8ed5253ad50e1f6f2d7a9774824e2886.ppt
- Количество слайдов: 34

The Biolux DEB Evidence and the Biolux Program Ralf Langhoff, MD Center for Vascular Medicine Berlin-Wilmersdorf St. Gertrauden Hospital Charité, CC 11 Academic Teaching Hospitals ― Charité Berlin
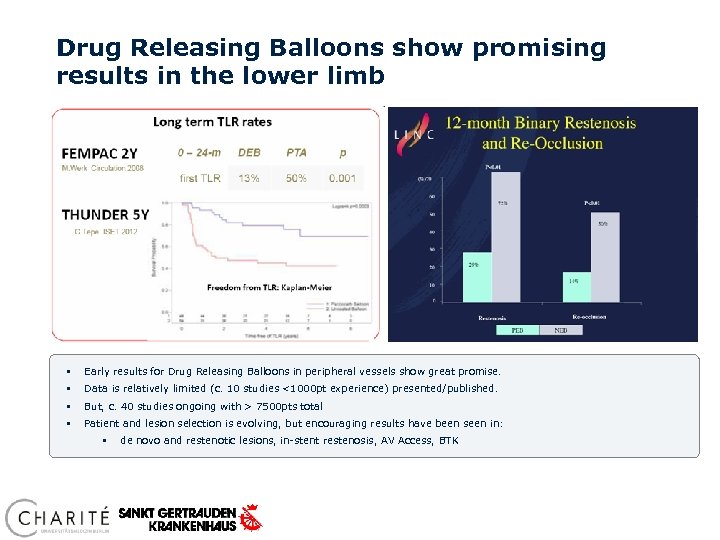
Drug Releasing Balloons show promising results in the lower limb § Early results for Drug Releasing Balloons in peripheral vessels show great promise. § Data is relatively limited (c. 10 studies <1000 pt experience) presented/published. § But, c. 40 studies ongoing with > 7500 pts total § Patient and lesion selection is evolving, but encouraging results have been seen in: § de novo and restenotic lesions, in-stent restenosis, AV Access, BTK
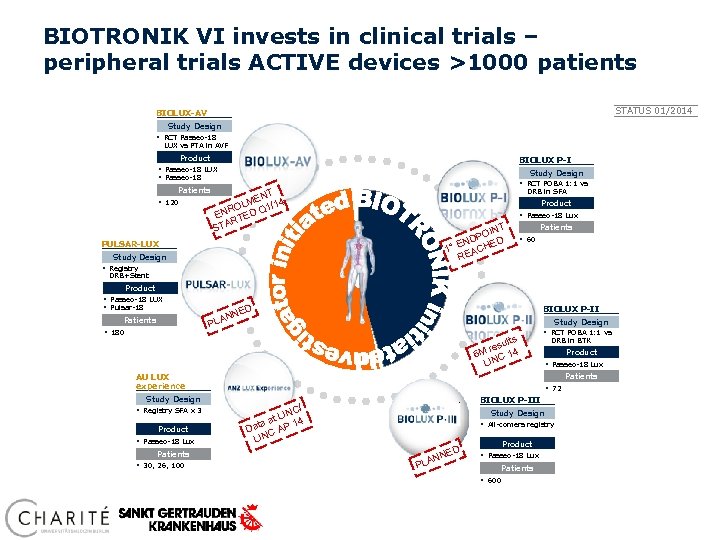
BIOTRONIK VI invests in clinical trials – peripheral trials ACTIVE devices >1000 patients STATUS 01/2014 BIOLUX-AV Study Design § RCT Passeo-18 LUX vs PTA in AVF Product BIOLUX P-I § Passeo-18 LUX § Passeo-18 Patients § 120 Study Design § RCT POBA 1: 1 vs DRB in SFA T EN 4 OLM Q 1/1 R EN TED AR ST Product § Passeo-18 Lux T OIN DP ED N 1° E ACH RE PULSAR-LUX Study Design Patients § 60 § Registry DRB+Stent Product § Passeo-18 LUX § Pulsar-18 Patients § 180 D BIOLUX P-II NE N PLA Study Design ults res 4 6 M C 1 LIN § RCT POBA 1: 1 vs Study Design Product § Passeo-18 Lux Patients § 30, 26, 100 Product § Passeo-18 Lux Patients AU LUX experience § Registry SFA x 3 DRB in BTK § 72 BIOLUX P-III / NC t LI 4 1 aa Dat C AP N LI Study Design § All-comers registry D PLA E NN Product § Passeo-18 Lux Patients § 600
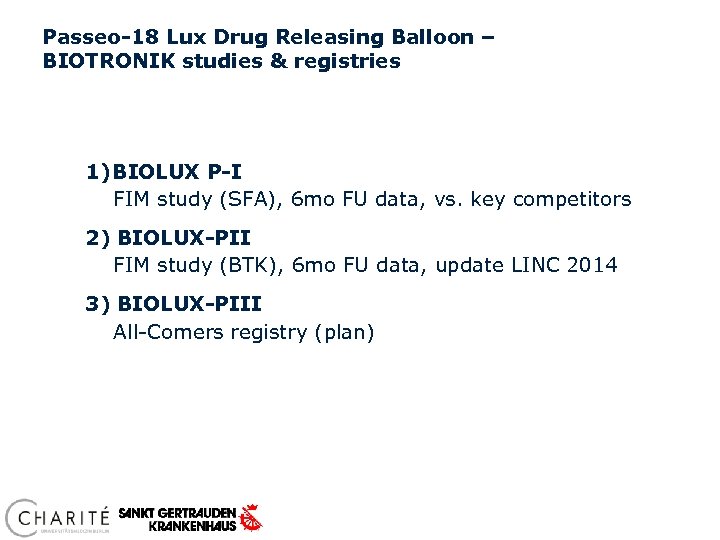
Passeo-18 Lux Drug Releasing Balloon – BIOTRONIK studies & registries 1) BIOLUX P-I FIM study (SFA), 6 mo FU data, vs. key competitors 2) BIOLUX-PII FIM study (BTK), 6 mo FU data, update LINC 2014 3) BIOLUX-PIII All-Comers registry (plan)

Passeo-18 Lux The ideal platform Size range Ø: 3 -7 mm L: 40, 80, 120 mm Compatibility Features and benefits 4 F: 3 -4 mm 5 F: 5 -7 mm Highly pushable, highly flexible catheter for optimal delivery Innovative insertion aid to reduce loss of drug coating.
