3765198acae2a721606ddf60a3e3fdbc.ppt
- Количество слайдов: 40
 The diversity of safety reporting requirements and Systems in Asia 2010. 05. 17 Beijing China 1
The diversity of safety reporting requirements and Systems in Asia 2010. 05. 17 Beijing China 1
 Presenter Jean-Christophe DELUMEAU MD Ph. D Head of Pharmacovigilance Asia-Pacific Bayer Healthcare Global R&D Center Asia, Beijing +86 10 6536 0829 office phone +86 13910420935 Blackberry phone http: //www. linkedin. com/in/delumeau. phv@me. com Disclaimer; The views and opinions expressed in the following slides are those of the individual presenter and should not be attributed to DIA or Bayer Healthcare. 2
Presenter Jean-Christophe DELUMEAU MD Ph. D Head of Pharmacovigilance Asia-Pacific Bayer Healthcare Global R&D Center Asia, Beijing +86 10 6536 0829 office phone +86 13910420935 Blackberry phone http: //www. linkedin. com/in/delumeau. phv@me. com Disclaimer; The views and opinions expressed in the following slides are those of the individual presenter and should not be attributed to DIA or Bayer Healthcare. 2
 Harmonization in Pharmacovigilance ¢ ICSR submission requirements ¢ Electronic submission (E 2 B standards) ¢ Aggregate reports submission requirements ¢ Coding dictionary ¢ Risk Management Plan and Risk mitigation requirements 3
Harmonization in Pharmacovigilance ¢ ICSR submission requirements ¢ Electronic submission (E 2 B standards) ¢ Aggregate reports submission requirements ¢ Coding dictionary ¢ Risk Management Plan and Risk mitigation requirements 3
 Harmonization in Pharmacovigilance ICH countries and beyond ¢ ICH Guidelines and MEDDRA dictionary implemented in USA, EU and Japan and more or less followed by other countries ¢ Specific Risk Management requirements ¢ Europe ¢ USA ¢ Specific ICSR submission requirements e. g. ¢ France-specific causality assessment (imputabilite) ¢ Spain: Mandatory reporting in Spanish ¢ E 2 B submission of ICSRs from Global pharma databases ¢ USA and Canada ¢ All 27 countries of the European Union + Norway, Iceland Croatia 4
Harmonization in Pharmacovigilance ICH countries and beyond ¢ ICH Guidelines and MEDDRA dictionary implemented in USA, EU and Japan and more or less followed by other countries ¢ Specific Risk Management requirements ¢ Europe ¢ USA ¢ Specific ICSR submission requirements e. g. ¢ France-specific causality assessment (imputabilite) ¢ Spain: Mandatory reporting in Spanish ¢ E 2 B submission of ICSRs from Global pharma databases ¢ USA and Canada ¢ All 27 countries of the European Union + Norway, Iceland Croatia 4
 E 2 B systems for ICSR management 5
E 2 B systems for ICSR management 5
 WHO-UMC International Safety Survey ¢ Uppsala Monitoring Center appointed by WHO ¢ Preferred ICSR transmission standard: E 2 B ¢ Alternative: old INTIS standard 6
WHO-UMC International Safety Survey ¢ Uppsala Monitoring Center appointed by WHO ¢ Preferred ICSR transmission standard: E 2 B ¢ Alternative: old INTIS standard 6
