ffd2f280efa5e8bd5891219cd30c0211.ppt
- Количество слайдов: 38

The Basics of Drug Development Science Nothing to be scared of! Or Which Way to the Clinic? IRMA Webinar September 28, 2010 Jim A. Turpin, Ph. D. Preclinical Team Leader Topical Microbicide Branch Prevention Science Program NIAID, Division of AIDS,

DHHS/NIH Required Disclaimer The views expressed are those of the presenter and do not necessarily reflect the official policies of the Department of Health and Human Services (HHS), nor does mention of trade names, commercial practices, or organizations imply endorsement by the U. S. Government DHHS/NIH/NIAID/DAIDS

Today's talk Touch on the following : What is drug development? Basic Science behind drug development Will use prevention and microbicides as a context for discussion Everything I say can be applied to other drug development efforts with appropriate modifications A complex process but will take the 20, 000 ft view DHHS/NIH/NIAID/DAIDS

Definition Lead or Candidate A compound or proposed new chemical entity (NCE) with properties or characteristics that make it appropriate for further development The terms can be applied interchangeably to any stage of the drug development process Emphasize that a selection (elimination) has occurred DHHS/NIH/NIAID/DAIDS
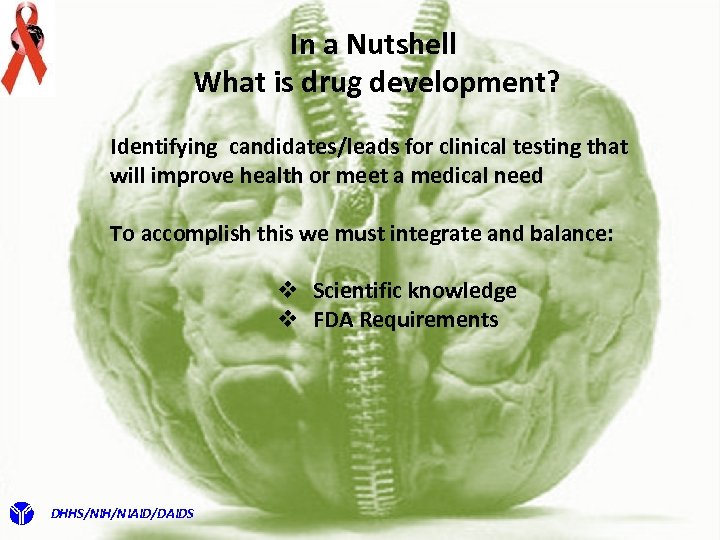
In a Nutshell What is drug development? Identifying candidates/leads for clinical testing that will improve health or meet a medical need To accomplish this we must integrate and balance: v Scientific knowledge v FDA Requirements DHHS/NIH/NIAID/DAIDS
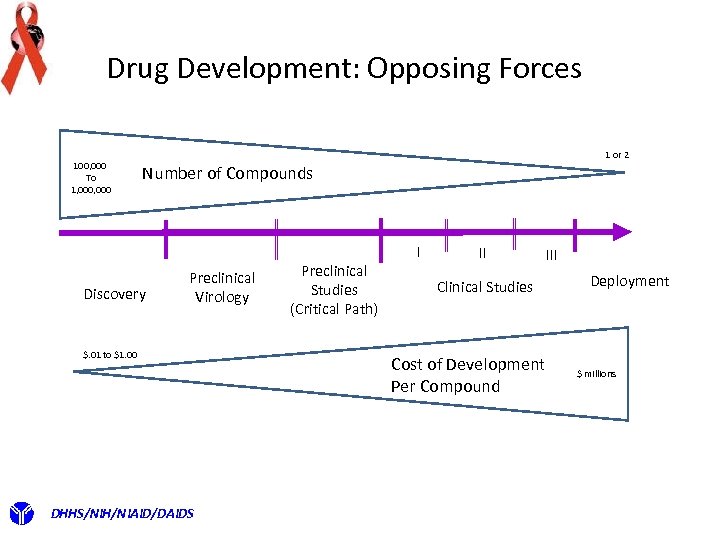
Drug Development: Opposing Forces 100, 000 To 1, 000 1 or 2 Number of Compounds Discovery Preclinical Virology $. 01 to $1. 00 DHHS/NIH/NIAID/DAIDS Preclinical Studies (Critical Path) I II III Clinical Studies Cost of Development Per Compound Deployment $ millions

