75c14d1584e2a9d7b407dd2414d5a15f.ppt
- Количество слайдов: 16
 The Description of Non-Covalent Interactions in Terms of Bent´s Rule Sławomir J. Grabowski Faculty of Chemistry, University of the Basque Country UPV/EHU, and Donostia International Physics Center (DIPC), P. K. 1072, 20080 Donostia, Spain IKERBASQUE, Basque Foundation for Science, 48011 Bilbao, Spain e-mail: s. grabowski@ikerbasque. org
The Description of Non-Covalent Interactions in Terms of Bent´s Rule Sławomir J. Grabowski Faculty of Chemistry, University of the Basque Country UPV/EHU, and Donostia International Physics Center (DIPC), P. K. 1072, 20080 Donostia, Spain IKERBASQUE, Basque Foundation for Science, 48011 Bilbao, Spain e-mail: s. grabowski@ikerbasque. org
 Studies on non-covalent interactions Rovira, C. ; Novoa, J. J. Strength and Directianolity of the S…S Intermolecular Interactions, Chem. Eur. J. 1999, 5, 3689 -3697 Braga, D. ; Bazzi, C. ; Grepioni, F. ; Novoa, J. J. Electrostatic compression on non-covalent interactions : the case of π stacks involving ions, New. J. Chem. 1999, 23, 577. Müller-Dethlefs , Hobza, P, Noncovalent Interactions: A Challenge for Experiment and Theory, Chem. Rev. 2000, 143 -167 Hobza, P. ; Müller-Dethlefs, K. Non-Covalent Interactions, Theory and Experiment, Royal Society of Chemistry, Thomas Graham House, Science Park, Milton Road, Cambridge, 2010.
Studies on non-covalent interactions Rovira, C. ; Novoa, J. J. Strength and Directianolity of the S…S Intermolecular Interactions, Chem. Eur. J. 1999, 5, 3689 -3697 Braga, D. ; Bazzi, C. ; Grepioni, F. ; Novoa, J. J. Electrostatic compression on non-covalent interactions : the case of π stacks involving ions, New. J. Chem. 1999, 23, 577. Müller-Dethlefs , Hobza, P, Noncovalent Interactions: A Challenge for Experiment and Theory, Chem. Rev. 2000, 143 -167 Hobza, P. ; Müller-Dethlefs, K. Non-Covalent Interactions, Theory and Experiment, Royal Society of Chemistry, Thomas Graham House, Science Park, Milton Road, Cambridge, 2010.
 Lewis acid – Lewis base Electron charge transfer A-H+ …- B hydrogen bond A-H+ …- H-B dihydrogen bond A+ …- H-B hydride bond C-X+ …- H-B halogen-hydride bond C-X+ …- B halogen bond P. Lipkowski, S. J. Grabowski, J. Leszczynski J. Phys. Chem. A 2006, 110, 10296.
Lewis acid – Lewis base Electron charge transfer A-H+ …- B hydrogen bond A-H+ …- H-B dihydrogen bond A+ …- H-B hydride bond C-X+ …- H-B halogen-hydride bond C-X+ …- B halogen bond P. Lipkowski, S. J. Grabowski, J. Leszczynski J. Phys. Chem. A 2006, 110, 10296.