 High degree of diversity in Asia ¢ Diversity of ICSR submission requirements ¢ Diversity of aggregate reports submission requirements ¢ Only a few countries are requesting RMPs ¢ Most countries are still using WHO-ART ¢ Only Japan is has an E 2 B safety data base, but E 2 B-J è Most AP countries are still using INTIS to forward ICSRs to the UMC è Electronic submission is not possible è An increasing number of countries request entering ICSRs into a country-specific web-based system 7
High degree of diversity in Asia ¢ Diversity of ICSR submission requirements ¢ Diversity of aggregate reports submission requirements ¢ Only a few countries are requesting RMPs ¢ Most countries are still using WHO-ART ¢ Only Japan is has an E 2 B safety data base, but E 2 B-J è Most AP countries are still using INTIS to forward ICSRs to the UMC è Electronic submission is not possible è An increasing number of countries request entering ICSRs into a country-specific web-based system 7
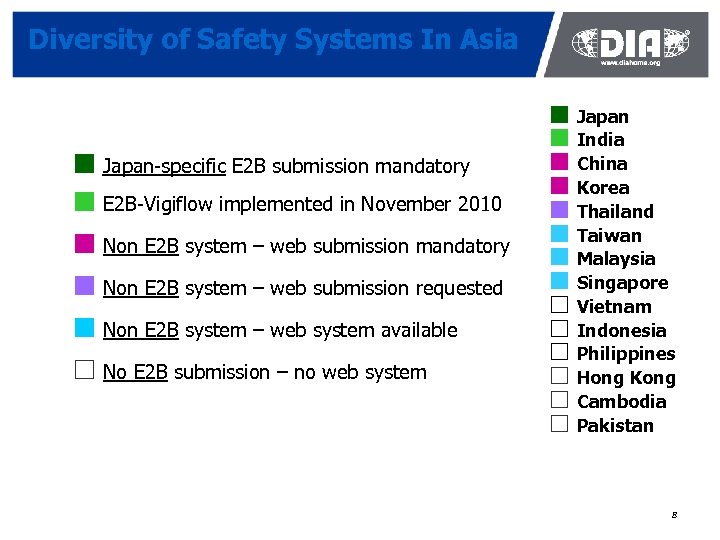 Diversity of Safety Systems In Asia ¢ Japan-specific E 2 B submission mandatory ¢ E 2 B-Vigiflow implemented in November 2010 ¢ Non E 2 B system – web submission mandatory ¢ Non E 2 B system – web submission requested ¢ Non E 2 B system – web system available £ No E 2 B submission – no web system ¢ Japan ¢ India ¢ China ¢ Korea ¢ Thailand ¢ Taiwan ¢ Malaysia ¢ Singapore £ Vietnam £ Indonesia £ Philippines £ Hong Kong £ Cambodia £ Pakistan 8
Diversity of Safety Systems In Asia ¢ Japan-specific E 2 B submission mandatory ¢ E 2 B-Vigiflow implemented in November 2010 ¢ Non E 2 B system – web submission mandatory ¢ Non E 2 B system – web submission requested ¢ Non E 2 B system – web system available £ No E 2 B submission – no web system ¢ Japan ¢ India ¢ China ¢ Korea ¢ Thailand ¢ Taiwan ¢ Malaysia ¢ Singapore £ Vietnam £ Indonesia £ Philippines £ Hong Kong £ Cambodia £ Pakistan 8
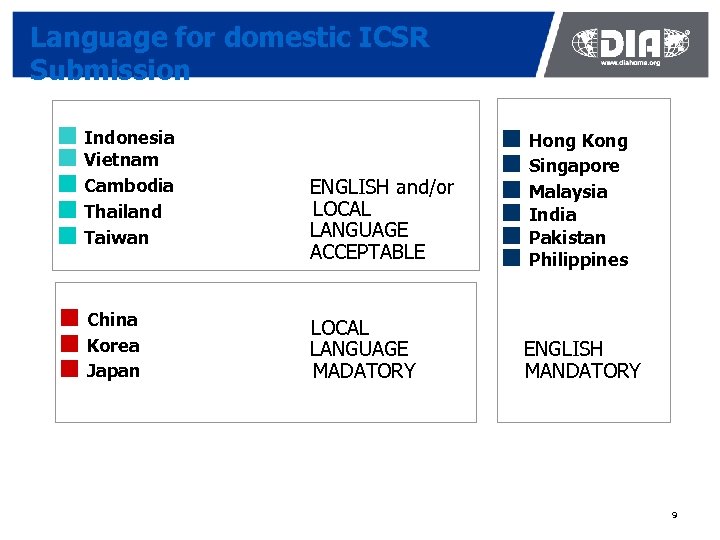 Language for domestic ICSR Submission ¢ Indonesia ¢ Vietnam ¢ Cambodia ¢ Thailand ¢ Taiwan ¢ China ¢ Korea ¢ Japan ENGLISH and/or LOCAL LANGUAGE ACCEPTABLE LOCAL LANGUAGE MADATORY ¢ Hong Kong ¢ Singapore ¢ Malaysia ¢ India ¢ Pakistan ¢ Philippines ENGLISH MANDATORY 9
Language for domestic ICSR Submission ¢ Indonesia ¢ Vietnam ¢ Cambodia ¢ Thailand ¢ Taiwan ¢ China ¢ Korea ¢ Japan ENGLISH and/or LOCAL LANGUAGE ACCEPTABLE LOCAL LANGUAGE MADATORY ¢ Hong Kong ¢ Singapore ¢ Malaysia ¢ India ¢ Pakistan ¢ Philippines ENGLISH MANDATORY 9
 ICSR submission requirements in Asia ¢ ICSR submission requirements vary considerably across countries depending upon ¢ Language requested for submission ¢ Domestic or foreign case ¢ Solicited or non-solicited ¢ Seriousness ¢ Causality ¢ Submission time frame ¢ Definition of the clock start ¢ Age of the product on the market ¢ Reference used for listedness 10
ICSR submission requirements in Asia ¢ ICSR submission requirements vary considerably across countries depending upon ¢ Language requested for submission ¢ Domestic or foreign case ¢ Solicited or non-solicited ¢ Seriousness ¢ Causality ¢ Submission time frame ¢ Definition of the clock start ¢ Age of the product on the market ¢ Reference used for listedness 10