Drug Development : Thy Name is Attrition FACTOIDS Average time from Discovery to Phase III trials : 12 years Probabilities of Success Discovery to Clinical Testing: ~11 % Initial lead identification, i. e. screen random library Chances of success ~ 1: 10, 000 that a compound will go to clinical testing Can raise to 1: 1000 if working on a series of compounds Clinical testing: 38% drop out in Phase I 60% percent drop out in Phase II 40% fail in Phase III Of successful Phase III candidates only 23% are approved as drugs by the FDA DHHS/NIH/NIAID/DAIDS
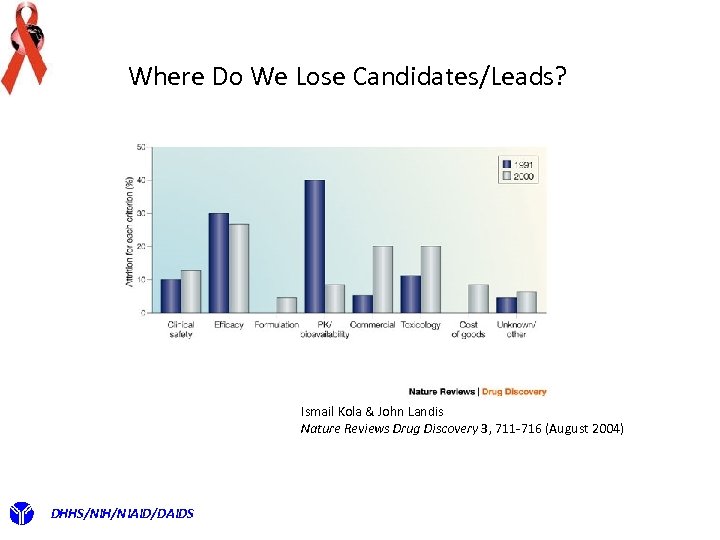
Where Do We Lose Candidates/Leads? Ismail Kola & John Landis Nature Reviews Drug Discovery 3, 711 -716 (August 2004) DHHS/NIH/NIAID/DAIDS

Drug Development and the Pipeline Concept Everyone talks about pipelines --Adjectives galore: robust, vibrant, sustainable A pipeline is a way to describe and conceptualize a drug development process involving many candidates/leads DHHS/NIH/NIAID/DAIDS

