4500ca4528344284cd797fb3e16b2072.ppt
- Количество слайдов: 53
 The Antiproteinuric Action of Cyclosporine Jai Radhakrishnan 09 -23 -2008
The Antiproteinuric Action of Cyclosporine Jai Radhakrishnan 09 -23 -2008
 Objectives • Clinical significance of proteinuria • Podocyte biology • The molecular biology of cyclosporine effect • Synaptopodin is a novel calcineurin substrate in the podocyte
Objectives • Clinical significance of proteinuria • Podocyte biology • The molecular biology of cyclosporine effect • Synaptopodin is a novel calcineurin substrate in the podocyte
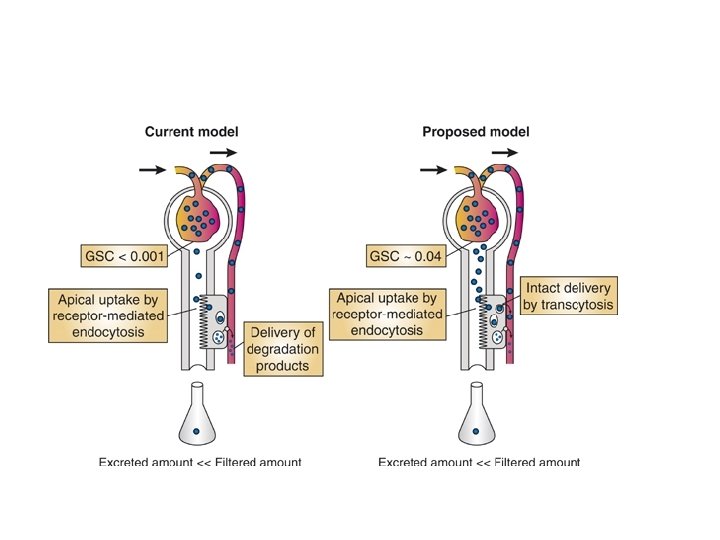
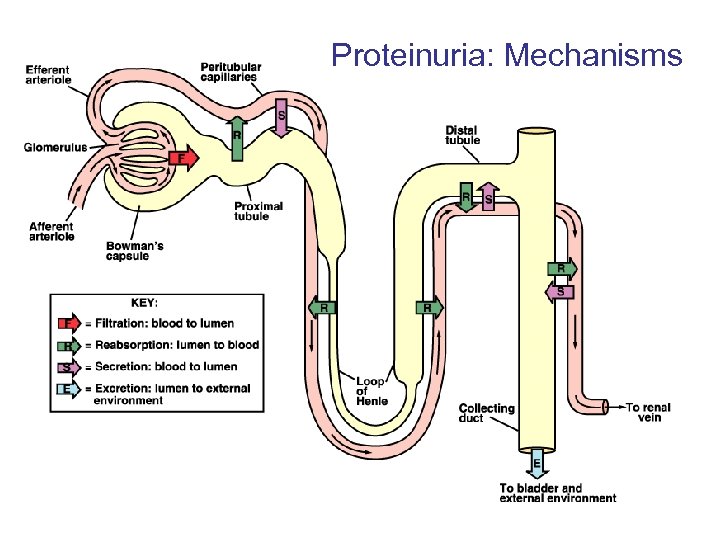 Proteinuria: Mechanisms
Proteinuria: Mechanisms
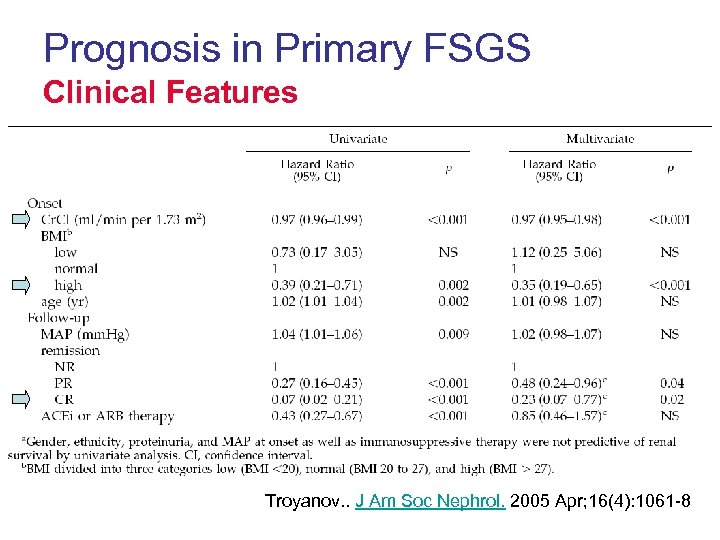 Prognosis in Primary FSGS Clinical Features Troyanov. . J Am Soc Nephrol. 2005 Apr; 16(4): 1061 -8
Prognosis in Primary FSGS Clinical Features Troyanov. . J Am Soc Nephrol. 2005 Apr; 16(4): 1061 -8
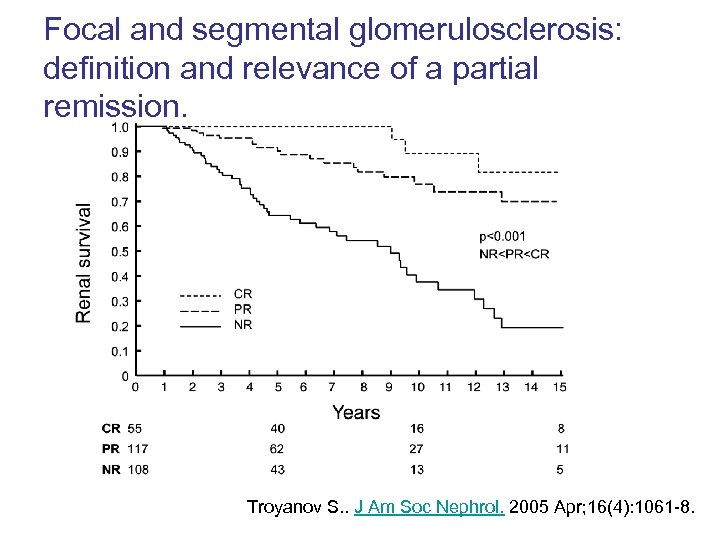 Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. Troyanov S. . J Am Soc Nephrol. 2005 Apr; 16(4): 1061 -8.
Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. Troyanov S. . J Am Soc Nephrol. 2005 Apr; 16(4): 1061 -8.
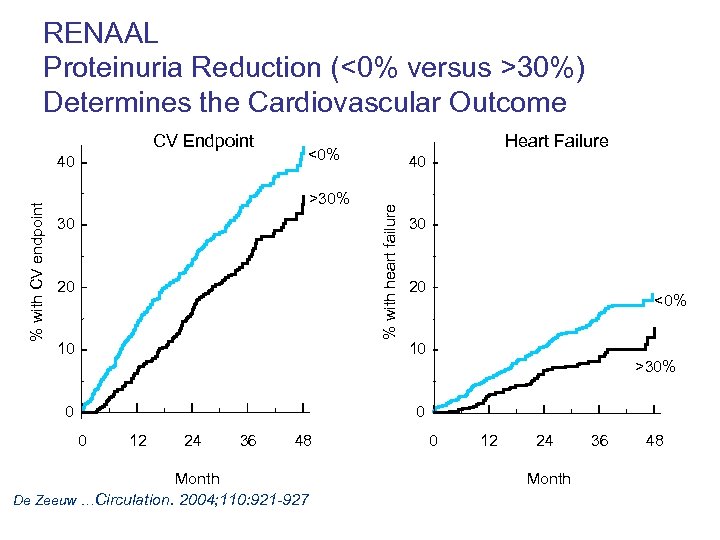 RENAAL Proteinuria Reduction (<0% versus >30%) Determines the Cardiovascular Outcome % with CV endpoint 40 Heart Failure <0% >30% 30 20 10 0 40 % with heart failure CV Endpoint 30 20 <0% 10 >30% 0 0 12 24 36 48 Month De Zeeuw …Circulation. 2004; 110: 921 -927 0 12 24 Month 36 48
RENAAL Proteinuria Reduction (<0% versus >30%) Determines the Cardiovascular Outcome % with CV endpoint 40 Heart Failure <0% >30% 30 20 10 0 40 % with heart failure CV Endpoint 30 20 <0% 10 >30% 0 0 12 24 36 48 Month De Zeeuw …Circulation. 2004; 110: 921 -927 0 12 24 Month 36 48
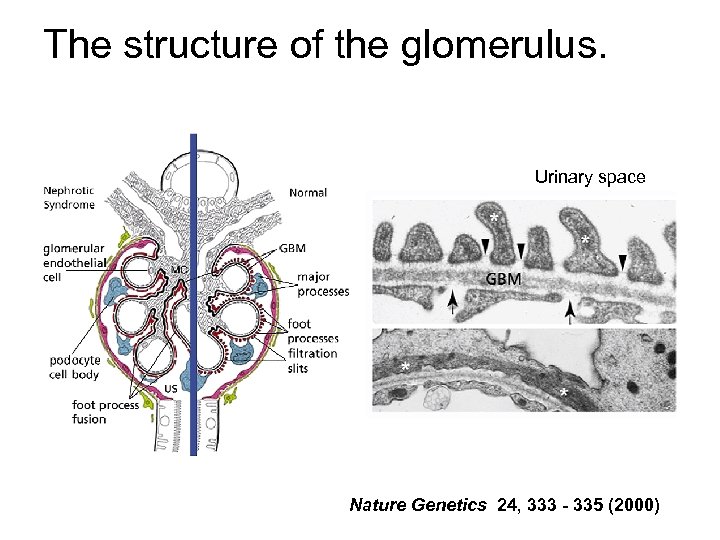 The structure of the glomerulus. Urinary space Nature Genetics 24, 333 - 335 (2000)
The structure of the glomerulus. Urinary space Nature Genetics 24, 333 - 335 (2000)
 Scanning electron micrograph Mouse glomerulus Physiol Rev. 2008 Apr; 88(2): 451 -87
Scanning electron micrograph Mouse glomerulus Physiol Rev. 2008 Apr; 88(2): 451 -87