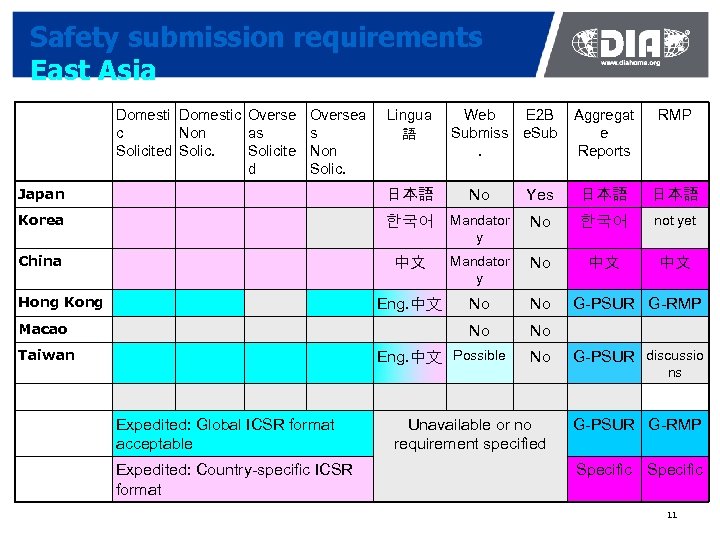 Safety submission requirements East Asia Domestic Overse c Non as Solicited Solicite d Oversea s Non Solic. Lingua 語 Web E 2 B Submiss e. Sub. Aggregat e Reports RMP Japan 日本語 No Yes 日本語 Korea 한국어 Mandator y No 한국어 not yet China 中文 Mandator y No 中文 中文 Eng. 中文 No No Hong Kong Macao Taiwan Eng. 中文 Possible Expedited: Global ICSR format acceptable Expedited: Country-specific ICSR format G-PSUR G-RMP No G-PSUR discussio Unavailable or no requirement specified G-PSUR G-RMP ns Specific 11
Safety submission requirements East Asia Domestic Overse c Non as Solicited Solicite d Oversea s Non Solic. Lingua 語 Web E 2 B Submiss e. Sub. Aggregat e Reports RMP Japan 日本語 No Yes 日本語 Korea 한국어 Mandator y No 한국어 not yet China 中文 Mandator y No 中文 中文 Eng. 中文 No No Hong Kong Macao Taiwan Eng. 中文 Possible Expedited: Global ICSR format acceptable Expedited: Country-specific ICSR format G-PSUR G-RMP No G-PSUR discussio Unavailable or no requirement specified G-PSUR G-RMP ns Specific 11
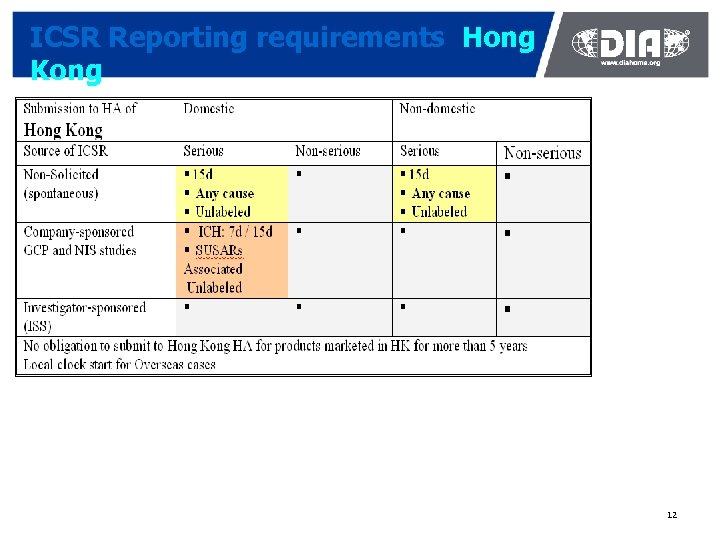 ICSR Reporting requirements Hong Kong 12
ICSR Reporting requirements Hong Kong 12
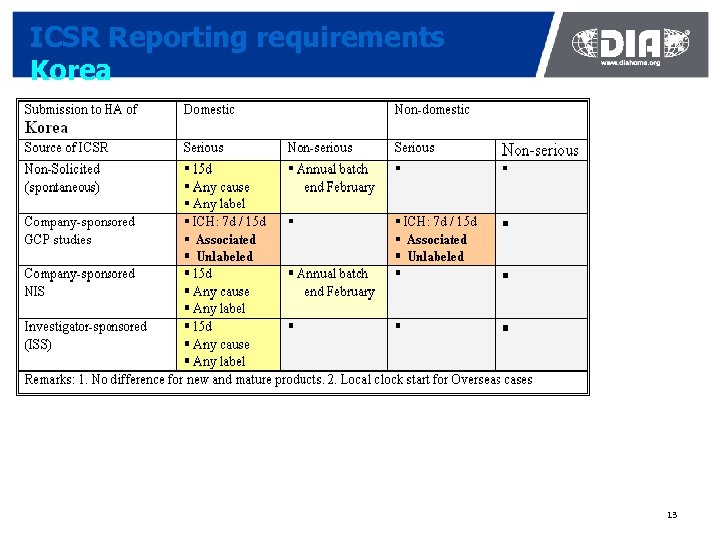 ICSR Reporting requirements Korea 13
ICSR Reporting requirements Korea 13
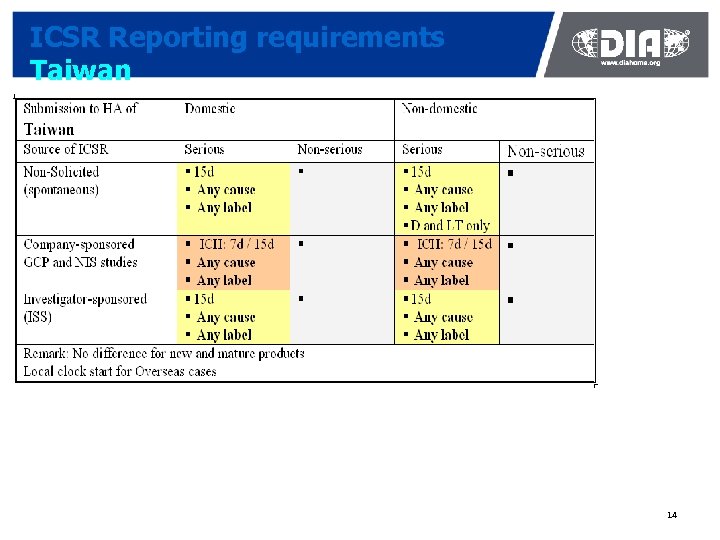 ICSR Reporting requirements Taiwan 14
ICSR Reporting requirements Taiwan 14
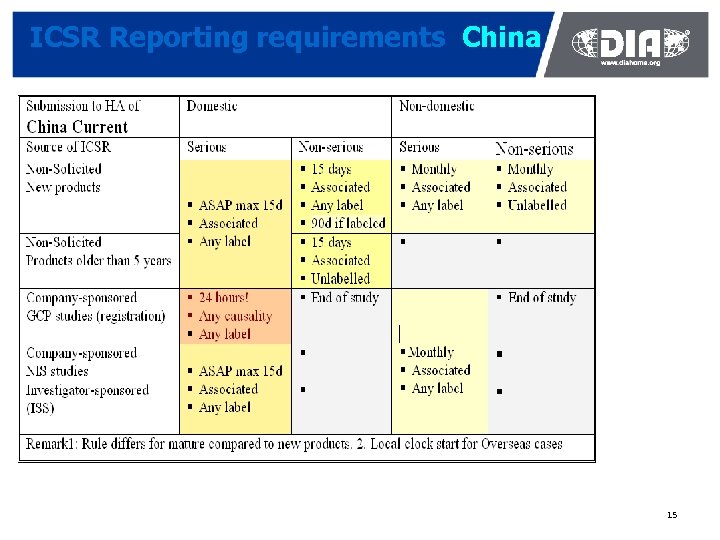 ICSR Reporting requirements China 15
ICSR Reporting requirements China 15
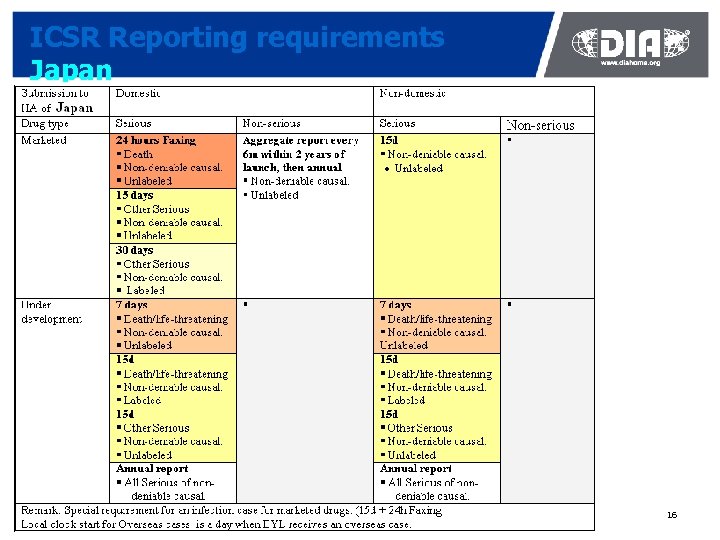 ICSR Reporting requirements Japan 16
ICSR Reporting requirements Japan 16
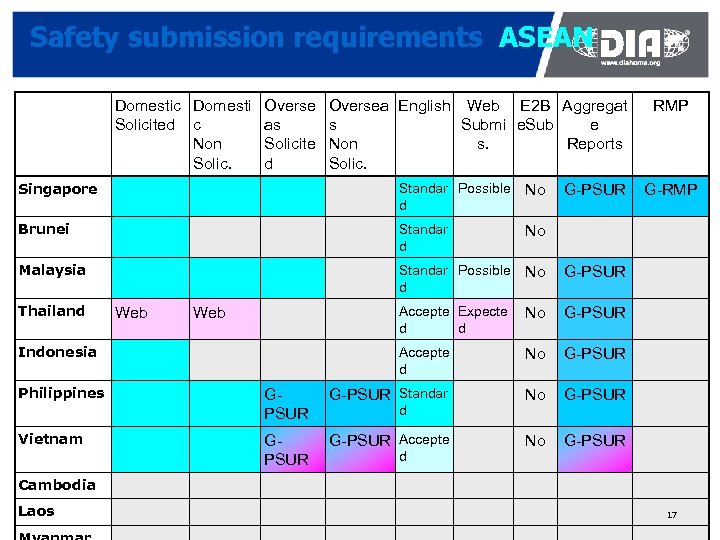 Safety submission requirements ASEAN Domestic Domesti Overse Solicited c as Non Solicite Solic. d Oversea English Web E 2 B Aggregat s Submi e. Sub e Non s. Reports Solic. Singapore Standar Possible d No Brunei Standar d No Malaysia Standar Possible d No G-PSUR Accepte Expecte d d No G-PSUR Accepte d No G-PSUR Standar No G-PSUR Accepte No RMP G-PSUR Thailand Web Indonesia Philippines Vietnam d d G-PSUR G-RMP Cambodia Laos 17
Safety submission requirements ASEAN Domestic Domesti Overse Solicited c as Non Solicite Solic. d Oversea English Web E 2 B Aggregat s Submi e. Sub e Non s. Reports Solic. Singapore Standar Possible d No Brunei Standar d No Malaysia Standar Possible d No G-PSUR Accepte Expecte d d No G-PSUR Accepte d No G-PSUR Standar No G-PSUR Accepte No RMP G-PSUR Thailand Web Indonesia Philippines Vietnam d d G-PSUR G-RMP Cambodia Laos 17
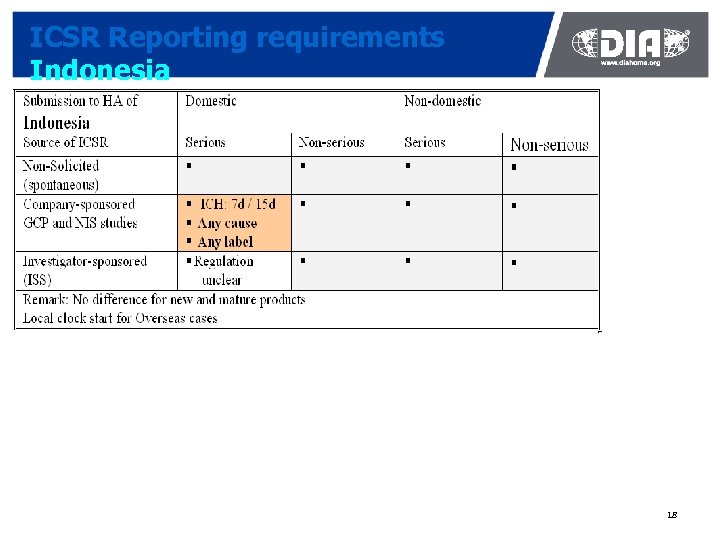 ICSR Reporting requirements Indonesia 18
ICSR Reporting requirements Indonesia 18
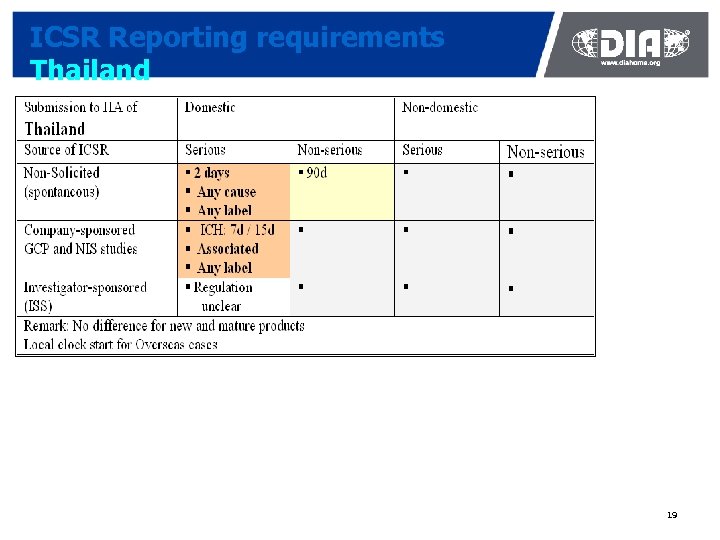 ICSR Reporting requirements Thailand 19
ICSR Reporting requirements Thailand 19
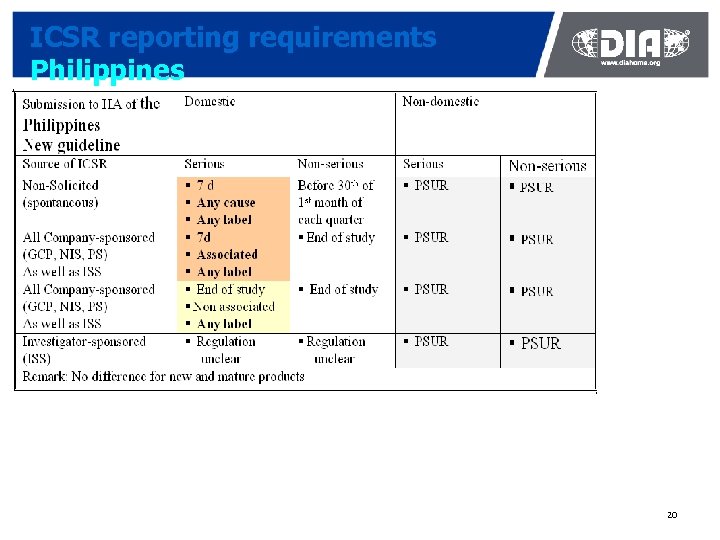 ICSR reporting requirements Philippines 20
ICSR reporting requirements Philippines 20
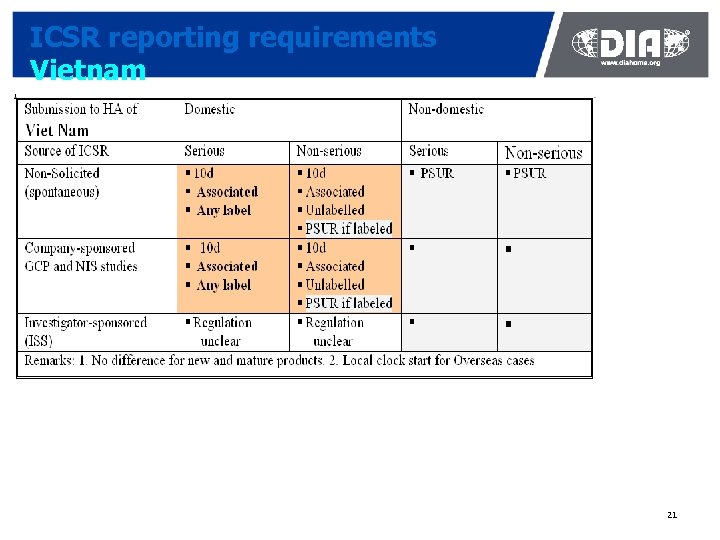 ICSR reporting requirements Vietnam 21
ICSR reporting requirements Vietnam 21
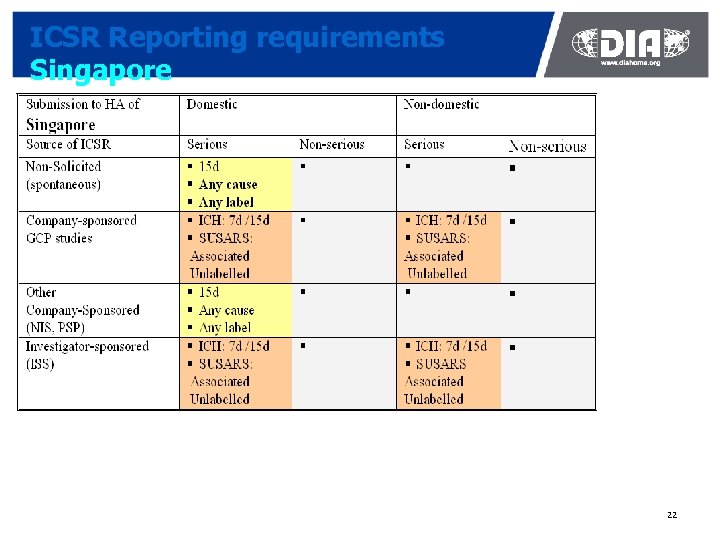 ICSR Reporting requirements Singapore 22