Pipelines are Defined by Their Goal Microbicides as the Example Must protect healthy and HIV infected men and women when used vaginally, penile and/or rectally without harm Harm= any effect that increases infection susceptibility or changes disease course for the worse, directly or indirectly May be used episodically or continually by individuals for decades Drug development strategy is used to identify suitable compounds to meet the above requirements proactively and efficiently to create a sustainable effort that: Generates new candidates/leads Eliminates the unsuitable DHHS/NIH/NIAID/DAIDS
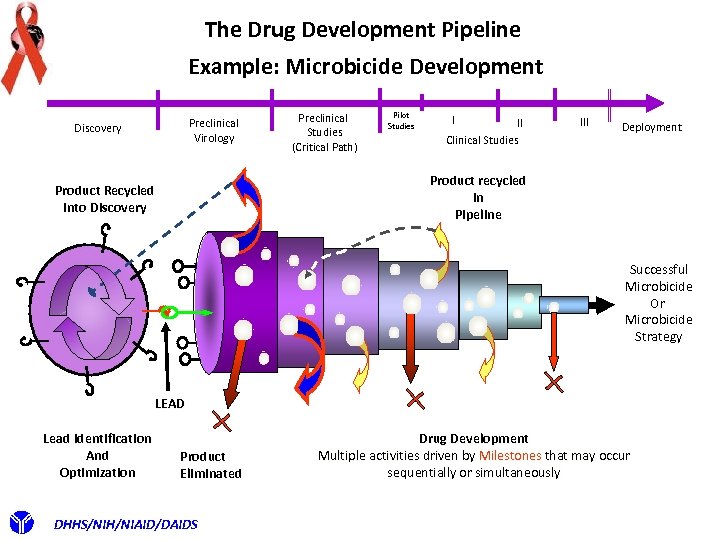
The Drug Development Pipeline Example: Microbicide Development Preclinical Virology Discovery Preclinical Studies (Critical Path) Pilot Studies I II Clinical Studies III Deployment Product recycled In Pipeline Product Recycled Into Discovery Successful Microbicide Or Microbicide Strategy LEAD Lead Identification And Optimization Product Eliminated DHHS/NIH/NIAID/DAIDS Drug Development Multiple activities driven by Milestones that may occur sequentially or simultaneously
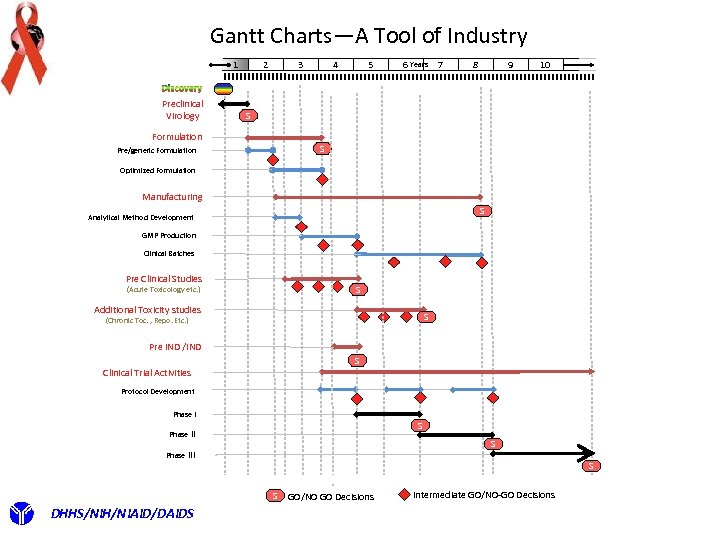
Gantt Charts—A Tool of Industry 2 1 Preclinical Virology 4 3 5 6 Years 7 8 9 10 S Formulation S Pre/generic Formulation Optimized Formulation Manufacturing S Analytical Method Development GMP Production Clinical Batches Pre Clinical Studies S (Acute Toxicology etc. ) Additional Toxicity studies S (Chronic Toc. , Repo. Etc. ) Pre IND /IND S Clinical Trial Activities Protocol Development Phase I S Phase III S S DHHS/NIH/NIAID/DAIDS GO/NO GO Decisions Intermediate GO/NO-GO Decisions
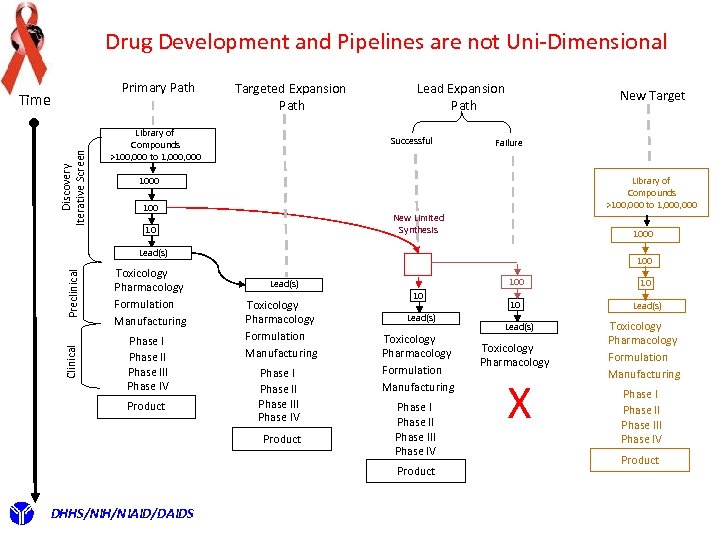
Drug Development and Pipelines are not Uni-Dimensional Primary Path Discovery Iterative Screen Time Targeted Expansion Path Library of Compounds >100, 000 to 1, 000 Lead Expansion Path Successful New Target Failure Library of Compounds >100, 000 to 1, 000 1000 100 New Limited Synthesis 10 1000 Clinical Preclinical Lead(s) Toxicology Pharmacology Formulation Manufacturing Phase II Phase III Phase IV Product 100 10 Lead(s) Toxicology Pharmacology Formulation Manufacturing Phase III Phase IV Product DHHS/NIH/NIAID/DAIDS 10 10 Lead(s) Toxicology Pharmacology X Toxicology Pharmacology Formulation Manufacturing Phase III Phase IV Product

The Challenge of the Basic Science of Drug Development is Finding the right needle in the right hay stack And once it is found confirming the needle you found is the one you were looking for Doing all of this in sustainable fashion DHHS/NIH/NIAID/DAIDS

The Foundation of Drug Development is: Scientific knowledge We must understand the disease to be able to find candidates/leads to prevent or treat it DHHS/NIH/NIAID/DAIDS

HIV drug development has to account for: What we do not know In HIV Infection 1. What form of the virus infects? 1. Cell –free 2. Cell-associated 3. Both 2. How does virus get to its target cells? 1. Micro-trauma 2. Translocation 3. Permeation 4. Cell capture 3. What is the first cell infected? DHHS/NIH/NIAID/DAIDS

