7c76dc3654fbbfdbcdd6030fb14fde48.ppt
- Количество слайдов: 34
 The 67 th International Symposium on Molecular Spectroscopy June 18 -22, 2012 Confirmed Assignments of Isomeric Dimethylbenzyl Radicals Generated by Corona Discharge (TI 10) Young Wook Yoon and Sang Kuk Lee sklee@pusan. ac. kr Department of Chemistry Pusan National University Pusan 609 -735, Korea A part of this work appears in J. Chem. Phys. 2011, 135, 214305. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 1
The 67 th International Symposium on Molecular Spectroscopy June 18 -22, 2012 Confirmed Assignments of Isomeric Dimethylbenzyl Radicals Generated by Corona Discharge (TI 10) Young Wook Yoon and Sang Kuk Lee sklee@pusan. ac. kr Department of Chemistry Pusan National University Pusan 609 -735, Korea A part of this work appears in J. Chem. Phys. 2011, 135, 214305. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 1
 Characteristics of Transient Molecules Ø Molecular radicals, molecular ions, and highly excited molecules. Ø Very short lifetime (less than 10 -6 sec), highly reactive in chemical reaction. Ø Cannot exist at ordinary condition. Need a special care for preservation. Ø Determine reaction pathway at the transition state as reaction intermediates. Ø Less than 2, 000 transient species among more than 18, 000 molecules in American Chemical Society Database. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 2
Characteristics of Transient Molecules Ø Molecular radicals, molecular ions, and highly excited molecules. Ø Very short lifetime (less than 10 -6 sec), highly reactive in chemical reaction. Ø Cannot exist at ordinary condition. Need a special care for preservation. Ø Determine reaction pathway at the transition state as reaction intermediates. Ø Less than 2, 000 transient species among more than 18, 000 molecules in American Chemical Society Database. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 2