ICSR Reporting requirements Singapore 22
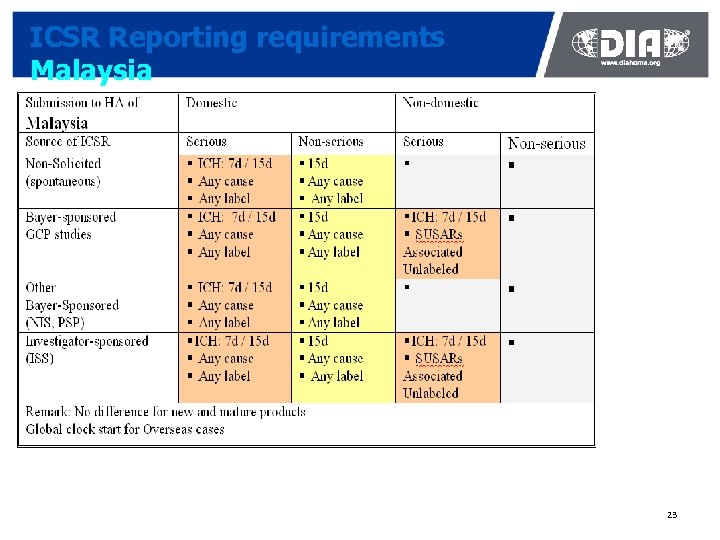 ICSR Reporting requirements Malaysia 23
ICSR Reporting requirements Malaysia 23
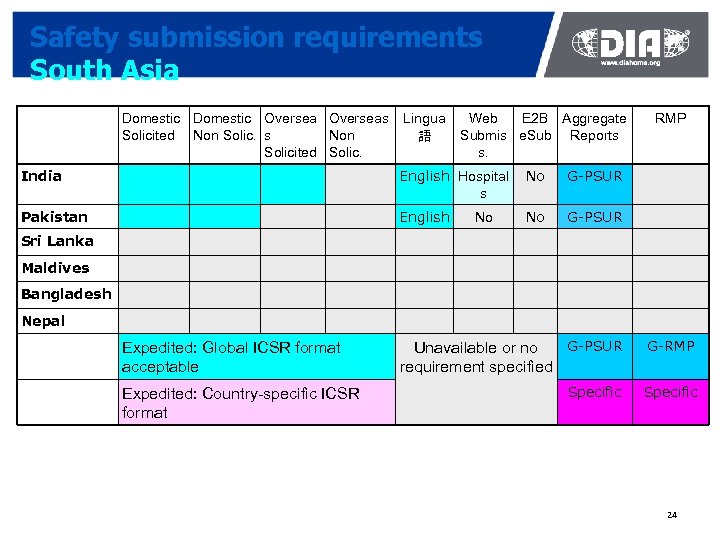 Safety submission requirements South Asia Domestic Overseas Solicited Non Solic. s Non Solicited Solic. Lingua 語 Web E 2 B Aggregate Submis e. Sub Reports s. India English Hospital s No G-PSUR Pakistan English No RMP G-PSUR No Sri Lanka Maldives Bangladesh Nepal Expedited: Global ICSR format acceptable Expedited: Country-specific ICSR format G-PSUR Unavailable or no requirement specified G-RMP Specific 24
Safety submission requirements South Asia Domestic Overseas Solicited Non Solic. s Non Solicited Solic. Lingua 語 Web E 2 B Aggregate Submis e. Sub Reports s. India English Hospital s No G-PSUR Pakistan English No RMP G-PSUR No Sri Lanka Maldives Bangladesh Nepal Expedited: Global ICSR format acceptable Expedited: Country-specific ICSR format G-PSUR Unavailable or no requirement specified G-RMP Specific 24
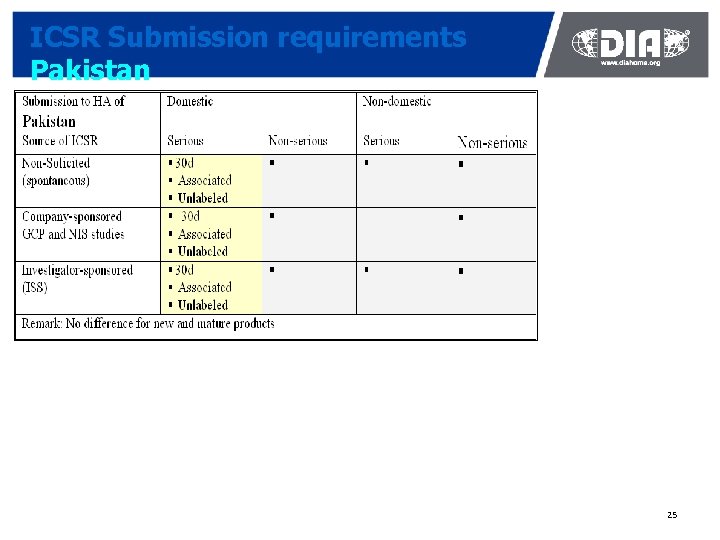 ICSR Submission requirements Pakistan 25
ICSR Submission requirements Pakistan 25
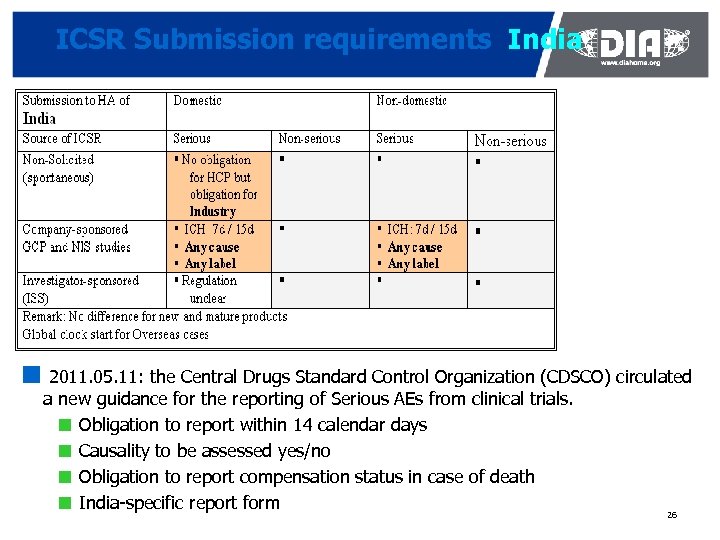 ICSR Submission requirements India ¢ 2011. 05. 11: the Central Drugs Standard Control Organization (CDSCO) circulated a new guidance for the reporting of Serious AEs from clinical trials. ¢ Obligation to report within 14 calendar days ¢ Causality to be assessed yes/no ¢ Obligation to report compensation status in case of death ¢ India-specific report form 26
ICSR Submission requirements India ¢ 2011. 05. 11: the Central Drugs Standard Control Organization (CDSCO) circulated a new guidance for the reporting of Serious AEs from clinical trials. ¢ Obligation to report within 14 calendar days ¢ Causality to be assessed yes/no ¢ Obligation to report compensation status in case of death ¢ India-specific report form 26
 ICSR Submission requirements India ¢ New reporting form proposed by the CDSCO 27
ICSR Submission requirements India ¢ New reporting form proposed by the CDSCO 27
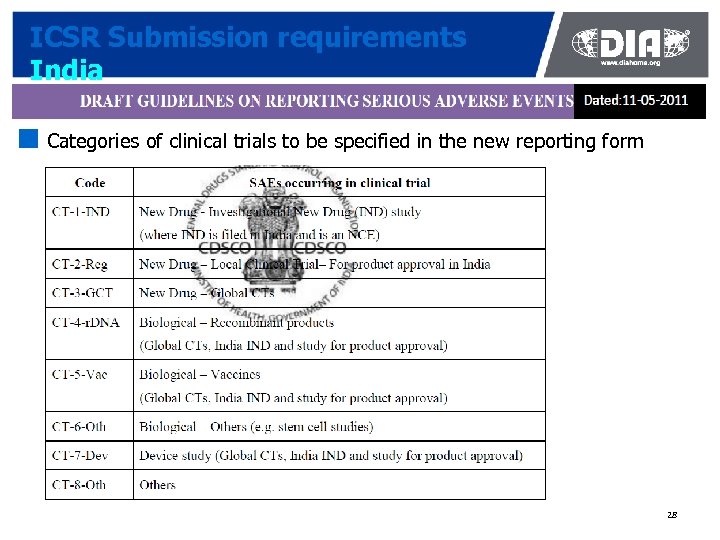 ICSR Submission requirements India ¢ Categories of clinical trials to be specified in the new reporting form 28
ICSR Submission requirements India ¢ Categories of clinical trials to be specified in the new reporting form 28
 Impact of country-specific requirements ¢ ICSR submission from global safety system is possible ¢ e-submission: not available in any AP country so far ¢ Printed on PDF or paper from the global company safety database ¢ ICSR submission via country-specific E 2 B system is possible ¢ Submission to the PMDA in Japan (日本語) ¢ ICSR submission via National web system is mandatory ¢ China (中文) ¢ Korea (한국어) ¢ Thailand (English acceptable) è Concern: more and more counties will make it mandatory 29