HIV drug development must also account for: What we think we know The data, models and observations we use to describe the process of HIV transmission and infection are subject to change as we learn more DHHS/NIH/NIAID/DAIDS
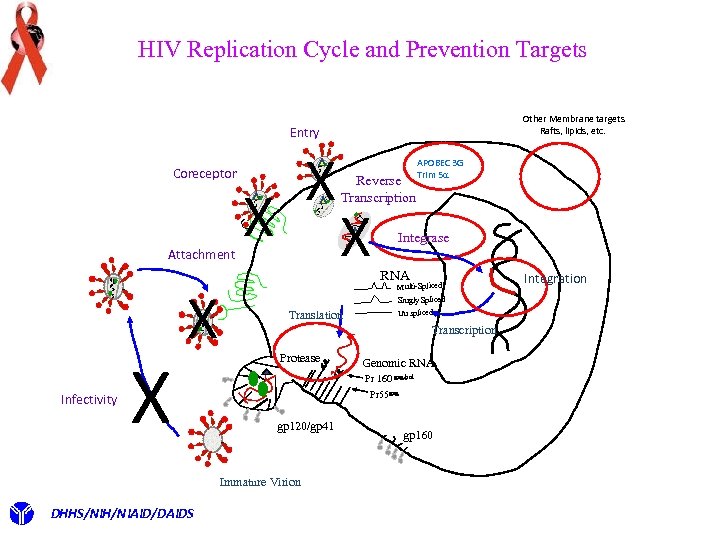
HIV Replication Cycle and Prevention Targets Other Membrane targets Rafts, lipids, etc. Entry Coreceptor Attachment Infectivity X X X Integrase RNA Multi-Spliced Translation Singly Spliced Un spliced Transcription Protease Genomic RNA Pr 160 gag/pol Pr 55 gag gp 120/gp 41 Immature Virion DHHS/NIH/NIAID/DAIDS Reverse Transcription APOBEC 3 G Trim 5 a gp 160 Integration
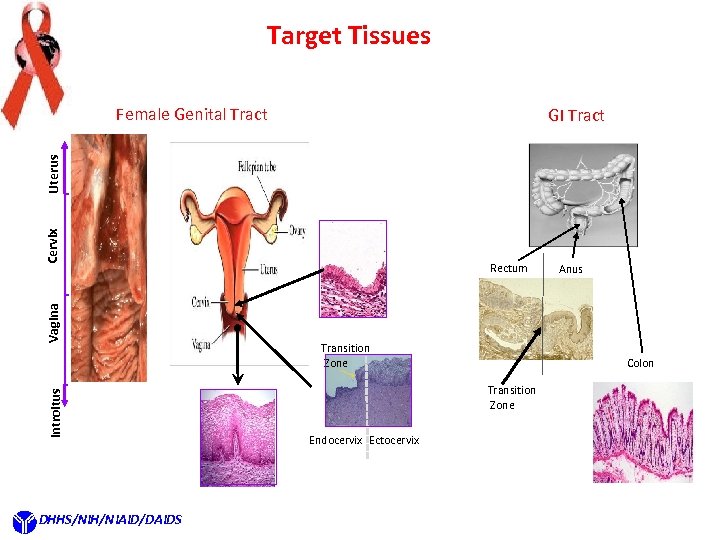
Target Tissues Female Genital Tract Introitus Vagina Cervix Uterus GI Tract DHHS/NIH/NIAID/DAIDS Rectum Transition Zone Colon Transition Zone Endocervix Ectocervix Anus
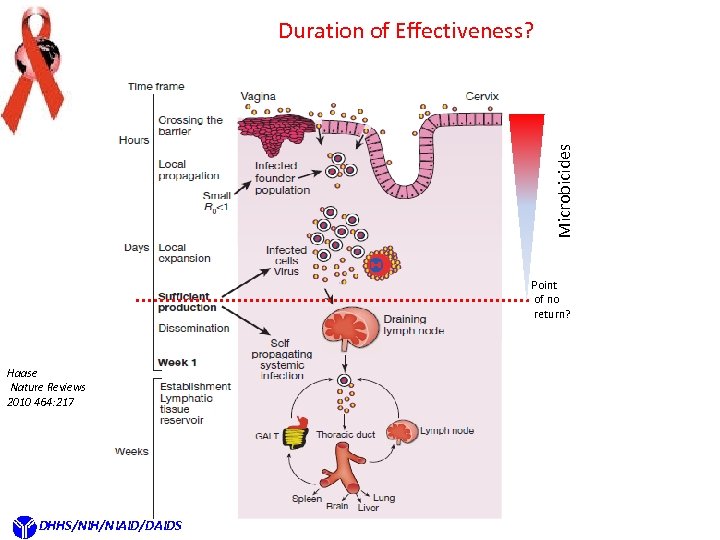
Microbicides Duration of Effectiveness? Point of no return? Haase Nature Reviews 2010 464: 217 DHHS/NIH/NIAID/DAIDS
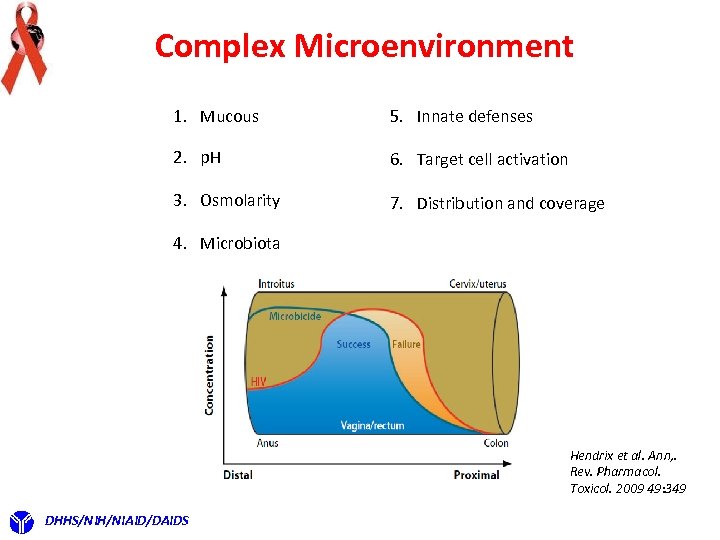
Complex Microenvironment 1. Mucous 5. Innate defenses 2. p. H 6. Target cell activation 3. Osmolarity 7. Distribution and coverage 4. Microbiota Hendrix et al. Ann, . Rev. Pharmacol. Toxicol. 2009 49: 349 DHHS/NIH/NIAID/DAIDS

