af6d5dee50ac27cc49f58bcdef450fa2.ppt
- Количество слайдов: 11

The Bio. Builder Lab Experience: i. Tune Device Present Prepare Perform
PRESENT can it fit? Where The Big Idea: Evaluate promoter and RBS combinations to optimize β-galactosidase output Objectives: Explain the functioning of the lac operon and relate it to this system. Measure a kinetic chemical reaction. Culture bacteria using appropriate microbiology techniques. Properly use synthetic biology and molecular genetics terms. Microbiology Molecular Genetics Operon Activity Transcription/Translation Enrichment/Extension
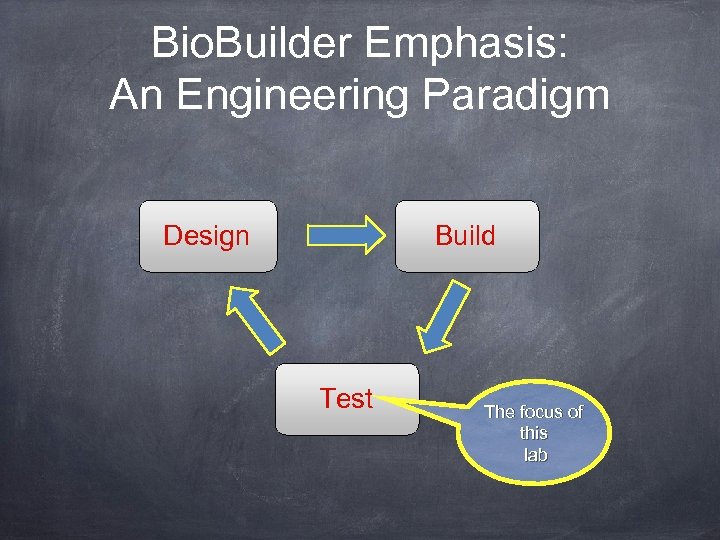
Bio. Builder Emphasis: An Engineering Paradigm Design Build Test The focus of this lab
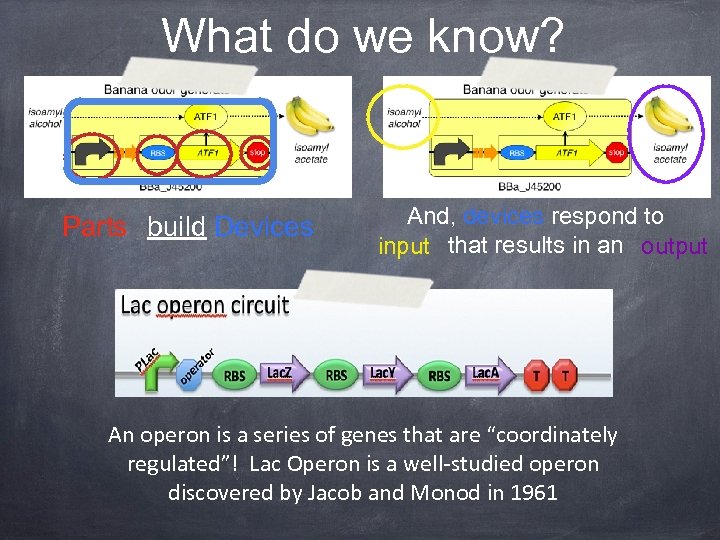
What do we know? Parts build Devices And, devices respond to input that results in an output An operon is a series of genes that are “coordinately regulated”! Lac Operon is a well-studied operon discovered by Jacob and Monod in 1961
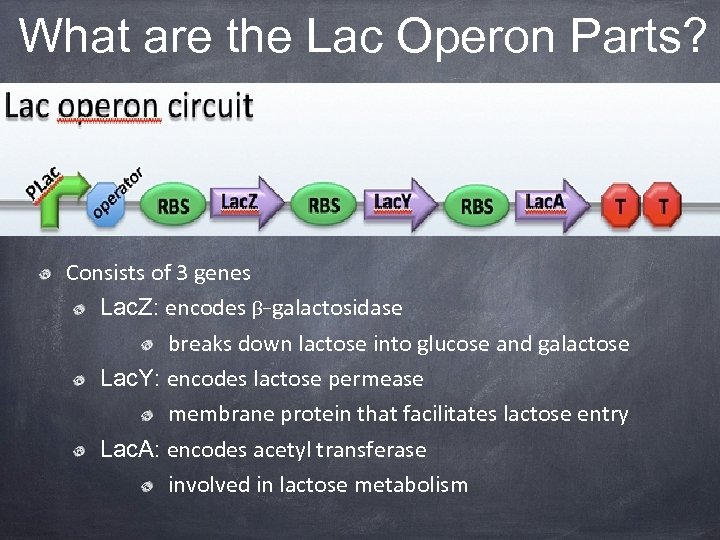
What are the Lac Operon Parts? Consists of 3 genes Lac. Z: encodes β-galactosidase breaks down lactose into glucose and galactose Lac. Y: encodes lactose permease membrane protein that facilitates lactose entry Lac. A: encodes acetyl transferase involved in lactose metabolism