Impact of country-specific requirements ¢ ICSR submission from global safety system is possible ¢ e-submission: not available in any AP country so far ¢ Printed on PDF or paper from the global company safety database ¢ ICSR submission via country-specific E 2 B system is possible ¢ Submission to the PMDA in Japan (日本語) ¢ ICSR submission via National web system is mandatory ¢ China (中文) ¢ Korea (한국어) ¢ Thailand (English acceptable) è Concern: more and more counties will make it mandatory 29
 Impact of country-specific requirements Japan ¢ ICSR submitted electronically to the PMDA (E 2 B-J standard) ¢ Japan-specific requirements e. g. Japanese language, specific reporting form, specific causality assessment rules ¢ Most companies use a J-specific system e. g. Perceive, Clinical Works in addition to their Global PV system thus duplicating data entry work Solutions to mitigate the impact ¢ Data transfer bridging applications to minimize duplicating data entry. ¢ Global system perspectives ¢ Alice (HP): only global system operating submission to PMDA ¢ Aris/Aris-J may be connected ¢ Argus/Argus-J (Oracle) not connectable so far but connecting the two platforms may be considered 30
Impact of country-specific requirements Japan ¢ ICSR submitted electronically to the PMDA (E 2 B-J standard) ¢ Japan-specific requirements e. g. Japanese language, specific reporting form, specific causality assessment rules ¢ Most companies use a J-specific system e. g. Perceive, Clinical Works in addition to their Global PV system thus duplicating data entry work Solutions to mitigate the impact ¢ Data transfer bridging applications to minimize duplicating data entry. ¢ Global system perspectives ¢ Alice (HP): only global system operating submission to PMDA ¢ Aris/Aris-J may be connected ¢ Argus/Argus-J (Oracle) not connectable so far but connecting the two platforms may be considered 30
 Impact of country-specific requirements Korea ¢ All domestic ICSRs must be entered in Korean language ¢ Into the web system Easydrug (except GCP trial cases) ¢ e-submission not yet possible ¢ KFDA is able to forward ICSRs to WHO’s UMC on E 2 B format ¢ No Korean version of Meddra to map Korean vs English medical terms Solutions to mitigate the impact ¢ Parallel data entry into global system and Easydrug ¢ Developing E 2 B transmission and Meddra capability è Need for collaboration between the KFDA, Software providers and Industry 31
Impact of country-specific requirements Korea ¢ All domestic ICSRs must be entered in Korean language ¢ Into the web system Easydrug (except GCP trial cases) ¢ e-submission not yet possible ¢ KFDA is able to forward ICSRs to WHO’s UMC on E 2 B format ¢ No Korean version of Meddra to map Korean vs English medical terms Solutions to mitigate the impact ¢ Parallel data entry into global system and Easydrug ¢ Developing E 2 B transmission and Meddra capability è Need for collaboration between the KFDA, Software providers and Industry 31