 Motivation Ø We have developed a technique of Corona Excited Supersonic Expansion (CESE) which is a laser-free spectroscopic tool for observation of vibronic emission spectra of transient species. Ø Benzyl-type radicals are excellent candidates for CESE system because they show strong visible emission of the D 1 → D 0 transition. Ø We want to see the substituent effect on electronic energy by substitution of methyl group, fluorine, chlorine atoms into benzene ring. This talk WI 12 Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 3
Motivation Ø We have developed a technique of Corona Excited Supersonic Expansion (CESE) which is a laser-free spectroscopic tool for observation of vibronic emission spectra of transient species. Ø Benzyl-type radicals are excellent candidates for CESE system because they show strong visible emission of the D 1 → D 0 transition. Ø We want to see the substituent effect on electronic energy by substitution of methyl group, fluorine, chlorine atoms into benzene ring. This talk WI 12 Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 3
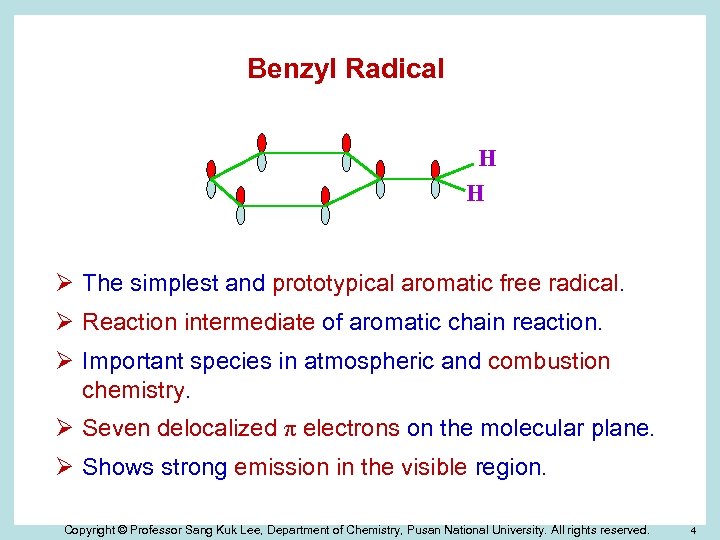 Benzyl Radical H H Ø The simplest and prototypical aromatic free radical. Ø Reaction intermediate of aromatic chain reaction. Ø Important species in atmospheric and combustion chemistry. Ø Seven delocalized π electrons on the molecular plane. Ø Shows strong emission in the visible region. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 4