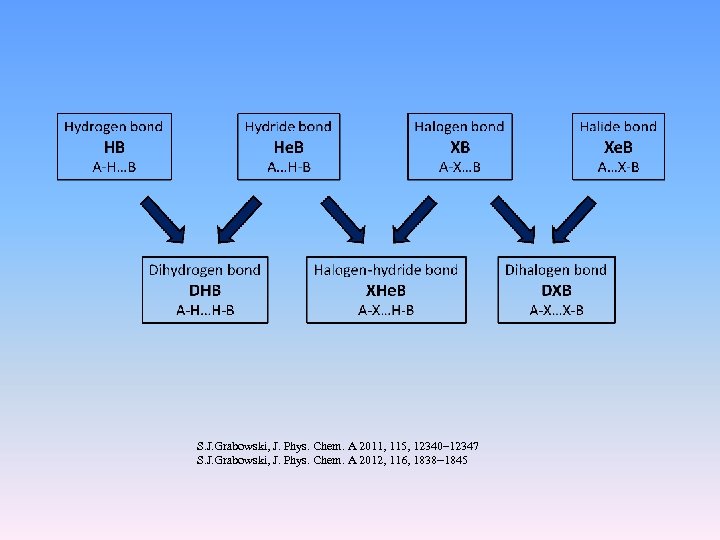 S. J. Grabowski, J. Phys. Chem. A 2011, 115, 12340– 12347 S. J. Grabowski, J. Phys. Chem. A 2012, 116, 1838− 1845
S. J. Grabowski, J. Phys. Chem. A 2011, 115, 12340– 12347 S. J. Grabowski, J. Phys. Chem. A 2012, 116, 1838− 1845
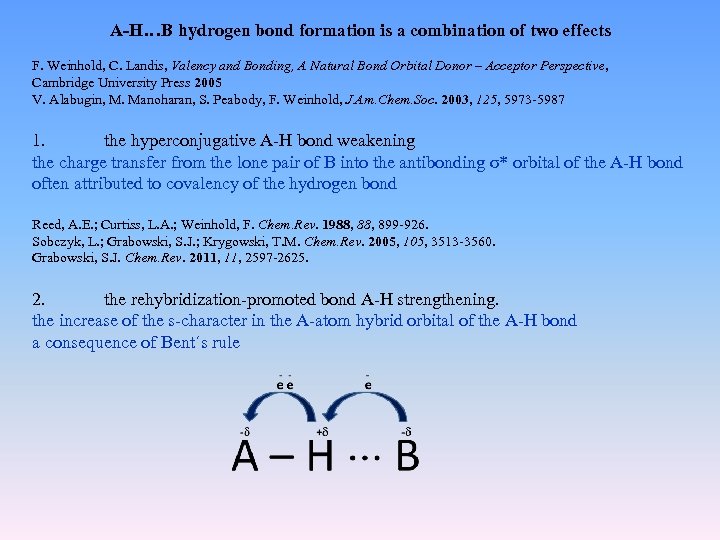 A-H…B hydrogen bond formation is a combination of two effects F. Weinhold, C. Landis, Valency and Bonding, A Natural Bond Orbital Donor – Acceptor Perspective, Cambridge University Press 2005 V. Alabugin, M. Manoharan, S. Peabody, F. Weinhold, J. Am. Chem. Soc. 2003, 125, 5973 -5987 1. the hyperconjugative A-H bond weakening the charge transfer from the lone pair of B into the antibonding σ* orbital of the A-H bond often attributed to covalency of the hydrogen bond Reed, A. E. ; Curtiss, L. A. ; Weinhold, F. Chem. Rev. 1988, 899 -926. Sobczyk, L. ; Grabowski, S. J. ; Krygowski, T. M. Chem. Rev. 2005, 105, 3513 -3560. Grabowski, S. J. Chem. Rev. 2011, 2597 -2625. 2. the rehybridization-promoted bond A-H strengthening. the increase of the s-character in the A-atom hybrid orbital of the A-H bond a consequence of Bent´s rule
A-H…B hydrogen bond formation is a combination of two effects F. Weinhold, C. Landis, Valency and Bonding, A Natural Bond Orbital Donor – Acceptor Perspective, Cambridge University Press 2005 V. Alabugin, M. Manoharan, S. Peabody, F. Weinhold, J. Am. Chem. Soc. 2003, 