cd7b9cc2c2eddfd4edae517e6bded61a.ppt
- Количество слайдов: 42
 The Failing Clinical Trial Enterprise in the US: Efforts of CTTI to Improve our National and Global Evidence Generating Capability Robert M Califf MD Vice Chancellor for Clinical Research, Duke University
The Failing Clinical Trial Enterprise in the US: Efforts of CTTI to Improve our National and Global Evidence Generating Capability Robert M Califf MD Vice Chancellor for Clinical Research, Duke University
 The Official Talk
The Official Talk
 US Clinical trials in crisis w. Trial start-up times lengthening w. Enrollment slowing w. Costs increasing w. Many investigators pulling out of clinical research 3
US Clinical trials in crisis w. Trial start-up times lengthening w. Enrollment slowing w. Costs increasing w. Many investigators pulling out of clinical research 3
 4
4
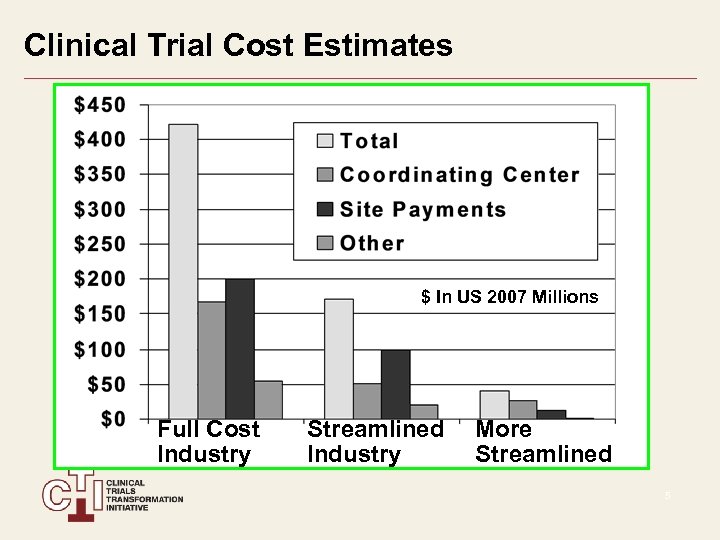 Clinical Trial Cost Estimates $ In US 2007 Millions Full Cost Industry Streamlined Industry More Streamlined 5
Clinical Trial Cost Estimates $ In US 2007 Millions Full Cost Industry Streamlined Industry More Streamlined 5
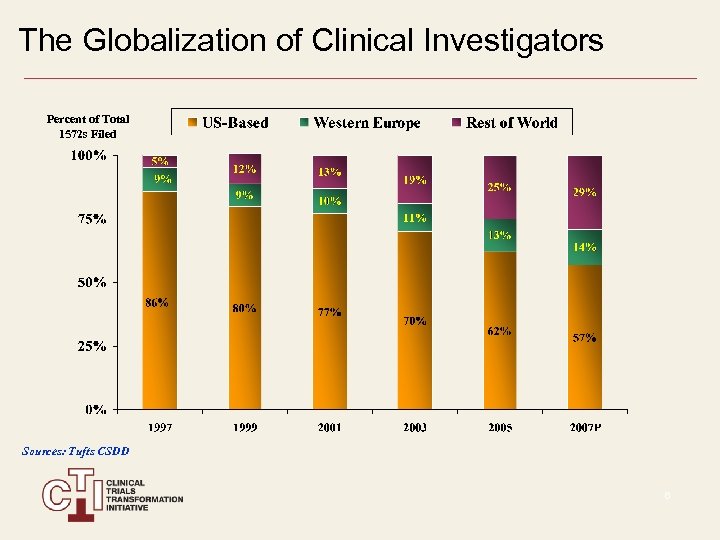 The Globalization of Clinical Investigators Percent of Total 1572 s Filed Sources: Tufts CSDD 6
The Globalization of Clinical Investigators Percent of Total 1572 s Filed Sources: Tufts CSDD 6
 Which Treatment is Best for Whom? High-Quality Evidence is Scarce 7 Tricoci P et al. JAMA 2009; 301: 831 -41 7
Which Treatment is Best for Whom? High-Quality Evidence is Scarce 7 Tricoci P et al. JAMA 2009; 301: 831 -41 7
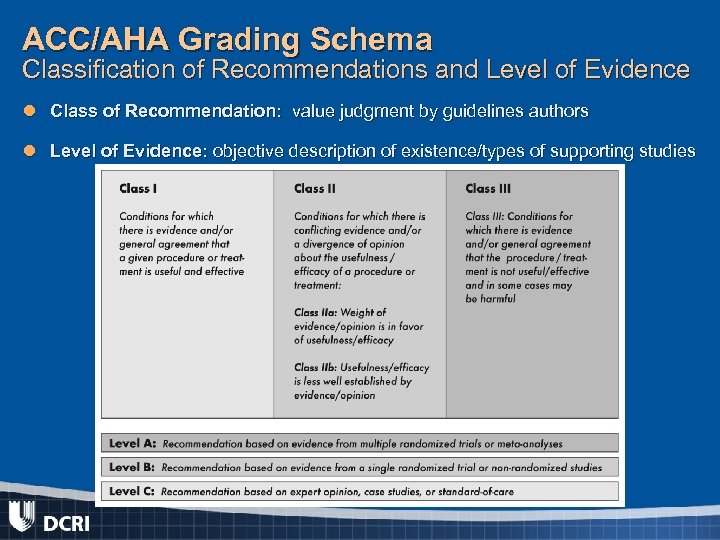 ACC/AHA Grading Schema Classification of Recommendations and Level of Evidence l Class of Recommendation: value judgment by guidelines authors l Level of Evidence: objective description of existence/types of supporting studies
ACC/AHA Grading Schema Classification of Recommendations and Level of Evidence l Class of Recommendation: value judgment by guidelines authors l Level of Evidence: objective description of existence/types of supporting studies
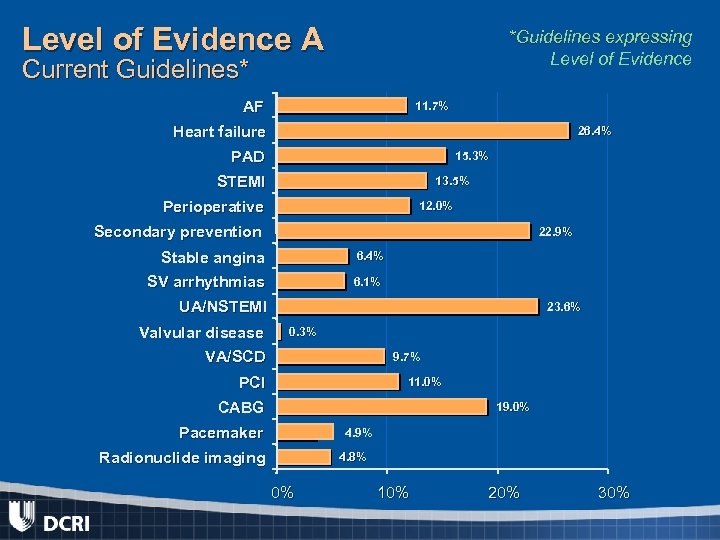 Level of Evidence A *Guidelines expressing Level of Evidence Current Guidelines* AF 11. 7% Heart failure 26. 4% PAD 15. 3% STEMI 13. 5% Perioperative 12. 0% Secondary prevention 22. 9% Stable angina 6. 4% SV arrhythmias 6. 1% UA/NSTEMI Valvular disease 23. 6% 0. 3% VA/SCD 9. 7% PCI 11. 0% CABG 19. 0% Pacemaker 4. 9% Radionuclide imaging 4. 8% 0% 10% 20% 30%
Level of Evidence A *Guidelines expressing Level of Evidence Current Guidelines* AF 11. 7% Heart failure 26. 4% PAD 15. 3% STEMI 13. 5% Perioperative 12. 0% Secondary prevention 22. 9% Stable angina 6. 4% SV arrhythmias 6. 1% UA/NSTEMI Valvular disease 23. 6% 0. 3% VA/SCD 9. 7% PCI 11. 0% CABG 19. 0% Pacemaker 4. 9% Radionuclide imaging 4. 8% 0% 10% 20% 30%
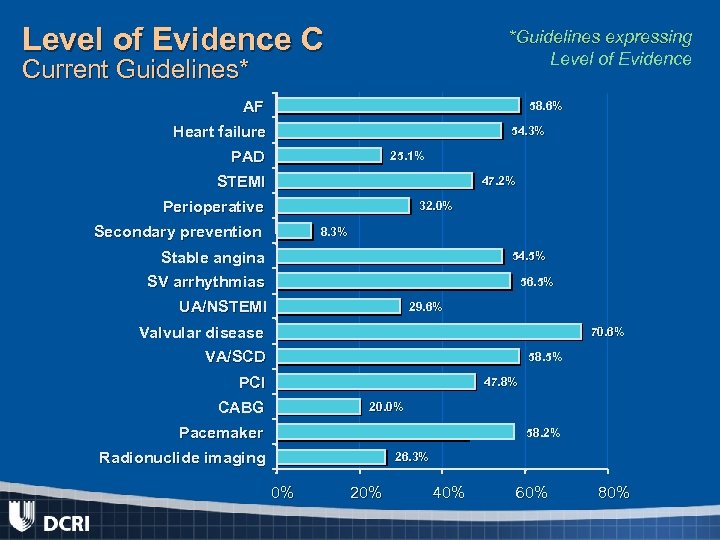 Level of Evidence C *Guidelines expressing Level of Evidence Current Guidelines* AF 58. 6% Heart failure 54. 3% PAD 25. 1% STEMI 47. 2% Perioperative 32. 0% Secondary prevention 8. 3% Stable angina 54. 5% SV arrhythmias 56. 5% UA/NSTEMI 29. 6% Valvular disease 70. 6% VA/SCD 58. 5% PCI 47. 8% CABG 20. 0% Pacemaker 58. 2% Radionuclide imaging 26. 3% 0% 20% 40% 60% 80%