Benzyl Radical H H Ø The simplest and prototypical aromatic free radical. Ø Reaction intermediate of aromatic chain reaction. Ø Important species in atmospheric and combustion chemistry. Ø Seven delocalized π electrons on the molecular plane. Ø Shows strong emission in the visible region. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 4
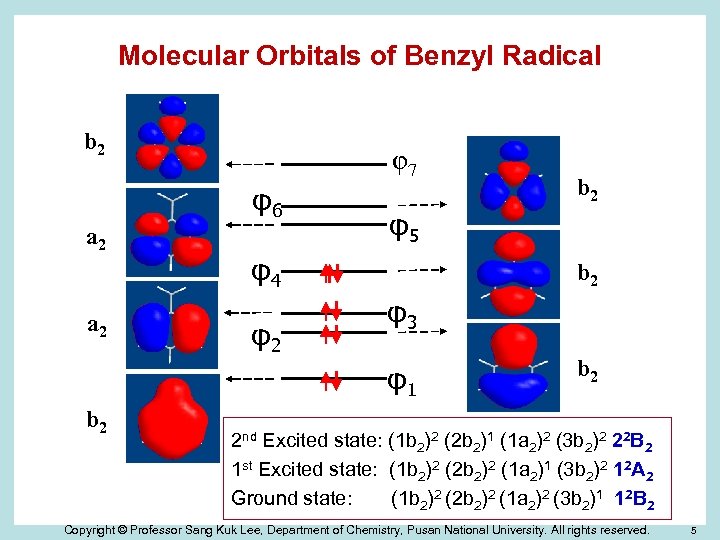 Molecular Orbitals of Benzyl Radical b 2 φ7 φ6 a 2 φ5 φ4 φ2 b 2 φ3 φ1 b 2 b 2 2 nd Excited state: (1 b 2)2 (2 b 2)1 (1 a 2)2 (3 b 2)2 22 B 2 1 st Excited state: (1 b 2)2 (2 b 2)2 (1 a 2)1 (3 b 2)2 12 A 2 Ground state: (1 b 2)2 (2 b 2)2 (1 a 2)2 (3 b 2)1 12 B 2 Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 5
Molecular Orbitals of Benzyl Radical b 2 φ7 φ6 a 2 φ5 φ4 φ2 b 2 φ3 φ1 b 2 b 2 2 nd Excited state: (1 b 2)2 (2 b 2)1 (1 a 2)2 (3 b 2)2 22 B 2 1 st Excited state: (1 b 2)2 (2 b 2)2 (1 a 2)1 (3 b 2)2 12 A 2 Ground state: (1 b 2)2 (2 b 2)2 (1 a 2)2 (3 b 2)1 12 B 2 Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 5
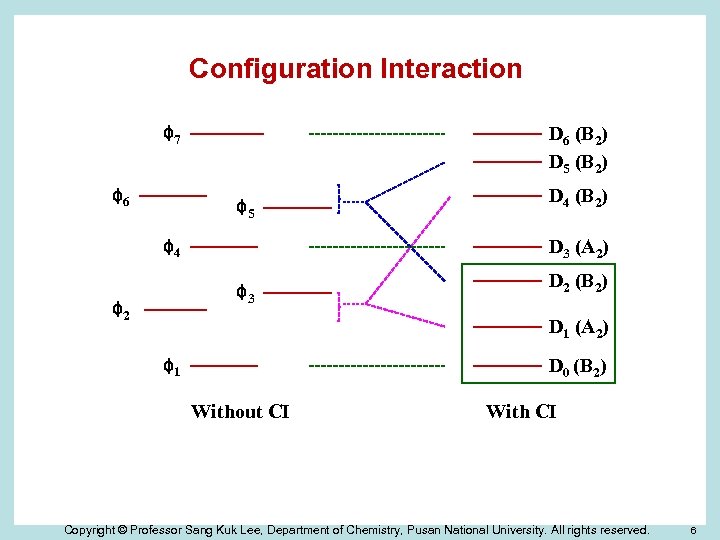 Configuration Interaction D 6 (B 2) D 5 (B 2) φ7 φ6 φ5 D 3 (A 2) φ4 φ3 φ2 D 4 (B 2) D 2 (B 2) D 1 (A 2) D 0 (B 2) φ1 Without CI With CI Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 6
Configuration Interaction D 6 (B 2) D 5 (B 2) φ7 φ6 φ5 D 3 (A 2) φ4 φ3 φ2 D 4 (B 2) D 2 (B 2) D 1 (A 2) D 0 (B 2) φ1 Without CI With CI Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 6
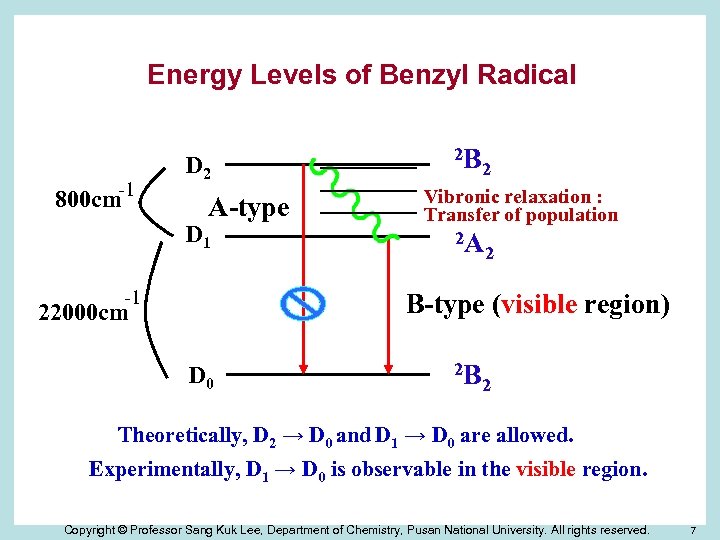 Energy Levels of Benzyl Radical 800 cm-1 D 2 A-type D 1 -1 2 B 2 Vibronic relaxation : Transfer of population 2 A 2 B-type (visible region) 22000 cm D 0 2 B 2 Theoretically, D 2 → D 0 and D 1 → D 0 are allowed. Experimentally, D 1 → D 0 is observable in the visible region. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 7