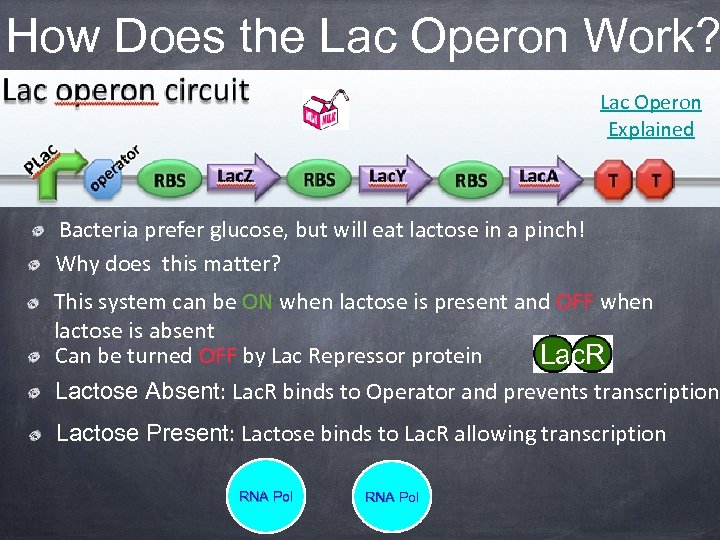
How Does the Lac Operon Work? Lac Operon Explained Bacteria prefer glucose, but will eat lactose in a pinch! Why does this matter? This system can be ON when lactose is present and OFF when lactose is absent Can be turned OFF by Lac Repressor protein Lac. R Lactose Absent: Lac. R binds to Operator and prevents transcription Lactose Present: Lactose binds to Lac. R allowing transcription RNA Pol
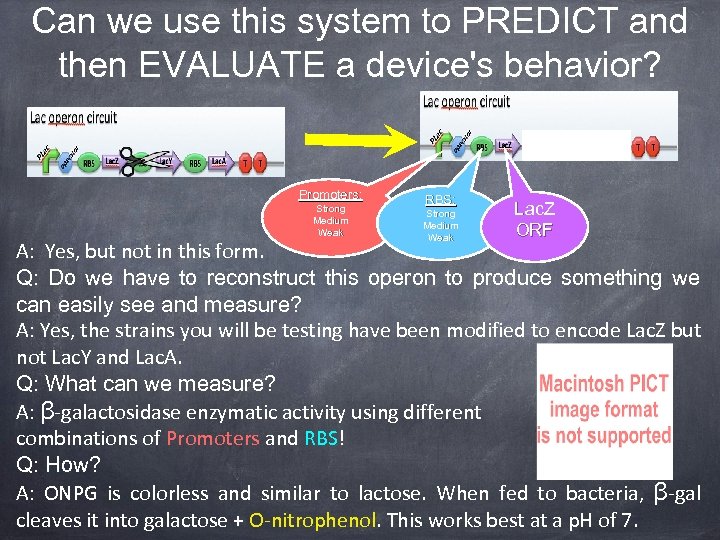
Can we use this system to PREDICT and then EVALUATE a device's behavior? Promoters: Strong Medium Weak RBS: Strong Medium Weak Lac. Z ORF A: Yes, but not in this form. Q: Do we have to reconstruct this operon to produce something we can easily see and measure? A: Yes, the strains you will be testing have been modified to encode Lac. Z but not Lac. Y and Lac. A. Q: What can we measure? A: β-galactosidase enzymatic activity using different combinations of Promoters and RBS! Q: How? A: ONPG is colorless and similar to lactose. When fed to bacteria, β-gal cleaves it into galactose + O-nitrophenol. This works best at a p. H of 7.