 Impact of country-specific requirements China ¢ Domestic ICSRs to be entered in Chinese into ADR center web system ¢ e-submission of ICSRs impossible (Not E 2 B compatible) ¢ WHO-ART still used although a Chinese version of MEDDRA exists ¢ Causality and Seriousness categories are specific to China ¢ Need to submit China-specific aggregate reports ¢ Not fully consistent with documents submitted to EMA and FDA ¢ Implications ¢ Duplication of data entry work for multinational companies and Chinese hospitals participating into international safety research ¢ To forward ICSRs to the UMC, the Chinese ADR Center needs to use the old INTIS or convert cases into E 2 B format 32
Impact of country-specific requirements China ¢ Domestic ICSRs to be entered in Chinese into ADR center web system ¢ e-submission of ICSRs impossible (Not E 2 B compatible) ¢ WHO-ART still used although a Chinese version of MEDDRA exists ¢ Causality and Seriousness categories are specific to China ¢ Need to submit China-specific aggregate reports ¢ Not fully consistent with documents submitted to EMA and FDA ¢ Implications ¢ Duplication of data entry work for multinational companies and Chinese hospitals participating into international safety research ¢ To forward ICSRs to the UMC, the Chinese ADR Center needs to use the old INTIS or convert cases into E 2 B format 32
 Impact of country-specific requirements China ¢ Revised Drug Affair Law at final stage of approval at Ministry of Health anticipated to be released in 2011 but still awaited ¢ New National ADR center database with web-based facility expected to become available by June 2011 ¢ The need to make the replacement Chinese safety database compatible with the E 2 B world seems now recognized by the SFDA and ADR who are looking for bridging solutions 33
Impact of country-specific requirements China ¢ Revised Drug Affair Law at final stage of approval at Ministry of Health anticipated to be released in 2011 but still awaited ¢ New National ADR center database with web-based facility expected to become available by June 2011 ¢ The need to make the replacement Chinese safety database compatible with the E 2 B world seems now recognized by the SFDA and ADR who are looking for bridging solutions 33
 Advantages for moving toward E 2 B ¢ Advantage for Health Authorities ¢ Recommended by the WHO-UMC for data transfer ¢ Facilitate ontribution to international safety research ¢ Enable using advanced signal detection systems e. g Empirica or Vigi. Mine ¢ Larger sample size for signal detection in specific ethnic groups ¢ Advantage for the pharmaceutical industry ¢ Avoid duplication of data entry workload ¢ Focus resources on signal detection and benefit/risk optimization 34
Advantages for moving toward E 2 B ¢ Advantage for Health Authorities ¢ Recommended by the WHO-UMC for data transfer ¢ Facilitate ontribution to international safety research ¢ Enable using advanced signal detection systems e. g Empirica or Vigi. Mine ¢ Larger sample size for signal detection in specific ethnic groups ¢ Advantage for the pharmaceutical industry ¢ Avoid duplication of data entry workload ¢ Focus resources on signal detection and benefit/risk optimization 34
