f48b3c84b71843100e18a107113eb3c8.ppt
- Количество слайдов: 100
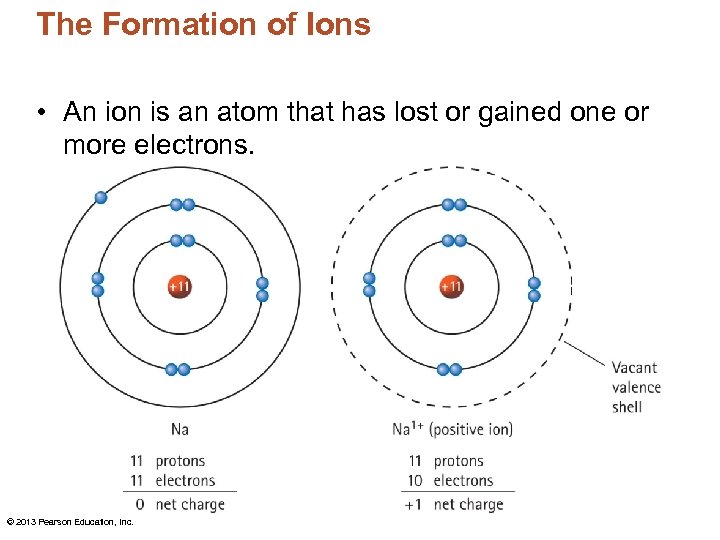 The Formation of Ions • An ion is an atom that has lost or gained one or more electrons. © 2013 Pearson Education, Inc.
The Formation of Ions • An ion is an atom that has lost or gained one or more electrons. © 2013 Pearson Education, Inc.
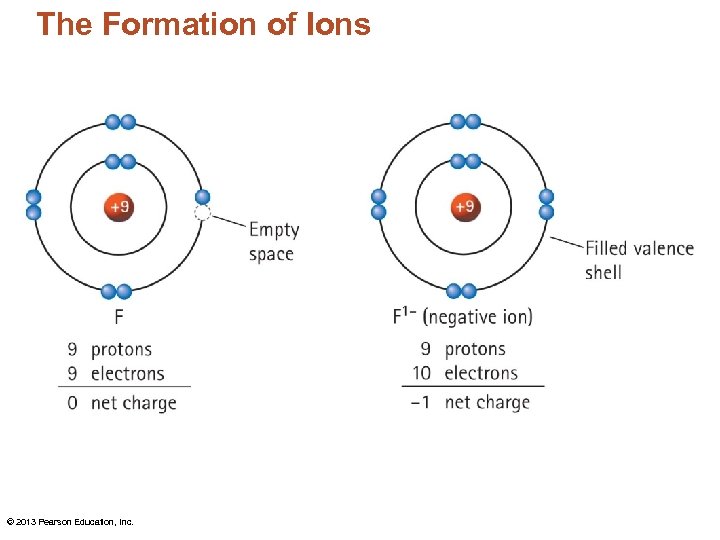 The Formation of Ions © 2013 Pearson Education, Inc.
The Formation of Ions © 2013 Pearson Education, Inc.
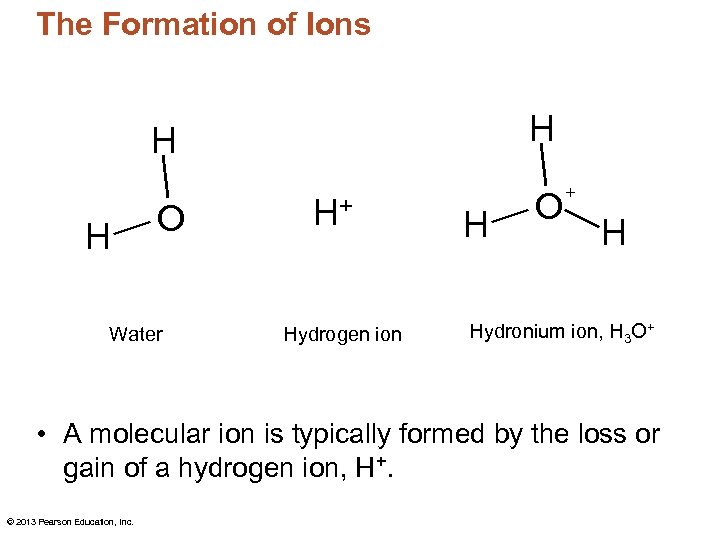 The Formation of Ions H H H O Water H+ Hydrogen ion H O + H Hydronium ion, H 3 O+ • A molecular ion is typically formed by the loss or gain of a hydrogen ion, H+. © 2013 Pearson Education, Inc.
The Formation of Ions H H H O Water H+ Hydrogen ion H O + H Hydronium ion, H 3 O+ • A molecular ion is typically formed by the loss or gain of a hydrogen ion, H+. © 2013 Pearson Education, Inc.
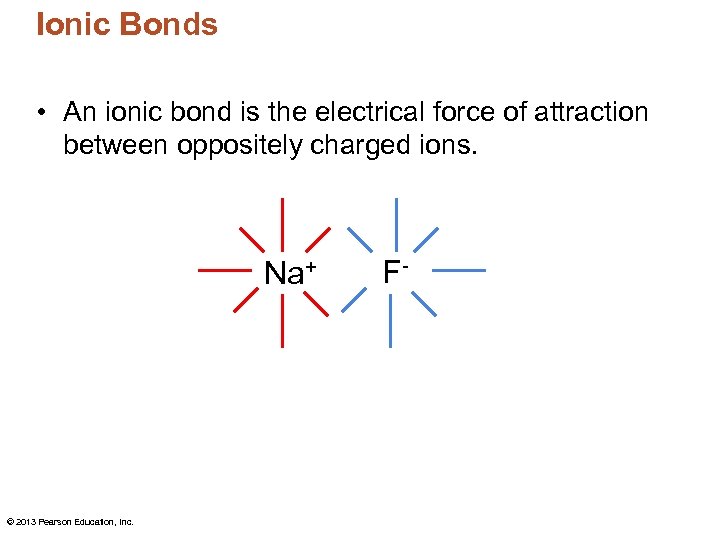 Ionic Bonds • An ionic bond is the electrical force of attraction between oppositely charged ions. Na+ © 2013 Pearson Education, Inc. F-
Ionic Bonds • An ionic bond is the electrical force of attraction between oppositely charged ions. Na+ © 2013 Pearson Education, Inc. F-
 Ionic Bonding • Used to show ration of ions. – Uses element symbols to indicate atoms present in the compound and subscripts to indicate ratios of atoms. • Example: Aluminum oxide Al 2 O 3 – 2 aluminum ions for every 3 oxide ions. • Example: Lithium oxide Li 2 O – 2 lithium ions for every oxide ion. – If no subscript 1 is assumed. • Not all compounds found in 1: 1 ration. • Ratio depends on the charges of the ions. – The compound has to be neutral.
Ionic Bonding • Used to show ration of ions. – Uses element symbols to indicate atoms present in the compound and subscripts to indicate ratios of atoms. • Example: Aluminum oxide Al 2 O 3 – 2 aluminum ions for every 3 oxide ions. • Example: Lithium oxide Li 2 O – 2 lithium ions for every oxide ion. – If no subscript 1 is assumed. • Not all compounds found in 1: 1 ration. • Ratio depends on the charges of the ions. – The compound has to be neutral.
 Ionic Bonding • Example: – Calcium forms ions with: • 2+ charge – Fluorine forms ions with: • 1 - charge • You need two fluorine atoms for every one calcium atom to get a neutral compound. – Ca. F 2
Ionic Bonding • Example: – Calcium forms ions with: • 2+ charge – Fluorine forms ions with: • 1 - charge • You need two fluorine atoms for every one calcium atom to get a neutral compound. – Ca. F 2
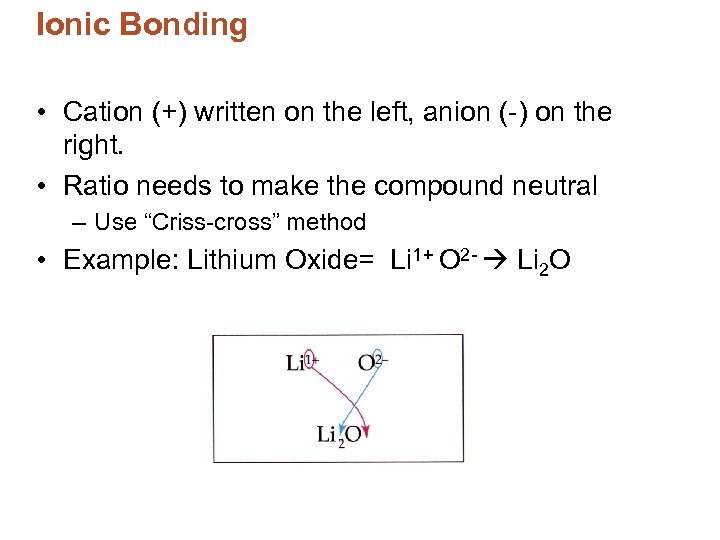 Ionic Bonding • Cation (+) written on the left, anion (-) on the right. • Ratio needs to make the compound neutral – Use “Criss-cross” method • Example: Lithium Oxide= Li 1+ O 2 - Li 2 O
Ionic Bonding • Cation (+) written on the left, anion (-) on the right. • Ratio needs to make the compound neutral – Use “Criss-cross” method • Example: Lithium Oxide= Li 1+ O 2 - Li 2 O
 Ionic Bonding • If possible the formula should be simplified to show the smallest whole-number ratio of the ions: – Mg 2+ + O 2 - Mg 2 O 2 Mg. O • For compounds with polyatomic ion, the polyatomic ion should be placed in parenthesis: – Ca 2+ + NO 3 - Ca(NO 3)2
Ionic Bonding • If possible the formula should be simplified to show the smallest whole-number ratio of the ions: – Mg 2+ + O 2 - Mg 2 O 2 Mg. O • For compounds with polyatomic ion, the polyatomic ion should be placed in parenthesis: – Ca 2+ + NO 3 - Ca(NO 3)2
 © 2013 Pearson Education, Inc.
© 2013 Pearson Education, Inc.
 Sample Problems • Write the formulas for: Sodium Bromide Magnesium Phosphate Potassium Iodide
Sample Problems • Write the formulas for: Sodium Bromide Magnesium Phosphate Potassium Iodide
 Ionic Bonds CHECK YOUR NEIGHBOR What is the chemical formula for a compound made of aluminum ions, Al 3+, and oxygen ions, O 2–? A. B. C. D. Al. O Al 3 O 2 Al 2 O 3 Al 6 O 6 Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
Ionic Bonds CHECK YOUR NEIGHBOR What is the chemical formula for a compound made of aluminum ions, Al 3+, and oxygen ions, O 2–? A. B. C. D. Al. O Al 3 O 2 Al 2 O 3 Al 6 O 6 Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
 Ionic Bonds CHECK YOUR NEIGHBOR What is the chemical formula for a compound made of magnesium ions, Mg 2+, and oxygen ions, O 2–? A. B. C. D. Mg. O Mg 2 O 2 Mg 4 O 4 any of the above Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
Ionic Bonds CHECK YOUR NEIGHBOR What is the chemical formula for a compound made of magnesium ions, Mg 2+, and oxygen ions, O 2–? A. B. C. D. Mg. O Mg 2 O 2 Mg 4 O 4 any of the above Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
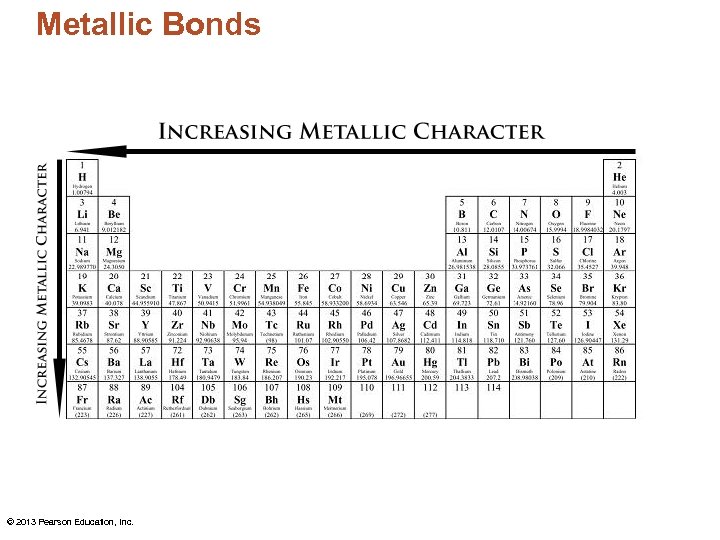
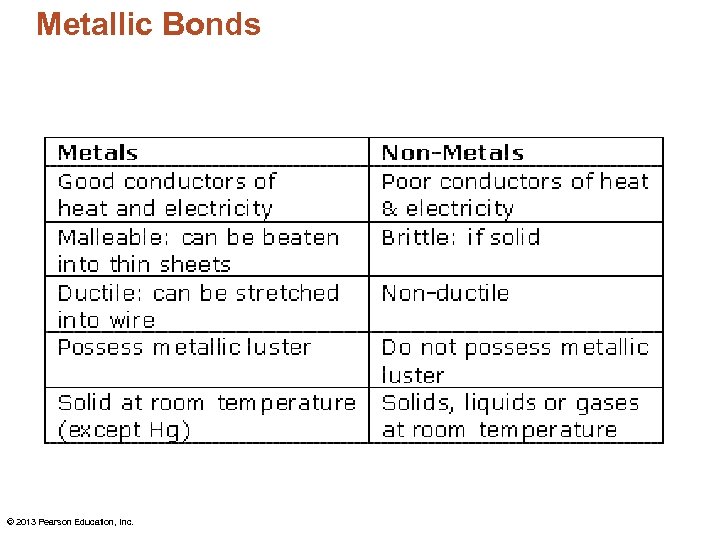
 Metallic Bonds • Outer electrons in metal atoms are held only weakly by the nucleus. • This weak attraction allows the electrons to move about quite freely. – So what affects one atom affects all the atoms bonded together. • This mobility of electrons accounts for many metallic properties. © 2013 Pearson Education, Inc.