Level of Evidence C *Guidelines expressing Level of Evidence Current Guidelines* AF 58. 6% Heart failure 54. 3% PAD 25. 1% STEMI 47. 2% Perioperative 32. 0% Secondary prevention 8. 3% Stable angina 54. 5% SV arrhythmias 56. 5% UA/NSTEMI 29. 6% Valvular disease 70. 6% VA/SCD 58. 5% PCI 47. 8% CABG 20. 0% Pacemaker 58. 2% Radionuclide imaging 26. 3% 0% 20% 40% 60% 80%
 It’s a “Systems Problem” All members of the clinical research enterprise have played a part in this problem Fixing it will require a collaborative effort w. FDA/global regulators w. Industry w. Academia/NIH w. Investigators in clinical practice w. Consumers 11
It’s a “Systems Problem” All members of the clinical research enterprise have played a part in this problem Fixing it will require a collaborative effort w. FDA/global regulators w. Industry w. Academia/NIH w. Investigators in clinical practice w. Consumers 11
 A collaborative effort to find solutions U. S. FDA’s Office of Critical Path Programs established a public-private partnership: The Clinical Trials Transformation Initiative (CTTI) All stakeholders involved Through a memorandum of understanding with FDA, Duke University serves as the host of CTTI 12
A collaborative effort to find solutions U. S. FDA’s Office of Critical Path Programs established a public-private partnership: The Clinical Trials Transformation Initiative (CTTI) All stakeholders involved Through a memorandum of understanding with FDA, Duke University serves as the host of CTTI 12
 Executive Committee Co-Chairs: Rob Califf(Duke) and Rachel Behrman(FDA) Academia: David De. Mets At-large representative: Ken Getz FDA: Bob Temple, CDER and Bram Zuckerman, CDRH Industry: Glenn Gormley, Jay Siegel, Susan Alpert, Alberto Grignolo Patient representative: Nancy Roach NIH liaison: Amy Patterson Non-US regulatory liaison: Hans-Georg Eichler, EMEA Ex-Officio Members: James Ferguson and Briggs Morrison, Steering Committee Co-chairs; Judith Kramer, Executive Director 13
Executive Committee Co-Chairs: Rob Califf(Duke) and Rachel Behrman(FDA) Academia: David De. Mets At-large representative: Ken Getz FDA: Bob Temple, CDER and Bram Zuckerman, CDRH Industry: Glenn Gormley, Jay Siegel, Susan Alpert, Alberto Grignolo Patient representative: Nancy Roach NIH liaison: Amy Patterson Non-US regulatory liaison: Hans-Georg Eichler, EMEA Ex-Officio Members: James Ferguson and Briggs Morrison, Steering Committee Co-chairs; Judith Kramer, Executive Director 13
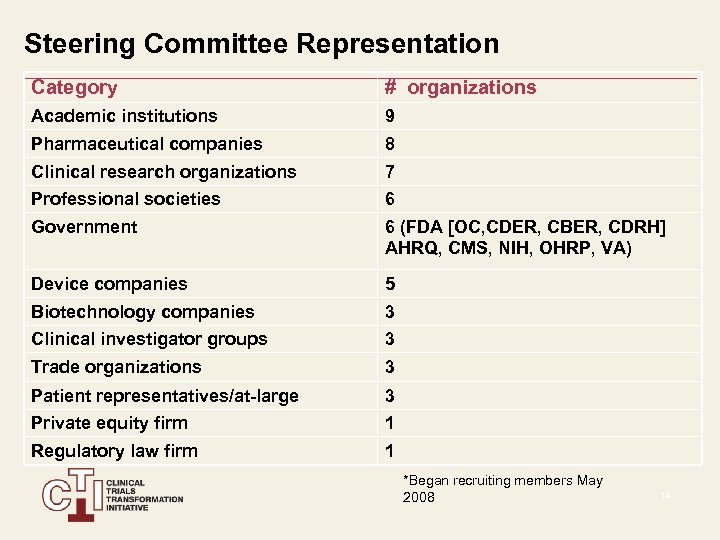 Steering Committee Representation Category # organizations Academic institutions 9 Pharmaceutical companies 8 Clinical research organizations 7 Professional societies 6 Government 6 (FDA [OC, CDER, CBER, CDRH] AHRQ, CMS, NIH, OHRP, VA) Device companies 5 Biotechnology companies 3 Clinical investigator groups 3 Trade organizations 3 Patient representatives/at-large 3 Private equity firm 1 Regulatory law firm 1 *Began recruiting members May 2008 14
Steering Committee Representation Category # organizations Academic institutions 9 Pharmaceutical companies 8 Clinical research organizations 7 Professional societies 6 Government 6 (FDA [OC, CDER, CBER, CDRH] AHRQ, CMS, NIH, OHRP, VA) Device companies 5 Biotechnology companies 3 Clinical investigator groups 3 Trade organizations 3 Patient representatives/at-large 3 Private equity firm 1 Regulatory law firm 1 *Began recruiting members May 2008 14
 Mission To identify practices that through broad adoption will increase the quality and efficiency of clinical trials 15