How do we decide what testing to do? DHHS/NIH/NIAID/DAIDS

First must ask: What Are the Optimal “Drug” Characteristics of a Candidate/Lead? 1. Ultimately driven by regulatory requirements to conduct clinical trials 2. FDA enforces regulations and provides guidance 3. No FDA guidance specific to microbicides 4. Most relevant FDA preclinical guidance is for antivirals: Guidance for Industry Antiviral Product Development http: //www. fda. gov/OHRMS/DOCKETS/98 fr/05 d-0183 -gdl 0002 -01. pdf 5. The needed properties can be divided into 4 general categories 1. Safety 3. Acceptability 2. Efficacy 4. Feasibility/Delivery DHHS/NIH/NIAID/DAIDS
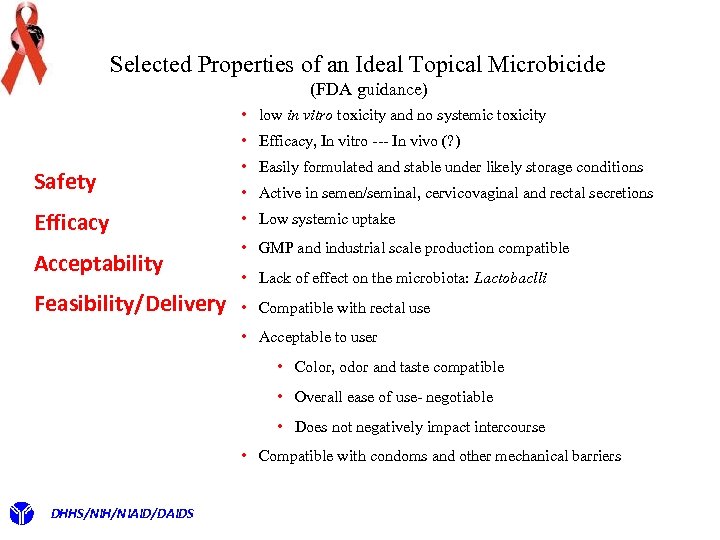
Selected Properties of an Ideal Topical Microbicide (FDA guidance) Safety Efficacy Acceptability Feasibility/Delivery • • • low in vitro toxicity and no systemic toxicity Efficacy, In vitro --- In vivo (? ) Easily formulated and stable under likely storage conditions Active in semen/seminal, cervicovaginal and rectal secretions Low systemic uptake • GMP and industrial scale production compatible • Lack of effect on the microbiota: Lactobaclli • Compatible with rectal use • Acceptable to user • Color, odor and taste compatible • Overall ease of use- negotiable • Does not negatively impact intercourse • Compatible with condoms and other mechanical barriers DHHS/NIH/NIAID/DAIDS