Metallic Bonds • Outer electrons in metal atoms are held only weakly by the nucleus. • This weak attraction allows the electrons to move about quite freely. – So what affects one atom affects all the atoms bonded together. • This mobility of electrons accounts for many metallic properties. © 2013 Pearson Education, Inc.
 Metallic Bonds © 2013 Pearson Education, Inc.
Metallic Bonds © 2013 Pearson Education, Inc.
 Metallic Bonds © 2013 Pearson Education, Inc.
Metallic Bonds © 2013 Pearson Education, Inc.
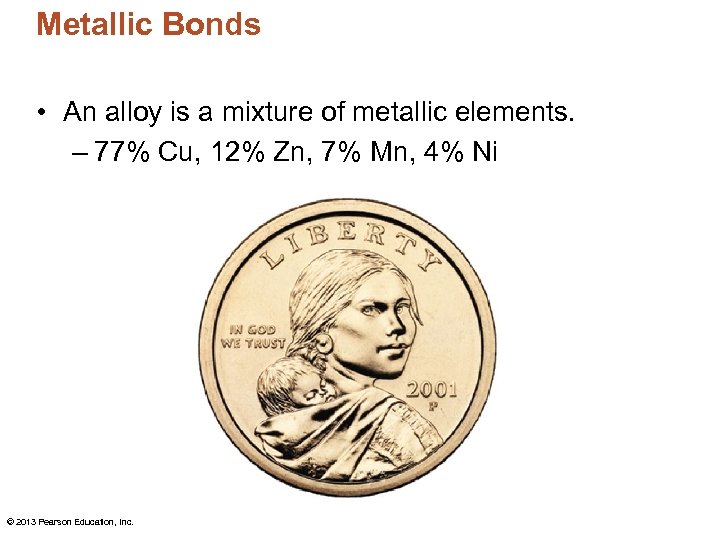 Metallic Bonds • An alloy is a mixture of metallic elements. – 77% Cu, 12% Zn, 7% Mn, 4% Ni © 2013 Pearson Education, Inc.
Metallic Bonds • An alloy is a mixture of metallic elements. – 77% Cu, 12% Zn, 7% Mn, 4% Ni © 2013 Pearson Education, Inc.
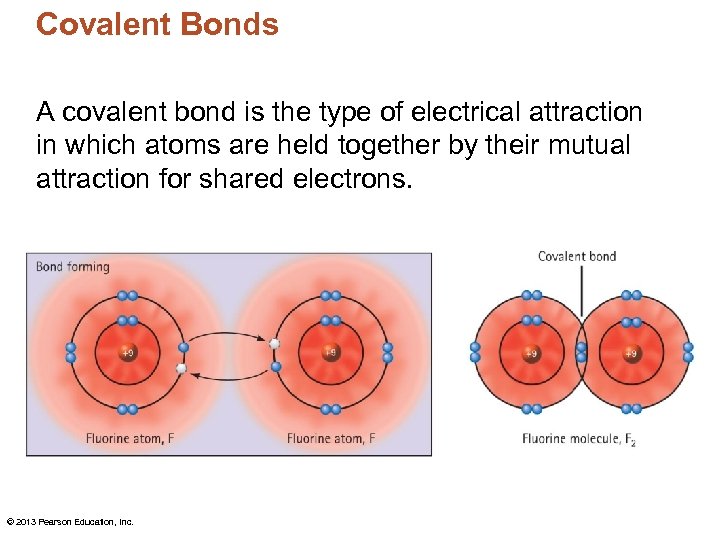 Covalent Bonds A covalent bond is the type of electrical attraction in which atoms are held together by their mutual attraction for shared electrons. © 2013 Pearson Education, Inc.
Covalent Bonds A covalent bond is the type of electrical attraction in which atoms are held together by their mutual attraction for shared electrons. © 2013 Pearson Education, Inc.
 Covalent Bonds • Electrons are shared not transferred. • Covalent bond – formed by a shared pair of electrons between two atoms. • Found in paper, plastic, plant matter, human tissue…. .
Covalent Bonds • Electrons are shared not transferred. • Covalent bond – formed by a shared pair of electrons between two atoms. • Found in paper, plastic, plant matter, human tissue…. .
 Describing Covalent Bonds • Described by the octet rule • Example: – A Fluorine atoms has 7 valence electrons – Each fluorine atom in molecular fluorine shares 1 electron with the other fluorine atom – results in a covalent bond. • Ammonia (NH 3)
Describing Covalent Bonds • Described by the octet rule • Example: – A Fluorine atoms has 7 valence electrons – Each fluorine atom in molecular fluorine shares 1 electron with the other fluorine atom – results in a covalent bond. • Ammonia (NH 3)
 Multiple Bonds • Single covalent bonds – single bonds – 2 atoms share exactly one pair of electrons • Double covalent bonds – double bond – Two pairs of shared electrons • Triple covalent bond – triple bond – Three pairs of shared electrons
Multiple Bonds • Single covalent bonds – single bonds – 2 atoms share exactly one pair of electrons • Double covalent bonds – double bond – Two pairs of shared electrons • Triple covalent bond – triple bond – Three pairs of shared electrons
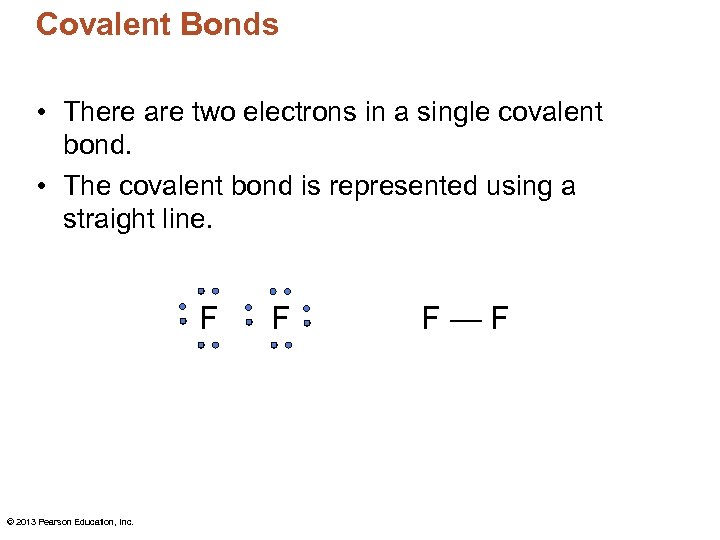 Covalent Bonds • There are two electrons in a single covalent bond. • The covalent bond is represented using a straight line. F © 2013 Pearson Education, Inc. F F—F
Covalent Bonds • There are two electrons in a single covalent bond. • The covalent bond is represented using a straight line. F © 2013 Pearson Education, Inc. F F—F
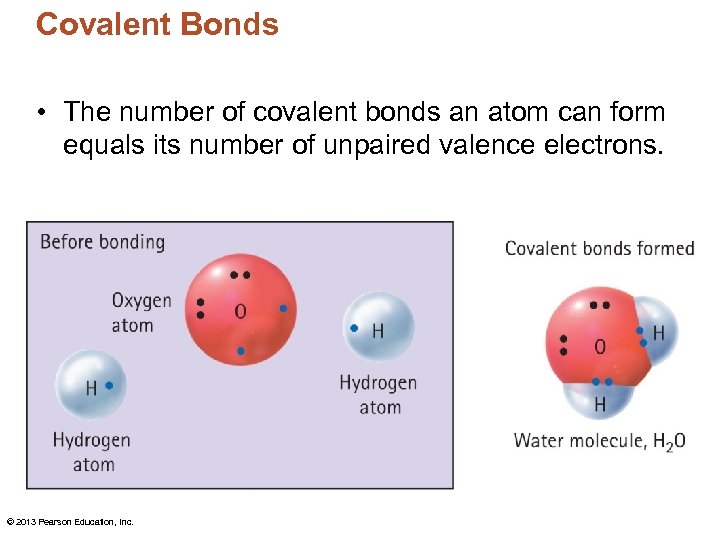 Covalent Bonds • The number of covalent bonds an atom can form equals its number of unpaired valence electrons. © 2013 Pearson Education, Inc.
Covalent Bonds • The number of covalent bonds an atom can form equals its number of unpaired valence electrons. © 2013 Pearson Education, Inc.
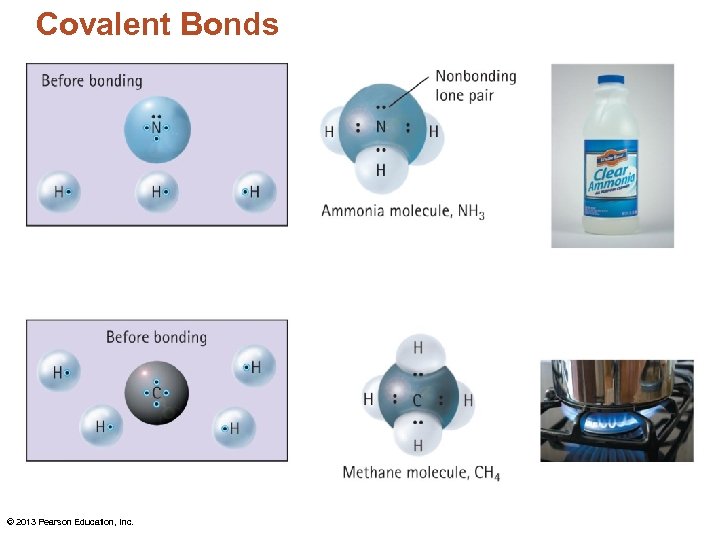 Covalent Bonds © 2013 Pearson Education, Inc.
Covalent Bonds © 2013 Pearson Education, Inc.
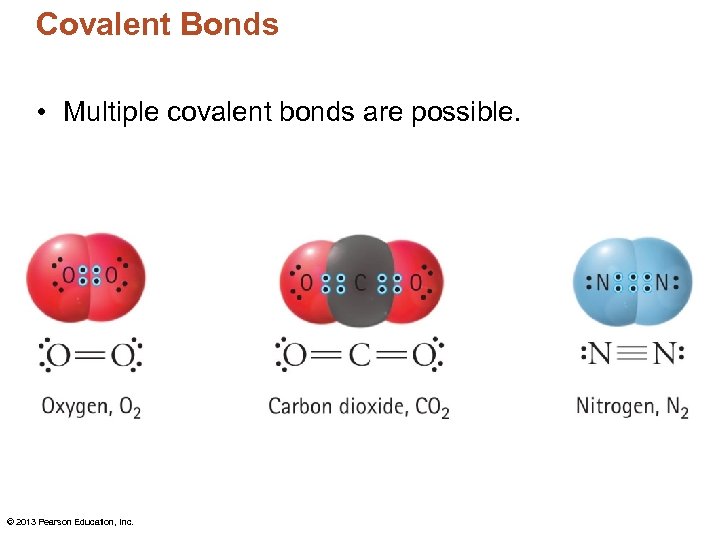 Covalent Bonds • Multiple covalent bonds are possible. © 2013 Pearson Education, Inc.