Energy Levels of Benzyl Radical 800 cm-1 D 2 A-type D 1 -1 2 B 2 Vibronic relaxation : Transfer of population 2 A 2 B-type (visible region) 22000 cm D 0 2 B 2 Theoretically, D 2 → D 0 and D 1 → D 0 are allowed. Experimentally, D 1 → D 0 is observable in the visible region. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 7
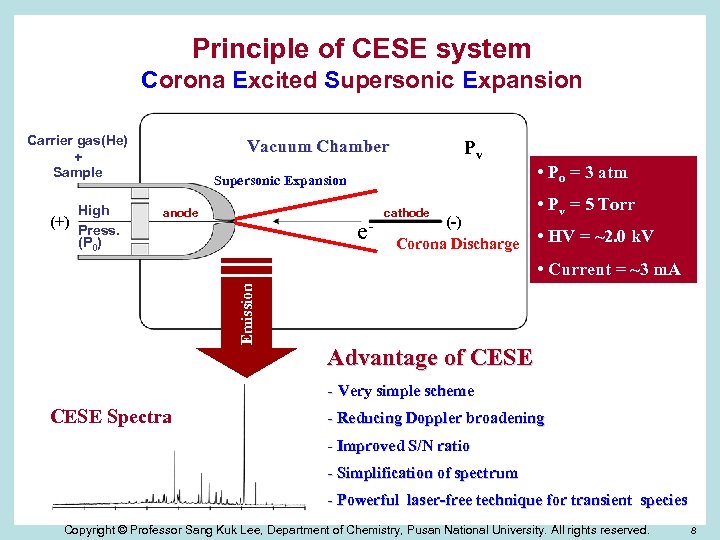 Principle of CESE system Corona Excited Supersonic Expansion Carrier gas(He) + Sample High (+) Press. (P 0) Vacuum Chamber Pv Supersonic Expansion P 0 anode e- cathode (-) Corona Discharge • Po = 3 atm • Pv = 5 Torr • HV = ~2. 0 k. V Emission • Current = ~3 m. A Advantage of CESE - Very simple scheme CESE Spectra - Reducing Doppler broadening - Improved S/N ratio - Simplification of spectrum - Powerful laser-free technique for transient species Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 8
Principle of CESE system Corona Excited Supersonic Expansion Carrier gas(He) + Sample High (+) Press. (P 0) Vacuum Chamber Pv Supersonic Expansion P 0 anode e- cathode (-) Corona Discharge • Po = 3 atm • Pv = 5 Torr • HV = ~2. 0 k. V Emission • Current = ~3 m. A Advantage of CESE - Very simple scheme CESE Spectra - Reducing Doppler broadening - Improved S/N ratio - Simplification of spectrum - Powerful laser-free technique for transient species Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 8
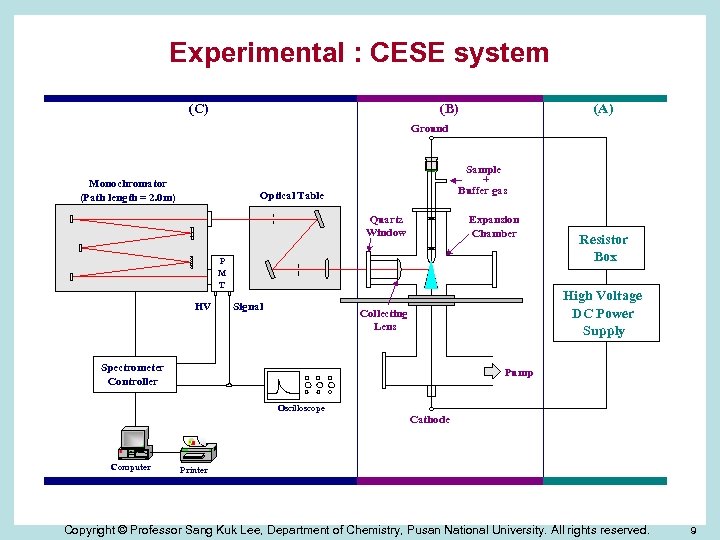 Experimental : CESE system (C) (B) (A) Ground Monochromator (Path length = 2. 0 m) Sample + Buffer gas Optical Table Quartz Window Expansion Chamber P M T HV Signal Resistor Box High Voltage DC Power Supply Collecting Lens Spectrometer Controller Pump Oscilloscope Cathode Computer Printer Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 9