125, 5973 -5987 1. the hyperconjugative A-H bond weakening the charge transfer from the lone pair of B into the antibonding σ* orbital of the A-H bond often attributed to covalency of the hydrogen bond Reed, A. E. ; Curtiss, L. A. ; Weinhold, F. Chem. Rev. 1988, 899 -926. Sobczyk, L. ; Grabowski, S. J. ; Krygowski, T. M. Chem. Rev. 2005, 105, 3513 -3560. Grabowski, S. J. Chem. Rev. 2011, 2597 -2625. 2. the rehybridization-promoted bond A-H strengthening. the increase of the s-character in the A-atom hybrid orbital of the A-H bond a consequence of Bent´s rule
 According to Bent´s rule atoms maximize the s-character in hybrid orbitals aimed toward electropositive substituents and maximize their p-character in such orbitals aimed toward electronegative substituents H. A. Bent, Chem. Rev. 1961, 275 -311
According to Bent´s rule atoms maximize the s-character in hybrid orbitals aimed toward electropositive substituents and maximize their p-character in such orbitals aimed toward electronegative substituents H. A. Bent, Chem. Rev. 1961, 275 -311
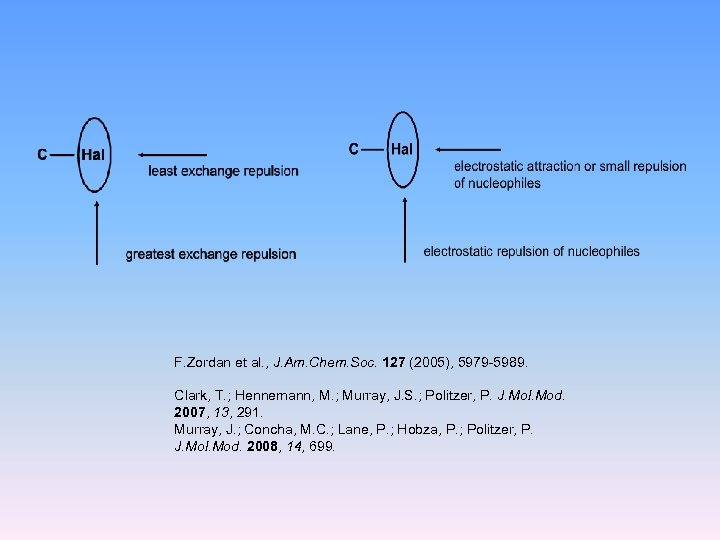 F. Zordan et al. , J. Am. Chem. Soc. 127 (2005), 5979 -5989. Clark, T. ; Hennemann, M. ; Murray, J. S. ; Politzer, P. J. Mol. Mod. 2007, 13, 291. Murray, J. ; Concha, M. C. ; Lane, P. ; Hobza, P. ; Politzer, P. J. Mol. Mod. 2008, 14, 699.