Covalent Bonds • Multiple covalent bonds are possible. © 2013 Pearson Education, Inc.
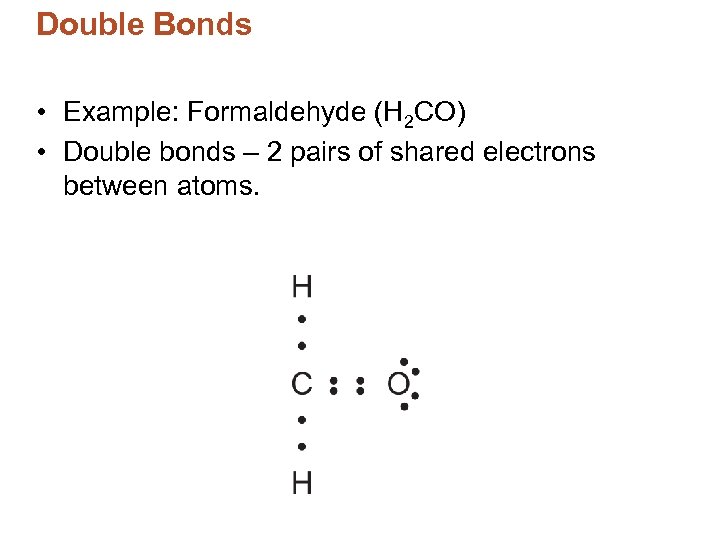 Double Bonds • Example: Formaldehyde (H 2 CO) • Double bonds – 2 pairs of shared electrons between atoms.
Double Bonds • Example: Formaldehyde (H 2 CO) • Double bonds – 2 pairs of shared electrons between atoms.
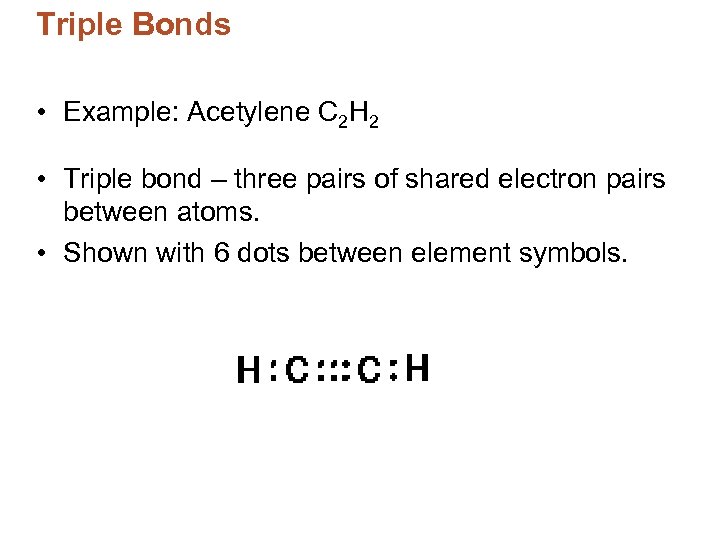 Triple Bonds • Example: Acetylene C 2 H 2 • Triple bond – three pairs of shared electron pairs between atoms. • Shown with 6 dots between element symbols.
Triple Bonds • Example: Acetylene C 2 H 2 • Triple bond – three pairs of shared electron pairs between atoms. • Shown with 6 dots between element symbols.
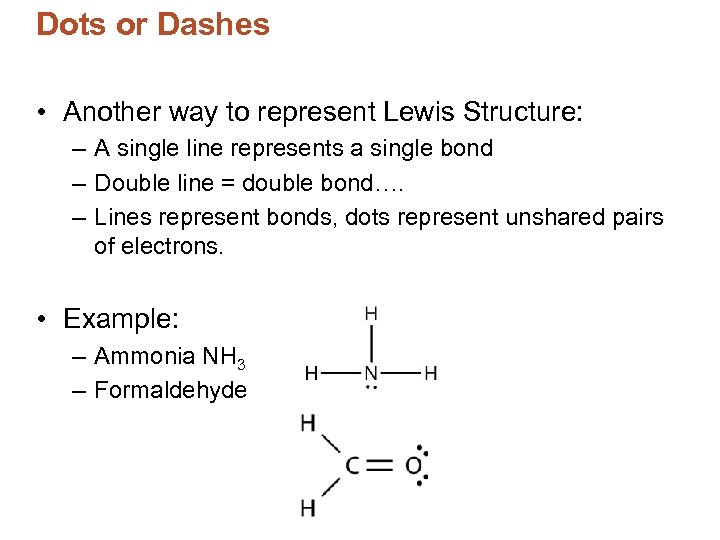 Dots or Dashes • Another way to represent Lewis Structure: – A single line represents a single bond – Double line = double bond…. – Lines represent bonds, dots represent unshared pairs of electrons. • Example: – Ammonia NH 3 – Formaldehyde
Dots or Dashes • Another way to represent Lewis Structure: – A single line represents a single bond – Double line = double bond…. – Lines represent bonds, dots represent unshared pairs of electrons. • Example: – Ammonia NH 3 – Formaldehyde
 Properties of Covalent Bonds • A covalent bond is a shared pair of electrons between 2 atoms. – Electrons are not always shared equally – Different atoms have different electronegativity • Measure of an atom’s attraction for electrons. – Shared electrons are more strongly attracted to the atom that is more electronegative.
Properties of Covalent Bonds • A covalent bond is a shared pair of electrons between 2 atoms. – Electrons are not always shared equally – Different atoms have different electronegativity • Measure of an atom’s attraction for electrons. – Shared electrons are more strongly attracted to the atom that is more electronegative.
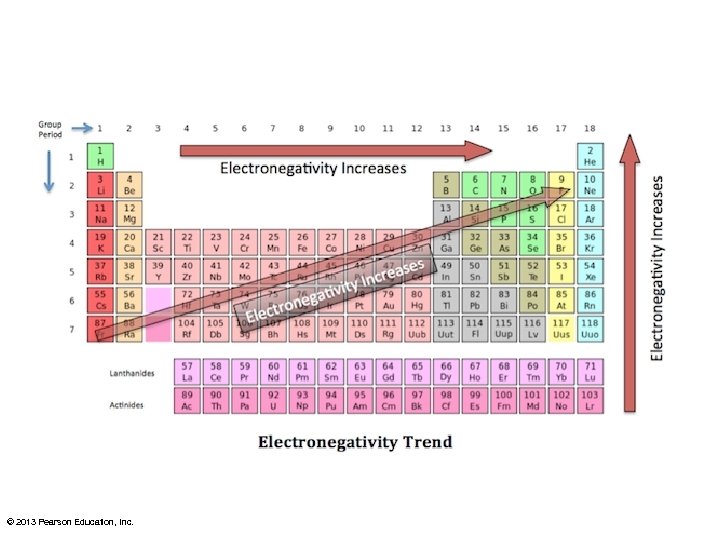
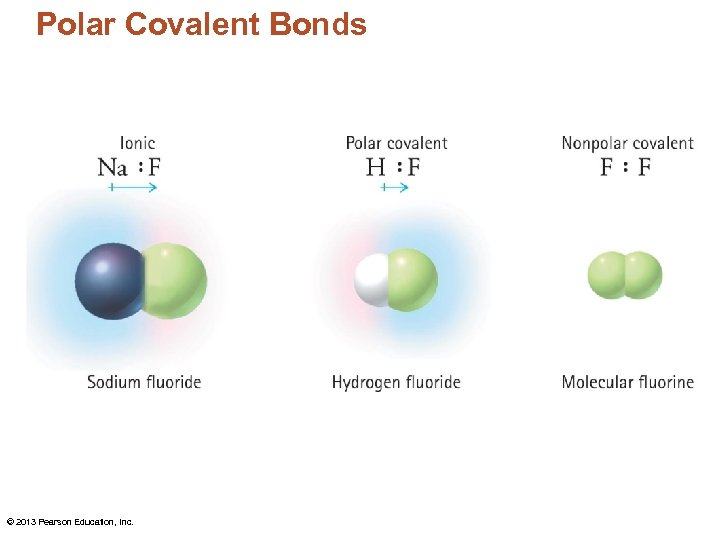
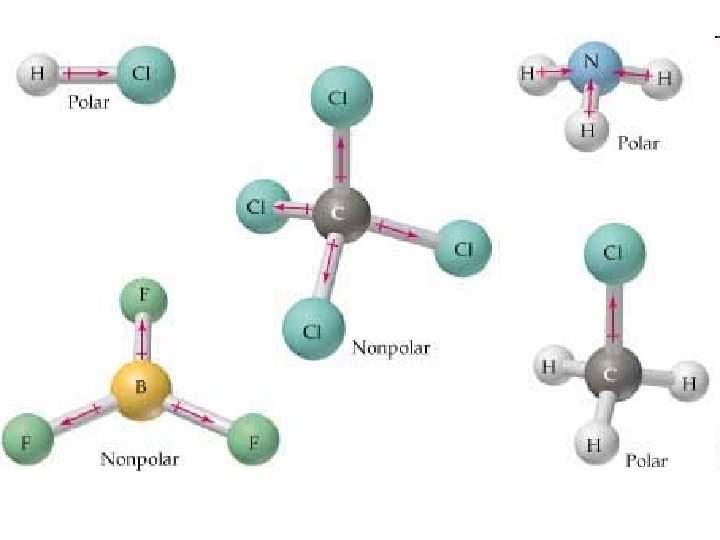
 Properties of Covalent Bonds • When one atom is significantly more electronegative than another the covalent bond is polar. – The atom with greater electronegativity gains a slightly negative charge because it has a slight excess of electron density – The shared electrons spend more time next to the more electronegative atom.
Properties of Covalent Bonds • When one atom is significantly more electronegative than another the covalent bond is polar. – The atom with greater electronegativity gains a slightly negative charge because it has a slight excess of electron density – The shared electrons spend more time next to the more electronegative atom.
 © 2013 Pearson Education, Inc.
© 2013 Pearson Education, Inc.
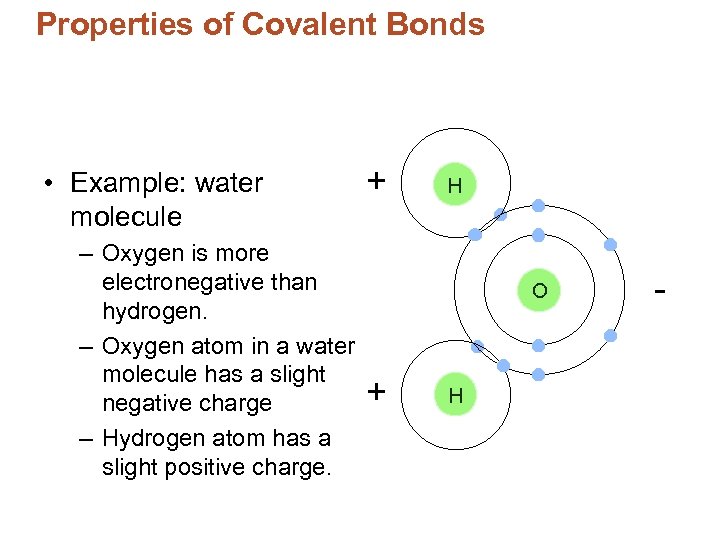 Properties of Covalent Bonds • Example: water molecule – Oxygen is more electronegative than hydrogen. – Oxygen atom in a water molecule has a slight negative charge – Hydrogen atom has a slight positive charge.
Properties of Covalent Bonds • Example: water molecule – Oxygen is more electronegative than hydrogen. – Oxygen atom in a water molecule has a slight negative charge – Hydrogen atom has a slight positive charge.
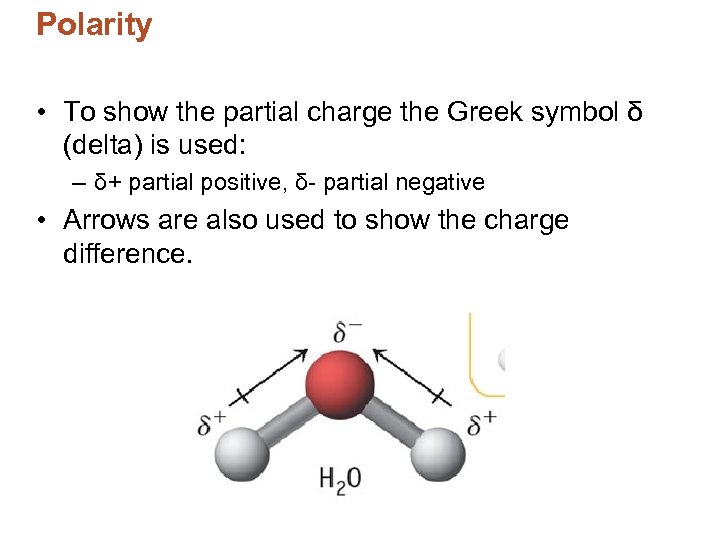 Polarity • To show the partial charge the Greek symbol δ (delta) is used: – δ+ partial positive, δ- partial negative • Arrows are also used to show the charge difference.
