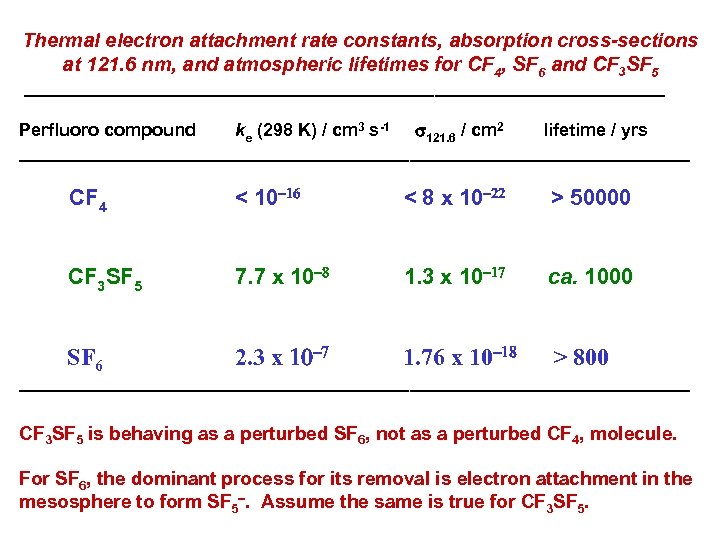
Thermal electron attachment rate constants, absorption cross-sections at 121. 6 nm, and atmospheric lifetimes for CF 4, SF 6 and CF 3 SF 5 ________________________________ Perfluoro compound ke (298 K) / cm 3 s-1 s 121. 6 / cm 2 lifetime / yrs __________________________________ CF 4 < 10 16 < 8 x 10 22 > 50000 CF 3 SF 5 7. 7 x 10 8 SF 6 2. 3 x 10 7 1. 3 x 10 17 ca. 1000 1. 76 x 10 18 > 800 __________________________________ CF 3 SF 5 is behaving as a perturbed SF 6, not as a perturbed CF 4, molecule. For SF 6, the dominant process for its removal is electron attachment in the mesosphere to form SF 5. Assume the same is true for CF 3 SF 5.

Ravishankara and Lovejoy, JCS Faraday Trans. , (1994) 90, 2159 [written six years before the CF 3 SF 5 story began] ‘When CFCs were invented and released into the atmosphere, their deleterious effects were not known. Fortunately, CFCs are relatively short-lived (ca. 100 years) compared to perfluorocarbons, Cx. Fy (ca. 1000 years) ; it will take only about a century for CFCs to be removed from the atmosphere once their emissions are curtailed. The release of any very long-lived species into the atmosphere should be viewed with great concern. PFC (and CF 3 SF 5) lifetimes, though long on historical timescales, are short compared to evolutionary timescales. Life on Earth may not be able to adopt to the changes these emissions may cause. Thus, it seems prudent to ask if a long-lived molecule should be considered ‘guilty’, unless proven otherwise. ’ or my view : Don’t put a long-lived pollutant up into the atmosphere in the first place. Attack problem at source.