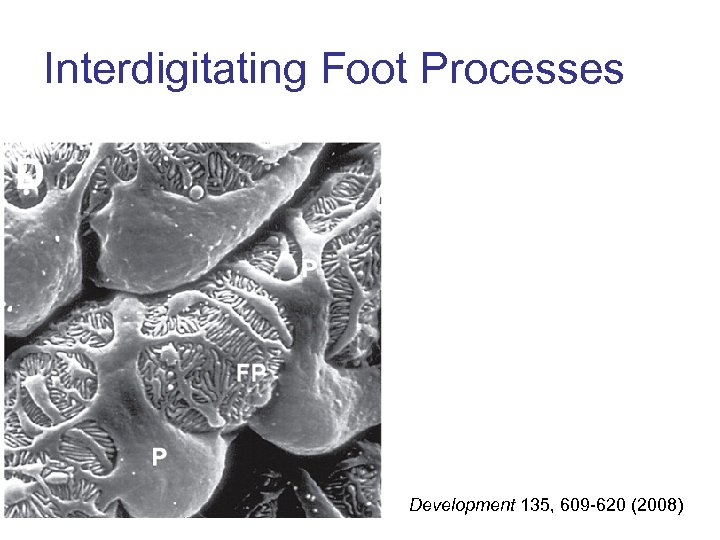 Interdigitating Foot Processes Development 135, 609 -620 (2008)
Interdigitating Foot Processes Development 135, 609 -620 (2008)
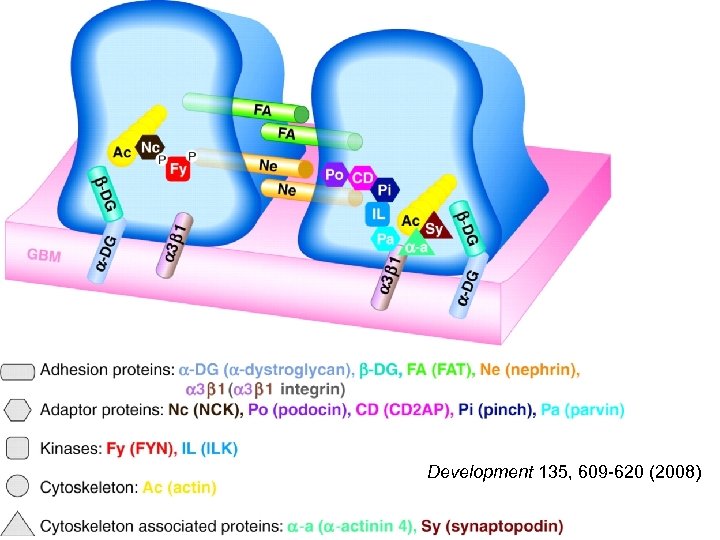 Schematic of the slit diaphragm and other important proteins involved in maintaining foot process assembly. Development 135, 609 -620 (2008)
Schematic of the slit diaphragm and other important proteins involved in maintaining foot process assembly. Development 135, 609 -620 (2008)
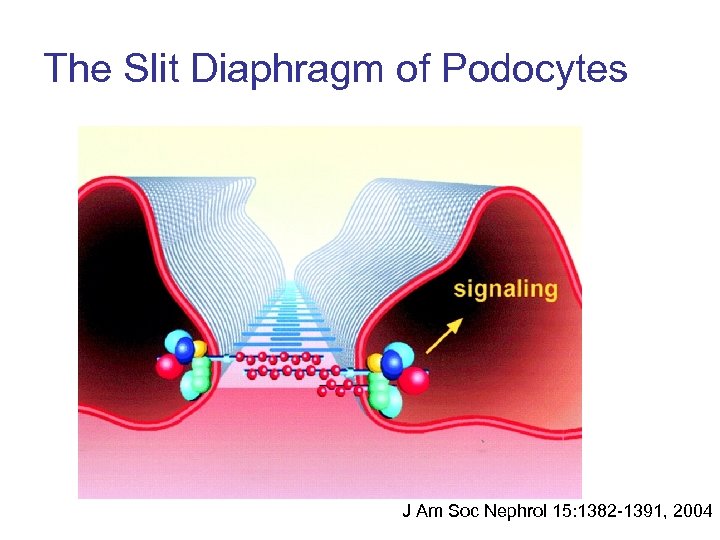 The Slit Diaphragm of Podocytes J Am Soc Nephrol 15: 1382 -1391, 2004
The Slit Diaphragm of Podocytes J Am Soc Nephrol 15: 1382 -1391, 2004
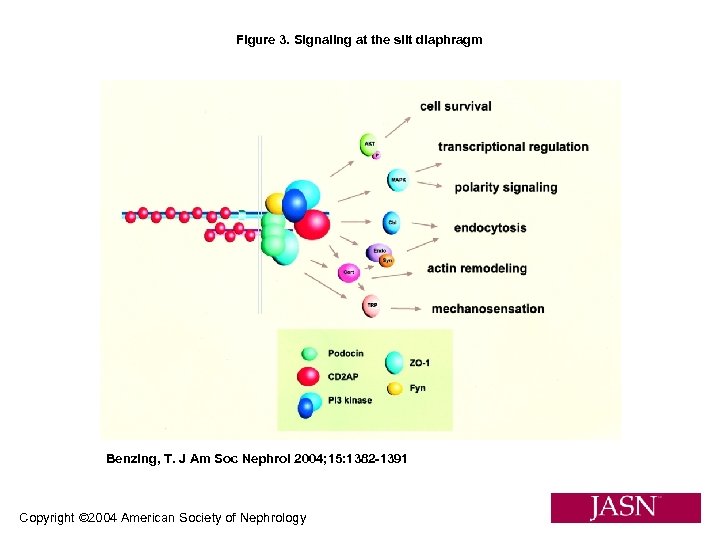 Figure 3. Signaling at the slit diaphragm Benzing, T. J Am Soc Nephrol 2004; 15: 1382 -1391 Copyright © 2004 American Society of Nephrology
Figure 3. Signaling at the slit diaphragm Benzing, T. J Am Soc Nephrol 2004; 15: 1382 -1391 Copyright © 2004 American Society of Nephrology
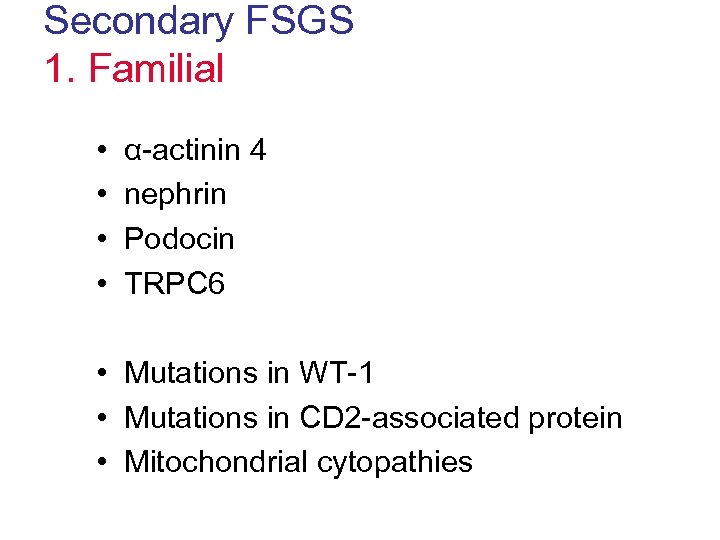 Secondary FSGS 1. Familial • • α-actinin 4 nephrin Podocin TRPC 6 • Mutations in WT-1 • Mutations in CD 2 -associated protein • Mitochondrial cytopathies
Secondary FSGS 1. Familial • • α-actinin 4 nephrin Podocin TRPC 6 • Mutations in WT-1 • Mutations in CD 2 -associated protein • Mitochondrial cytopathies
 Calcineurin Inhibitors: Cyclosporine
Calcineurin Inhibitors: Cyclosporine
 Steroid-resistant FSGS Cyclosporine (North American Collaborative Trial) • 49 patients with steroid-resistant FSGS • 26 weeks of CYA + low-dose prednisone vs placebo plus prednisone. • F/U 200 weeks Cattran DC, Appel GB, . . Kidney Int. 1999 Dec; 56(6): 2220 -6.
Steroid-resistant FSGS Cyclosporine (North American Collaborative Trial) • 49 patients with steroid-resistant FSGS • 26 weeks of CYA + low-dose prednisone vs placebo plus prednisone. • F/U 200 weeks Cattran DC, Appel GB, . . Kidney Int. 1999 Dec; 56(6): 2220 -6.
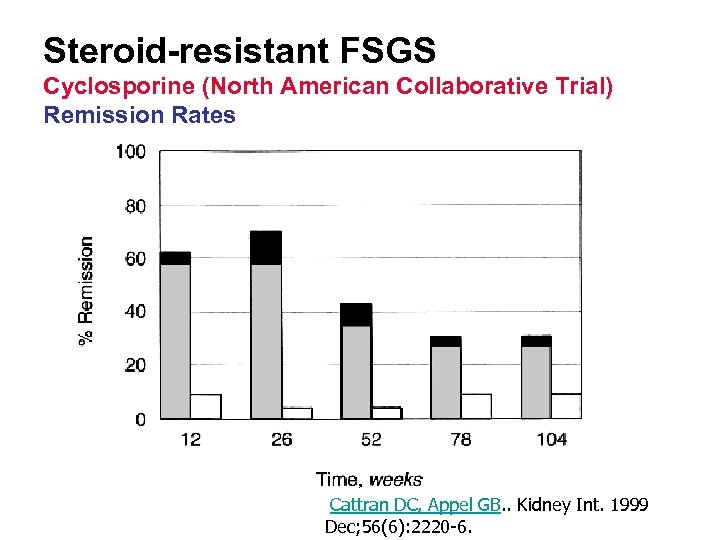 Steroid-resistant FSGS Cyclosporine (North American Collaborative Trial) Remission Rates Cattran DC, Appel GB. . Kidney Int. 1999 Dec; 56(6): 2220 -6.
Steroid-resistant FSGS Cyclosporine (North American Collaborative Trial) Remission Rates Cattran DC, Appel GB. . Kidney Int. 1999 Dec; 56(6): 2220 -6.