Polarity • To show the partial charge the Greek symbol δ (delta) is used: – δ+ partial positive, δ- partial negative • Arrows are also used to show the charge difference.
 Polarity • A bond between 2 atoms that have similar electronegativities will be nonpolar. – Have relatively weak attractions to other nonpolar molecules – Nonpolar covalent bond – F-F
Polarity • A bond between 2 atoms that have similar electronegativities will be nonpolar. – Have relatively weak attractions to other nonpolar molecules – Nonpolar covalent bond – F-F
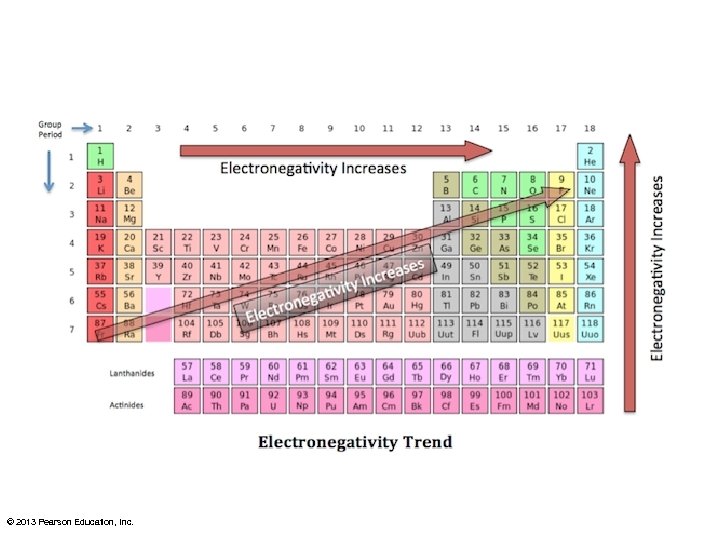
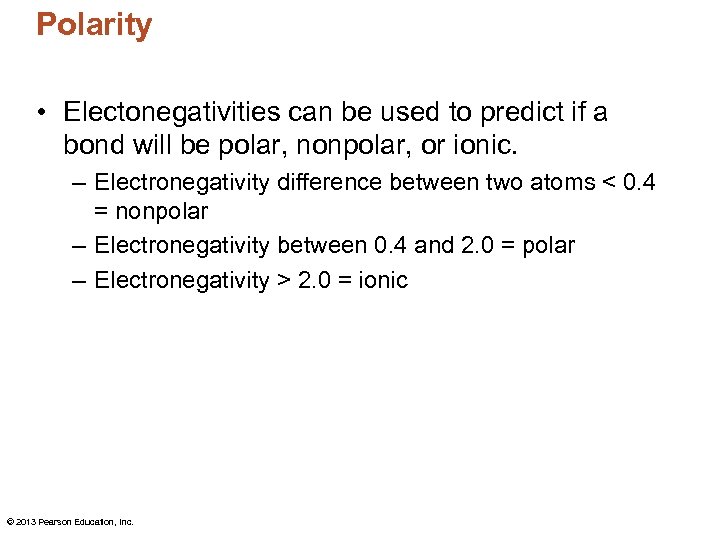 Polarity • Electonegativities can be used to predict if a bond will be polar, nonpolar, or ionic. – Electronegativity difference between two atoms < 0. 4 = nonpolar – Electronegativity between 0. 4 and 2. 0 = polar – Electronegativity > 2. 0 = ionic © 2013 Pearson Education, Inc.
Polarity • Electonegativities can be used to predict if a bond will be polar, nonpolar, or ionic. – Electronegativity difference between two atoms < 0. 4 = nonpolar – Electronegativity between 0. 4 and 2. 0 = polar – Electronegativity > 2. 0 = ionic © 2013 Pearson Education, Inc.
 © 2013 Pearson Education, Inc.
© 2013 Pearson Education, Inc.
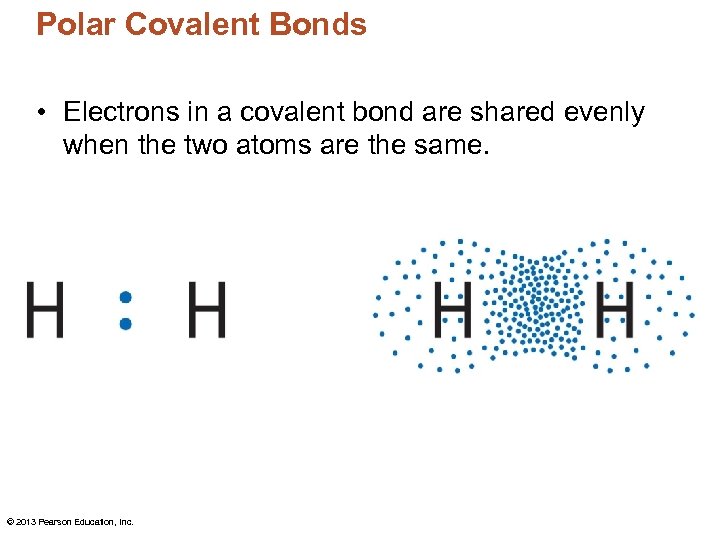 Polar Covalent Bonds • Electrons in a covalent bond are shared evenly when the two atoms are the same. © 2013 Pearson Education, Inc.
Polar Covalent Bonds • Electrons in a covalent bond are shared evenly when the two atoms are the same. © 2013 Pearson Education, Inc.
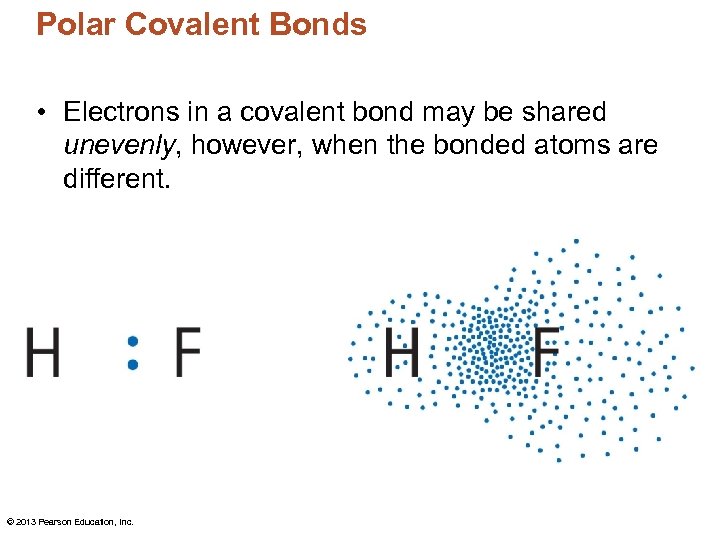 Polar Covalent Bonds • Electrons in a covalent bond may be shared unevenly, however, when the bonded atoms are different. © 2013 Pearson Education, Inc.
Polar Covalent Bonds • Electrons in a covalent bond may be shared unevenly, however, when the bonded atoms are different. © 2013 Pearson Education, Inc.
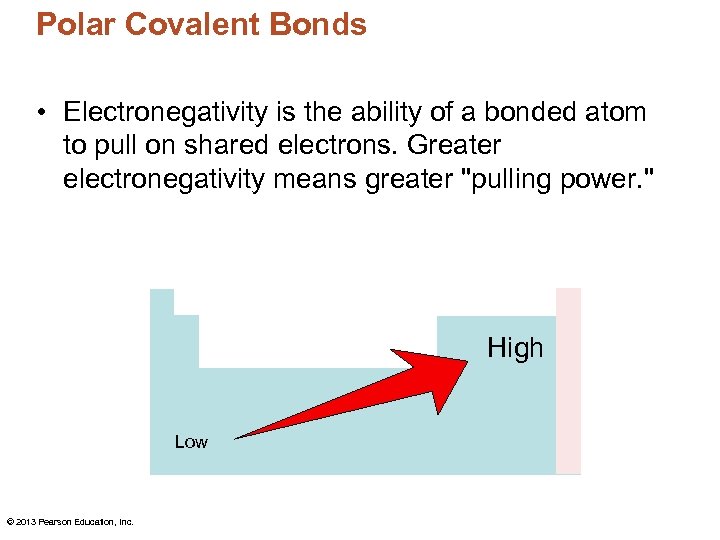 Polar Covalent Bonds • Electronegativity is the ability of a bonded atom to pull on shared electrons. Greater electronegativity means greater "pulling power. " High Low © 2013 Pearson Education, Inc.
Polar Covalent Bonds • Electronegativity is the ability of a bonded atom to pull on shared electrons. Greater electronegativity means greater "pulling power. " High Low © 2013 Pearson Education, Inc.
 © 2013 Pearson Education, Inc.
© 2013 Pearson Education, Inc.
 Polar Covalent Bonds © 2013 Pearson Education, Inc.
Polar Covalent Bonds © 2013 Pearson Education, Inc.
 Polar Covalent Bonds CHECK YOUR NEIGHBOR Which is heavier: carbon dioxide, CO 2, or water, H 2 O? A. Carbon dioxide is heavier. B. Water is heavier. C. Both have the same number of atoms, so they weigh the same. D. It depends on other factors. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
Polar Covalent Bonds CHECK YOUR NEIGHBOR Which is heavier: carbon dioxide, CO 2, or water, H 2 O? A. Carbon dioxide is heavier. B. Water is heavier. C. Both have the same number of atoms, so they weigh the same. D. It depends on other factors. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
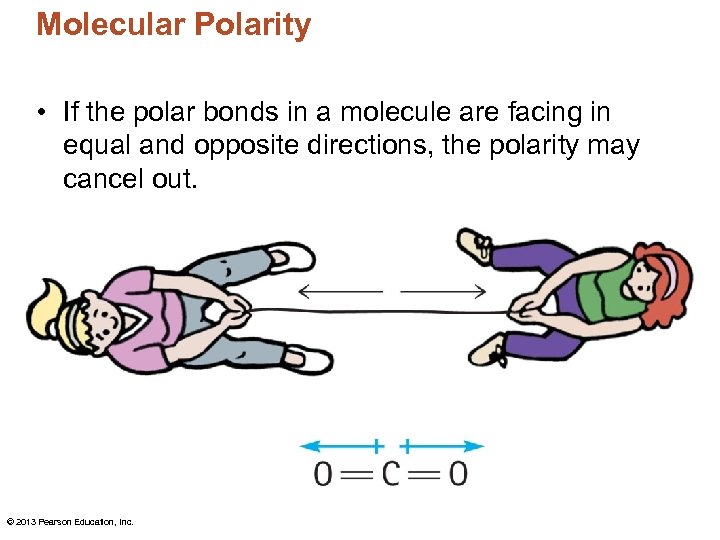 Molecular Polarity • If the polar bonds in a molecule are facing in equal and opposite directions, the polarity may cancel out. © 2013 Pearson Education, Inc.
Molecular Polarity • If the polar bonds in a molecule are facing in equal and opposite directions, the polarity may cancel out. © 2013 Pearson Education, Inc.
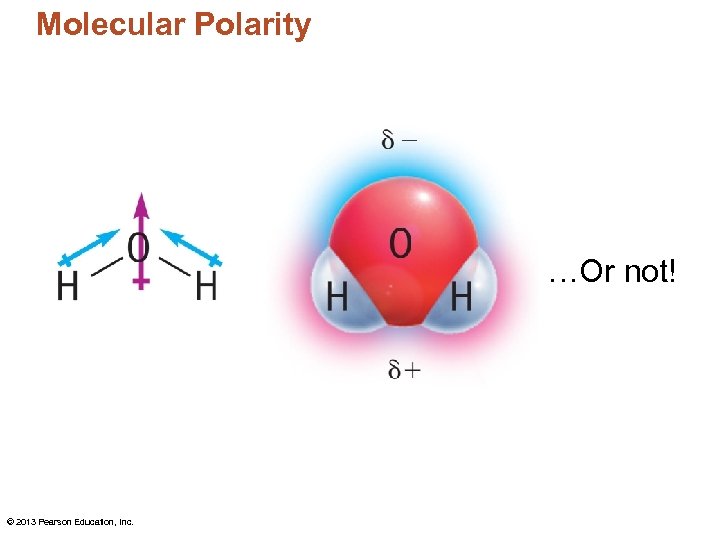 Molecular Polarity …Or not! © 2013 Pearson Education, Inc.