Influence of enviromental issues on UK policy ? Energy, nuclear (already happened) Transport, obvious (within 1 -2 years) Individual carbon allowances (coming soon) Retail, Sunday trading (? ) On international policy ? Carbon trading, morality ? Population, realistic ? But time is short : 10 -20 years only The stratospheric ozone ‘story’ may give us optimism
![The Ozone story : distribution in the stratosphere before ca. 1960 [O 3]max = The Ozone story : distribution in the stratosphere before ca. 1960 [O 3]max =](https://present5.com/presentation/fe5ecdec85f9b95d53ada16606fc7c33/image-41.jpg)
The Ozone story : distribution in the stratosphere before ca. 1960 [O 3]max = 4× 1012 cm-3 at 30 km
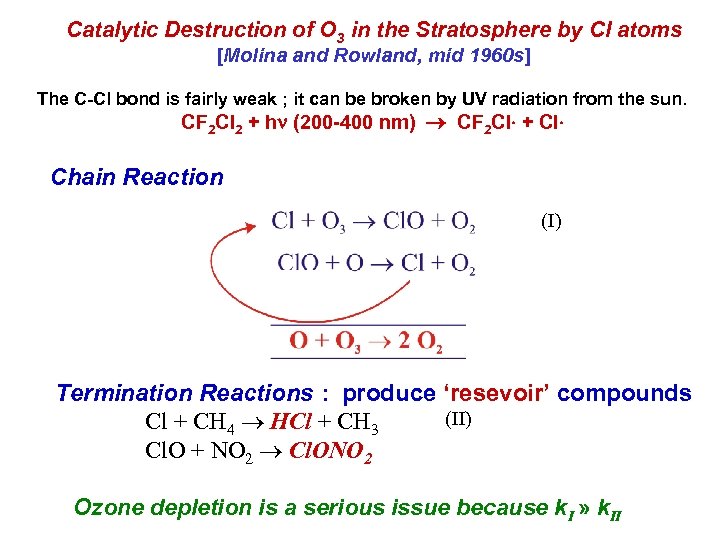
Catalytic Destruction of O 3 in the Stratosphere by Cl atoms [Molina and Rowland, mid 1960 s] The C-Cl bond is fairly weak ; it can be broken by UV radiation from the sun. CF 2 Cl 2 + hn (200 -400 nm) CF 2 Cl + Cl Chain Reaction (I) Termination Reactions : produce ‘resevoir’ compounds (II) Cl + CH 4 HCl + CH 3 Cl. O + NO 2 Cl. ONO 2 Ozone depletion is a serious issue because k. I » k. II
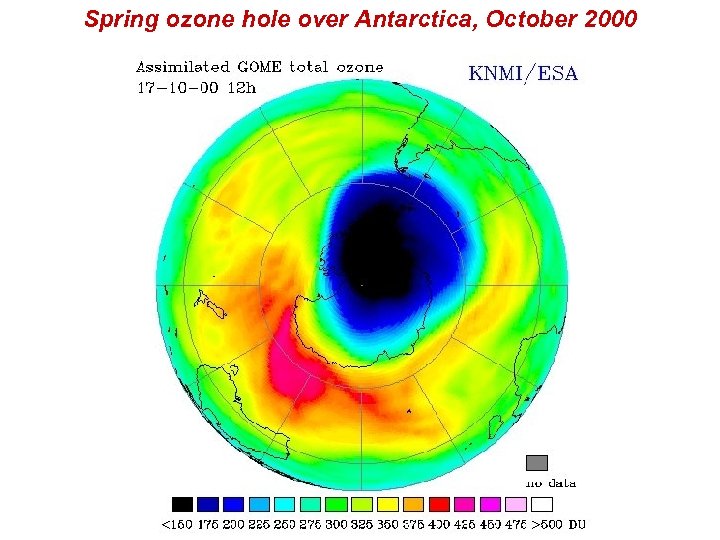
Spring ozone hole over Antarctica, October 2000
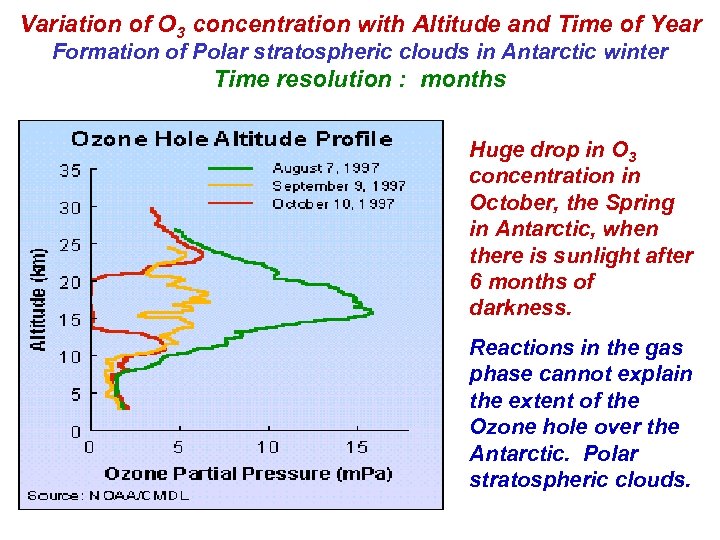
Variation of O 3 concentration with Altitude and Time of Year Formation of Polar stratospheric clouds in Antarctic winter Time resolution : months Huge drop in O 3 concentration in October, the Spring in Antarctic, when there is sunlight after 6 months of darkness. Reactions in the gas phase cannot explain the extent of the Ozone hole over the Antarctic. Polar stratospheric clouds.

Success story for atmospheric scientists ? 1930 s Large-scale production of chlorofluorocarbons begins 1964 First prediction by Molina and Rowland of O 3 destruction 1980 First observation of ozone hole in Antarctica 1987 36 nations sign Montreal Protocol 1988 Du Pont stop production of CFCs 1992 93 nations sign Copenhagen Protocol 2006 First reports that ozone hole may be recovering ; timescale 50 100 years (e. g. Nature (2006) 441, 39 -45) ***************************************** Cause for pessimism : CFCs do not affect the ‘standard’ of most peoples’ lives. Reduction in CO 2 and CH 4 concentrations may.
fe5ecdec85f9b95d53ada16606fc7c33.ppt