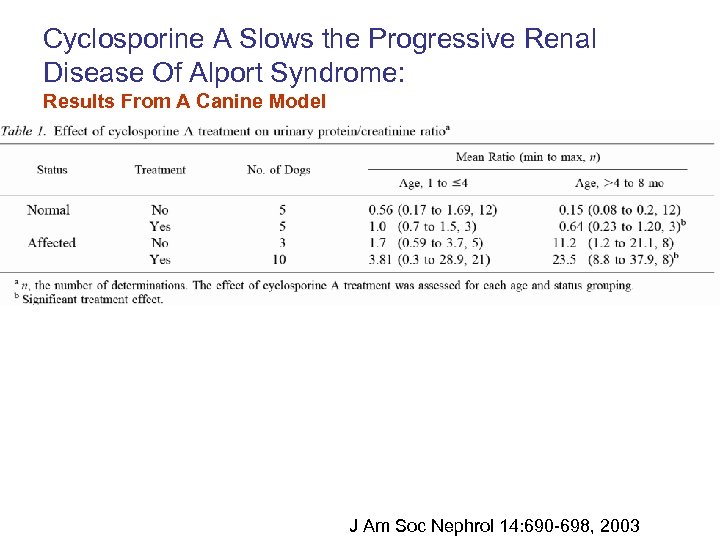 Cyclosporine A Slows the Progressive Renal Disease Of Alport Syndrome: Results From A Canine Model J Am Soc Nephrol 14: 690 -698, 2003
Cyclosporine A Slows the Progressive Renal Disease Of Alport Syndrome: Results From A Canine Model J Am Soc Nephrol 14: 690 -698, 2003
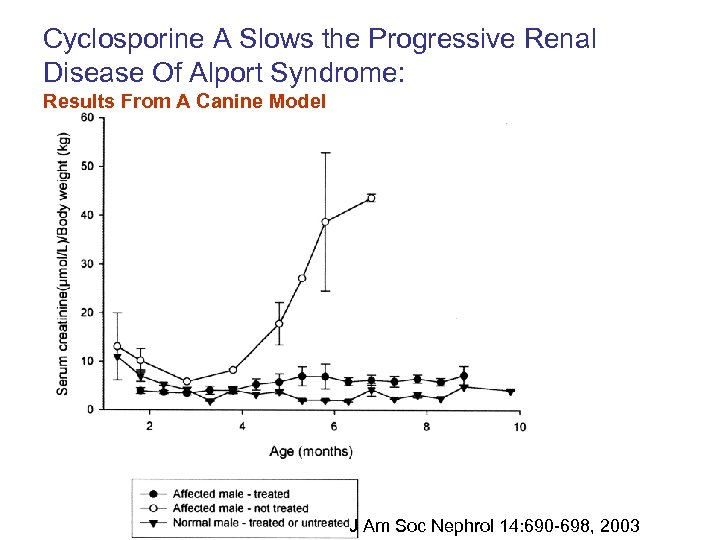 Cyclosporine A Slows the Progressive Renal Disease Of Alport Syndrome: Results From A Canine Model J Am Soc Nephrol 14: 690 -698, 2003
Cyclosporine A Slows the Progressive Renal Disease Of Alport Syndrome: Results From A Canine Model J Am Soc Nephrol 14: 690 -698, 2003
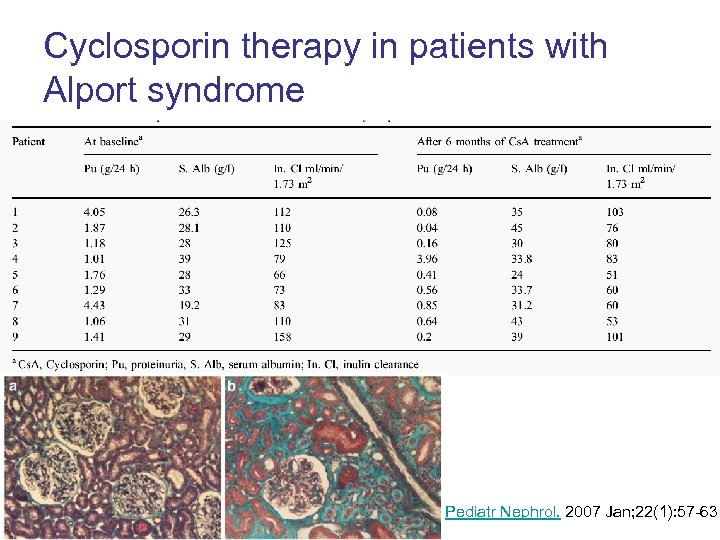 Cyclosporin therapy in patients with Alport syndrome Pediatr Nephrol. 2007 Jan; 22(1): 57 -63
Cyclosporin therapy in patients with Alport syndrome Pediatr Nephrol. 2007 Jan; 22(1): 57 -63
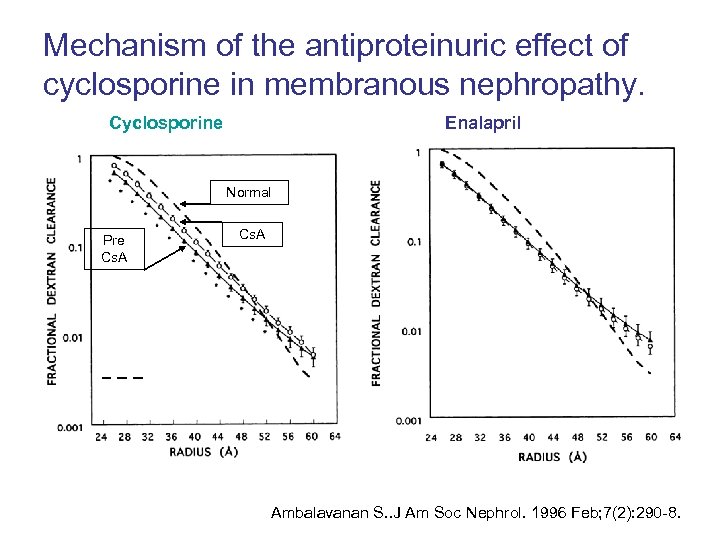 Mechanism of the antiproteinuric effect of cyclosporine in membranous nephropathy. Cyclosporine Enalapril Normal Pre Cs. A Ambalavanan S. . J Am Soc Nephrol. 1996 Feb; 7(2): 290 -8.
Mechanism of the antiproteinuric effect of cyclosporine in membranous nephropathy. Cyclosporine Enalapril Normal Pre Cs. A Ambalavanan S. . J Am Soc Nephrol. 1996 Feb; 7(2): 290 -8.
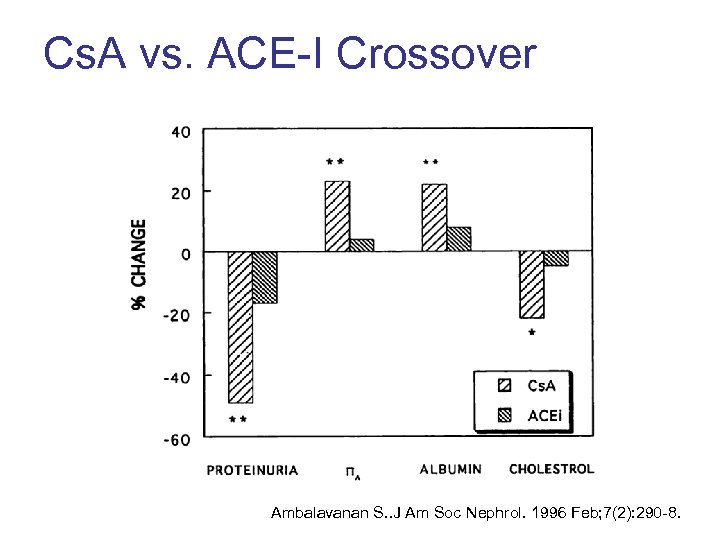 Cs. A vs. ACE-I Crossover Ambalavanan S. . J Am Soc Nephrol. 1996 Feb; 7(2): 290 -8.
Cs. A vs. ACE-I Crossover Ambalavanan S. . J Am Soc Nephrol. 1996 Feb; 7(2): 290 -8.
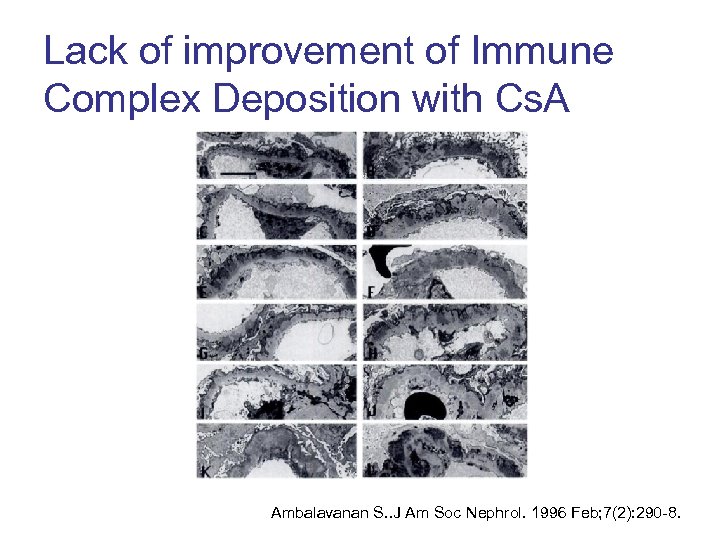 Lack of improvement of Immune Complex Deposition with Cs. A Ambalavanan S. . J Am Soc Nephrol. 1996 Feb; 7(2): 290 -8.
Lack of improvement of Immune Complex Deposition with Cs. A Ambalavanan S. . J Am Soc Nephrol. 1996 Feb; 7(2): 290 -8.