Molecular Polarity …Or not! © 2013 Pearson Education, Inc.
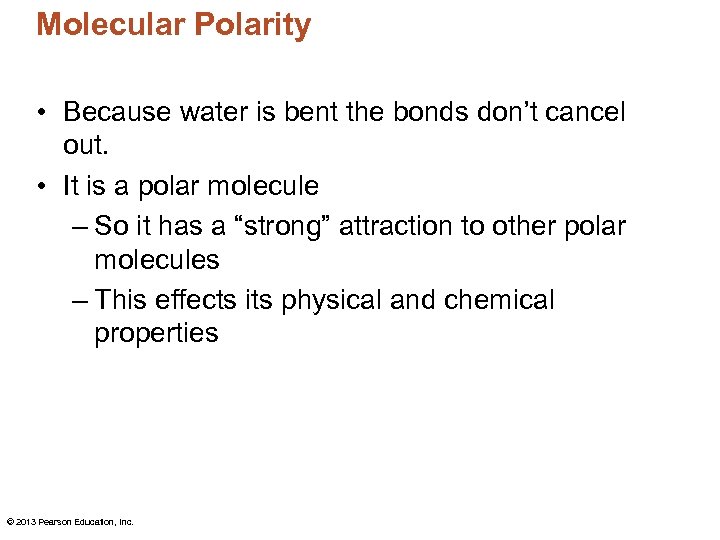 Molecular Polarity • Because water is bent the bonds don’t cancel out. • It is a polar molecule – So it has a “strong” attraction to other polar molecules – This effects its physical and chemical properties © 2013 Pearson Education, Inc.
Molecular Polarity • Because water is bent the bonds don’t cancel out. • It is a polar molecule – So it has a “strong” attraction to other polar molecules – This effects its physical and chemical properties © 2013 Pearson Education, Inc.
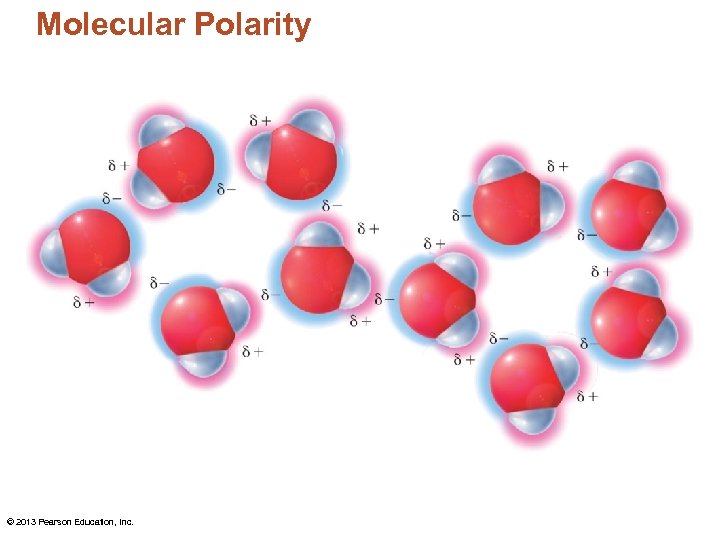 Molecular Polarity © 2013 Pearson Education, Inc.
Molecular Polarity © 2013 Pearson Education, Inc.
 Molecular Polarity CHECK YOUR NEIGHBOR Water has such a relatively high boiling point because water A. B. C. D. is such a heavy substance. is transparent so that heat passes right through it. contains three atoms per molecules are so sticky. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
Molecular Polarity CHECK YOUR NEIGHBOR Water has such a relatively high boiling point because water A. B. C. D. is such a heavy substance. is transparent so that heat passes right through it. contains three atoms per molecules are so sticky. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
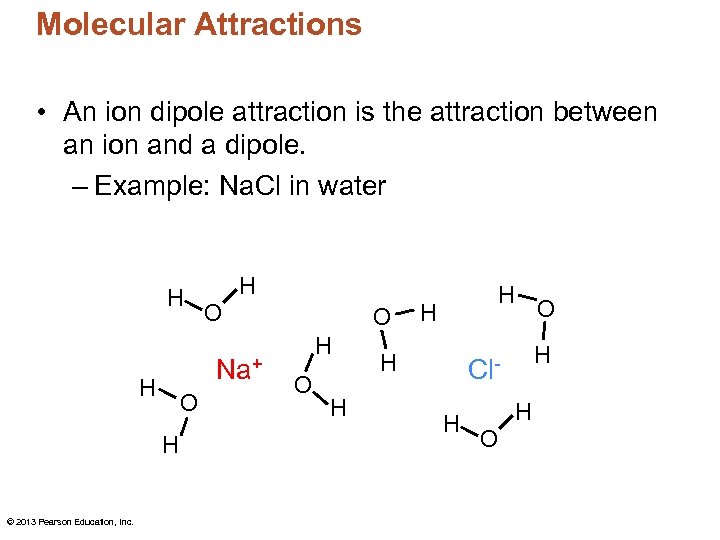 Molecular Attractions • An ion dipole attraction is the attraction between an ion and a dipole. – Example: Na. Cl in water H O Na+ H O H © 2013 Pearson Education, Inc. H O H H H Cl. H H O O
Molecular Attractions • An ion dipole attraction is the attraction between an ion and a dipole. – Example: Na. Cl in water H O Na+ H O H © 2013 Pearson Education, Inc. H O H H H Cl. H H O O
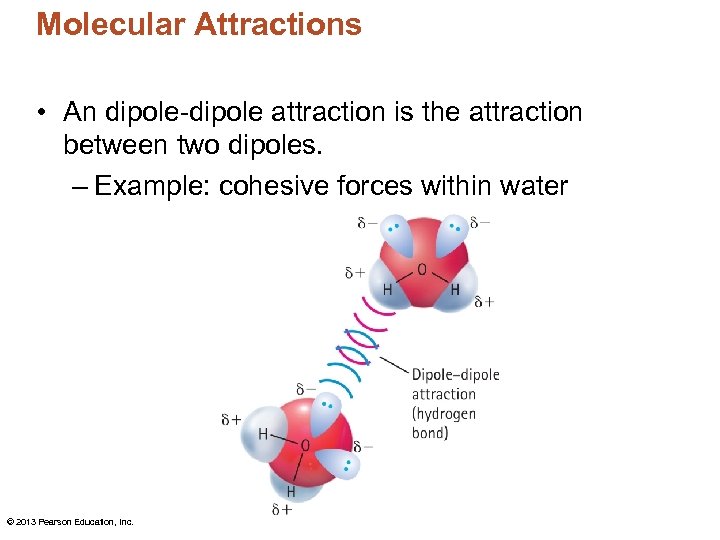 Molecular Attractions • An dipole-dipole attraction is the attraction between two dipoles. – Example: cohesive forces within water © 2013 Pearson Education, Inc.
Molecular Attractions • An dipole-dipole attraction is the attraction between two dipoles. – Example: cohesive forces within water © 2013 Pearson Education, Inc.
 Dipoles • A dipole can also attract a molecule that is ordinarily not a dipole. • When a dipole approaches a nonpolar molecule, its partial charge either attracts or repels the electrons of the other particle.
Dipoles • A dipole can also attract a molecule that is ordinarily not a dipole. • When a dipole approaches a nonpolar molecule, its partial charge either attracts or repels the electrons of the other particle.
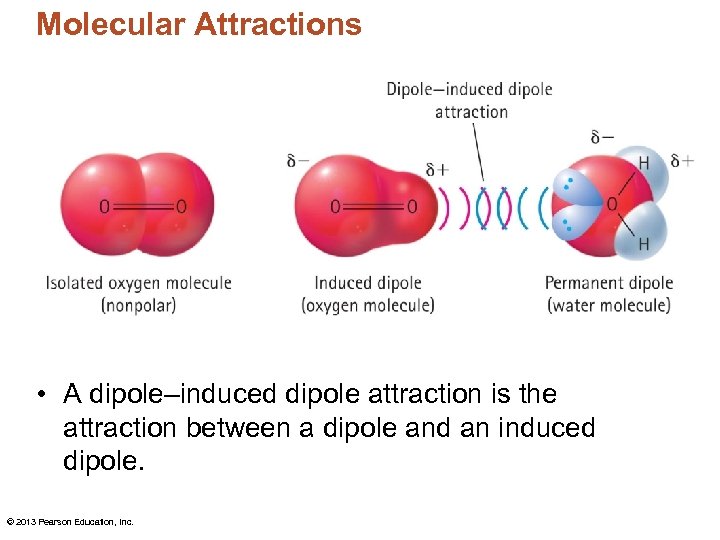 Molecular Attractions • A dipole–induced dipole attraction is the attraction between a dipole and an induced dipole. © 2013 Pearson Education, Inc.
Molecular Attractions • A dipole–induced dipole attraction is the attraction between a dipole and an induced dipole. © 2013 Pearson Education, Inc.
 Molecular Attractions CHECK YOUR NEIGHBOR Is it possible for a fish to drown? A. B. C. D. no, because fish breathe water yes, when the water contains too little oxygen no, because water is 88. 8% oxygen by mass yes, when the water is not moving Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
Molecular Attractions CHECK YOUR NEIGHBOR Is it possible for a fish to drown? A. B. C. D. no, because fish breathe water yes, when the water contains too little oxygen no, because water is 88. 8% oxygen by mass yes, when the water is not moving Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
 Molecular Attractions CHECK YOUR NEIGHBOR A nonpolar material, such as oxygen, O 2, is soluble in a polar material, such as water, H 2 O, by way of A. B. C. D. ion–dipole attractions. dipole–induced dipole attractions. all of the above Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
Molecular Attractions CHECK YOUR NEIGHBOR A nonpolar material, such as oxygen, O 2, is soluble in a polar material, such as water, H 2 O, by way of A. B. C. D. ion–dipole attractions. dipole–induced dipole attractions. all of the above Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
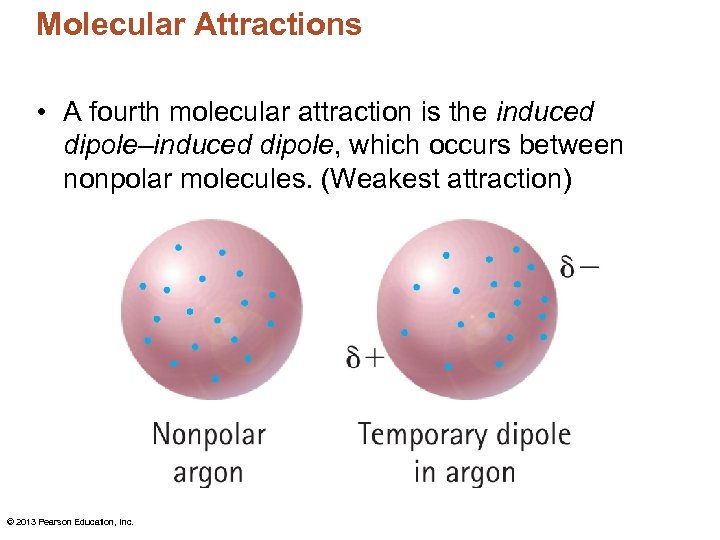 Molecular Attractions • A fourth molecular attraction is the induced dipole–induced dipole, which occurs between nonpolar molecules. (Weakest attraction) © 2013 Pearson Education, Inc.
Molecular Attractions • A fourth molecular attraction is the induced dipole–induced dipole, which occurs between nonpolar molecules. (Weakest attraction) © 2013 Pearson Education, Inc.
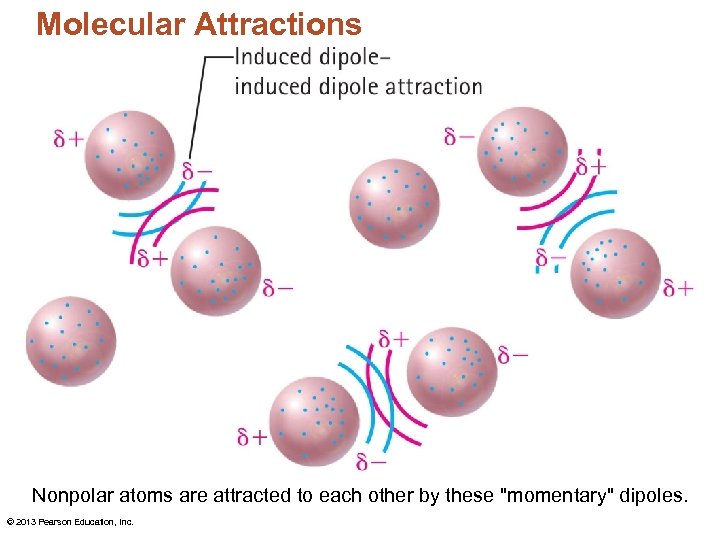 Molecular Attractions Nonpolar atoms are attracted to each other by these "momentary" dipoles. © 2013 Pearson Education, Inc.