Mission To identify practices that through broad adoption will increase the quality and efficiency of clinical trials 15
 Scope CTTI will generate evidence about how to improve the design and execution of clinical trials CTTI will foster widespread change based on evidence CTTI was created to address a crisis for US clinical research, however… w. Trials and issues are global w. CTTI seeks to identify practice improvements that can be applied internationally w. CTTI is engaging international collaborators 16
Scope CTTI will generate evidence about how to improve the design and execution of clinical trials CTTI will foster widespread change based on evidence CTTI was created to address a crisis for US clinical research, however… w. Trials and issues are global w. CTTI seeks to identify practice improvements that can be applied internationally w. CTTI is engaging international collaborators 16
 Strategy to Accomplish our Mission Aggressively pursue development of evidence w. Conduct “research on research” w. Pursue collaborative activities with other organizations sharing similar goals Parallel activities: w. Systematically analyze the clinical trials process and potential impact of our activities w. Maintain awareness of other efforts w. Promote adoption of CTTI recommendations 17
Strategy to Accomplish our Mission Aggressively pursue development of evidence w. Conduct “research on research” w. Pursue collaborative activities with other organizations sharing similar goals Parallel activities: w. Systematically analyze the clinical trials process and potential impact of our activities w. Maintain awareness of other efforts w. Promote adoption of CTTI recommendations 17
 Initial Priority Areas* for Research on Research Design principles Data quality and quantity (including monitoring) Study start-up Adverse event reporting *Defined by CTTI’s Executive Committee 18
Initial Priority Areas* for Research on Research Design principles Data quality and quantity (including monitoring) Study start-up Adverse event reporting *Defined by CTTI’s Executive Committee 18
 Status of CTTI Projects 2 Ongoing Projects (with 7 workstreams) w. Effective and efficient monitoring as a component of quality assurance in the conduct of clinical trials w. Improving the system of reporting and interpreting unexpected serious adverse events (SAEs) to investigators conducting research under an IND 2 Project plans in development Executive and Steering Committees held a brainstorming session in September 3 Collaborations established 19
Status of CTTI Projects 2 Ongoing Projects (with 7 workstreams) w. Effective and efficient monitoring as a component of quality assurance in the conduct of clinical trials w. Improving the system of reporting and interpreting unexpected serious adverse events (SAEs) to investigators conducting research under an IND 2 Project plans in development Executive and Steering Committees held a brainstorming session in September 3 Collaborations established 19
 Effective and Efficient Monitoring Project Goal w Identify best practices and provide sensible criteria to help sponsors select the most appropriate monitoring methods for a trial, thereby improving quality while optimizing resources Specific objectives 1. Describe the range of current monitoring practices and examine factors that drive their adoption 2. Define key quality objectives for clinical trials 3. Illustrate strengths and weaknesses of the various monitoring practices in meeting quality objectives for a range of clinical trial settings 20