Experimental : CESE system (C) (B) (A) Ground Monochromator (Path length = 2. 0 m) Sample + Buffer gas Optical Table Quartz Window Expansion Chamber P M T HV Signal Resistor Box High Voltage DC Power Supply Collecting Lens Spectrometer Controller Pump Oscilloscope Cathode Computer Printer Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 9
 Overview of CESE system Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 10
Overview of CESE system Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 10
 Emission in CESE system Demonstration with Helium Discharge in CESE 1. 2 cm Electrode Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 11
Emission in CESE system Demonstration with Helium Discharge in CESE 1. 2 cm Electrode Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 11
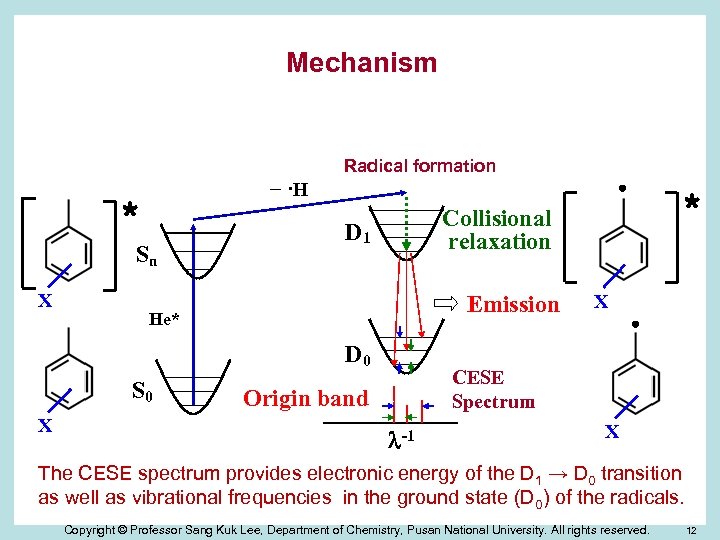 Mechanism Radical formation − ·H * Sn X D 1 Emission He* D 0 S 0 X * Collisional relaxation X CESE Spectrum Origin band -1 X The CESE spectrum provides electronic energy of the D 1 → D 0 transition as well as vibrational frequencies in the ground state (D 0) of the radicals. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 12
Mechanism Radical formation − ·H * Sn X D 1 Emission He* D 0 S 0 X * Collisional relaxation X CESE Spectrum Origin band -1 X The CESE spectrum provides electronic energy of the D 1 → D 0 transition as well as vibrational frequencies in the ground state (D 0) of the radicals. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 12
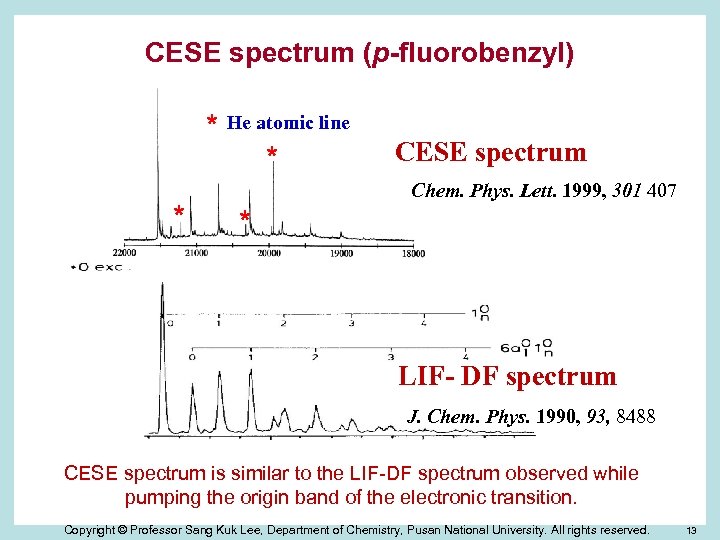 CESE spectrum (p-fluorobenzyl) * He atomic line * * CESE spectrum Chem. Phys. Lett. 1999, 301 407 * LIF- DF spectrum J. Chem. Phys. 1990, 93, 8488 CESE spectrum is similar to the LIF-DF spectrum observed while pumping the origin band of the electronic transition. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 13