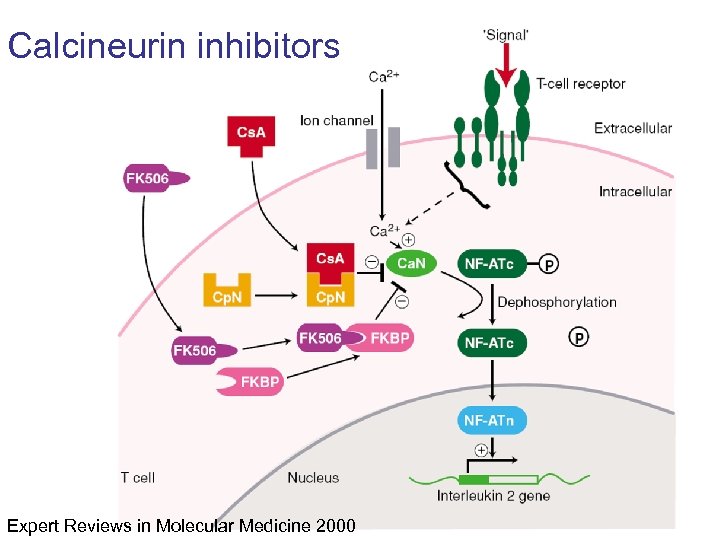 Calcineurin inhibitors Expert Reviews in Molecular Medicine 2000
Calcineurin inhibitors Expert Reviews in Molecular Medicine 2000
 Nature Medicine 14, 931 - 938 (2008)
Nature Medicine 14, 931 - 938 (2008)
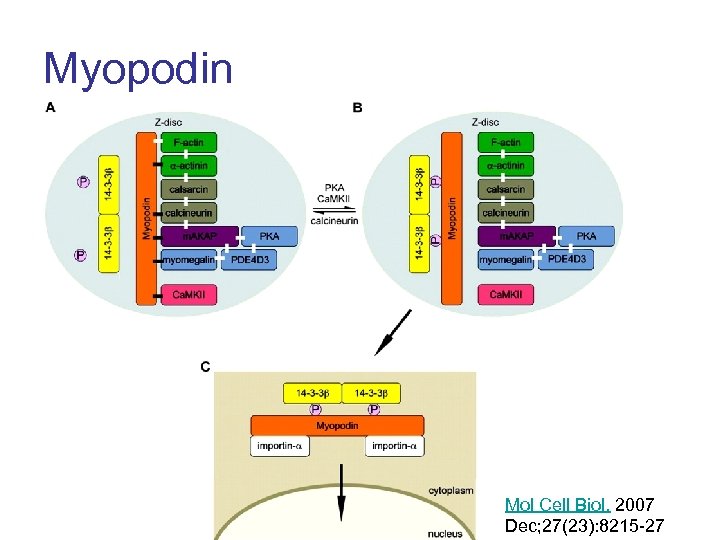 Myopodin Mol Cell Biol. 2007 Dec; 27(23): 8215 -27
Myopodin Mol Cell Biol. 2007 Dec; 27(23): 8215 -27
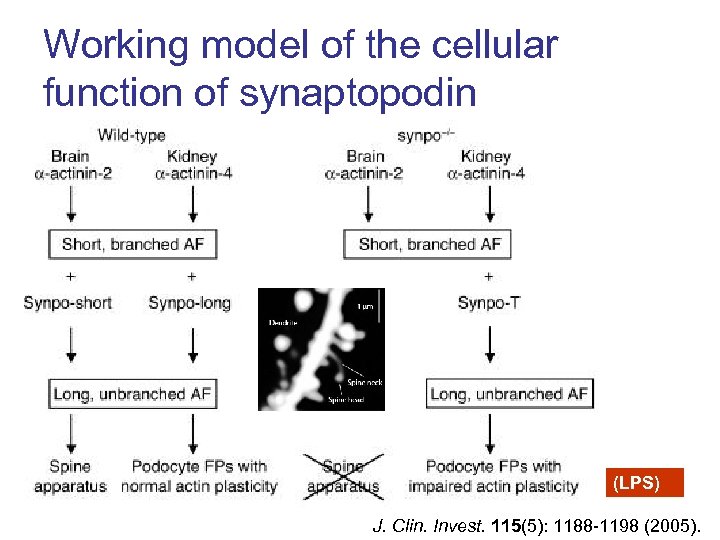 Working model of the cellular function of synaptopodin (LPS) J. Clin. Invest. 115(5): 1188 -1198 (2005).
Working model of the cellular function of synaptopodin (LPS) J. Clin. Invest. 115(5): 1188 -1198 (2005).
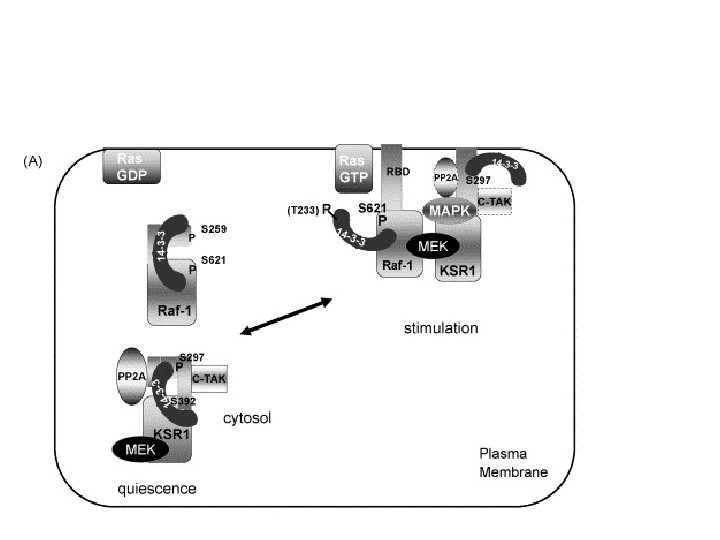
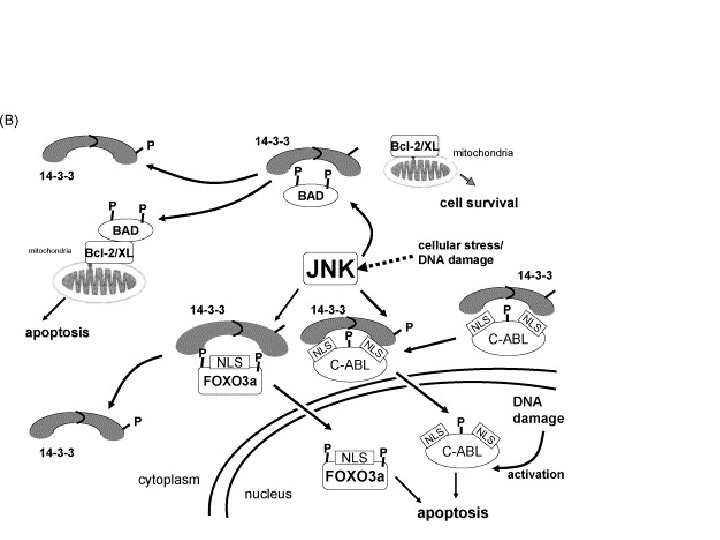
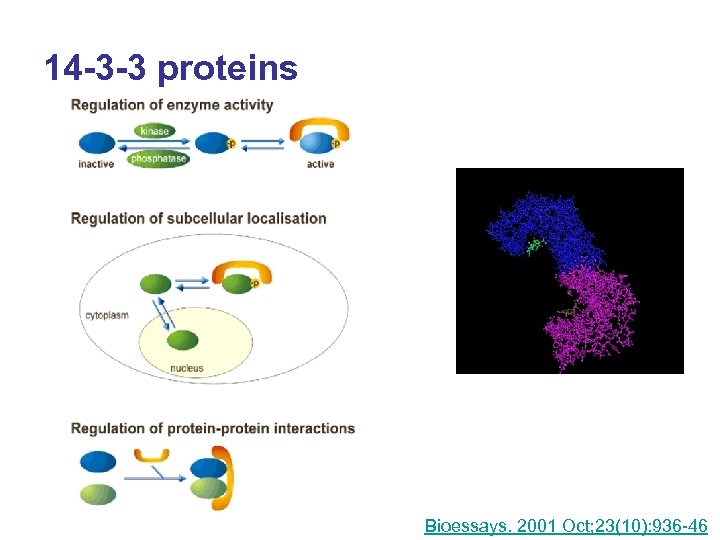 14 -3 -3 proteins Bioessays. 2001 Oct; 23(10): 936 -46
14 -3 -3 proteins Bioessays. 2001 Oct; 23(10): 936 -46
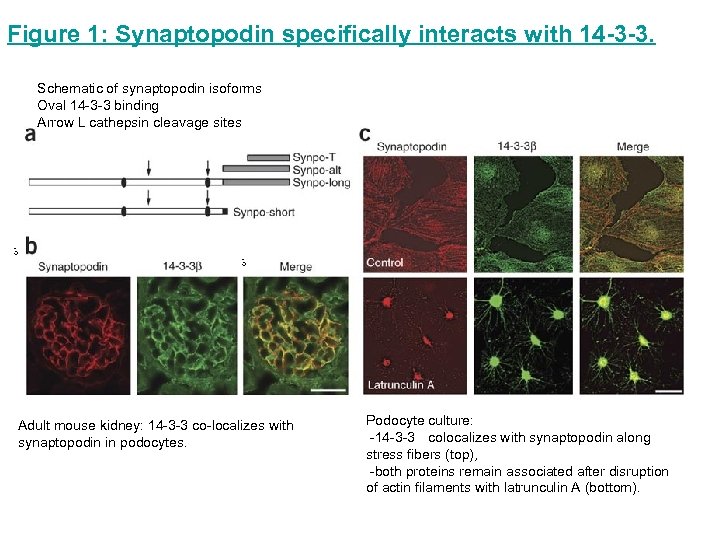 Figure 1: Synaptopodin specifically interacts with 14 -3 -3. Schematic of synaptopodin isoforms Oval 14 -3 -3 binding Arrow L cathepsin cleavage sites Adult mouse kidney: 14 -3 -3 co-localizes with synaptopodin in podocytes. Podocyte culture: -14 -3 -3 colocalizes with synaptopodin along stress fibers (top), -both proteins remain associated after disruption of actin filaments with latrunculin A (bottom).
Figure 1: Synaptopodin specifically interacts with 14 -3 -3. Schematic of synaptopodin isoforms Oval 14 -3 -3 binding Arrow L cathepsin cleavage sites Adult mouse kidney: 14 -3 -3 co-localizes with synaptopodin in podocytes. Podocyte culture: -14 -3 -3 colocalizes with synaptopodin along stress fibers (top), -both proteins remain associated after disruption of actin filaments with latrunculin A (bottom).
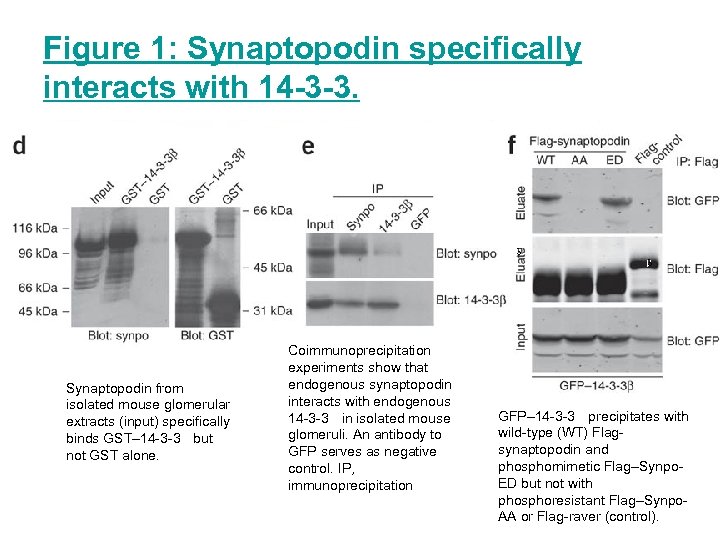 Figure 1: Synaptopodin specifically interacts with 14 -3 -3. Synaptopodin from isolated mouse glomerular extracts (input) specifically binds GST– 14 -3 -3 but not GST alone. Coimmunoprecipitation experiments show that endogenous synaptopodin interacts with endogenous 14 -3 -3 in isolated mouse glomeruli. An antibody to GFP serves as negative control. IP, immunoprecipitation GFP– 14 -3 -3 precipitates with wild-type (WT) Flagsynaptopodin and phosphomimetic Flag–Synpo. ED but not with phosphoresistant Flag–Synpo. AA or Flag-raver (control).