PREPARATION Goal: To evaluate promoter and RBS combinations to optimize βgalactosidase output Advanced Prep. . . 1. Streak strains from stabs onto plates. You can view how to prep this here: Streaking from Stabs 2. Grow strains from plates as liquid overnights. You can view how to prep this here: Liquid Overnight Cultures We’re Ready to Assay. . . Are you? 3. Set up Mc. Farland Standards if Spec 20 is unavailable 4. Prepare solutions for β-galactosidase assay: a. Bicarbonate Buffer b. ONPG (START) c. 1 M Sodium Carbonate (STOP) 5. To buy or not to buy. . . chloroform? ? ?
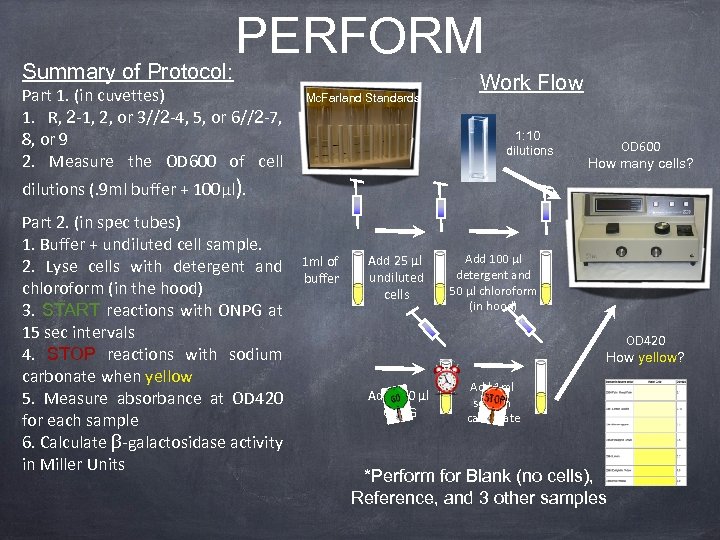
Summary of Protocol: PERFORM Part 1. (in cuvettes) 1. R, 2 -1, 2, or 3//2 -4, 5, or 6//2 -7, 8, or 9 2. Measure the OD 600 of cell Mc. Farland Standards Work Flow 1: 10 dilutions OD 600 How many cells? dilutions (. 9 ml buffer + 100µl). Part 2. (in spec tubes) 1. Buffer + undiluted cell sample. 2. Lyse cells with detergent and chloroform (in the hood) 3. START reactions with ONPG at 15 sec intervals 4. STOP reactions with sodium carbonate when yellow 5. Measure absorbance at OD 420 for each sample 6. Calculate β-galactosidase activity in Miller Units 1 ml of buffer Add 25 µl undiluted cells Add 100 µl detergent and 50 µl chloroform (in hood) OD 420 How yellow? Add 100 µl ONPG Add 1 ml sodium carbonate *Perform for Blank (no cells), Reference, and 3 other samples
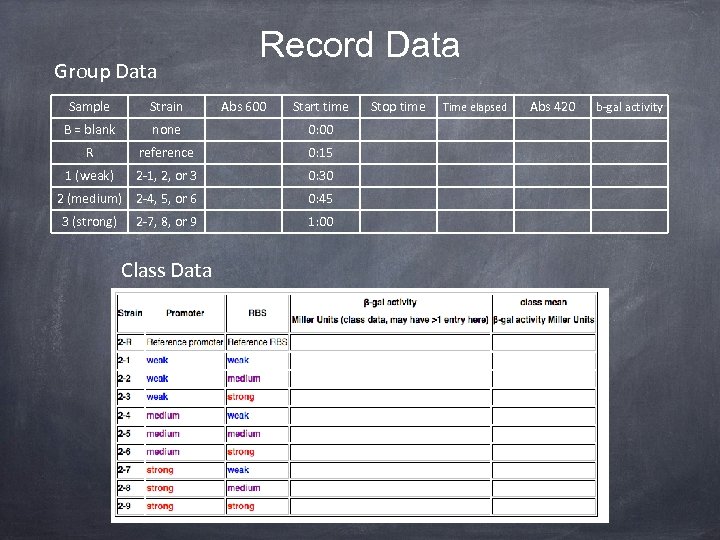
Group Data Record Data Sample Strain B = blank none 0: 00 R reference 0: 15 1 (weak) 2 -1, 2, or 3 0: 30 2 (medium) 2 -4, 5, or 6 0: 45 3 (strong) 2 -7, 8, or 9 Class Data Abs 600 Start time 1: 00 Stop time Time elapsed Abs 420 b-gal activity

Submit Your Data Here: http: //www. biobuilder. org/activities/ Password: natbioethics
af6d5dee50ac27cc49f58bcdef450fa2.ppt