Effective and Efficient Monitoring Project Goal w Identify best practices and provide sensible criteria to help sponsors select the most appropriate monitoring methods for a trial, thereby improving quality while optimizing resources Specific objectives 1. Describe the range of current monitoring practices and examine factors that drive their adoption 2. Define key quality objectives for clinical trials 3. Illustrate strengths and weaknesses of the various monitoring practices in meeting quality objectives for a range of clinical trial settings 20
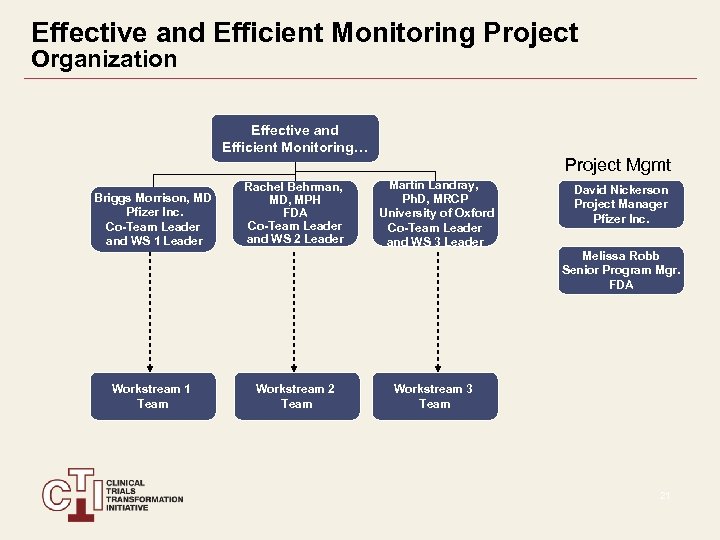 Effective and Efficient Monitoring Project Organization Effective and Efficient Monitoring… Project Mgmt Briggs Morrison, MD Pfizer Inc. Co-Team Leader and WS 1 Leader Rachel Behrman, MD, MPH FDA Co-Team Leader and WS 2 Leader Martin Landray, Ph. D, MRCP University of Oxford Co-Team Leader and WS 3 Leader David Nickerson Project Manager Pfizer Inc. Melissa Robb Senior Program Mgr. FDA Workstream 1 Team Workstream 2 Team Workstream 3 Team 21
Effective and Efficient Monitoring Project Organization Effective and Efficient Monitoring… Project Mgmt Briggs Morrison, MD Pfizer Inc. Co-Team Leader and WS 1 Leader Rachel Behrman, MD, MPH FDA Co-Team Leader and WS 2 Leader Martin Landray, Ph. D, MRCP University of Oxford Co-Team Leader and WS 3 Leader David Nickerson Project Manager Pfizer Inc. Melissa Robb Senior Program Mgr. FDA Workstream 1 Team Workstream 2 Team Workstream 3 Team 21
 Improving SAE Reporting to IND Investigators Goal: To generate empirical evidence about the current US system for reporting SAEs to investigators under an IND w Consider potential modifications of the current system to more efficiently and effectively inform investigators of these events 22
Improving SAE Reporting to IND Investigators Goal: To generate empirical evidence about the current US system for reporting SAEs to investigators under an IND w Consider potential modifications of the current system to more efficiently and effectively inform investigators of these events 22
 Improving SAE Reporting to IND Investigators Subprojects 1. Document current range of sponsor practices for: a. Reporting unexpected SAEs to investigators; b. Oversight of product safety (eg DSMBs, safety committees) 2. a. Quantify investigators’ time spent receiving, interpreting, and communicating individual expedited reports b. Assess perceived value to investigators of individual expedited reports in updating product’s risk profile 3. Compare current practice of submitting individual SAEs with an alternative approach based on European Commission’s guidance 4. Convene an expert group to integrate results and recommend ways to optimize reporting of SAEs to investigators and assure subject protection 23
Improving SAE Reporting to IND Investigators Subprojects 1. Document current range of sponsor practices for: a. Reporting unexpected SAEs to investigators; b. Oversight of product safety (eg DSMBs, safety committees) 2. a. Quantify investigators’ time spent receiving, interpreting, and communicating individual expedited reports b. Assess perceived value to investigators of individual expedited reports in updating product’s risk profile 3. Compare current practice of submitting individual SAEs with an alternative approach based on European Commission’s guidance 4. Convene an expert group to integrate results and recommend ways to optimize reporting of SAEs to investigators and assure subject protection 23