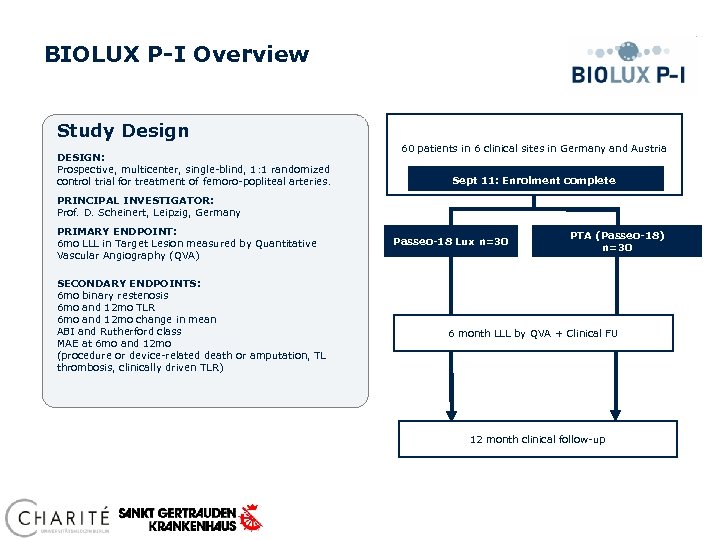
BIOLUX P-I Overview Study Design DESIGN: Prospective, multicenter, single-blind, 1: 1 randomized control trial for treatment of femoro-popliteal arteries. 60 patients in 6 clinical sites in Germany and Austria Sept 11: Enrolment complete PRINCIPAL INVESTIGATOR: Prof. D. Scheinert, Leipzig, Germany PRIMARY ENDPOINT: 6 mo LLL in Target Lesion measured by Quantitative Vascular Angiography (QVA) SECONDARY ENDPOINTS: 6 mo binary restenosis 6 mo and 12 mo TLR 6 mo and 12 mo change in mean ABI and Rutherford class MAE at 6 mo and 12 mo (procedure or device-related death or amputation, TL thrombosis, clinically driven TLR) Passeo-18 Lux n=30 PTA (Passeo-18) n=30 6 month LLL by QVA + Clinical FU 12 month clinical follow-up
Biolux P 1 TRIAL (POBA vs. DEB 7 - month Follow-up
Case report BIOLUX P 1 18 -month Follow-up
Case report BIOLUX P 1 Lesion
Case report BIOLUX P 1 Treatment
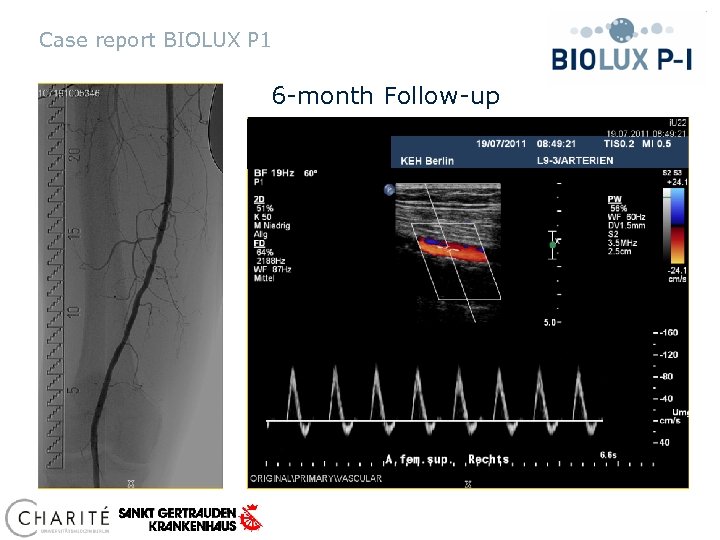
Case report BIOLUX P 1 6 -month Follow-up
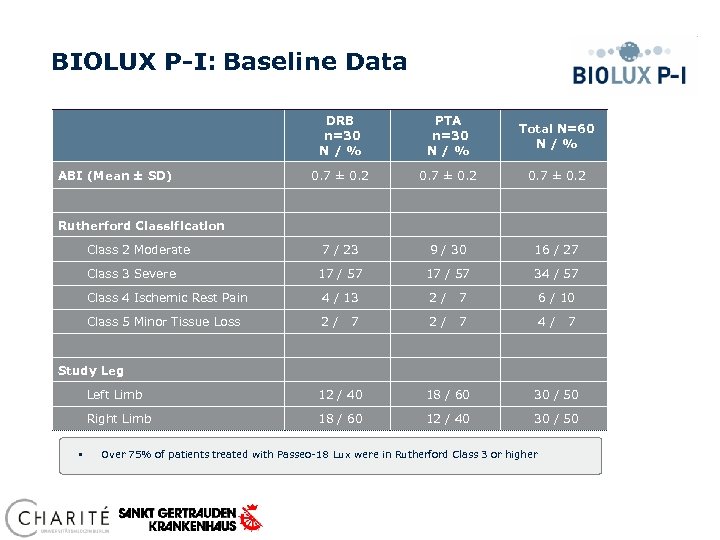
BIOLUX P-I: Baseline Data ABI (Mean ± SD) DRB n=30 N / % PTA n=30 N / % Total N=60 N / % 0. 7 ± 0. 2 7 / 23 9 / 30 16 / 27 17 / 57 34 / 57 Rutherford Classification Class 2 Moderate Class 3 Severe Class 4 Ischemic Rest Pain 4 / 13 2/ 7 6 / 10 Class 5 Minor Tissue Loss 2/ 2/ 7 4/ 7 7 Study Leg Left Limb 18 / 60 30 / 50 Right Limb § 12 / 40 18 / 60 12 / 40 30 / 50 Over 75% of patients treated with Passeo-18 Lux were in Rutherford Class 3 or higher
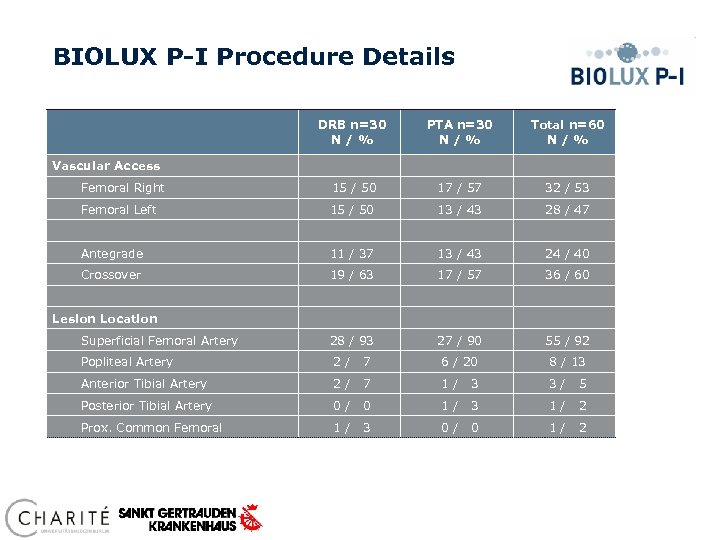
BIOLUX P-I Procedure Details DRB n=30 N / % PTA n=30 N / % Total n=60 N / % Femoral Right 15 / 50 17 / 57 32 / 53 Femoral Left 15 / 50 13 / 43 28 / 47 Antegrade 11 / 37 13 / 43 24 / 40 Crossover 19 / 63 17 / 57 36 / 60 Superficial Femoral Artery 28 / 93 27 / 90 55 / 92 Popliteal Artery 2/ 7 6 / 20 8 / 13 Anterior Tibial Artery 2/ 7 1/ 3 3/ 5 Posterior Tibial Artery 0/ 0 1/ 3 1/ 2 Prox. Common Femoral 1/ 3 0/ 0 1/ 2 Vascular Access Lesion Location