How do you Guidance into Drug Development? The Role of the Algorithm: A set of rules or steps that results in achieving the right answer in the shortest period of time Algorithms and the Basic Science of Drug Development 1. Use best assays to pick the best candidates/leads 2. Basic Science driven 3. May include approaches to address gaps in knowledge 4. In Prevention---everyone has one 5. Tailored to the objectives and goals of the individual drug development program DHHS/NIH/NIAID/DAIDS
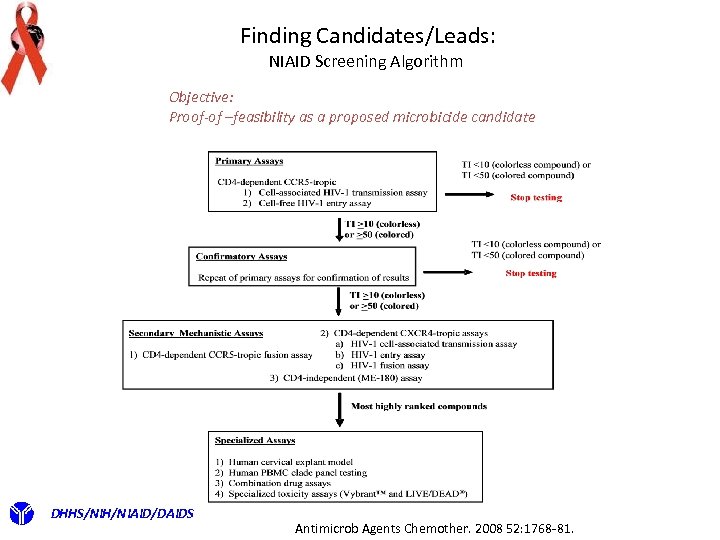
Finding Candidates/Leads: NIAID Screening Algorithm Objective: Proof-of –feasibility as a proposed microbicide candidate DHHS/NIH/NIAID/DAIDS Antimicrob Agents Chemother. 2008 52: 1768 -81.
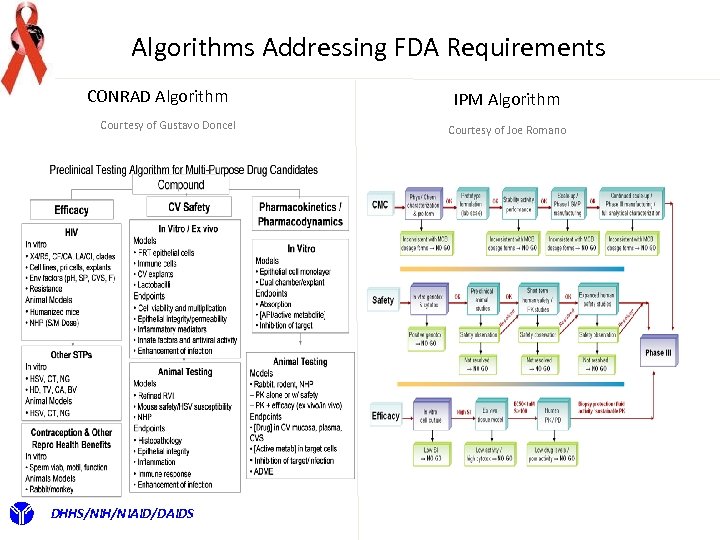
Algorithms Addressing FDA Requirements CONRAD Algorithm Courtesy of Gustavo Doncel DHHS/NIH/NIAID/DAIDS IPM Algorithm Courtesy of Joe Romano