Figure 1: Synaptopodin specifically interacts with 14 -3 -3. Synaptopodin from isolated mouse glomerular extracts (input) specifically binds GST– 14 -3 -3 but not GST alone. Coimmunoprecipitation experiments show that endogenous synaptopodin interacts with endogenous 14 -3 -3 in isolated mouse glomeruli. An antibody to GFP serves as negative control. IP, immunoprecipitation GFP– 14 -3 -3 precipitates with wild-type (WT) Flagsynaptopodin and phosphomimetic Flag–Synpo. ED but not with phosphoresistant Flag–Synpo. AA or Flag-raver (control).
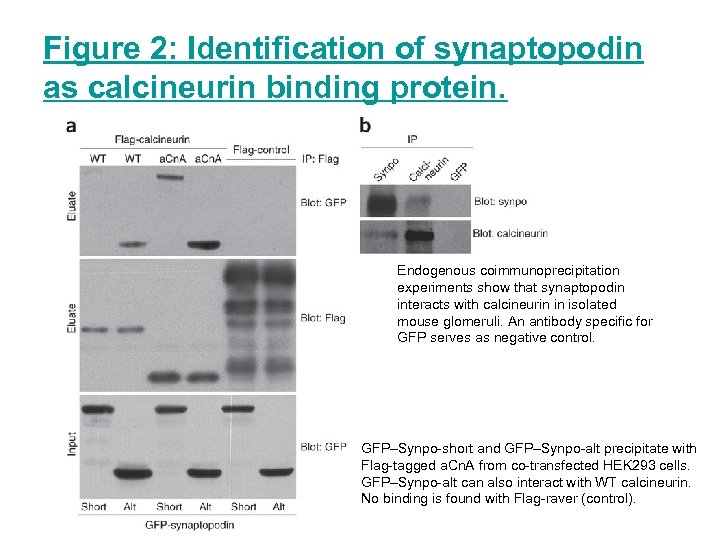 Figure 2: Identification of synaptopodin as calcineurin binding protein. Endogenous coimmunoprecipitation experiments show that synaptopodin interacts with calcineurin in isolated mouse glomeruli. An antibody specific for GFP serves as negative control. GFP–Synpo-short and GFP–Synpo-alt precipitate with Flag-tagged a. Cn. A from co-transfected HEK 293 cells. GFP–Synpo-alt can also interact with WT calcineurin. No binding is found with Flag-raver (control).
Figure 2: Identification of synaptopodin as calcineurin binding protein. Endogenous coimmunoprecipitation experiments show that synaptopodin interacts with calcineurin in isolated mouse glomeruli. An antibody specific for GFP serves as negative control. GFP–Synpo-short and GFP–Synpo-alt precipitate with Flag-tagged a. Cn. A from co-transfected HEK 293 cells. GFP–Synpo-alt can also interact with WT calcineurin. No binding is found with Flag-raver (control).
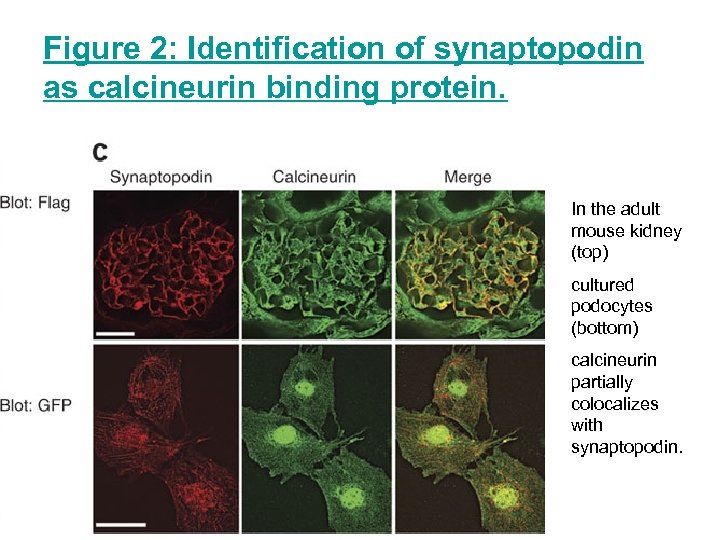 Figure 2: Identification of synaptopodin as calcineurin binding protein. In the adult mouse kidney (top) cultured podocytes (bottom) calcineurin partially colocalizes with synaptopodin.
Figure 2: Identification of synaptopodin as calcineurin binding protein. In the adult mouse kidney (top) cultured podocytes (bottom) calcineurin partially colocalizes with synaptopodin.
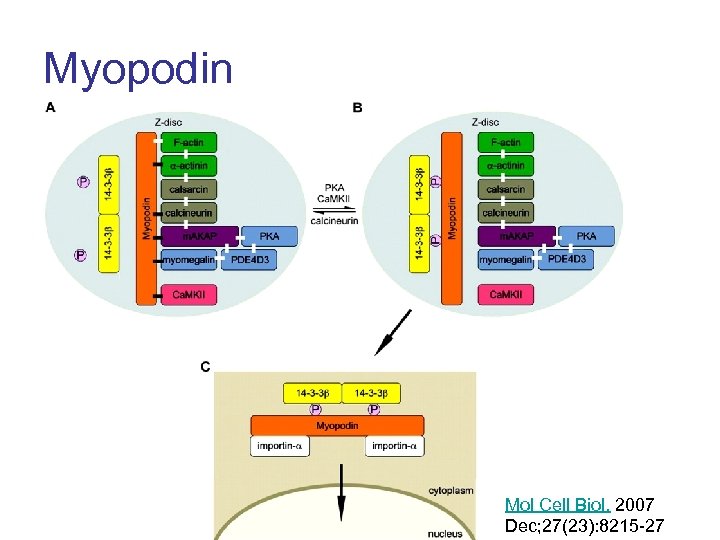 Myopodin Mol Cell Biol. 2007 Dec; 27(23): 8215 -27
Myopodin Mol Cell Biol. 2007 Dec; 27(23): 8215 -27
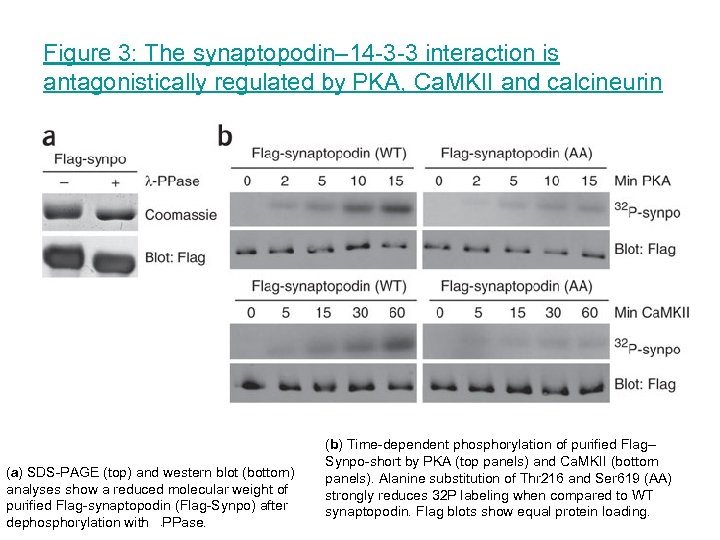 Figure 3: The synaptopodin– 14 -3 -3 interaction is antagonistically regulated by PKA, Ca. MKII and calcineurin (a) SDS-PAGE (top) and western blot (bottom) analyses show a reduced molecular weight of purified Flag-synaptopodin (Flag-Synpo) after dephosphorylation with PPase. - (b) Time-dependent phosphorylation of purified Flag– Synpo-short by PKA (top panels) and Ca. MKII (bottom panels). Alanine substitution of Thr 216 and Ser 619 (AA) strongly reduces 32 P labeling when compared to WT synaptopodin. Flag blots show equal protein loading.
Figure 3: The synaptopodin– 14 -3 -3 interaction is antagonistically regulated by PKA, Ca. MKII and calcineurin (a) SDS-PAGE (top) and western blot (bottom) analyses show a reduced molecular weight of purified Flag-synaptopodin (Flag-Synpo) after dephosphorylation with PPase. - (b) Time-dependent phosphorylation of purified Flag– Synpo-short by PKA (top panels) and Ca. MKII (bottom panels). Alanine substitution of Thr 216 and Ser 619 (AA) strongly reduces 32 P labeling when compared to WT synaptopodin. Flag blots show equal protein loading.
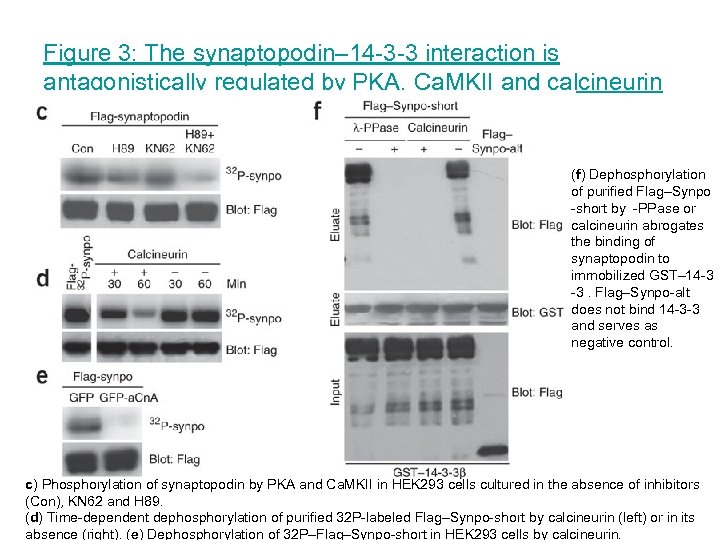 Figure 3: The synaptopodin– 14 -3 -3 interaction is antagonistically regulated by PKA, Ca. MKII and calcineurin (f) Dephosphorylation of purified Flag–Synpo -short by -PPase or calcineurin abrogates the binding of synaptopodin to immobilized GST– 14 -3 -3. Flag–Synpo-alt does not bind 14 -3 -3 and serves as negative control. c) Phosphorylation of synaptopodin by PKA and Ca. MKII in HEK 293 cells cultured in the absence of inhibitors (Con), KN 62 and H 89. (d) Time-dependent dephosphorylation of purified 32 P-labeled Flag–Synpo-short by calcineurin (left) or in its absence (right). (e) Dephosphorylation of 32 P–Flag–Synpo-short in HEK 293 cells by calcineurin.