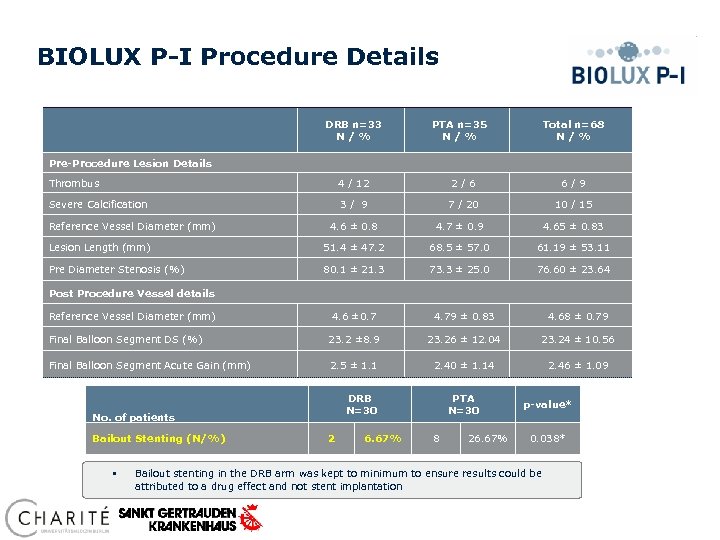
BIOLUX P-I Procedure Details DRB n=33 N / % PTA n=35 N / % Total n=68 N / % Pre-Procedure Lesion Details Thrombus 4 / 12 2/6 6/9 Severe Calcification 3/ 9 7 / 20 10 / 15 Reference Vessel Diameter (mm) 4. 6 ± 0. 8 4. 7 ± 0. 9 4. 65 ± 0. 83 Lesion Length (mm) 51. 4 ± 47. 2 68. 5 ± 57. 0 61. 19 ± 53. 11 Pre Diameter Stenosis (%) 80. 1 ± 21. 3 73. 3 ± 25. 0 76. 60 ± 23. 64 Post Procedure Vessel details Reference Vessel Diameter (mm) 4. 6 ± 0. 7 4. 79 ± 0. 83 4. 68 ± 0. 79 Final Balloon Segment DS (%) 23. 2 ± 8. 9 23. 26 ± 12. 04 23. 24 ± 10. 56 Final Balloon Segment Acute Gain (mm) 2. 5 ± 1. 1 2. 40 ± 1. 14 2. 46 ± 1. 09 DRB N=30 No. of patients Bailout Stenting (N/%) § 2 6. 67% PTA N=30 8 26. 67% p-value* 0. 038* Bailout stenting in the DRB arm was kept to minimum to ensure results could be attributed to a drug effect and not stent implantation
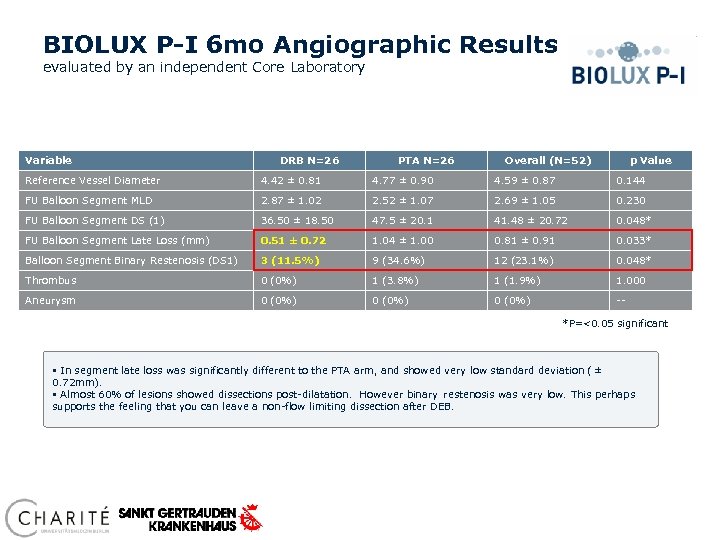
BIOLUX P-I 6 mo Angiographic Results evaluated by an independent Core Laboratory Variable DRB N=26 PTA N=26 Overall (N=52) p Value Reference Vessel Diameter 4. 42 ± 0. 81 4. 77 ± 0. 90 4. 59 ± 0. 87 0. 144 FU Balloon Segment MLD 2. 87 ± 1. 02 2. 52 ± 1. 07 2. 69 ± 1. 05 0. 230 FU Balloon Segment DS (1) 36. 50 ± 18. 50 47. 5 ± 20. 1 41. 48 ± 20. 72 0. 048* FU Balloon Segment Late Loss (mm) 0. 51 ± 0. 72 1. 04 ± 1. 00 0. 81 ± 0. 91 0. 033* Balloon Segment Binary Restenosis (DS 1) 3 (11. 5%) 9 (34. 6%) 12 (23. 1%) 0. 048* Thrombus 0 (0%) 1 (3. 8%) 1 (1. 9%) 1. 000 Aneurysm 0 (0%) -*P=<0. 05 significant § In segment late loss was significantly different to the PTA arm, and showed very low standard deviation ( ± 0. 72 mm). § Almost 60% of lesions showed dissections post-dilatation. However binary restenosis was very low. This perhaps supports the feeling that you can leave a non-flow limiting dissection after DEB.