 Collaborations Use of clinical trials in evaluation of comparative effectiveness w. Collaboration between the Center for Medical Technology Policy (CMTP), Pragmatic Approaches to Comparative Effectiveness (PACE), and CTTI w. Expert meeting held May 6, 2009—directed to policymakers — New approaches to clinical trials will make them more attractive for comparative effectiveness research — Manuscript proposing increased operational efficiency, analytical efficiency, and generalizability of clinical trials published in Aug. 4 th issue Annals of Internal Medicine 24
Collaborations Use of clinical trials in evaluation of comparative effectiveness w. Collaboration between the Center for Medical Technology Policy (CMTP), Pragmatic Approaches to Comparative Effectiveness (PACE), and CTTI w. Expert meeting held May 6, 2009—directed to policymakers — New approaches to clinical trials will make them more attractive for comparative effectiveness research — Manuscript proposing increased operational efficiency, analytical efficiency, and generalizability of clinical trials published in Aug. 4 th issue Annals of Internal Medicine 24
 Collaborations (continued) FDA-initiated training course directed to clinical investigators Collaborative effort to standardize definitions and data collection methods/case report forms for cardiovascular trials w. FDA-initiated effort involving academics, industry, CDISC, and HL 7 25
Collaborations (continued) FDA-initiated training course directed to clinical investigators Collaborative effort to standardize definitions and data collection methods/case report forms for cardiovascular trials w. FDA-initiated effort involving academics, industry, CDISC, and HL 7 25
 How do we effect widespread change? Problems with clinical trials widely recognized Need to go beyond elucidation of problems to effect change w. Current behavior often driven by incentives and fears not consistent with the goal of increased efficiency w. Decision makers at different levels have variable understanding of the “big picture” — Organizations engage in activities that may not add value — Trade off of cost vs. value not explicit w. Wide spread perception that clinical trials just take that long and cost that much! 26
How do we effect widespread change? Problems with clinical trials widely recognized Need to go beyond elucidation of problems to effect change w. Current behavior often driven by incentives and fears not consistent with the goal of increased efficiency w. Decision makers at different levels have variable understanding of the “big picture” — Organizations engage in activities that may not add value — Trade off of cost vs. value not explicit w. Wide spread perception that clinical trials just take that long and cost that much! 26
 How do we effect widespread change? CTTI’s Approach Involve all sectors in selection, conduct, and interpretation of projects Explore the business case for change from different perspectives Keep dialogue open across sectors Provide evidence that can influence regulatory guidance Attempt to create a “level playing field” when recommending change (i. e. don’t place a single organization or sector at risk) 27