Molecular Attractions Nonpolar atoms are attracted to each other by these "momentary" dipoles. © 2013 Pearson Education, Inc.
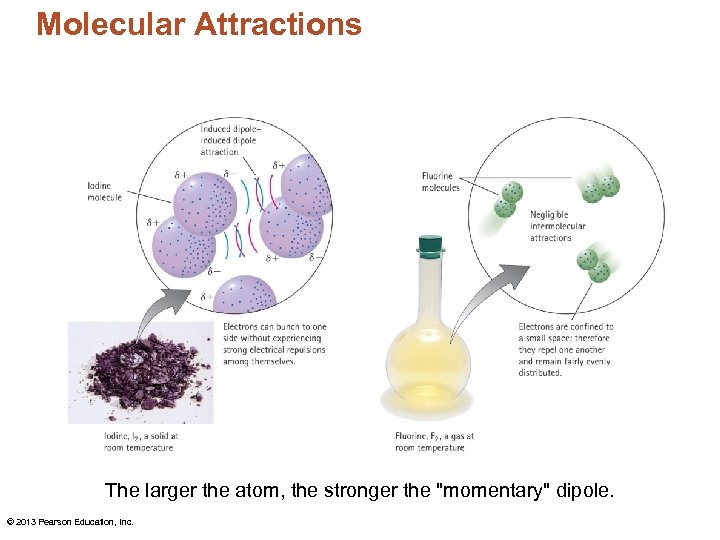 Molecular Attractions The larger the atom, the stronger the "momentary" dipole. © 2013 Pearson Education, Inc.
Molecular Attractions The larger the atom, the stronger the "momentary" dipole. © 2013 Pearson Education, Inc.
 Molecular Attractions The tiny nonpolar fluorine atoms in Teflon provide very weak attractions, which is why Teflon provides a "nonstick" surface. © 2013 Pearson Education, Inc.
Molecular Attractions The tiny nonpolar fluorine atoms in Teflon provide very weak attractions, which is why Teflon provides a "nonstick" surface. © 2013 Pearson Education, Inc.
 Molecular Attractions • Small nonpolar molecules are easier to pull apart then large nonpolar molecules • Like small pieces of velcro vs large pieces of velcro • Why do you need soap to clean your clothes? © 2013 Pearson Education, Inc.
Molecular Attractions • Small nonpolar molecules are easier to pull apart then large nonpolar molecules • Like small pieces of velcro vs large pieces of velcro • Why do you need soap to clean your clothes? © 2013 Pearson Education, Inc.
 Molecular Attractions So, how do the gecko's sticky feet stay so clean? © 2013 Pearson Education, Inc.
Molecular Attractions So, how do the gecko's sticky feet stay so clean? © 2013 Pearson Education, Inc.
 Molecular Attractions CHECK YOUR NEIGHBOR Which type of molecular attraction takes the least amount of energy to break apart? A. B. C. D. ion–dipole attractions dipole–induced dipole attractions induced dipole–induced dipole attractions Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
Molecular Attractions CHECK YOUR NEIGHBOR Which type of molecular attraction takes the least amount of energy to break apart? A. B. C. D. ion–dipole attractions dipole–induced dipole attractions induced dipole–induced dipole attractions Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
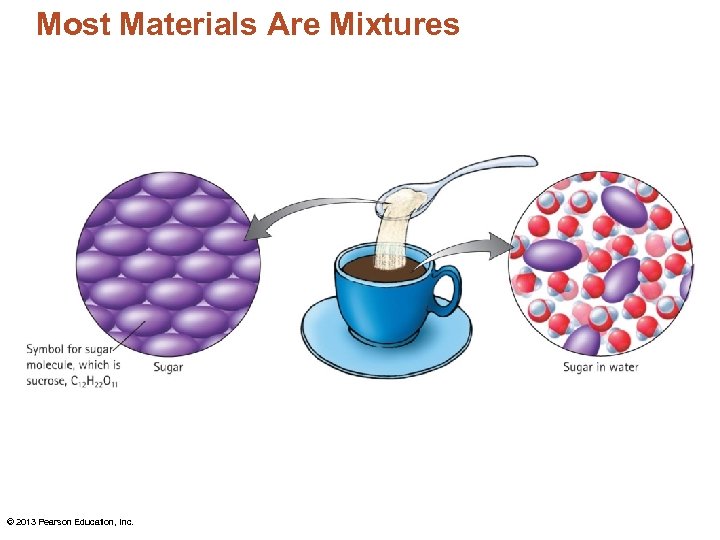
 Most Materials Are Mixtures • A pure substance is a material that consists of only one type of element or compound. • A mixture is a collection of two or more pure substances. • It can be separated by physical means. © 2013 Pearson Education, Inc.
Most Materials Are Mixtures • A pure substance is a material that consists of only one type of element or compound. • A mixture is a collection of two or more pure substances. • It can be separated by physical means. © 2013 Pearson Education, Inc.
 Most Materials Are Mixtures © 2013 Pearson Education, Inc.
Most Materials Are Mixtures © 2013 Pearson Education, Inc.
 The Chemist's Classification of Matter © 2013 Pearson Education, Inc.
The Chemist's Classification of Matter © 2013 Pearson Education, Inc.
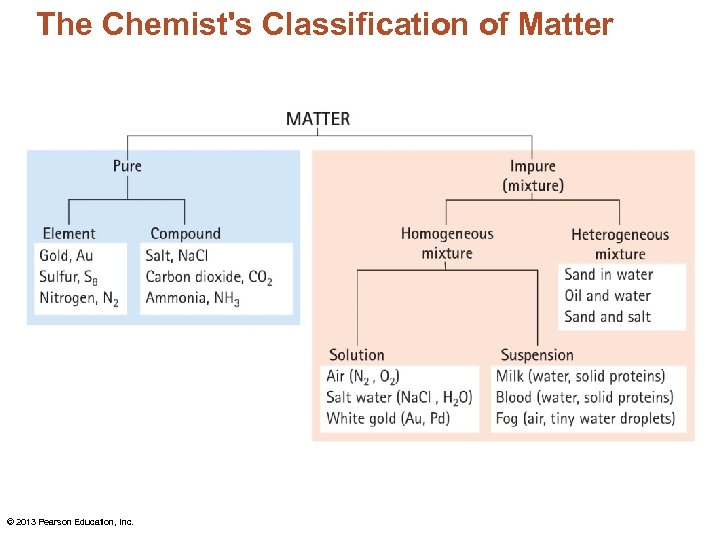
 The Chemist's Classification of Matter • Pure materials consist of a single element or compound. • Impure materials consist of two or more elements or compounds. • Mixtures may be heterogeneous or homogeneous. © 2013 Pearson Education, Inc.
The Chemist's Classification of Matter • Pure materials consist of a single element or compound. • Impure materials consist of two or more elements or compounds. • Mixtures may be heterogeneous or homogeneous. © 2013 Pearson Education, Inc.
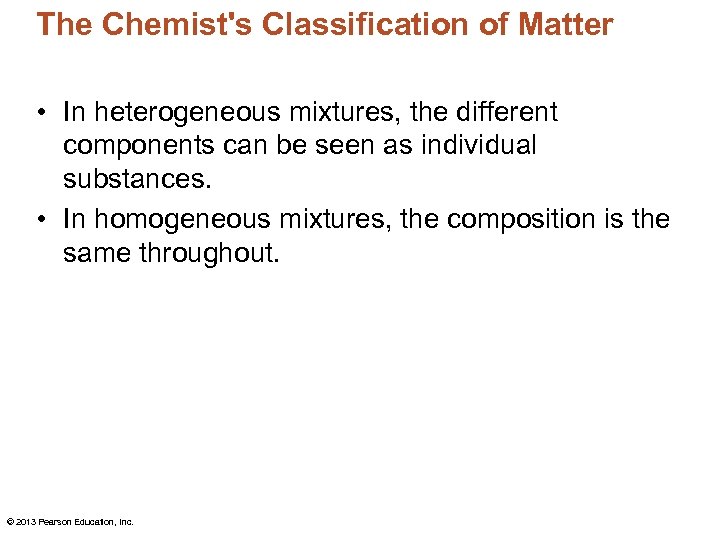 The Chemist's Classification of Matter • In heterogeneous mixtures, the different components can be seen as individual substances. • In homogeneous mixtures, the composition is the same throughout. © 2013 Pearson Education, Inc.
The Chemist's Classification of Matter • In heterogeneous mixtures, the different components can be seen as individual substances. • In homogeneous mixtures, the composition is the same throughout. © 2013 Pearson Education, Inc.
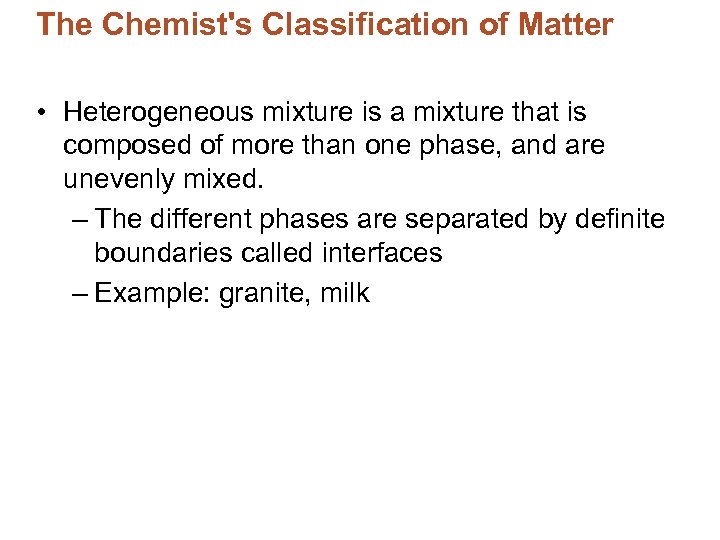 The Chemist's Classification of Matter • Heterogeneous mixture is a mixture that is composed of more than one phase, and are unevenly mixed. – The different phases are separated by definite boundaries called interfaces – Example: granite, milk
The Chemist's Classification of Matter • Heterogeneous mixture is a mixture that is composed of more than one phase, and are unevenly mixed. – The different phases are separated by definite boundaries called interfaces – Example: granite, milk
 • Two or more substances that can be easily separated by physical means form a mixture. – Heterogeneous mixture—mixture of different and easily distinguishable materials
• Two or more substances that can be easily separated by physical means form a mixture. – Heterogeneous mixture—mixture of different and easily distinguishable materials
 The Chemist's Classification of Matter © 2013 Pearson Education, Inc.
The Chemist's Classification of Matter © 2013 Pearson Education, Inc.
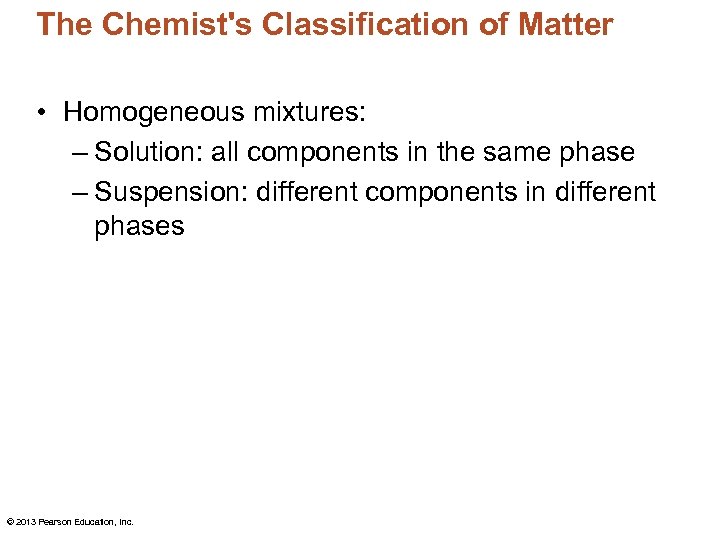 The Chemist's Classification of Matter • Homogeneous mixtures: – Solution: all components in the same phase – Suspension: different components in different phases © 2013 Pearson Education, Inc.
The Chemist's Classification of Matter • Homogeneous mixtures: – Solution: all components in the same phase – Suspension: different components in different phases © 2013 Pearson Education, Inc.