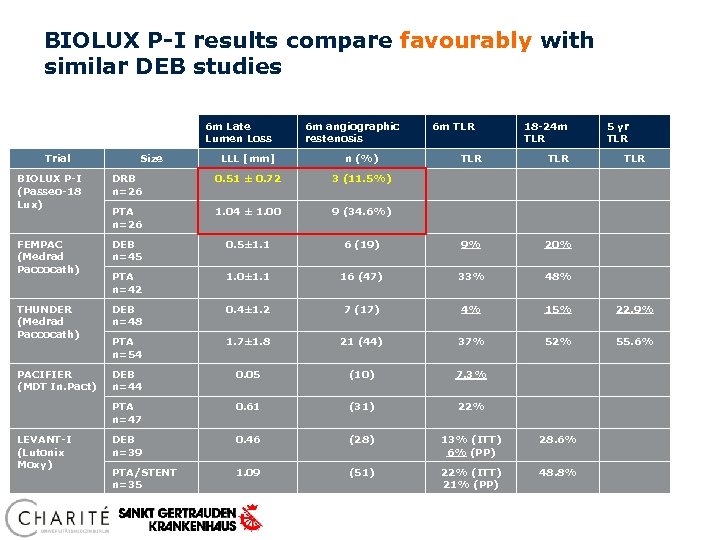
BIOLUX P-I results compare favourably with similar DEB studies 6 m Late Lumen Loss Trial Size 6 m angiographic restenosis LLL [mm] n (%) 6 m TLR 18 -24 m TLR TLR 5 yr TLR BIOLUX P-I (Passeo-18 Lux) DRB n=26 0. 51 ± 0. 72 3 (11. 5%) PTA n=26 1. 04 ± 1. 00 9 (34. 6%) FEMPAC (Medrad Paccocath) DEB n=45 0. 5± 1. 1 6 (19) 9% 20% PTA n=42 1. 0± 1. 1 16 (47) 33% 48% THUNDER (Medrad Paccocath) DEB n=48 0. 4± 1. 2 7 (17) 4% 15% 22. 9% PTA n=54 1. 7± 1. 8 21 (44) 37% 52% 55. 6% PACIFIER (MDT In. Pact) DEB n=44 0. 05 (10) 7. 3% PTA n=47 0. 61 (31) 22% DEB n=39 0. 46 (28) 13% (ITT) 6% (PP) 28. 6% PTA/STENT n=35 1. 09 (51) 22% (ITT) 21% (PP) 48. 8% LEVANT-I (Lutonix Moxy)
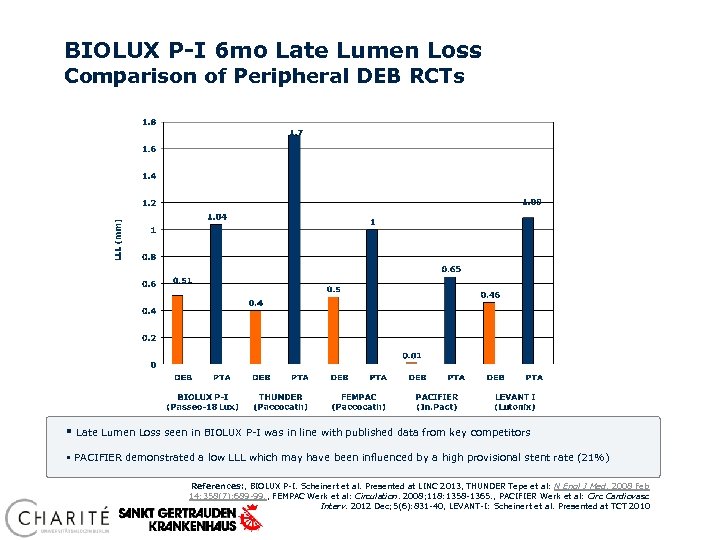
BIOLUX P-I 6 mo Late Lumen Loss Comparison of Peripheral DEB RCTs § Late Lumen Loss seen in BIOLUX P-I was in line with published data from key competitors § PACIFIER demonstrated a low LLL which may have been influenced by a high provisional stent rate (21%) References: , BIOLUX P-I. Scheinert et al. Presented at LINC 2013, THUNDER Tepe et al: N Engl J Med. 2008 Feb 14; 358(7): 689 -99. , FEMPAC Werk et al: Circulation. 2008; 118: 1358 -1365. , PACIFIER Werk et al: Circ Cardiovasc Interv. 2012 Dec; 5(6): 831 -40, LEVANT-I: Scheinert et al. Presented at TCT 2010
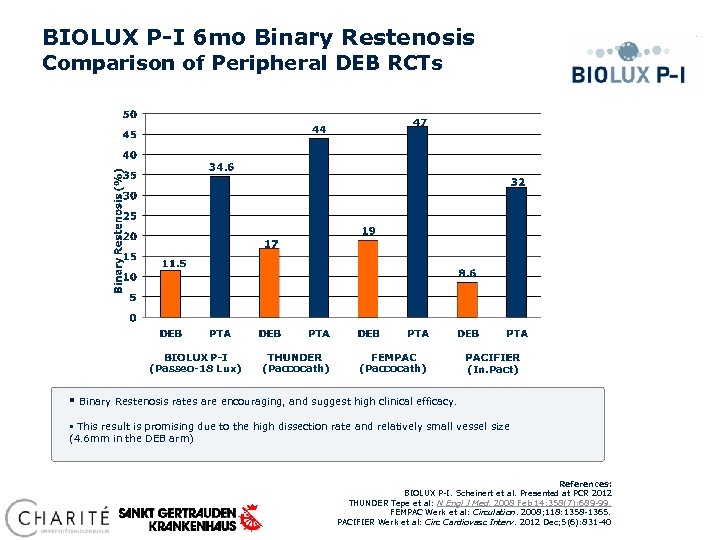
BIOLUX P-I 6 mo Binary Restenosis Comparison of Peripheral DEB RCTs (Passeo-18 Lux) § (Paccocath) (In. Pact) Binary Restenosis rates are encouraging, and suggest high clinical efficacy. § This result is promising due to the high dissection rate and relatively small vessel size (4. 6 mm in the DEB arm) References: BIOLUX P-I. Scheinert et al. Presented at PCR 2012 THUNDER Tepe et al: N Engl J Med. 2008 Feb 14; 358(7): 689 -99. FEMPAC Werk et al: Circulation. 2008; 118: 1358 -1365. PACIFIER Werk et al: Circ Cardiovasc Interv. 2012 Dec; 5(6): 831 -40
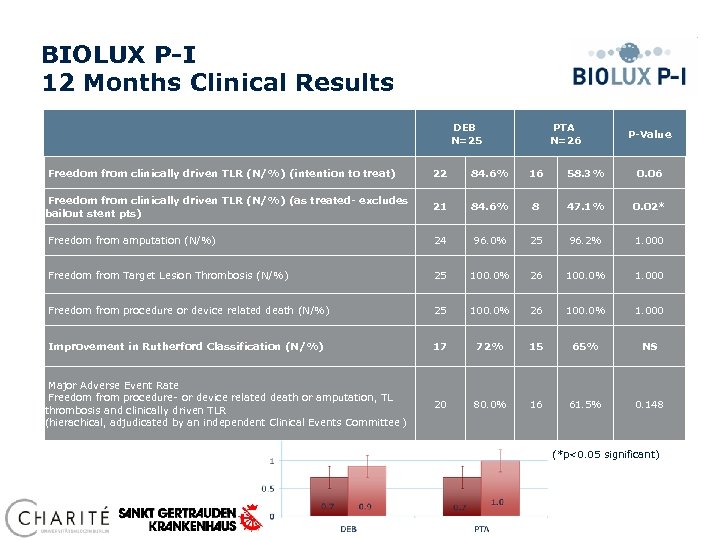
BIOLUX P-I 12 Months Clinical Results DEB N=25 PTA N=26 P-Value Freedom from clinically driven TLR (N/%) (intention to treat) 22 84. 6% 16 58. 3% 0. 06 Freedom from clinically driven TLR (N/%) (as treated- excludes bailout stent pts) 21 84. 6% 8 47. 1% 0. 02* Freedom from amputation (N/%) 24 96. 0% 25 96. 2% 1. 000 Freedom from Target Lesion Thrombosis (N/%) 25 100. 0% 26 100. 0% 1. 000 Freedom from procedure or device related death (N/%) 25 100. 0% 26 100. 0% 1. 000 Improvement in Rutherford Classification (N/%) 17 72% 15 65% NS Major Adverse Event Rate Freedom from procedure- or device related death or amputation, TL thrombosis and clinically driven TLR (hierachical, adjudicated by an independent Clinical Events Committee ) 20 80. 0% 16 61. 5% 0. 148 Ankle Brachial Index (*p<0. 05 significant)