How do we effect widespread change? CTTI’s Approach Involve all sectors in selection, conduct, and interpretation of projects Explore the business case for change from different perspectives Keep dialogue open across sectors Provide evidence that can influence regulatory guidance Attempt to create a “level playing field” when recommending change (i. e. don’t place a single organization or sector at risk) 27
 For more information…. CTTI Website-Home wwww. trialstransformation. org Projects wwww. trialstransformation. org/projects Member organizations wwww. trialstransformation. org/members/member-organizations/ How to join wwww. trialstransformation. org/join 28
For more information…. CTTI Website-Home wwww. trialstransformation. org Projects wwww. trialstransformation. org/projects Member organizations wwww. trialstransformation. org/members/member-organizations/ How to join wwww. trialstransformation. org/join 28
 Clinical Trials 2008; 5: 38 -39 Sensible Guidelines Conference January 25 -26, 2007 Sensible guidelines for the conduct of large scientific method randomized trials n “For aof evidence-basedthat is at the heart medicine, no n Large trials are important n Complexity and cost increasing good evidence that the layers of complexity, approvals, processes, and laws to protect subjects have actually achieved their purpose. n Excess data, number of visits, onsite monitoring n Layers of ethics approvals What is clear is that such processes are extremely expensive and delay studies. n Goal: stimulate reform and simplification of clinical trials procedures, while enhancing patient safety and autonomy, improving the scientific validity and integrity of trials and making them more affordable. ” GCP ≠ good, or clinically relevant, or even practical n n
Clinical Trials 2008; 5: 38 -39 Sensible Guidelines Conference January 25 -26, 2007 Sensible guidelines for the conduct of large scientific method randomized trials n “For aof evidence-basedthat is at the heart medicine, no n Large trials are important n Complexity and cost increasing good evidence that the layers of complexity, approvals, processes, and laws to protect subjects have actually achieved their purpose. n Excess data, number of visits, onsite monitoring n Layers of ethics approvals What is clear is that such processes are extremely expensive and delay studies. n Goal: stimulate reform and simplification of clinical trials procedures, while enhancing patient safety and autonomy, improving the scientific validity and integrity of trials and making them more affordable. ” GCP ≠ good, or clinically relevant, or even practical n n
 Clinical and Translational Science Award (CTSA) At peak a $500 million investment by the NIH in translating scientific discoveries to better human health
Clinical and Translational Science Award (CTSA) At peak a $500 million investment by the NIH in translating scientific discoveries to better human health
 My Opinion—This does not represent CTTI
My Opinion—This does not represent CTTI
 Disruptive innovation is needed to create a very different system based on electronic data collection in practice with quality built in through a systematic approach.