 • Homogeneous mixture consist of only one phase, and are evenly mixed. – Example: sugar, salt, seawater, quartz, air • Homogeneous mixture—contains two or more gaseous, liquid, or solid substances blended evenly; also called a solution
• Homogeneous mixture consist of only one phase, and are evenly mixed. – Example: sugar, salt, seawater, quartz, air • Homogeneous mixture—contains two or more gaseous, liquid, or solid substances blended evenly; also called a solution
 The Chemist's Classification of Matter CHECK YOUR NEIGHBOR Is the air in your house a homogeneous or a heterogeneous mixture? A. homogeneous, because it is mixed very well B. heterogeneous, because of the dust particles it contains C. homogeneous, because it is all at the same temperature D. heterogeneous, because it consists of different types of molecules Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
The Chemist's Classification of Matter CHECK YOUR NEIGHBOR Is the air in your house a homogeneous or a heterogeneous mixture? A. homogeneous, because it is mixed very well B. heterogeneous, because of the dust particles it contains C. homogeneous, because it is all at the same temperature D. heterogeneous, because it consists of different types of molecules Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
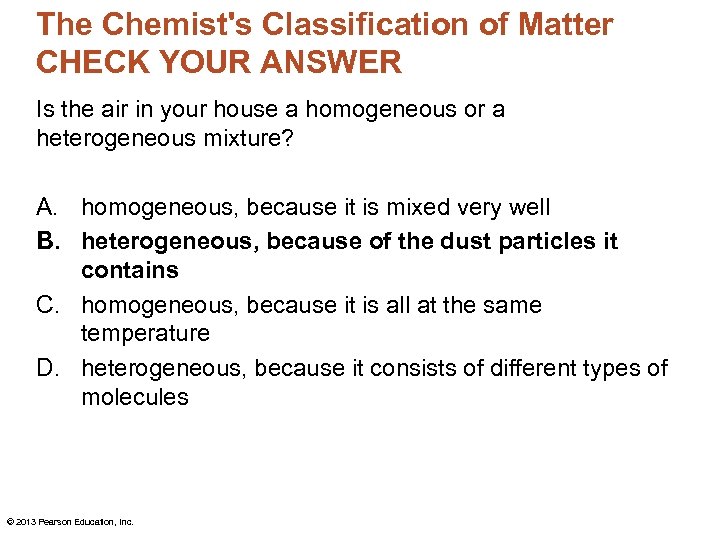 The Chemist's Classification of Matter CHECK YOUR ANSWER Is the air in your house a homogeneous or a heterogeneous mixture? A. homogeneous, because it is mixed very well B. heterogeneous, because of the dust particles it contains C. homogeneous, because it is all at the same temperature D. heterogeneous, because it consists of different types of molecules © 2013 Pearson Education, Inc.
The Chemist's Classification of Matter CHECK YOUR ANSWER Is the air in your house a homogeneous or a heterogeneous mixture? A. homogeneous, because it is mixed very well B. heterogeneous, because of the dust particles it contains C. homogeneous, because it is all at the same temperature D. heterogeneous, because it consists of different types of molecules © 2013 Pearson Education, Inc.
 Solutions • A solution is a homogenous mixture consisting of ions or molecules. • A solution consists of a solute (dissolved material) in a solvent (dissolving material) – A solvent is the major component of a solution. – A solute is the minor component of a solution. • If a solution is saturated, then no more solute will dissolve in it. © 2013 Pearson Education, Inc.
Solutions • A solution is a homogenous mixture consisting of ions or molecules. • A solution consists of a solute (dissolved material) in a solvent (dissolving material) – A solvent is the major component of a solution. – A solute is the minor component of a solution. • If a solution is saturated, then no more solute will dissolve in it. © 2013 Pearson Education, Inc.
 Solutions • Solutions are not necessarily always liquid – Both air and glass are solutions
Solutions • Solutions are not necessarily always liquid – Both air and glass are solutions
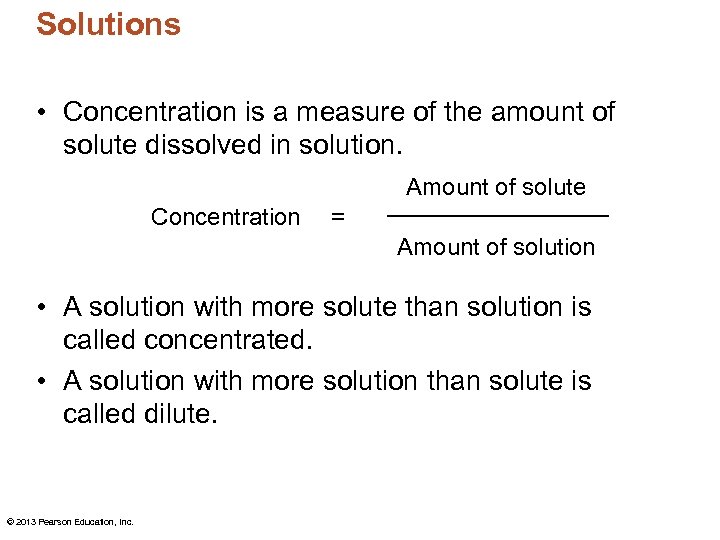 Solutions • Concentration is a measure of the amount of solute dissolved in solution. Amount of solute Concentration = Amount of solution • A solution with more solute than solution is called concentrated. • A solution with more solution than solute is called dilute. © 2013 Pearson Education, Inc.
Solutions • Concentration is a measure of the amount of solute dissolved in solution. Amount of solute Concentration = Amount of solution • A solution with more solute than solution is called concentrated. • A solution with more solution than solute is called dilute. © 2013 Pearson Education, Inc.
 • The factor-label method treats labels as factors. – These factors divide out. • There are two main rules to solving science problems with the factor-label method: – 1. Always carry along your units with any measurement you use. – 2. You need to form the appropriate labeled ratios (equalities).
• The factor-label method treats labels as factors. – These factors divide out. • There are two main rules to solving science problems with the factor-label method: – 1. Always carry along your units with any measurement you use. – 2. You need to form the appropriate labeled ratios (equalities).
 • SIGNIFICANT DIGITS • In addition and subtraction, the answer may contain only as many decimal places as the measurement having the least number of decimal places. • In multiplication and division, the answer may contain only as many significant digits as the measurement with the least number of significant digits.
• SIGNIFICANT DIGITS • In addition and subtraction, the answer may contain only as many decimal places as the measurement having the least number of decimal places. • In multiplication and division, the answer may contain only as many significant digits as the measurement with the least number of significant digits.
 Significant Digit Rules • • 1) ALL non-zero numbers (1, 2, 3, 4, 5, 6, 7, 8, 9) are ALWAYS significant. 2) ALL zeroes between non-zero numbers are ALWAYS significant. 3) ALL zeroes which are SIMULTANEOUSLY to the right of the decimal point AND at the end of the number are ALWAYS significant. 4) ALL zeroes which are to the left of a written decimal point and are in a number >= 10 are ALWAYS significant.
Significant Digit Rules • • 1) ALL non-zero numbers (1, 2, 3, 4, 5, 6, 7, 8, 9) are ALWAYS significant. 2) ALL zeroes between non-zero numbers are ALWAYS significant. 3) ALL zeroes which are SIMULTANEOUSLY to the right of the decimal point AND at the end of the number are ALWAYS significant. 4) ALL zeroes which are to the left of a written decimal point and are in a number >= 10 are ALWAYS significant.
 • Example Problem: – How many centimeters in 2 meters? – You will see from the metric conversion chart that 1 meter = 100 cm – we turn this into a ratio by writing it like this:
• Example Problem: – How many centimeters in 2 meters? – You will see from the metric conversion chart that 1 meter = 100 cm – we turn this into a ratio by writing it like this:
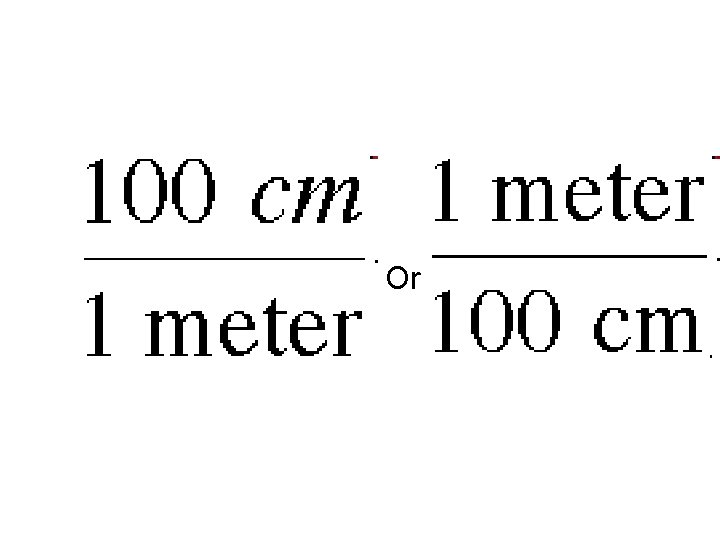 Or
Or
 • Once you have the equalities you must pick the one that will cancel out the units leaving the desired units. • Then multiply your starting quantity (2 meters) by the equality that will give your desired units.
• Once you have the equalities you must pick the one that will cancel out the units leaving the desired units. • Then multiply your starting quantity (2 meters) by the equality that will give your desired units.
 • As a rule of thumb your problem set up should look like this:
• As a rule of thumb your problem set up should look like this:
 Practice Problems • 1. How many wheels on 350 Ford pickups (use the equality 1 pickup = 4 tires) - the starting units are pickups, the ending units need to be wheels.
Practice Problems • 1. How many wheels on 350 Ford pickups (use the equality 1 pickup = 4 tires) - the starting units are pickups, the ending units need to be wheels.
 Practice Problems • 2. How many millimeters in 34 hectometers (use the equality 10, 000 mm = 1 hectometer)? • Sometimes you will need to multiply by more than one ratio to get to your desired units, you can do this by using linking units. Your setup will look like this:
Practice Problems • 2. How many millimeters in 34 hectometers (use the equality 10, 000 mm = 1 hectometer)? • Sometimes you will need to multiply by more than one ratio to get to your desired units, you can do this by using linking units. Your setup will look like this:
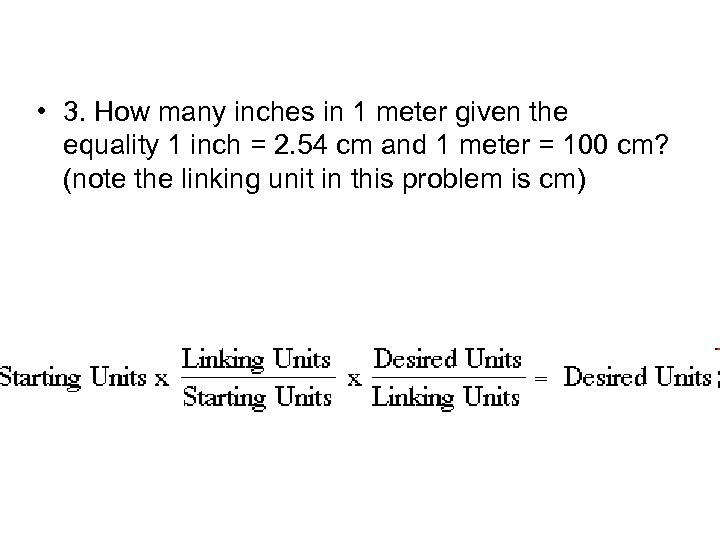 • 3. How many inches in 1 meter given the equality 1 inch = 2. 54 cm and 1 meter = 100 cm? (note the linking unit in this problem is cm)
• 3. How many inches in 1 meter given the equality 1 inch = 2. 54 cm and 1 meter = 100 cm? (note the linking unit in this problem is cm)
 • 4. How many grams in 150 pounds given the equalities 1 pound = 0. 454 kg and 1 kg = 1000 grams?
• 4. How many grams in 150 pounds given the equalities 1 pound = 0. 454 kg and 1 kg = 1000 grams?