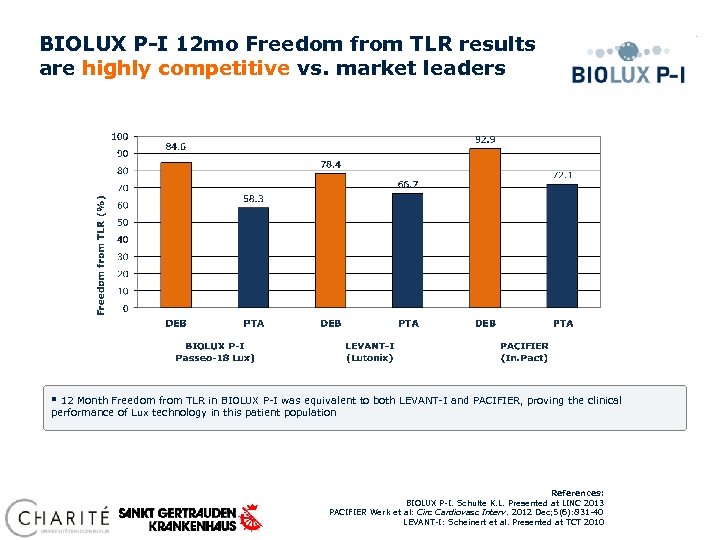
BIOLUX P-I 12 mo Freedom from TLR results are highly competitive vs. market leaders § 12 Month Freedom from TLR in BIOLUX P-I was equivalent to both LEVANT-I and PACIFIER, proving the clinical performance of Lux technology in this patient population References: BIOLUX P-I. Schulte K. L. Presented at LINC 2013 PACIFIER Werk et al: Circ Cardiovasc Interv. 2012 Dec; 5(6): 831 -40 LEVANT-I: Scheinert et al. Presented at TCT 2010
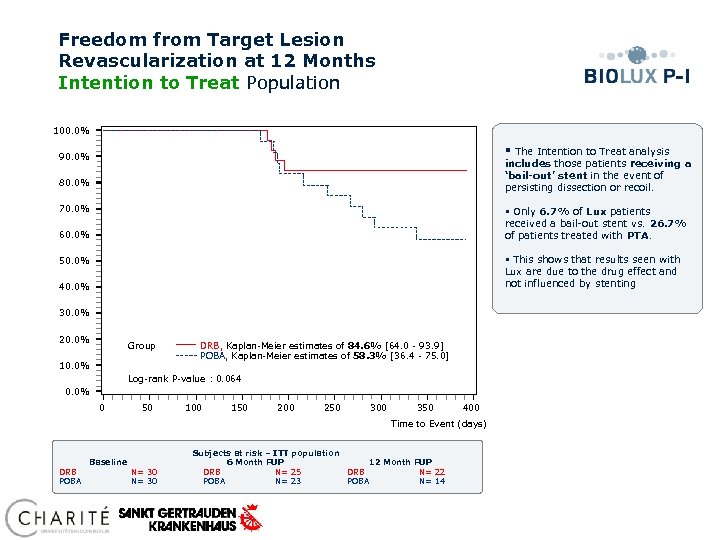
Freedom from Target Lesion Revascularization at 12 Months Intention to Treat Population 100. 0% § The Intention to Treat analysis includes those patients receiving a ‘bail-out’ stent in the event of persisting dissection or recoil. 90. 0% 80. 0% 70. 0% § Only 6. 7% of Lux patients received a bail-out stent vs. 26. 7% of patients treated with PTA. 60. 0% § This shows that results seen with Lux are due to the drug effect and not influenced by stenting 50. 0% 40. 0% 30. 0% 20. 0% Group 10. 0% DRB, Kaplan-Meier estimates of 84. 6% [64. 0 - 93. 9] POBA, Kaplan-Meier estimates of 58. 3% [36. 4 - 75. 0] Log-rank P-value : 0. 064 0. 0% 0 50 100 150 200 250 300 350 400 Time to Event (days) Subjects at risk – ITT population Baseline 6 Month FUP 12 Month FUP DRB N= 30 DRB N= 25 DRB N= 22 POBA N= 30 POBA N= 23 POBA N= 14
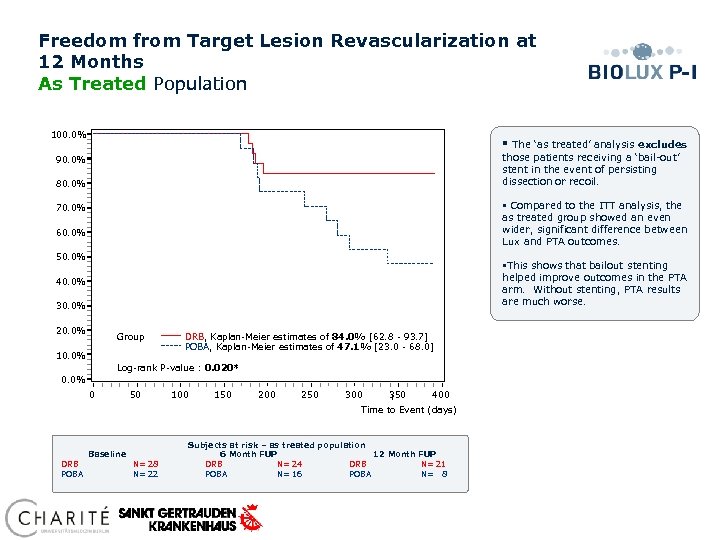
Freedom from Target Lesion Revascularization at 12 Months As Treated Population 100. 0% § The ‘as treated’ analysis excludes those patients receiving a ‘bail-out’ stent in the event of persisting dissection or recoil. 90. 0% 80. 0% § Compared to the ITT analysis, the as treated group showed an even wider, significant difference between Lux and PTA outcomes. 70. 0% 60. 0% 50. 0% §This shows that bailout stenting helped improve outcomes in the PTA arm. Without stenting, PTA results are much worse. 40. 0% 30. 0% 20. 0% Group 10. 0% DRB, Kaplan-Meier estimates of 84. 0% [62. 8 - 93. 7] POBA, Kaplan-Meier estimates of 47. 1% [23. 0 - 68. 0] Log-rank P-value : 0. 020* 0. 0% 0 50 100 150 200 250 300 350 400 Time to Event (days) Subjects at risk – as treated population Baseline 6 Month FUP 12 Month FUP DRB N= 28 DRB N= 24 DRB N= 21 POBA N= 22 POBA N= 16 POBA N= 8
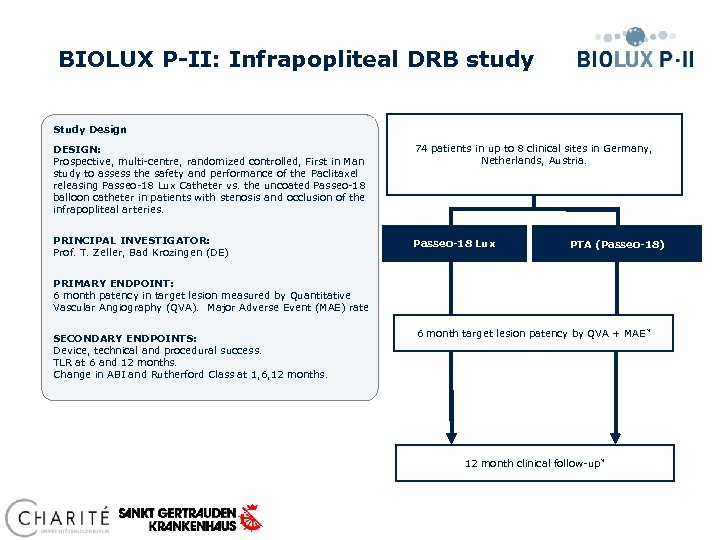