CESE spectrum (p-fluorobenzyl) * He atomic line * * CESE spectrum Chem. Phys. Lett. 1999, 301 407 * LIF- DF spectrum J. Chem. Phys. 1990, 93, 8488 CESE spectrum is similar to the LIF-DF spectrum observed while pumping the origin band of the electronic transition. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 13
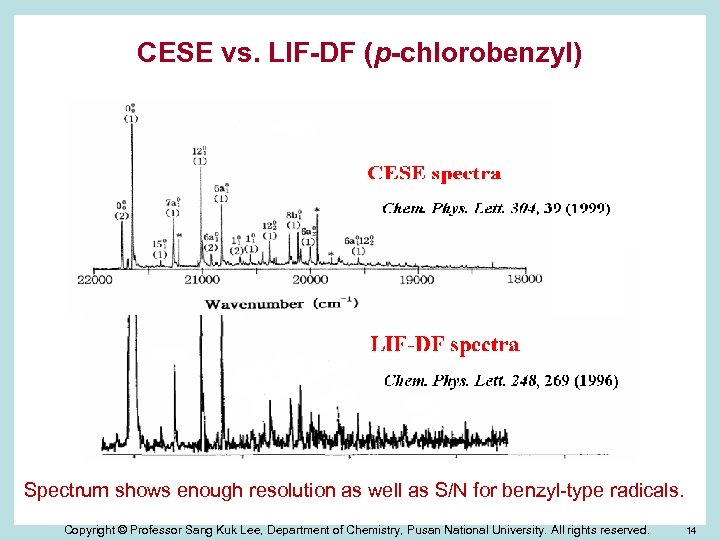 CESE vs. LIF-DF (p-chlorobenzyl) Spectrum shows enough resolution as well as S/N for benzyl-type radicals. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 14
CESE vs. LIF-DF (p-chlorobenzyl) Spectrum shows enough resolution as well as S/N for benzyl-type radicals. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 14
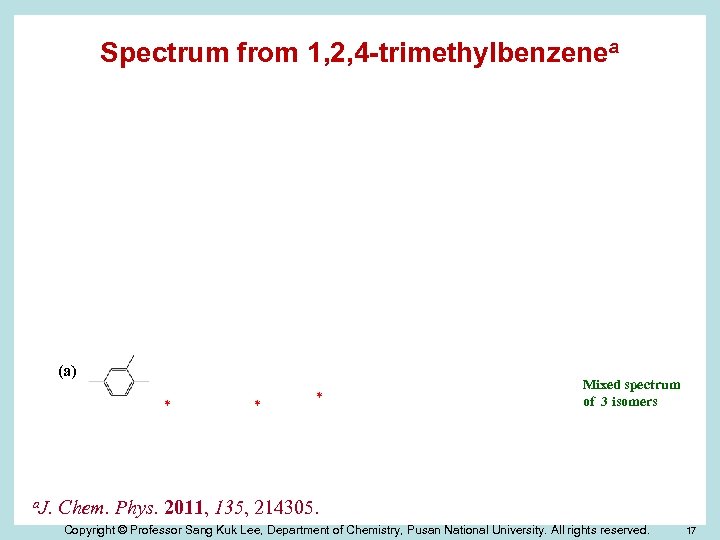
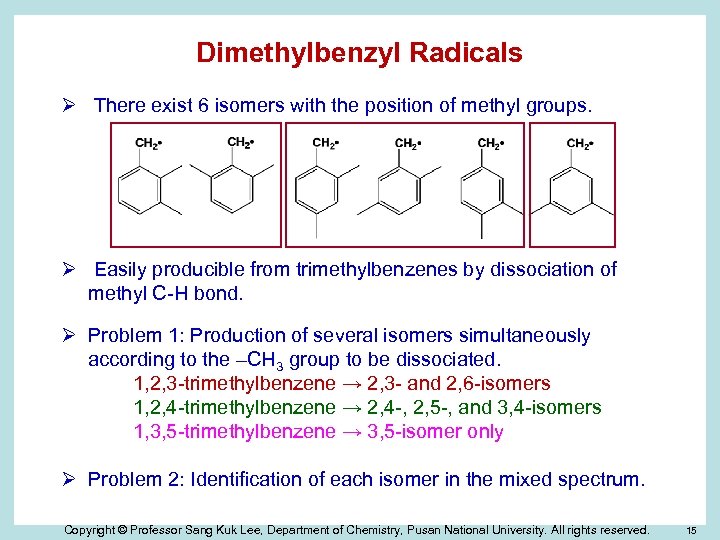 Dimethylbenzyl Radicals Ø There exist 6 isomers with the position of methyl groups. Ø Easily producible from trimethylbenzenes by dissociation of methyl C-H bond. Ø Problem 1: Production of several isomers simultaneously according to the –CH 3 group to be dissociated. 1, 2, 3 -trimethylbenzene → 2, 3 - and 2, 6 -isomers 1, 2, 4 -trimethylbenzene → 2, 4 -, 2, 5 -, and 3, 4 -isomers 1, 3, 5 -trimethylbenzene → 3, 5 -isomer only Ø Problem 2: Identification of each isomer in the mixed spectrum. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 15
Dimethylbenzyl Radicals Ø There exist 6 isomers with the position of methyl groups. Ø Easily producible from trimethylbenzenes by dissociation of methyl C-H bond. Ø Problem 1: Production of several isomers simultaneously according to the –CH 3 group to be dissociated. 1, 2, 3 -trimethylbenzene → 2, 3 - and 2, 6 -isomers 1, 2, 4 -trimethylbenzene → 2, 4 -, 2, 5 -, and 3, 4 -isomers 1, 3, 5 -trimethylbenzene → 3, 5 -isomer only Ø Problem 2: Identification of each isomer in the mixed spectrum. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 15