Figure 3: The synaptopodin– 14 -3 -3 interaction is antagonistically regulated by PKA, Ca. MKII and calcineurin (f) Dephosphorylation of purified Flag–Synpo -short by -PPase or calcineurin abrogates the binding of synaptopodin to immobilized GST– 14 -3 -3. Flag–Synpo-alt does not bind 14 -3 -3 and serves as negative control. c) Phosphorylation of synaptopodin by PKA and Ca. MKII in HEK 293 cells cultured in the absence of inhibitors (Con), KN 62 and H 89. (d) Time-dependent dephosphorylation of purified 32 P-labeled Flag–Synpo-short by calcineurin (left) or in its absence (right). (e) Dephosphorylation of 32 P–Flag–Synpo-short in HEK 293 cells by calcineurin.
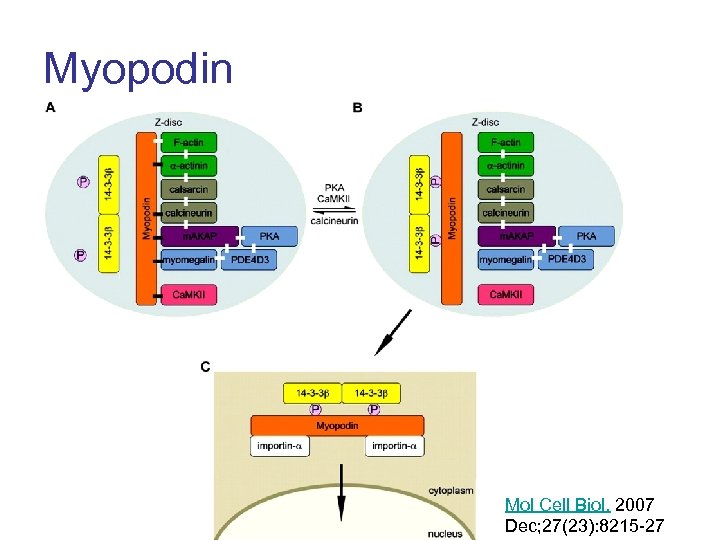 Myopodin Mol Cell Biol. 2007 Dec; 27(23): 8215 -27
Myopodin Mol Cell Biol. 2007 Dec; 27(23): 8215 -27
 Actin “stress fibers” • Stress fibers are a specific cytoskeletal organization of actin monomers. • Involved in cell shape and structural functions • Actin monomers form long polymers, which attach to the plasma membrane at focal adhesions. • Contraction of the actin stress fibers allows the cell to exert tension on the substratum (an important part of controlling morphogenesis. • Formation of stress fibers and focal adhesion complexes are a key regulatory event in cell growth and cell movement such as migration and invasion.
Actin “stress fibers” • Stress fibers are a specific cytoskeletal organization of actin monomers. • Involved in cell shape and structural functions • Actin monomers form long polymers, which attach to the plasma membrane at focal adhesions. • Contraction of the actin stress fibers allows the cell to exert tension on the substratum (an important part of controlling morphogenesis. • Formation of stress fibers and focal adhesion complexes are a key regulatory event in cell growth and cell movement such as migration and invasion.
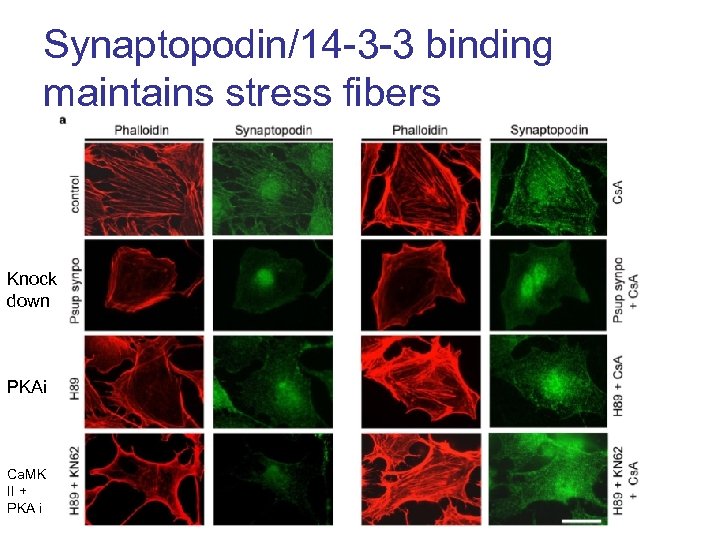 Synaptopodin/14 -3 -3 binding maintains stress fibers Knock down PKAi Ca. MK II + PKA i
Synaptopodin/14 -3 -3 binding maintains stress fibers Knock down PKAi Ca. MK II + PKA i
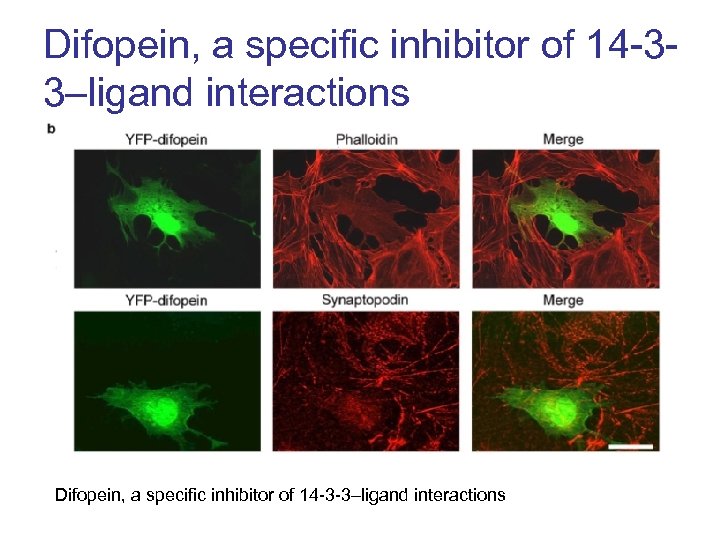 Difopein, a specific inhibitor of 14 -33–ligand interactions Difopein, a specific inhibitor of 14 -3 -3–ligand interactions
Difopein, a specific inhibitor of 14 -33–ligand interactions Difopein, a specific inhibitor of 14 -3 -3–ligand interactions
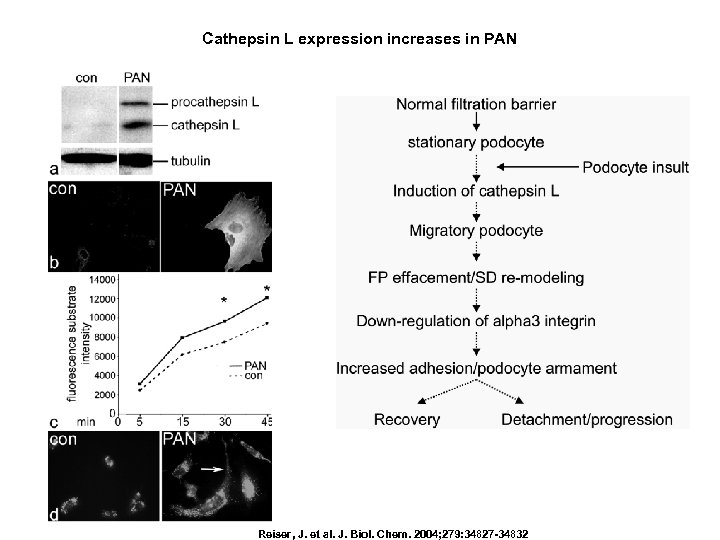 Cathepsin L expression increases in PAN Reiser, J. et al. J. Biol. Chem. 2004; 279: 34827 -34832
Cathepsin L expression increases in PAN Reiser, J. et al. J. Biol. Chem. 2004; 279: 34827 -34832
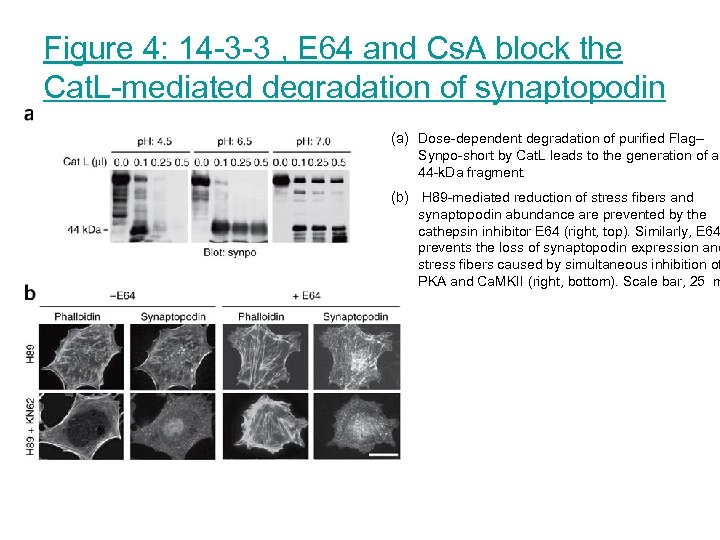 Figure 4: 14 -3 -3 , E 64 and Cs. A block the Cat. L-mediated degradation of synaptopodin (a) Dose-dependent degradation of purified Flag– Synpo-short by Cat. L leads to the generation of a 44 -k. Da fragment. (b) H 89 -mediated reduction of stress fibers and synaptopodin abundance are prevented by the cathepsin inhibitor E 64 (right, top). Similarly, E 64 prevents the loss of synaptopodin expression and stress fibers caused by simultaneous inhibition of PKA and Ca. MKII (right, bottom). Scale bar, 25 m
Figure 4: 14 -3 -3 , E 64 and Cs. A block the Cat. L-mediated degradation of synaptopodin (a) Dose-dependent degradation of purified Flag– Synpo-short by Cat. L leads to the generation of a 44 -k. Da fragment. (b) H 89 -mediated reduction of stress fibers and synaptopodin abundance are prevented by the cathepsin inhibitor E 64 (right, top). Similarly, E 64 prevents the loss of synaptopodin expression and stress fibers caused by simultaneous inhibition of PKA and Ca. MKII (right, bottom). Scale bar, 25 m
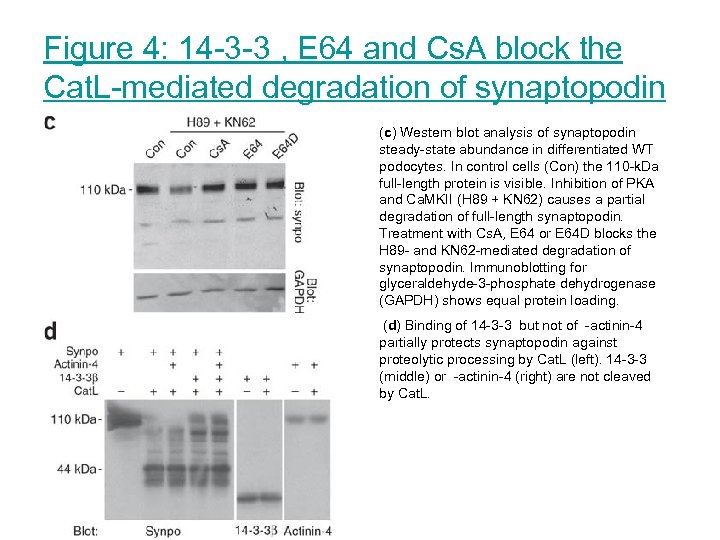 Figure 4: 14 -3 -3 , E 64 and Cs. A block the Cat. L-mediated degradation of synaptopodin (c) Western blot analysis of synaptopodin steady-state abundance in differentiated WT podocytes. In control cells (Con) the 110 -k. Da full-length protein is visible. Inhibition of PKA and Ca. MKII (H 89 + KN 62) causes a partial degradation of full-length synaptopodin. Treatment with Cs. A, E 64 or E 64 D blocks the H 89 - and KN 62 -mediated degradation of synaptopodin. Immunoblotting for glyceraldehyde-3 -phosphate dehydrogenase (GAPDH) shows equal protein loading. (d) Binding of 14 -3 -3 but not of -actinin-4 partially protects synaptopodin against proteolytic processing by Cat. L (left). 14 -3 -3 (middle) or -actinin-4 (right) are not cleaved by Cat. L.