BIOLUX P-II: Infrapopliteal DRB study Study Design DESIGN: Prospective, multi-centre, randomized controlled, First in Man study to assess the safety and performance of the Paclitaxel releasing Passeo-18 Lux Catheter vs. the uncoated Passeo-18 balloon catheter in patients with stenosis and occlusion of the infrapopliteal arteries. 74 patients in up to 8 clinical sites in Germany, Netherlands, Austria. PRINCIPAL INVESTIGATOR: Prof. T. Zeller, Bad Krozingen (DE) Passeo-18 Lux PTA (Passeo-18) PRIMARY ENDPOINT: 6 month patency in target lesion measured by Quantitative Vascular Angiography (QVA). Major Adverse Event (MAE) rate SECONDARY ENDPOINTS: Device, technical and procedural success. TLR at 6 and 12 months. Change in ABI and Rutherford Class at 1, 6, 12 months. 6 month target lesion patency by QVA + MAE * 12 month clinical follow-up *
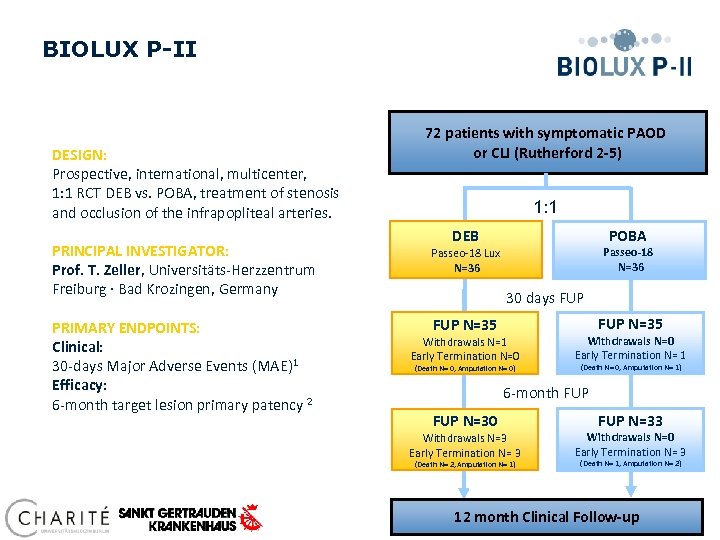
BIOLUX P-II DESIGN: Prospective, international, multicenter, 1: 1 RCT DEB vs. POBA, treatment of stenosis and occlusion of the infrapopliteal arteries. 72 patients with symptomatic PAOD or CLI (Rutherford 2 -5) 1: 1 POBA DEB PRINCIPAL INVESTIGATOR: Prof. T. Zeller, Universitäts-Herzzentrum Freiburg · Bad Krozingen, Germany Passeo-18 Lux N=36 PRIMARY ENDPOINTS: Clinical: 30 -days Major Adverse Events (MAE)1 Efficacy: 6 -month target lesion primary patency 2 FUP N=35 Passeo-18 N=36 30 days FUP N=35 Withdrawals N=1 Early Termination N=0 (Death N= 0, Amputation N= 0) Withdrawals N=0 Early Termination N= 1 (Death N= 0, Amputation N= 1) 6 -month FUP N=30 Withdrawals N=3 Early Termination N= 3 (Death N= 2, Amputation N= 1) FUP N=33 Withdrawals N=0 Early Termination N= 3 (Death N= 1, Amputation N= 2) 12 month Clinical Follow-up

Major Inclusion / Exclusion Criteria Inclusion Criteria Exclusion Criteria Single or sequential de novo or restenotic lesions (≥ 70%) in the infrapopliteal arteries ≥ 30 mm § Previously implanted stent in target lesion § Previous bypass surgery of target vessel § Lesions should not extend beyond the ankle joint § Acute thrombus in the target vessel left untreated § A maximum of 2 different vessels can be treated § Acute MI ≤ 3 months § Planned major amputation above the ankle of target limb, or any other planned major surgery within 30 days post-procedure § Flow-limiting (> 50% DS) inflow lesion proximal to target lesion, left untreated (DEB or DES not allowed for the treatment of inflow lesions) § § RVD 2. 0 – 4. 0 mm § At least one non-occluded crural vessel with angiographically documented run-off to the foot
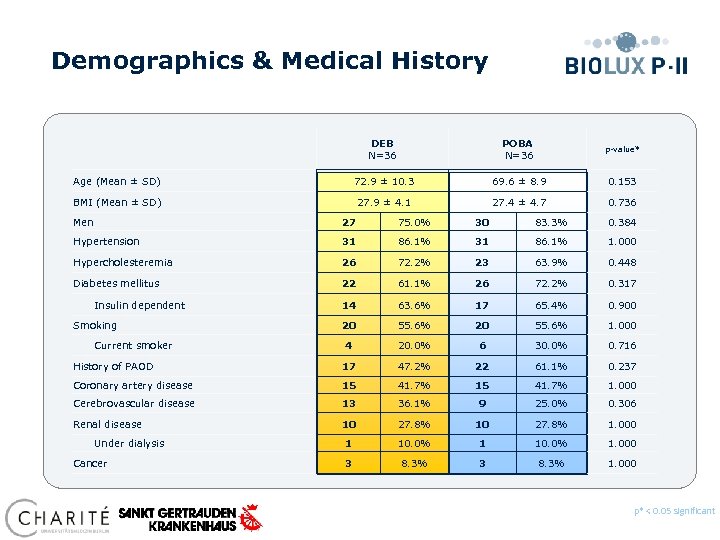