 E 2 B systems for ICSR management ¢ Used by the Pharma Industry e. g. ¢ AERS (Oracle) ¢ ARGUS and ARGUS-J (Oracle) ¢ ARISg and ARISj (Aris Global) ¢ Safety-Easy (ABcube) ¢ Company-specific (e. g. MSD, AZ etc. . ) ¢ For Health Authorities e. g. ¢ AERS (Oracle) used by the FDA ¢ Eudravigilance (EMA-specific) ¢ Vigiflow (UMC) used by Swiss. Medic ¢ ARISg used by the French Medicinal Agency ¢ Safety-Easy (ABcube) 35
E 2 B systems for ICSR management ¢ Used by the Pharma Industry e. g. ¢ AERS (Oracle) ¢ ARGUS and ARGUS-J (Oracle) ¢ ARISg and ARISj (Aris Global) ¢ Safety-Easy (ABcube) ¢ Company-specific (e. g. MSD, AZ etc. . ) ¢ For Health Authorities e. g. ¢ AERS (Oracle) used by the FDA ¢ Eudravigilance (EMA-specific) ¢ Vigiflow (UMC) used by Swiss. Medic ¢ ARISg used by the French Medicinal Agency ¢ Safety-Easy (ABcube) 35
 E 2 B solutions for National Heath Authorities ¢ ORACLE AERS ¢ Oracle actively promoting AERS for regulatory authorities ¢ For signal detection, following the acquisition of Phase Forward, Oracle is working on integrating Empirica Signal (developed in collaboration with the FDA) into the AERS platform ¢ In a second step, Empirica Signal will be integrated into the Argus platform as Argus Perceptive will be discontinued. 36
E 2 B solutions for National Heath Authorities ¢ ORACLE AERS ¢ Oracle actively promoting AERS for regulatory authorities ¢ For signal detection, following the acquisition of Phase Forward, Oracle is working on integrating Empirica Signal (developed in collaboration with the FDA) into the AERS platform ¢ In a second step, Empirica Signal will be integrated into the Argus platform as Argus Perceptive will be discontinued. 36
 E 2 B solutions for National Heath Authorities ¢ VIGIFLOW (WHO-UMC) may be set in 3 different ways ICSRs entered locally and forwarded to the central UMC database v ICSRs entered locally and forwarded to a Country-specific database container maintained by the UMC in Uppsala w Setting a Vigiflow system in the Country (full license needed) ¢ ¢ Vigiflow is designed to be customised to local languages The UMC is keen to support interfacing Vigiflow with E 2 B-compatible ICSR submission systems of the Pharmaceutical industry è Cost-effective way to open the door to ICSR e-submission in countries with limited budget to purchase and maintain a national safety database 37
E 2 B solutions for National Heath Authorities ¢ VIGIFLOW (WHO-UMC) may be set in 3 different ways ICSRs entered locally and forwarded to the central UMC database v ICSRs entered locally and forwarded to a Country-specific database container maintained by the UMC in Uppsala w Setting a Vigiflow system in the Country (full license needed) ¢ ¢ Vigiflow is designed to be customised to local languages The UMC is keen to support interfacing Vigiflow with E 2 B-compatible ICSR submission systems of the Pharmaceutical industry è Cost-effective way to open the door to ICSR e-submission in countries with limited budget to purchase and maintain a national safety database 37