Common Themes? Safety and Activity ü Safety- In vitro cytotoxicity ü Activity---Efficacy ü Effect in Ex Vivo Tissue Model (Explants)--Safety and Efficacy ü Animal Model--Efficacy ü FDA required testing—Safety (Critical Path) DHHS/NIH/NIAID/DAIDS
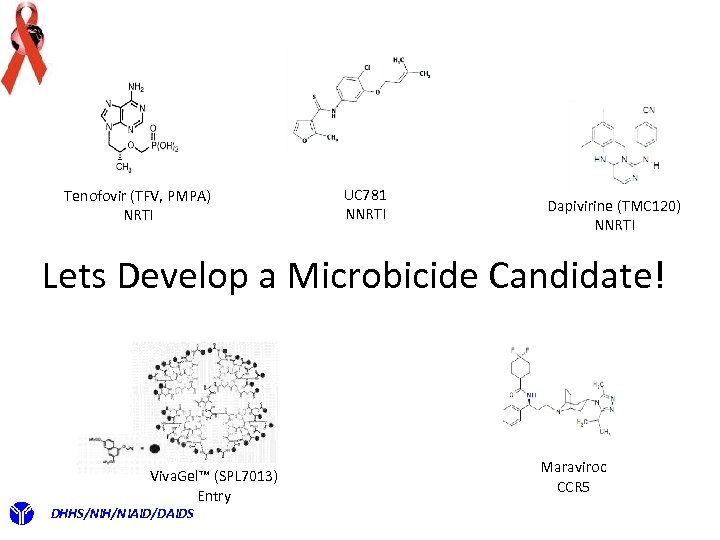
Tenofovir (TFV, PMPA) NRTI UC 781 NNRTI Dapivirine (TMC 120) NNRTI Lets Develop a Microbicide Candidate! Viva. Gel™ (SPL 7013) Entry DHHS/NIH/NIAID/DAIDS Maraviroc CCR 5
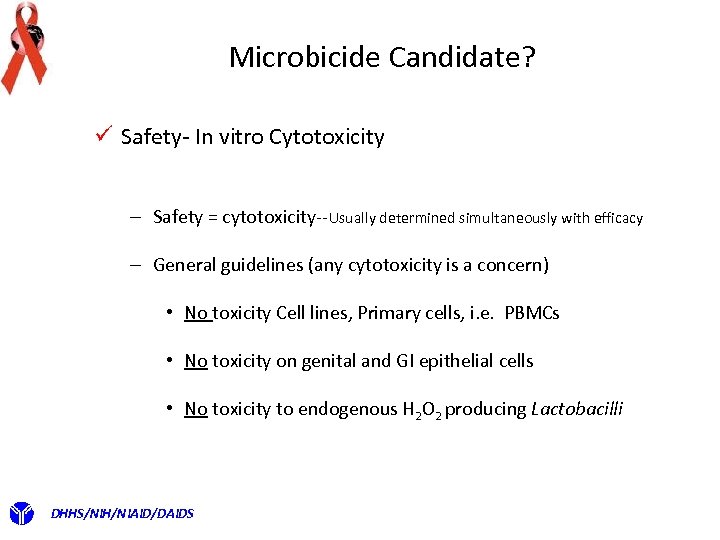
Microbicide Candidate? ü Safety- In vitro Cytotoxicity – Safety = cytotoxicity--Usually determined simultaneously with efficacy – General guidelines (any cytotoxicity is a concern) • No toxicity Cell lines, Primary cells, i. e. PBMCs • No toxicity on genital and GI epithelial cells • No toxicity to endogenous H 2 O 2 producing Lactobacilli DHHS/NIH/NIAID/DAIDS
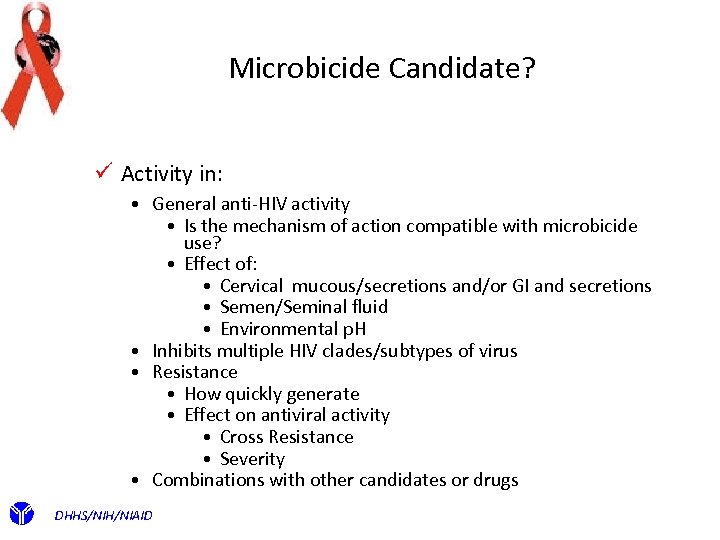
Microbicide Candidate? ü Activity in: • General anti-HIV activity • Is the mechanism of action compatible with microbicide use? • Effect of: • Cervical mucous/secretions and/or GI and secretions • Semen/Seminal fluid • Environmental p. H • Inhibits multiple HIV clades/subtypes of virus • Resistance • How quickly generate • Effect on antiviral activity • Cross Resistance • Severity • Combinations with other candidates or drugs DHHS/NIH/NIAID
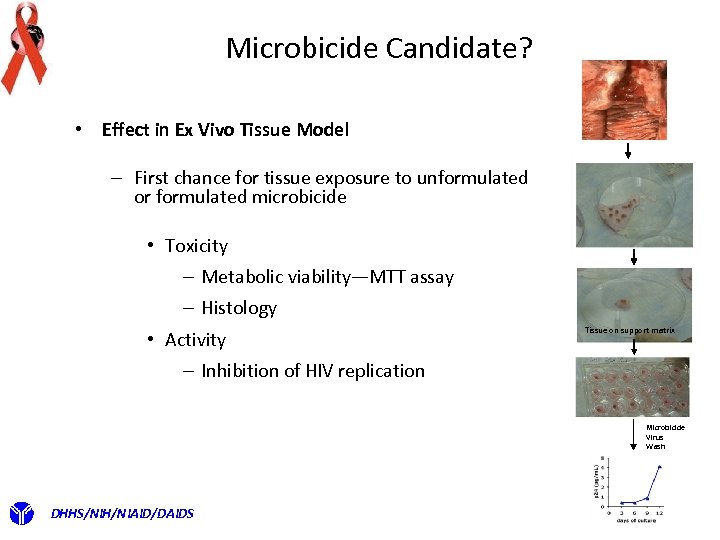
Microbicide Candidate? • Effect in Ex Vivo Tissue Model – First chance for tissue exposure to unformulated or formulated microbicide • Toxicity – Metabolic viability—MTT assay – Histology • Activity Tissue on support matrix – Inhibition of HIV replication Microbicide Virus Wash DHHS/NIH/NIAID/DAIDS
Microbicide Candidate? • Animal model testing (efficacy) • Humanized mice • NHP • Role of efficacy • Researcher--argument for “Biological Plausibility” • FDA--optional but preferred to be active DHHS/NIH/NIAID/DAIDS