Demographics & Medical History DEB N=36 POBA N=36 p-value* Age (Mean ± SD) 72. 9 ± 10. 3 69. 6 ± 8. 9 0. 153 BMI (Mean ± SD) 27. 9 ± 4. 1 27. 4 ± 4. 7 0. 736 Men 27 75. 0% 30 83. 3% 0. 384 Hypertension 31 86. 1% 31 86. 1% 1. 000 Hypercholesteremia 26 72. 2% 23 63. 9% 0. 448 Diabetes mellitus 22 61. 1% 26 72. 2% 0. 317 14 63. 6% 17 65. 4% 0. 900 20 55. 6% 20 55. 6% 1. 000 30. 0% 0. 716 Insulin dependent Smoking Current smoker 4 20. 0% 6 History of PAOD 17 47. 2% 22 61. 1% 0. 237 Coronary artery disease 15 41. 7% 1. 000 Cerebrovascular disease 13 36. 1% 9 25. 0% 0. 306 Renal disease 10 27. 8% 10 27. 8% 1. 000 1 10. 0% 1. 000 3 8. 3% 1. 000 Under dialysis Cancer 1 3 p* < 0. 05 significant Preliminary data, database not locked yet
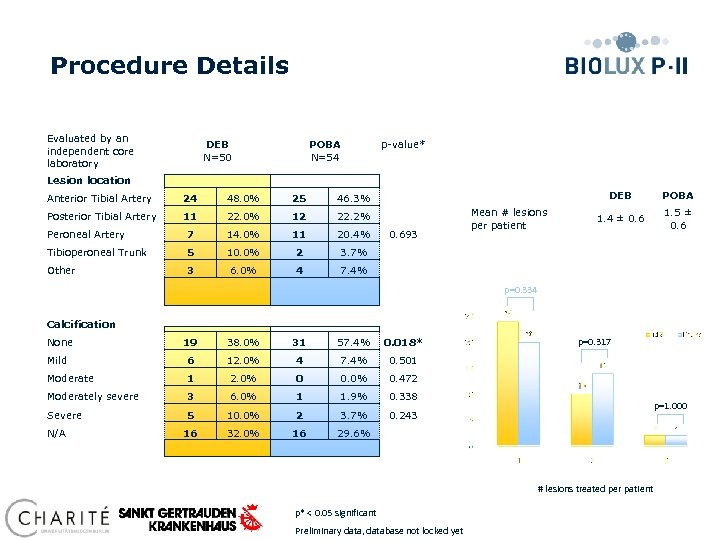
Procedure Details Evaluated by an independent core laboratory DEB N=50 POBA N=54 p-value* Lesion location Anterior Tibial Artery 24 48. 0% 25 Posterior Tibial Artery 11 22. 0% 12 22. 2% Peroneal Artery 7 14. 0% 11 20. 4% Tibioperoneal Trunk 5 10. 0% 2 3 6. 0% 4 POBA 1. 4 ± 0. 6 1. 5 ± 0. 6 3. 7% Other DEB 46. 3% 7. 4% 0. 693 Mean # lesions per patient p=0. 334 Calcification None 19 38. 0% 31 57. 4% 0. 018* Mild 6 12. 0% 4 7. 4% 0. 501 Moderate 1 2. 0% 0 0. 0% 0. 472 Moderately severe 3 6. 0% 1 1. 9% 0. 338 Severe 5 10. 0% 2 3. 7% 0. 243 16 32. 0% 16 p=0. 317 29. 6% N/A p=1. 000 # lesions treated per patient p* < 0. 05 significant Preliminary data, database not locked yet
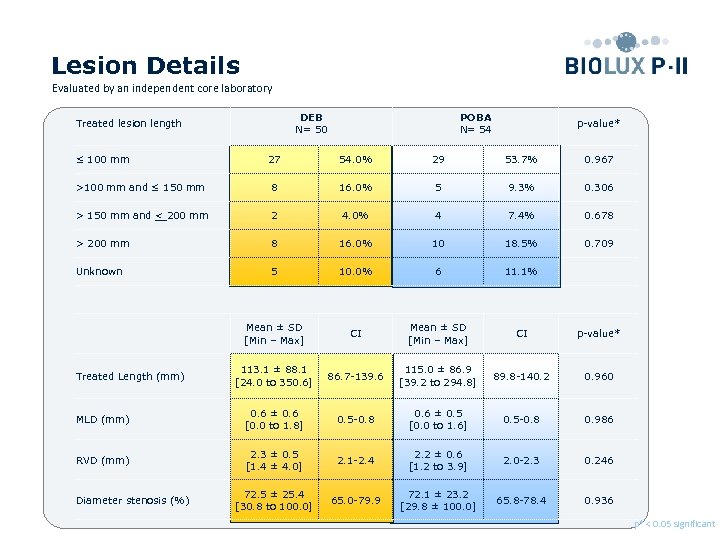
Lesion Details Evaluated by an independent core laboratory DEB N= 50 Treated lesion length ≤ 100 mm POBA N= 54 p-value* 27 54. 0% 29 53. 7% 0. 967 >100 mm and ≤ 150 mm 8 16. 0% 5 9. 3% 0. 306 > 150 mm and < 200 mm 2 4. 0% 4 7. 4% 0. 678 > 200 mm 8 16. 0% 10 18. 5% 0. 709 Unknown 5 10. 0% 6 11. 1% Mean ± SD [Min – Max] CI p-value* 113. 1 ± 88. 1 [24. 0 to 350. 6] 86. 7 -139. 6 115. 0 ± 86. 9 [39. 2 to 294. 8] 89. 8 -140. 2 0. 960 MLD (mm) 0. 6 ± 0. 6 [0. 0 to 1. 8] 0. 5 -0. 8 0. 6 ± 0. 5 [0. 0 to 1. 6] 0. 5 -0. 8 0. 986 RVD (mm) 2. 3 ± 0. 5 [1. 4 ± 4. 0] 2. 1 -2. 4 2. 2 ± 0. 6 [1. 2 to 3. 9] 2. 0 -2. 3 0. 246 72. 5 ± 25. 4 [30. 8 to 100. 0] 65. 0 -79. 9 72. 1 ± 23. 2 [29. 8 ± 100. 0] 65. 8 -78. 4 0. 936 Treated Length (mm) Diameter stenosis (%) p* < 0. 05 significant Preliminary data, database not locked yet
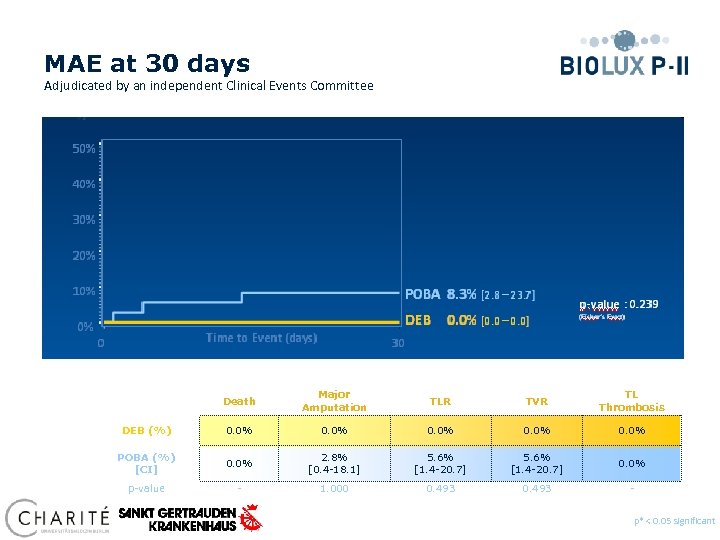
MAE at 30 days Adjudicated by an independent Clinical Events Committee 50% 40% Death Major Amputation TLR TVR TL Thrombosis DEB (%) 0. 0% POBA (%) [CI] 0. 0% 2. 8% [0. 4 -18. 1] 5. 6% [1. 4 -20. 7] 0. 0% p-value - 1. 000 0. 493 - p* < 0. 05 significant Preliminary data, database not locked yet
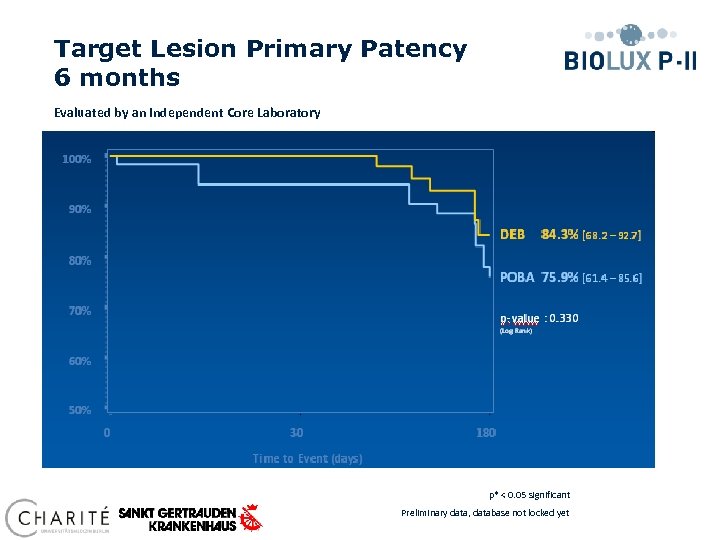
Target Lesion Primary Patency 6 months Evaluated by an independent Core Laboratory Time to Event (days) p* < 0. 05 significant Preliminary data, database not locked yet
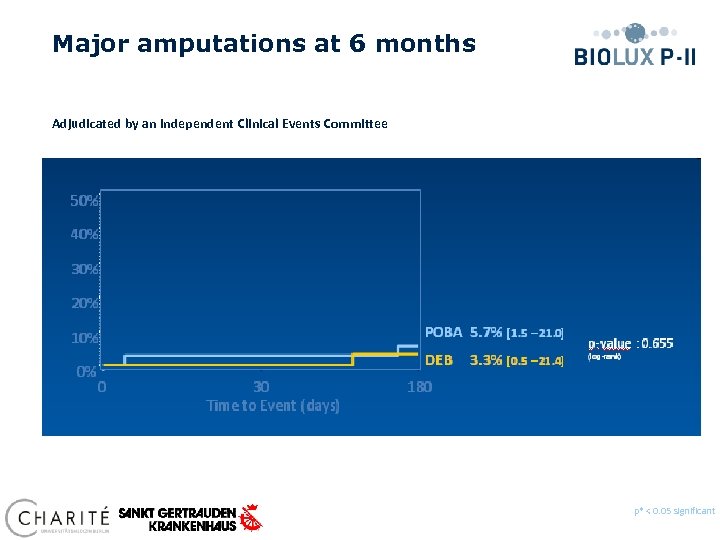
Major amputations at 6 months Adjudicated by an independent Clinical Events Committee p* < 0. 05 significant Preliminary data, database not locked yet