Disruptive innovation is needed to create a very different system based on electronic data collection in practice with quality built in through a systematic approach.
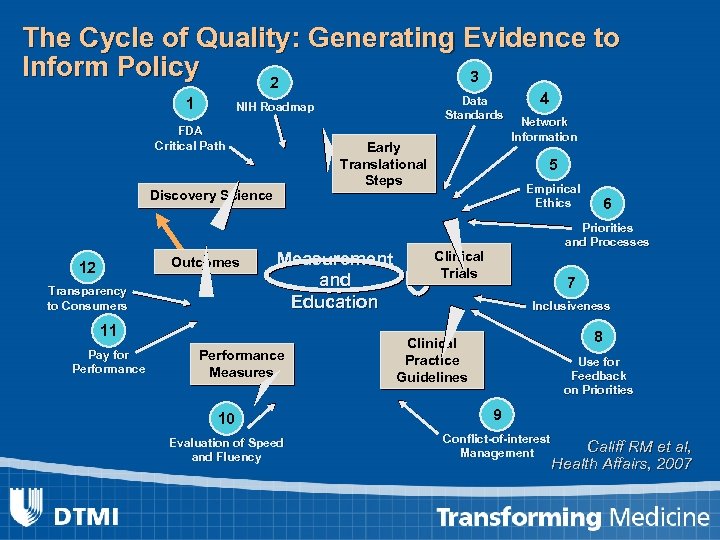 The Cycle of Quality: Generating Evidence to Inform Policy 3 2 1 FDA Critical Path Early Translational Steps Discovery Science Outcomes 12 Transparency to Consumers Measurement and Education 11 Pay for Performance Data Standards NIH Roadmap Performance Measures 4 Network Information 5 Empirical Ethics 6 Priorities and Processes Clinical Trials 7 Inclusiveness 8 Clinical Practice Guidelines Use for Feedback on Priorities 10 9 Evaluation of Speed and Fluency Conflict-of-interest Management Califf RM et al, Health Affairs, 2007
The Cycle of Quality: Generating Evidence to Inform Policy 3 2 1 FDA Critical Path Early Translational Steps Discovery Science Outcomes 12 Transparency to Consumers Measurement and Education 11 Pay for Performance Data Standards NIH Roadmap Performance Measures 4 Network Information 5 Empirical Ethics 6 Priorities and Processes Clinical Trials 7 Inclusiveness 8 Clinical Practice Guidelines Use for Feedback on Priorities 10 9 Evaluation of Speed and Fluency Conflict-of-interest Management Califf RM et al, Health Affairs, 2007
 The Learning Health System Ù Articulated goal of the Institute of Medicine Ù By implementing electronic health records, data warehouses and disease registries, every patient’s data will be used to further knowledge Ù This means that all places of practice will become research sites Ù Research must become a normal part of clinical practice, not something done separately from clinical practice (except for very special early phase and highly controlled types of studies)
The Learning Health System Ù Articulated goal of the Institute of Medicine Ù By implementing electronic health records, data warehouses and disease registries, every patient’s data will be used to further knowledge Ù This means that all places of practice will become research sites Ù Research must become a normal part of clinical practice, not something done separately from clinical practice (except for very special early phase and highly controlled types of studies)