Microbicide Candidate? SAFETY: The Critical Path Studies required by FDA International Conference on Harmonization (ICH) guidance are also used Goal of the Critical Path: Prepare for testing in humans by looking for any issues that will affect safety and/or feasibility DHHS/NIH/NIAID/DAIDS
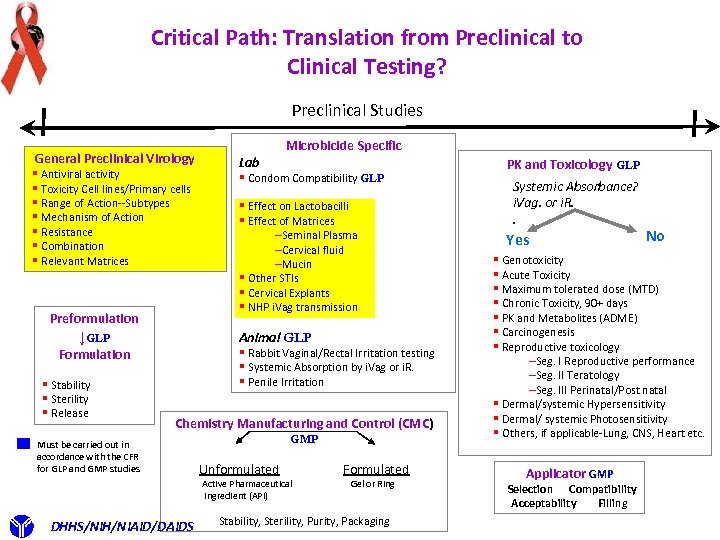
Critical Path: Translation from Preclinical to Clinical Testing? Preclinical Studies General Preclinical Virology § Antiviral activity § Toxicity Cell lines/Primary cells § Range of Action--Subtypes § Mechanism of Action § Resistance § Combination § Relevant Matrices Preformulation ↓GLP § Condom Compatibility GLP § Effect on Lactobacilli § Effect of Matrices –Seminal Plasma –Cervical fluid –Mucin § Other STIs § Cervical Explants § NHP i. Vag transmission Animal GLP § Rabbit Vaginal/Rectal Irritation testing § Systemic Absorption by i. Vag or i. R. § Penile Irritation Formulation § Stability § Sterility § Release Lab Microbicide Specific Chemistry Manufacturing and Control (CMC) Must be carried out in accordance with the CFR for GLP and GMP studies GMP Unformulated Active Pharmaceutical Ingredient (API) DHHS/NIH/NIAID/DAIDS Formulated Gel or Ring Stability, Sterility, Purity, Packaging PK and Toxicology GLP Systemic Absorbance? i. Vag. or i. R. . Yes No § Genotoxicity § Acute Toxicity § Maximum tolerated dose (MTD) § Chronic Toxicity, 90+ days § PK and Metabolites (ADME) § Carcinogenesis § Reproductive toxicology –Seg. I Reproductive performance –Seg. II Teratology –Seg. III Perinatal/Post natal § Dermal/systemic Hypersensitivity § Dermal/ systemic Photosensitivity § Others, if applicable-Lung, CNS, Heart etc. Applicator GMP Selection Compatibility Acceptability Filling

Take Home Messages Drug development is a process The process is controlled by multiple factors • FDA regulations and guidances • Basic science of HIV infection • Evolving knowledge of what might work The basic science of drug development is a process to select the candidate(s) with the best chance of success DHHS/NIH/NIAID/DAIDS

AND FINALLY: Jim Turpin’s slightly twisted final thoughts on the Basic Science of Drug Development Ø A challenge --not for the faint-hearted Ø Must embrace the Zen of Candidate Reduction It is never too early or late to eliminate a candidate and if you do-- kill it dead---put a stake through its heart, and bury it at midnight at a crossroads –No Regrets Allowed Ø The process is ever evolving—flexibility of mind is good! Ø Seems complicated but the roadmap is there, just gotta follow it Ø Sometimes its one of the most rewarding things you can do because it can change the world DHHS/NIH/NIAID/DAIDS

Thank You Questions? Timbuk 3 –The future is so bright I gotta wear shades! DHHS/NIH/NIAID/DAIDS
ffd2f280efa5e8bd5891219cd30c0211.ppt