F. Zordan et al. , J. Am. Chem. Soc. 127 (2005), 5979 -5989. Clark, T. ; Hennemann, M. ; Murray, J. S. ; Politzer, P. J. Mol. Mod. 2007, 13, 291. Murray, J. ; Concha, M. C. ; Lane, P. ; Hobza, P. ; Politzer, P. J. Mol. Mod. 2008, 14, 699.
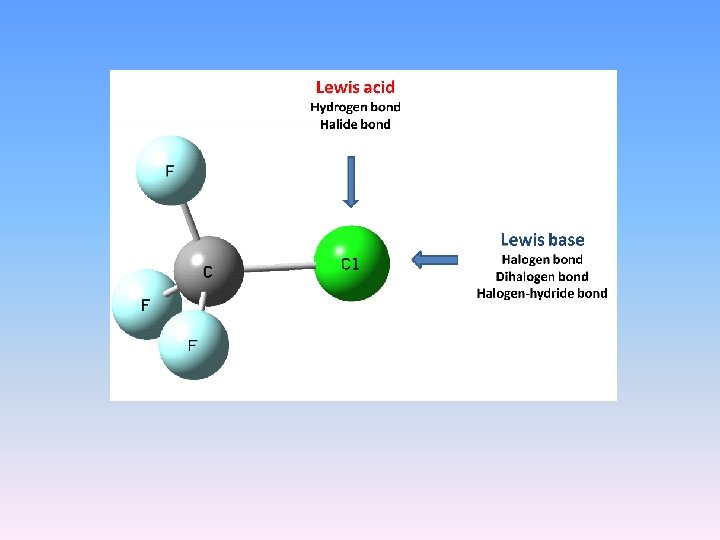
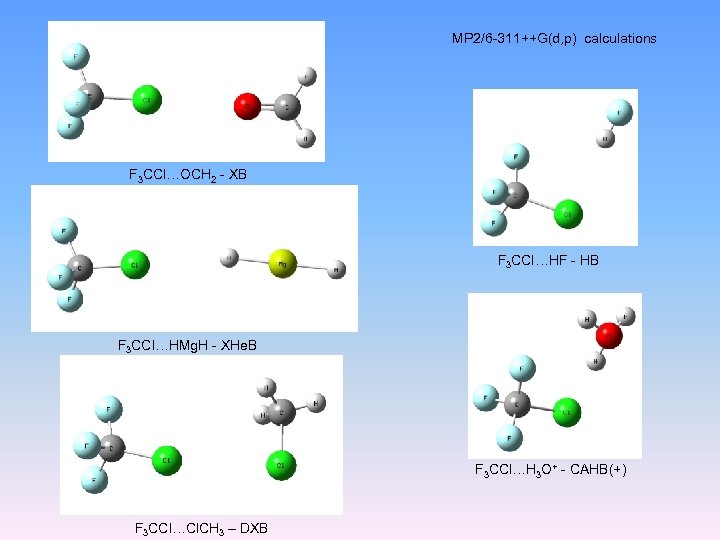 MP 2/6 -311++G(d, p) calculations F 3 CCl…OCH 2 - XB F 3 CCl…HF - HB F 3 CCl…HMg. H - XHe. B F 3 CCl…H 3 O+ - CAHB(+) F 3 CCl…Cl. CH 3 – DXB
MP 2/6 -311++G(d, p) calculations F 3 CCl…OCH 2 - XB F 3 CCl…HF - HB F 3 CCl…HMg. H - XHe. B F 3 CCl…H 3 O+ - CAHB(+) F 3 CCl…Cl. CH 3 – DXB
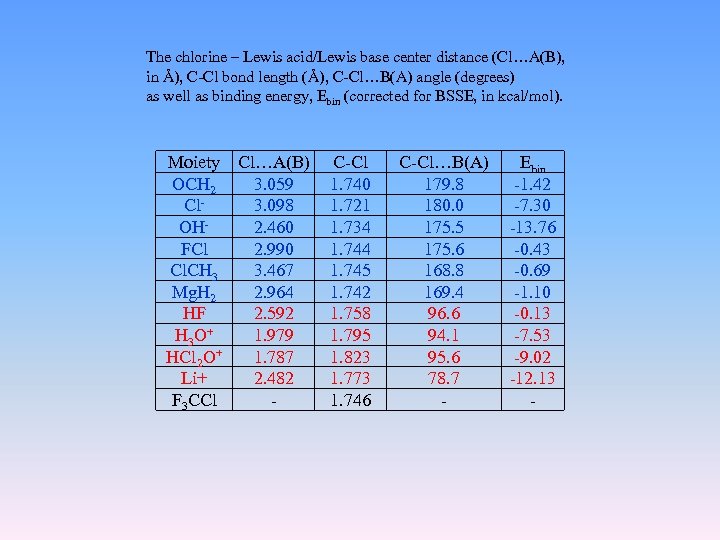 The chlorine – Lewis acid/Lewis base center distance (Cl…A(B), in Å), C-Cl bond length (Å), C-Cl…B(A) angle (degrees) as well as binding energy, Ebin (corrected for BSSE, in kcal/mol). Moiety Cl…A(B) OCH 2 3. 059 Cl 3. 098 OH 2. 460 FCl 2. 990 Cl. CH 3 3. 467 Mg. H 2 2. 964 HF 2. 592 H 3 O+ 1. 979 HCl 2 O+ 1. 787 Li+ 2. 482 F 3 CCl - C-Cl 1. 740 1. 721 1. 734 1. 745 1. 742 1. 758 1. 795 1. 823 1. 773 1. 746 C-Cl…B(A) 179. 8 180. 0 175. 5 175. 6 168. 8 169. 4 96. 6 94. 1 95. 6 78. 7 - Ebin -1. 42 -7. 30 -13. 76 -0. 43 -0. 69 -1. 10 -0. 13 -7. 53 -9. 02 -12. 13 -
The chlorine – Lewis acid/Lewis base center distance (Cl…A(B), in Å), C-Cl bond length (Å), C-Cl…B(A) angle (degrees) as well as binding energy, Ebin (corrected for BSSE, in kcal/mol). Moiety Cl…A(B) OCH 2 3. 059 Cl 3. 098 OH 2. 460 FCl 2. 990 Cl. CH 3 3. 467 Mg. H 2 2. 964 HF 2. 