Conclusions § At 30 days clinical results show MAE composite of 0. 0% for the Passeo 18 Lux vs. 8. 3% compared to the control PTA balloon (p=0. 239). § At 6 months angiographic follow-up, Passeo-18 Lux DRB demonstrated a target lesion primary patency of 84. 3% vs. 75. 9% compared to the control PTA balloon (p=0. 330). § At 6 months, 59% of patients improved in Rutherford Classification in the DEB group vs. 47% in the control group. No patients worsened in the DEB group, vs. 6% in the POBA group. § Improvement of Rutherford Class 5 patients at 6 months was significant in the DEB group (p=0. 002) compared to the POBA group. p* < 0. 05 significant Preliminary data, database not locked yet
BIOLUX P-III All-comers registry is necessary to strengthen our Peripheral DRB offering Study Design DESIGN: Prospective, international, multi-centre, open label, allcomers registry to expand understand the safety and efficacy data on the Passeo-18 Lux DRB in a real world population of subjects with obstructive disease of the infrainguinal arteries. PRINCIPAL INVESTIGATOR: tbd PRIMARY ENDPOINT: Freedom from clinically-driven target lesion revascularization (TLR) within 12 months post-index procedure. SECONDARY ENDPOINTS: (selected) Freedom from clinically-driven target lesion revascularization (TLR) within 24 months post-index procedure Primary patency at 12 and 24 months Major Adverse Events (MAE) at 6, 12 and 24 months Quality of Life (QOL) assessment questionnaires: Pain scale, SF-12, WIQ at 6, 12 and 24 months. 600 patients in c. 40 clinical sites in: CENEMEA and MIDWEST (pending ‘call for centres’) First patient inclusion: Q 2/3 -2014 Passeo-18 Lux 6 months: MAE, change in ABI, RC 12 months: freedom from TLR, primary patency, MAE, change in ABI 24 months: freedom from TLR, primary patency, MAE, change in ABI
Clinical Conclusions § At 12 months clinical results show freedom from TLR of 84. 6% for the Passeo-18 Lux vs 58. 3% compared to the control PTA balloon in the SFA in Biolux PI. • At 30 days clinical results show MAE composite of 0. 0% for the Passeo 18 Lux § At 6 month safety and efficacy for the Biolux balloon is shown in challenging BTK lesions. Improvement of Rutherford Class 5 patients at 6 months was significant in the DEB group (p=0. 002) compared to the POBA group. § Passeo-18 Lux drug releasing balloon demonstrated a significant reduction in late luminal loss and binary restenosis at 6 months compared to the control PTA balloon for SFA and BTK vs. 8. 3% compared to the control PTA balloon (p=0. 239) in Biolux PII for BTK. *P=<0. 05 significant
8ed5253ad50e1f6f2d7a9774824e2886.ppt