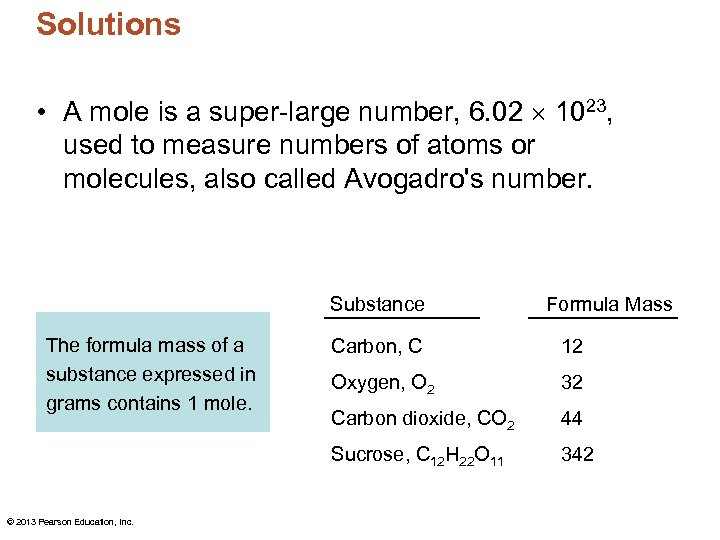 Solutions • A mole is a super-large number, 6. 02 1023, used to measure numbers of atoms or molecules, also called Avogadro's number. Substance The formula mass of a substance expressed in grams contains 1 mole. Formula Mass 12 Oxygen, O 2 32 Carbon dioxide, CO 2 44 Sucrose, C 12 H 22 O 11 © 2013 Pearson Education, Inc. Carbon, C 342
Solutions • A mole is a super-large number, 6. 02 1023, used to measure numbers of atoms or molecules, also called Avogadro's number. Substance The formula mass of a substance expressed in grams contains 1 mole. Formula Mass 12 Oxygen, O 2 32 Carbon dioxide, CO 2 44 Sucrose, C 12 H 22 O 11 © 2013 Pearson Education, Inc. Carbon, C 342
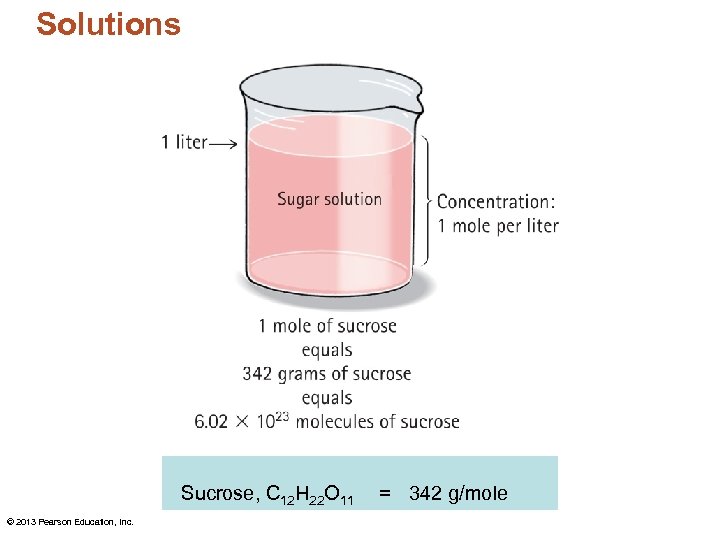 Solutions Sucrose, C 12 H 22 O 11 © 2013 Pearson Education, Inc. = 342 g/mole
Solutions Sucrose, C 12 H 22 O 11 © 2013 Pearson Education, Inc. = 342 g/mole
 Solutions CHECK YOUR NEIGHBOR Water, H 2 O, has a formula mass of 18. How many moles of water are there in 18 grams of water? A. B. C. D. 0. 5 mole 1 mole 9 moles 18 moles Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
Solutions CHECK YOUR NEIGHBOR Water, H 2 O, has a formula mass of 18. How many moles of water are there in 18 grams of water? A. B. C. D. 0. 5 mole 1 mole 9 moles 18 moles Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
 Solutions CHECK YOUR NEIGHBOR How many grams of water, H 2 O, are there in 2 moles of water? A. B. C. D. 1 gram 9 grams 18 grams 36 grams Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
Solutions CHECK YOUR NEIGHBOR How many grams of water, H 2 O, are there in 2 moles of water? A. B. C. D. 1 gram 9 grams 18 grams 36 grams Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
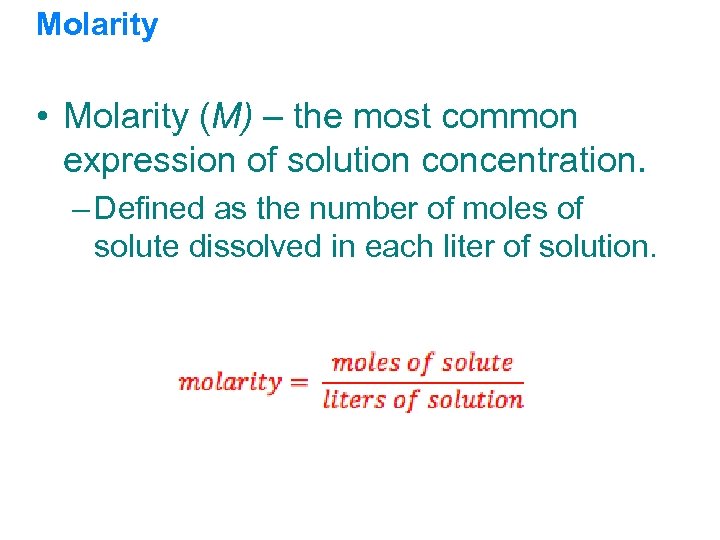 Molarity • Molarity (M) – the most common expression of solution concentration. – Defined as the number of moles of solute dissolved in each liter of solution.
Molarity • Molarity (M) – the most common expression of solution concentration. – Defined as the number of moles of solute dissolved in each liter of solution.
 Molarity • Example: – What is the molarity of a solution formed by mixing 10. 0 g H 2 SO 4 with enough water to make 100. 0 m. L of solution? - convert ml to L - complete the molarity equation - convert grams to moles
Molarity • Example: – What is the molarity of a solution formed by mixing 10. 0 g H 2 SO 4 with enough water to make 100. 0 m. L of solution? - convert ml to L - complete the molarity equation - convert grams to moles
 Practice Problems • What is the molarity of the solution produced when 145 g of sodium chloride is dissolved in sufficient water to prepare 2. 75 L of solution?
Practice Problems • What is the molarity of the solution produced when 145 g of sodium chloride is dissolved in sufficient water to prepare 2. 75 L of solution?
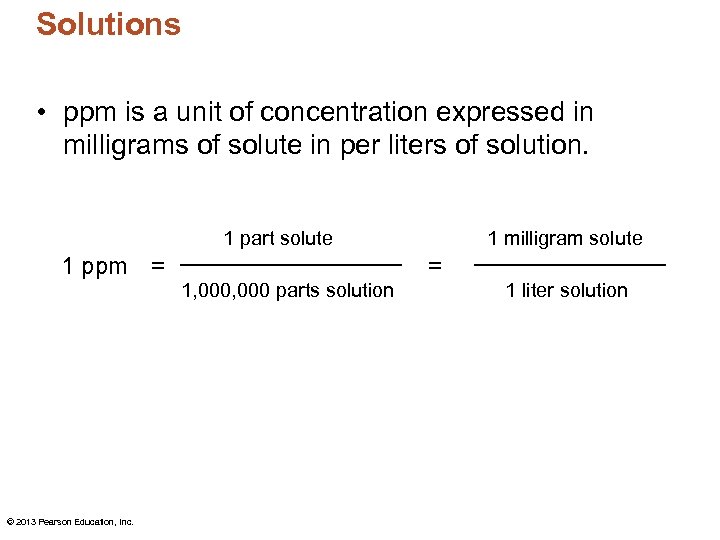 Solutions • ppm is a unit of concentration expressed in milligrams of solute in per liters of solution. 1 part solute 1 ppm = © 2013 Pearson Education, Inc. 1, 000 parts solution 1 milligram solute = 1 liter solution
Solutions • ppm is a unit of concentration expressed in milligrams of solute in per liters of solution. 1 part solute 1 ppm = © 2013 Pearson Education, Inc. 1, 000 parts solution 1 milligram solute = 1 liter solution
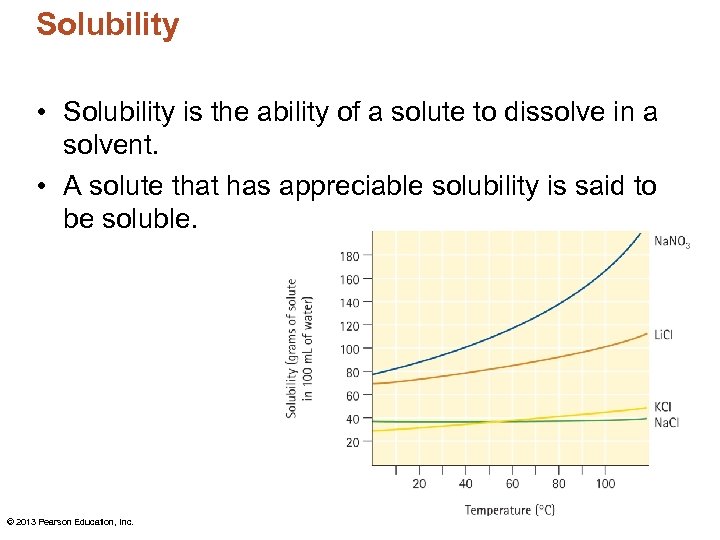 Solubility • Solubility is the ability of a solute to dissolve in a solvent. • A solute that has appreciable solubility is said to be soluble. © 2013 Pearson Education, Inc.
Solubility • Solubility is the ability of a solute to dissolve in a solvent. • A solute that has appreciable solubility is said to be soluble. © 2013 Pearson Education, Inc.
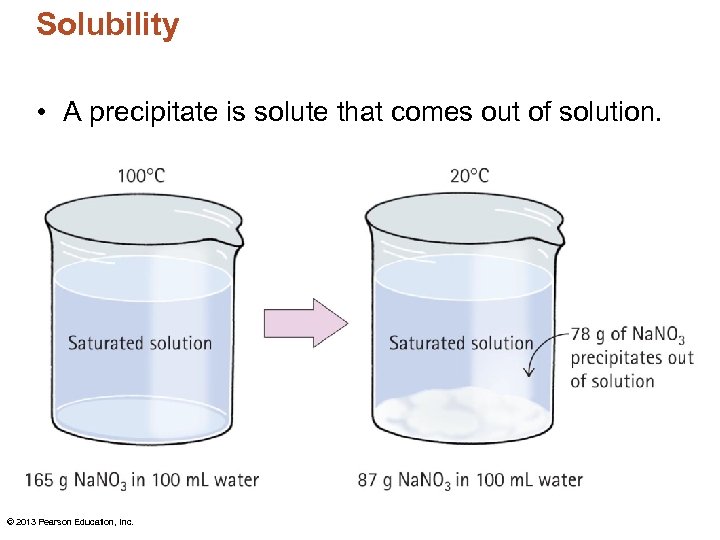 Solubility • A precipitate is solute that comes out of solution. © 2013 Pearson Education, Inc.
Solubility • A precipitate is solute that comes out of solution. © 2013 Pearson Education, Inc.
 Temperature • Solutions of gases in liquids are greatly affected by changes in temperature. – As temperature increases, the kinetic energy of the solute gas becomes greater. • As the temperature increases, the solubility of a gas in a liquid decreases.
Temperature • Solutions of gases in liquids are greatly affected by changes in temperature. – As temperature increases, the kinetic energy of the solute gas becomes greater. • As the temperature increases, the solubility of a gas in a liquid decreases.
 Temperature • Solubility of solids in liquids increases as the temperature increases. – Relationship depends on the energy change during solution formation. – If the temperature drops when the solute and solvent are mixed, raising the temperature will increase the solubility.
Temperature • Solubility of solids in liquids increases as the temperature increases. – Relationship depends on the energy change during solution formation. – If the temperature drops when the solute and solvent are mixed, raising the temperature will increase the solubility.
 Solubility CHECK YOUR NEIGHBOR The amount of oxygen, O 2, dissolved in the waters of the Arctic Ocean is –––– the amount of oxygen dissolved in warm tropical waters. A. B. C. D. greater than about equal to less than It depends on other factors. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
Solubility CHECK YOUR NEIGHBOR The amount of oxygen, O 2, dissolved in the waters of the Arctic Ocean is –––– the amount of oxygen dissolved in warm tropical waters. A. B. C. D. greater than about equal to less than It depends on other factors. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
 Chapter 12 Review CHECK YOUR NEIGHBOR By mass, water is 88. 88% oxygen. So, why can't we breathe water? Hint: What is the elemental formula for the oxygen we breathe and the chemical formula for water? Explain your answer to your neighbor. © 2013 Pearson Education, Inc.
Chapter 12 Review CHECK YOUR NEIGHBOR By mass, water is 88. 88% oxygen. So, why can't we breathe water? Hint: What is the elemental formula for the oxygen we breathe and the chemical formula for water? Explain your answer to your neighbor. © 2013 Pearson Education, Inc.