592 H 3 O+ 1. 979 HCl 2 O+ 1. 787 Li+ 2. 482 F 3 CCl - C-Cl 1. 740 1. 721 1. 734 1. 745 1. 742 1. 758 1. 795 1. 823 1. 773 1. 746 C-Cl…B(A) 179. 8 180. 0 175. 5 175. 6 168. 8 169. 4 96. 6 94. 1 95. 6 78. 7 - Ebin -1. 42 -7. 30 -13. 76 -0. 43 -0. 69 -1. 10 -0. 13 -7. 53 -9. 02 -12. 13 -
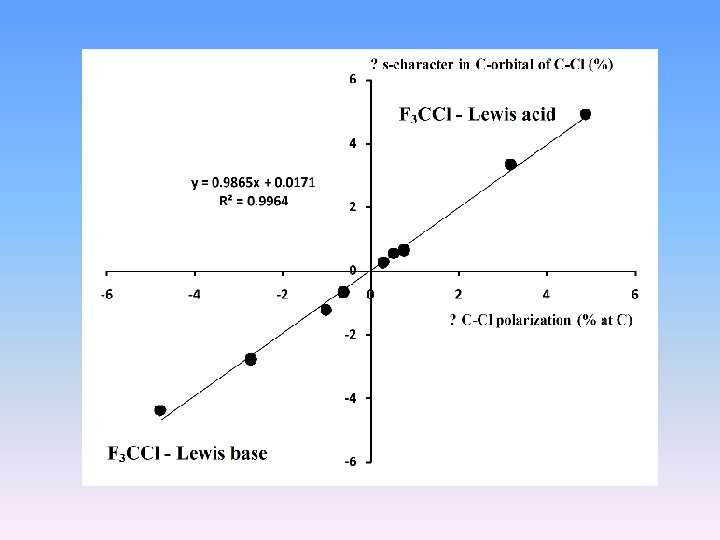
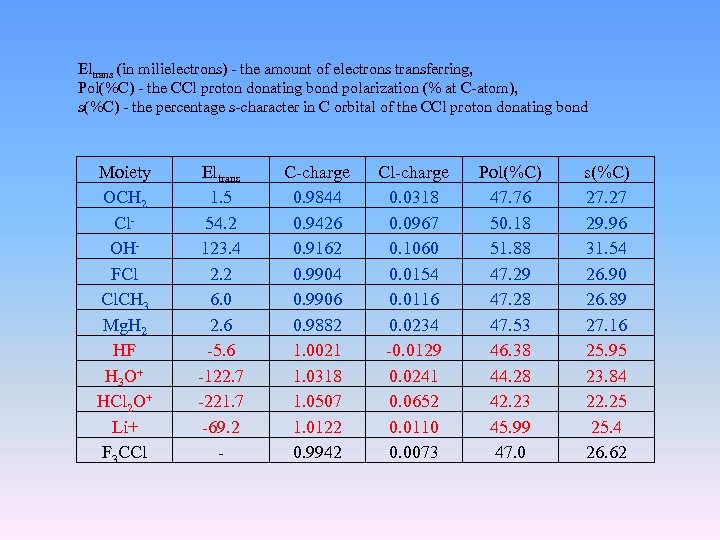 Eltrans (in milielectrons) - the amount of electrons transferring, Pol(%C) - the CCl proton donating bond polarization (% at C-atom), s(%C) - the percentage s-character in C orbital of the CCl proton donating bond Moiety OCH 2 Cl. OHFCl Cl. CH 3 Mg. H 2 HF H 3 O+ HCl 2 O+ Li+ F 3 CCl Eltrans 1. 5 54. 2 123. 4 2. 2 6. 0 2. 6 -5. 6 -122. 7 -221. 7 -69. 2 - C-charge 0. 9844 0. 9426 0. 9162 0. 9904 0. 9906 0. 9882 1. 0021 1. 0318 1. 0507 1. 0122 0. 9942 Cl-charge 0. 0318 0. 0967 0. 1060 0. 0154 0. 0116 0. 0234 -0. 0129 0. 0241 0. 0652 0. 0110 0. 0073 Pol(%C) 47. 76 50. 18 51. 88 47. 29 47. 28 47. 53 46. 38 44. 28 42. 23 45. 99 47. 0 s(%C) 27. 27 29. 96 31. 54 26. 90 26. 89 27. 16 25. 95 23. 84 22. 25 25. 4 26. 62
Eltrans (in milielectrons) - the amount of electrons transferring, Pol(%C) - the CCl proton donating bond polarization (% at C-atom), s(%C) - the percentage s-character in C orbital of the CCl proton donating bond Moiety OCH 2 Cl. OHFCl Cl. CH 3 Mg. H 2 HF H 3 O+ HCl 2 O+ Li+ F 3 CCl Eltrans 1. 5 54. 2 123. 4 2. 2 6. 