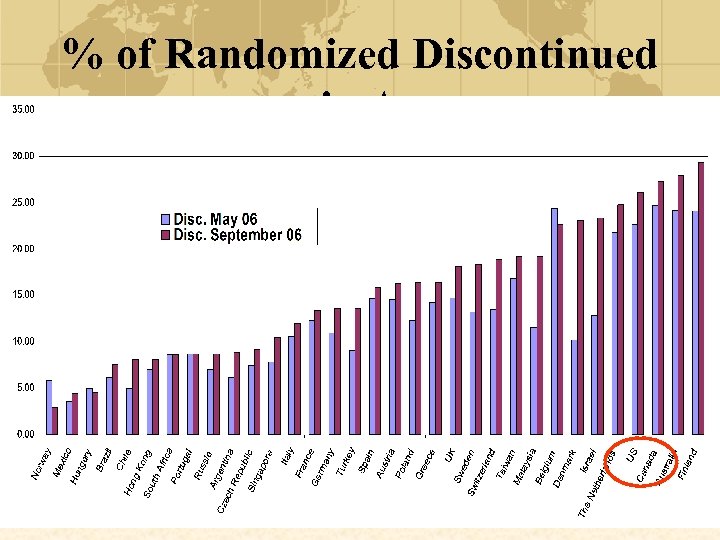 % of Randomized Discontinued in A
% of Randomized Discontinued in A
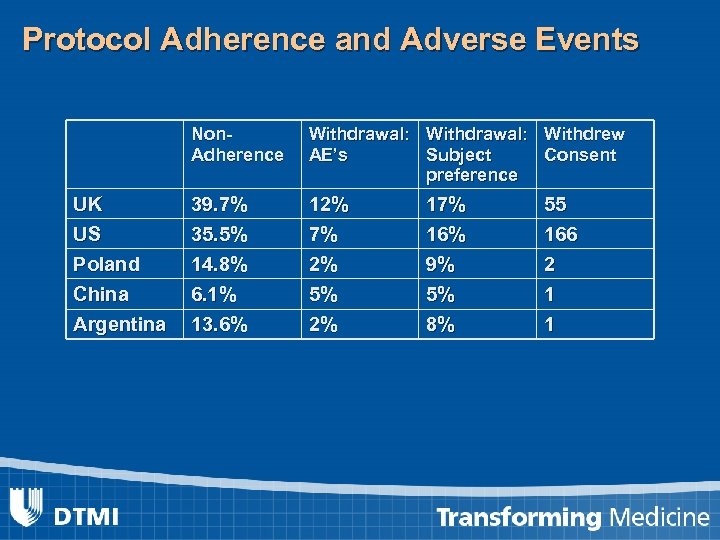 Protocol Adherence and Adverse Events Non. Adherence Withdrawal: Withdrew AE’s Subject Consent preference UK US 39. 7% 35. 5% 12% 7% 16% 55 166 Poland China 14. 8% 6. 1% 2% 5% 9% 5% 2 1 Argentina 13. 6% 2% 8% 1
Protocol Adherence and Adverse Events Non. Adherence Withdrawal: Withdrew AE’s Subject Consent preference UK US 39. 7% 35. 5% 12% 7% 16% 55 166 Poland China 14. 8% 6. 1% 2% 5% 9% 5% 2 1 Argentina 13. 6% 2% 8% 1
 Image Source: http: //www. pandora. ca/pictures 21/900666. jpg
Image Source: http: //www. pandora. ca/pictures 21/900666. jpg
 The Demise of Empires Ù Dominance at a point in time Ù Arrogance about superiority Ù Failure to pay attention to quality of work Ù Leaders content to “ride the wave” Ù Entrenched interests can buy stability through controlling laws and regulations Ù Inability to create or respond to innovation Ù Cost of transactions exceeds cost of actually doing the work!
The Demise of Empires Ù Dominance at a point in time Ù Arrogance about superiority Ù Failure to pay attention to quality of work Ù Leaders content to “ride the wave” Ù Entrenched interests can buy stability through controlling laws and regulations Ù Inability to create or respond to innovation Ù Cost of transactions exceeds cost of actually doing the work!
 Disruptive Innovation in Clinical Trials Ù Electronic health records Ù Data warehouses in integrated health systems Ù Learning health systems Ù Use same information for clinical care and research Ù Electronic permissions systems for participation in research Ù Evaluation of RESEARCH SITES on a periodic basis with constant electronic surveillance and periodic audits for cause
Disruptive Innovation in Clinical Trials Ù Electronic health records Ù Data warehouses in integrated health systems Ù Learning health systems Ù Use same information for clinical care and research Ù Electronic permissions systems for participation in research Ù Evaluation of RESEARCH SITES on a periodic basis with constant electronic surveillance and periodic audits for cause

 Thank you
Thank you