 Diversity of Safety Systems In Asia News as of 2011. 05. 17 ¢ India: Vigiflow implemented in November 2010 at the All India Institute of Medical Sciences (AIIMS) to support the Pharmacovigilance Program of India (Pv. PI). In April, the Ministry of Health appointed the Indian Pharmacopoeia Commission (IPC). IPC will also be using Vigiflow. New requirements for reporting SAEs from clinical trials ¢ China: The National ADR Center is planning to implement a new ADR-reporting web-based system in June 2011. The date of release is not confirmed. This system is not anticipated to be E 2 B compatible, however the ADR center has decided to look for solutions to bridge their new system with the E 2 B world ¢ Taiwan: In January 2011, Taiwanese ADR center sent a questionnaire to the Pharma industry inquiring on the benefit for moving to an E 2 B system ¢ Vietnam: The DI&ADR center is planning to set a locally designed countryspecific web-based ICSR reporting system ¢ Singapore, Malaysia, Thailand, Australia, New Zealand are considering acquiring an E 2 B system but there seem to be no clear decision so far 38
Diversity of Safety Systems In Asia News as of 2011. 05. 17 ¢ India: Vigiflow implemented in November 2010 at the All India Institute of Medical Sciences (AIIMS) to support the Pharmacovigilance Program of India (Pv. PI). In April, the Ministry of Health appointed the Indian Pharmacopoeia Commission (IPC). IPC will also be using Vigiflow. New requirements for reporting SAEs from clinical trials ¢ China: The National ADR Center is planning to implement a new ADR-reporting web-based system in June 2011. The date of release is not confirmed. This system is not anticipated to be E 2 B compatible, however the ADR center has decided to look for solutions to bridge their new system with the E 2 B world ¢ Taiwan: In January 2011, Taiwanese ADR center sent a questionnaire to the Pharma industry inquiring on the benefit for moving to an E 2 B system ¢ Vietnam: The DI&ADR center is planning to set a locally designed countryspecific web-based ICSR reporting system ¢ Singapore, Malaysia, Thailand, Australia, New Zealand are considering acquiring an E 2 B system but there seem to be no clear decision so far 38
 Different viewpoints but sharing similar goals ¢ National Health Authorities ¢ Domestic pharmaceutical companies ¢ International Organisation ¢ Multi-National pharmaceutical Companies ¢ All Pharmacovigilance professionals ¢ Aiming at ensuring the safe use of medicinal agents ¢ Facing Increasing amounts of ICSRs ¢ Dealing with limited resources è Harmonization helps avoiding duplicating work and focusing on Benefit versus Risk Management è Harmonisation for better health (ICH Mission Statement) 39
Different viewpoints but sharing similar goals ¢ National Health Authorities ¢ Domestic pharmaceutical companies ¢ International Organisation ¢ Multi-National pharmaceutical Companies ¢ All Pharmacovigilance professionals ¢ Aiming at ensuring the safe use of medicinal agents ¢ Facing Increasing amounts of ICSRs ¢ Dealing with limited resources è Harmonization helps avoiding duplicating work and focusing on Benefit versus Risk Management è Harmonisation for better health (ICH Mission Statement) 39
 Thank you for your attention 40
Thank you for your attention 40