0 2. 6 -5. 6 -122. 7 -221. 7 -69. 2 - C-charge 0. 9844 0. 9426 0. 9162 0. 9904 0. 9906 0. 9882 1. 0021 1. 0318 1. 0507 1. 0122 0. 9942 Cl-charge 0. 0318 0. 0967 0. 1060 0. 0154 0. 0116 0. 0234 -0. 0129 0. 0241 0. 0652 0. 0110 0. 0073 Pol(%C) 47. 76 50. 18 51. 88 47. 29 47. 28 47. 53 46. 38 44. 28 42. 23 45. 99 47. 0 s(%C) 27. 27 29. 96 31. 54 26. 90 26. 89 27. 16 25. 95 23. 84 22. 25 25. 4 26. 62
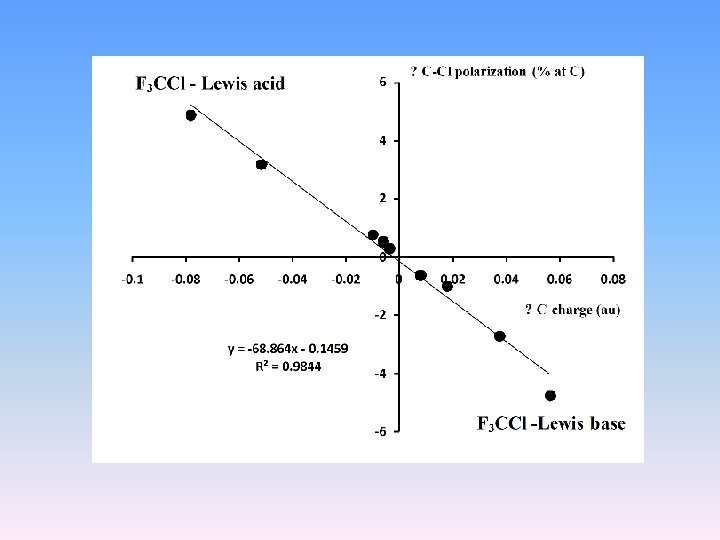
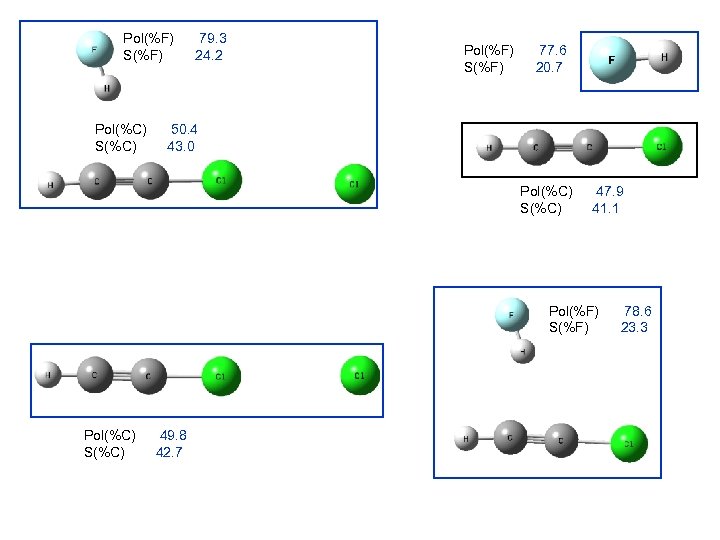 Pol(%F) S(%F) Pol(%C) S(%C) 79. 3 24. 2 Pol(%F) S(%F) 77. 6 20. 7 50. 4 43. 0 Pol(%C) S(%C) 47. 9 41. 1 Pol(%F) S(%F) Pol(%C) S(%C) 49. 8 42. 7 78. 6 23. 3
Pol(%F) S(%F) Pol(%C) S(%C) 79. 3 24. 2 Pol(%F) S(%F) 77. 6 20. 7 50. 4 43. 0 Pol(%C) S(%C) 47. 9 41. 1 Pol(%F) S(%F) Pol(%C) S(%C) 49. 8 42. 7 78. 6 23. 3
 Santiago de Compostela- WATOC 2011
Santiago de Compostela- WATOC 2011
 Thank you for attention Financial support comes from Eusko Jaurlaritza (GIC 07/85 IT-330 -07) and the Spanish Office for Scientific Research (CTQ 2011 -27374). Technical and human support provided by IZO-SGI SGIker (UPV/EHU, MICINN, GV/EJ, ESF) is gratefully acknowledged.
Thank you for attention Financial support comes from Eusko Jaurlaritza (GIC 07/85 IT-330 -07) and the Spanish Office for Scientific Research (CTQ 2011 -27374). Technical and human support provided by IZO-SGI SGIker (UPV/EHU, MICINN, GV/EJ, ESF) is gratefully acknowledged.