Figure 4: 14 -3 -3 , E 64 and Cs. A block the Cat. L-mediated degradation of synaptopodin (c) Western blot analysis of synaptopodin steady-state abundance in differentiated WT podocytes. In control cells (Con) the 110 -k. Da full-length protein is visible. Inhibition of PKA and Ca. MKII (H 89 + KN 62) causes a partial degradation of full-length synaptopodin. Treatment with Cs. A, E 64 or E 64 D blocks the H 89 - and KN 62 -mediated degradation of synaptopodin. Immunoblotting for glyceraldehyde-3 -phosphate dehydrogenase (GAPDH) shows equal protein loading. (d) Binding of 14 -3 -3 but not of -actinin-4 partially protects synaptopodin against proteolytic processing by Cat. L (left). 14 -3 -3 (middle) or -actinin-4 (right) are not cleaved by Cat. L.
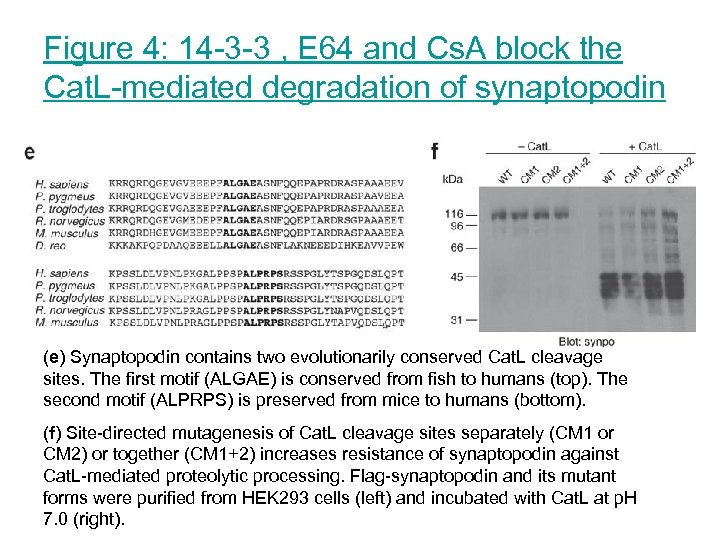 Figure 4: 14 -3 -3 , E 64 and Cs. A block the Cat. L-mediated degradation of synaptopodin (e) Synaptopodin contains two evolutionarily conserved Cat. L cleavage sites. The first motif (ALGAE) is conserved from fish to humans (top). The second motif (ALPRPS) is preserved from mice to humans (bottom). (f) Site-directed mutagenesis of Cat. L cleavage sites separately (CM 1 or CM 2) or together (CM 1+2) increases resistance of synaptopodin against Cat. L-mediated proteolytic processing. Flag-synaptopodin and its mutant forms were purified from HEK 293 cells (left) and incubated with Cat. L at p. H 7. 0 (right).
Figure 4: 14 -3 -3 , E 64 and Cs. A block the Cat. L-mediated degradation of synaptopodin (e) Synaptopodin contains two evolutionarily conserved Cat. L cleavage sites. The first motif (ALGAE) is conserved from fish to humans (top). The second motif (ALPRPS) is preserved from mice to humans (bottom). (f) Site-directed mutagenesis of Cat. L cleavage sites separately (CM 1 or CM 2) or together (CM 1+2) increases resistance of synaptopodin against Cat. L-mediated proteolytic processing. Flag-synaptopodin and its mutant forms were purified from HEK 293 cells (left) and incubated with Cat. L at p. H 7. 0 (right).
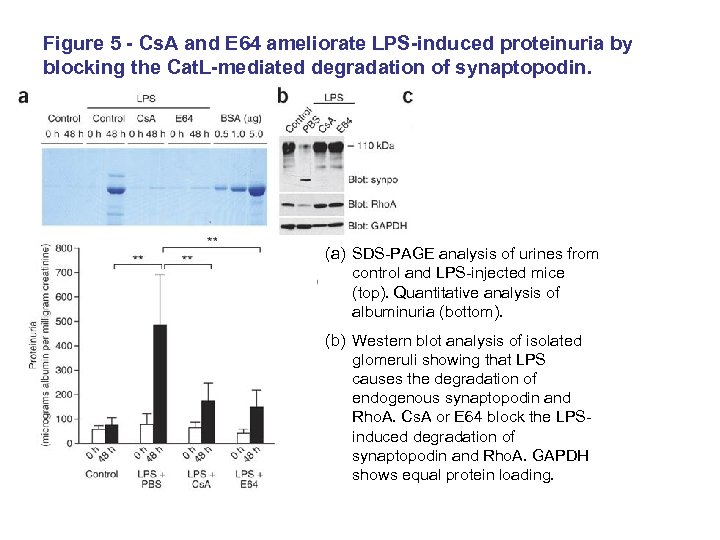 Figure 5 - Cs. A and E 64 ameliorate LPS-induced proteinuria by blocking the Cat. L-mediated degradation of synaptopodin. (a) SDS-PAGE analysis of urines from control and LPS-injected mice (top). Quantitative analysis of albuminuria (bottom). (b) Western blot analysis of isolated glomeruli showing that LPS causes the degradation of endogenous synaptopodin and Rho. A. Cs. A or E 64 block the LPSinduced degradation of synaptopodin and Rho. A. GAPDH shows equal protein loading.
Figure 5 - Cs. A and E 64 ameliorate LPS-induced proteinuria by blocking the Cat. L-mediated degradation of synaptopodin. (a) SDS-PAGE analysis of urines from control and LPS-injected mice (top). Quantitative analysis of albuminuria (bottom). (b) Western blot analysis of isolated glomeruli showing that LPS causes the degradation of endogenous synaptopodin and Rho. A. Cs. A or E 64 block the LPSinduced degradation of synaptopodin and Rho. A. GAPDH shows equal protein loading.
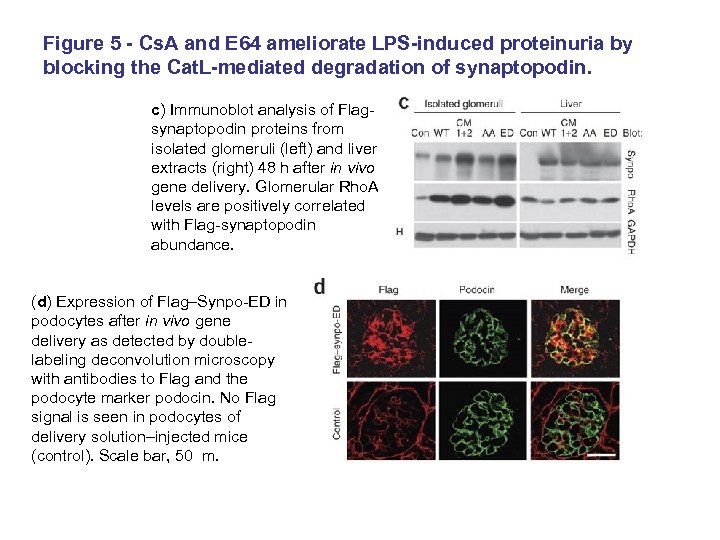 Figure 5 - Cs. A and E 64 ameliorate LPS-induced proteinuria by blocking the Cat. L-mediated degradation of synaptopodin. c) Immunoblot analysis of Flagsynaptopodin proteins from isolated glomeruli (left) and liver extracts (right) 48 h after in vivo gene delivery. Glomerular Rho. A levels are positively correlated with Flag-synaptopodin abundance. (d) Expression of Flag–Synpo-ED in podocytes after in vivo gene delivery as detected by doublelabeling deconvolution microscopy with antibodies to Flag and the podocyte marker podocin. No Flag signal is seen in podocytes of delivery solution–injected mice (control). Scale bar, 50 m.
Figure 5 - Cs. A and E 64 ameliorate LPS-induced proteinuria by blocking the Cat. L-mediated degradation of synaptopodin. c) Immunoblot analysis of Flagsynaptopodin proteins from isolated glomeruli (left) and liver extracts (right) 48 h after in vivo gene delivery. Glomerular Rho. A levels are positively correlated with Flag-synaptopodin abundance. (d) Expression of Flag–Synpo-ED in podocytes after in vivo gene delivery as detected by doublelabeling deconvolution microscopy with antibodies to Flag and the podocyte marker podocin. No Flag signal is seen in podocytes of delivery solution–injected mice (control). Scale bar, 50 m.
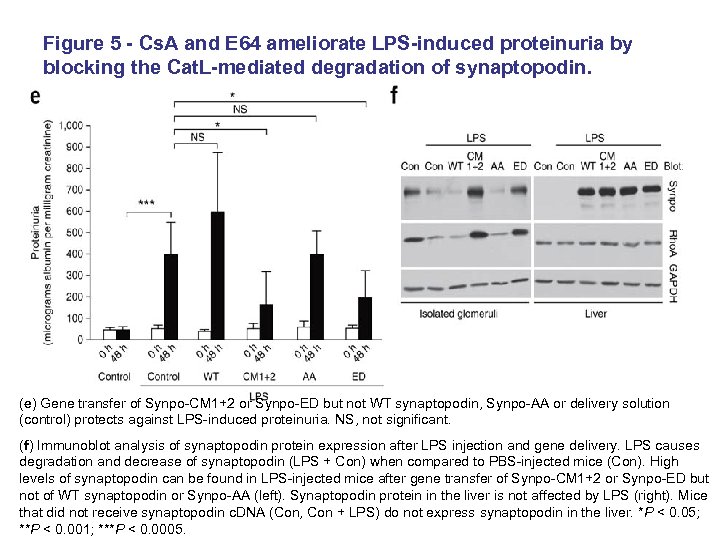 Figure 5 - Cs. A and E 64 ameliorate LPS-induced proteinuria by blocking the Cat. L-mediated degradation of synaptopodin. (e) Gene transfer of Synpo-CM 1+2 or Synpo-ED but not WT synaptopodin, Synpo-AA or delivery solution (control) protects against LPS-induced proteinuria. NS, not significant. (f) Immunoblot analysis of synaptopodin protein expression after LPS injection and gene delivery. LPS causes degradation and decrease of synaptopodin (LPS + Con) when compared to PBS-injected mice (Con). High levels of synaptopodin can be found in LPS-injected mice after gene transfer of Synpo-CM 1+2 or Synpo-ED but not of WT synaptopodin or Synpo-AA (left). Synaptopodin protein in the liver is not affected by LPS (right). Mice that did not receive synaptopodin c. DNA (Con, Con + LPS) do not express synaptopodin in the liver. *P < 0. 05; **P < 0. 001; ***P < 0. 0005.
Figure 5 - Cs. A and E 64 ameliorate LPS-induced proteinuria by blocking the Cat. L-mediated degradation of synaptopodin. (e) Gene transfer of Synpo-CM 1+2 or Synpo-ED but not WT synaptopodin, Synpo-AA or delivery solution (control) protects against LPS-induced proteinuria. NS, not significant. (f) Immunoblot analysis of synaptopodin protein expression after LPS injection and gene delivery. LPS causes degradation and decrease of synaptopodin (LPS + Con) when compared to PBS-injected mice (Con). High levels of synaptopodin can be found in LPS-injected mice after gene transfer of Synpo-CM 1+2 or Synpo-ED but not of WT synaptopodin or Synpo-AA (left). Synaptopodin protein in the liver is not affected by LPS (right). Mice that did not receive synaptopodin c. DNA (Con, Con + LPS) do not express synaptopodin in the liver. *P < 0. 05; **P < 0. 001; ***P < 0. 0005.
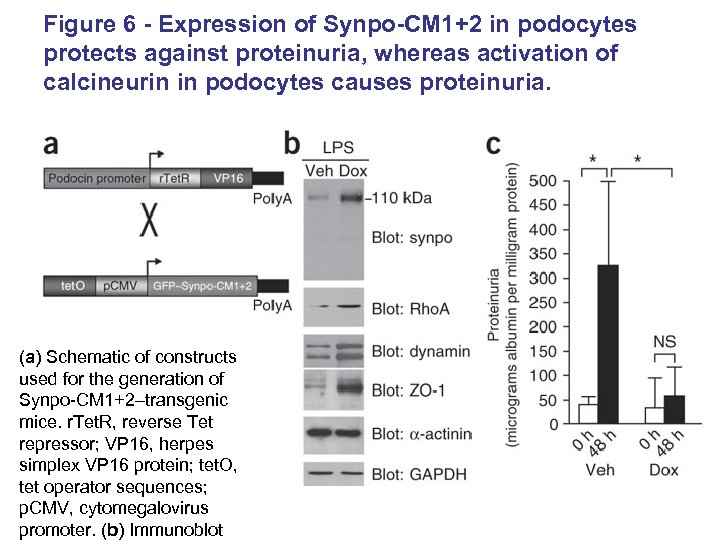 Figure 6 - Expression of Synpo-CM 1+2 in podocytes protects against proteinuria, whereas activation of calcineurin in podocytes causes proteinuria. (a) Schematic of constructs used for the generation of Synpo-CM 1+2–transgenic mice. r. Tet. R, reverse Tet repressor; VP 16, herpes simplex VP 16 protein; tet. O, tet operator sequences; p. CMV, cytomegalovirus promoter. (b) Immunoblot
Figure 6 - Expression of Synpo-CM 1+2 in podocytes protects against proteinuria, whereas activation of calcineurin in podocytes causes proteinuria. (a) Schematic of constructs used for the generation of Synpo-CM 1+2–transgenic mice. r. Tet. R, reverse Tet repressor; VP 16, herpes simplex VP 16 protein; tet. O, tet operator sequences; p. CMV, cytomegalovirus promoter. (b) Immunoblot
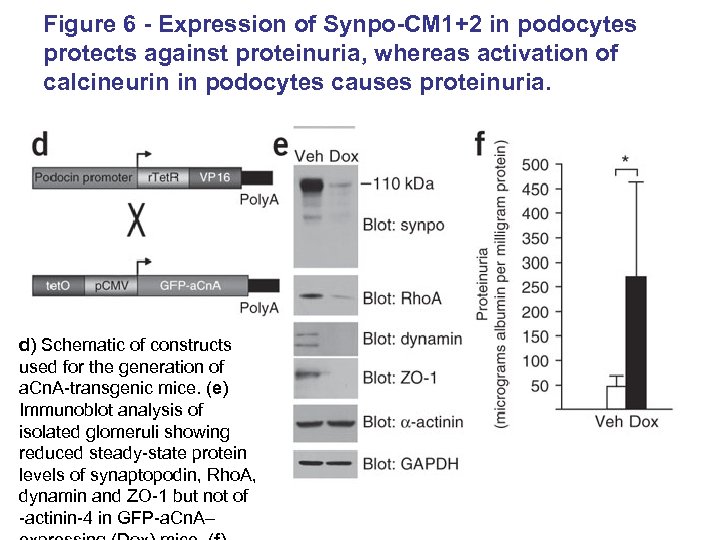 Figure 6 - Expression of Synpo-CM 1+2 in podocytes protects against proteinuria, whereas activation of calcineurin in podocytes causes proteinuria. d) Schematic of constructs used for the generation of a. Cn. A-transgenic mice. (e) Immunoblot analysis of isolated glomeruli showing reduced steady-state protein levels of synaptopodin, Rho. A, dynamin and ZO-1 but not of -actinin-4 in GFP-a. Cn. A–
Figure 6 - Expression of Synpo-CM 1+2 in podocytes protects against proteinuria, whereas activation of calcineurin in podocytes causes proteinuria. d) Schematic of constructs used for the generation of a. Cn. A-transgenic mice. (e) Immunoblot analysis of isolated glomeruli showing reduced steady-state protein levels of synaptopodin, Rho. A, dynamin and ZO-1 but not of -actinin-4 in GFP-a. Cn. A–
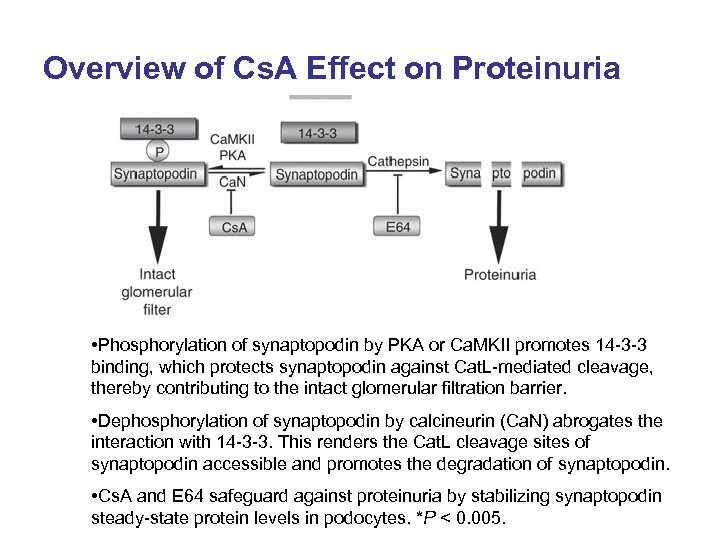 Overview of Cs. A Effect on Proteinuria • Phosphorylation of synaptopodin by PKA or Ca. MKII promotes 14 -3 -3 binding, which protects synaptopodin against Cat. L-mediated cleavage, thereby contributing to the intact glomerular filtration barrier. • Dephosphorylation of synaptopodin by calcineurin (Ca. N) abrogates the interaction with 14 -3 -3. This renders the Cat. L cleavage sites of synaptopodin accessible and promotes the degradation of synaptopodin. • Cs. A and E 64 safeguard against proteinuria by stabilizing synaptopodin steady-state protein levels in podocytes. *P < 0. 005.
Overview of Cs. A Effect on Proteinuria • Phosphorylation of synaptopodin by PKA or Ca. MKII promotes 14 -3 -3 binding, which protects synaptopodin against Cat. L-mediated cleavage, thereby contributing to the intact glomerular filtration barrier. • Dephosphorylation of synaptopodin by calcineurin (Ca. N) abrogates the interaction with 14 -3 -3. This renders the Cat. L cleavage sites of synaptopodin accessible and promotes the degradation of synaptopodin. • Cs. A and E 64 safeguard against proteinuria by stabilizing synaptopodin steady-state protein levels in podocytes. *P < 0. 005.