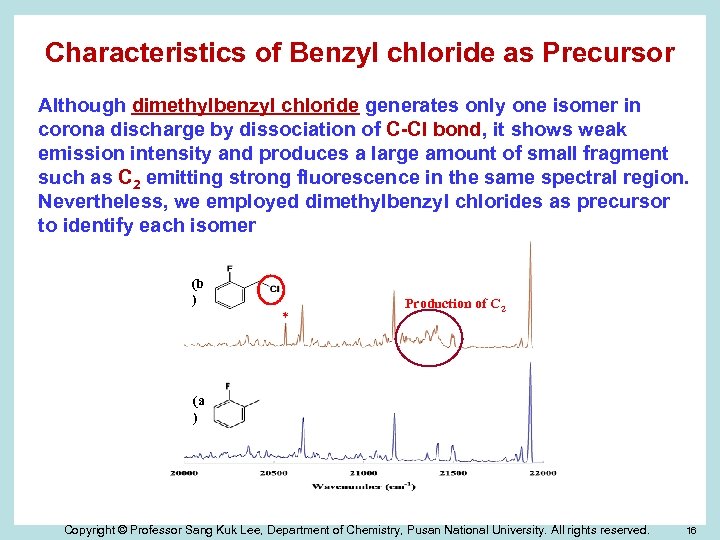 Characteristics of Benzyl chloride as Precursor Although dimethylbenzyl chloride generates only one isomer in corona discharge by dissociation of C-Cl bond, it shows weak emission intensity and produces a large amount of small fragment such as C 2 emitting strong fluorescence in the same spectral region. Nevertheless, we employed dimethylbenzyl chlorides as precursor to identify each isomer (b ) * Production of C 2 (a ) Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 16
Characteristics of Benzyl chloride as Precursor Although dimethylbenzyl chloride generates only one isomer in corona discharge by dissociation of C-Cl bond, it shows weak emission intensity and produces a large amount of small fragment such as C 2 emitting strong fluorescence in the same spectral region. Nevertheless, we employed dimethylbenzyl chlorides as precursor to identify each isomer (b ) * Production of C 2 (a ) Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 16
 Spectrum from 1, 2, 4 -trimethylbenzenea (d) * * (c) (b) * * * (a) * a. J. * * Mixed spectrum of 3 isomers Chem. Phys. 2011, 135, 214305. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 17
Spectrum from 1, 2, 4 -trimethylbenzenea (d) * * (c) (b) * * * (a) * a. J. * * Mixed spectrum of 3 isomers Chem. Phys. 2011, 135, 214305. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 17
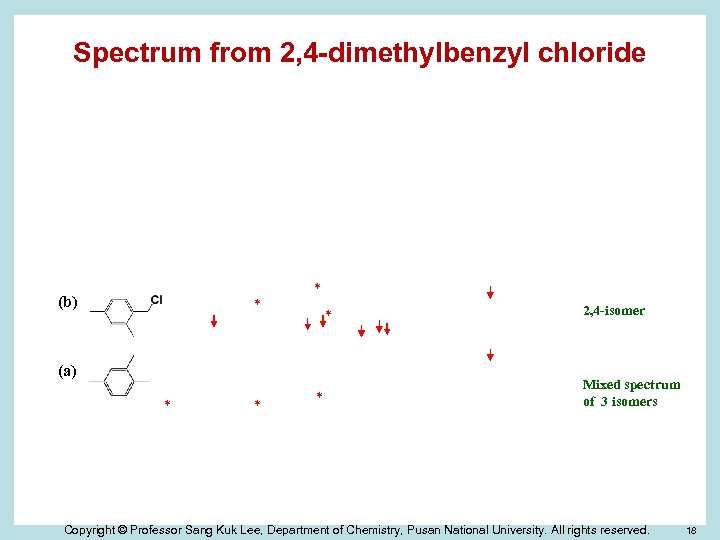 Spectrum from 2, 4 -dimethylbenzyl chloride (d) * * (c) (b) * * * (a) * * * 2, 4 -isomer Mixed spectrum of 3 isomers Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 18
Spectrum from 2, 4 -dimethylbenzyl chloride (d) * * (c) (b) * * * (a) * * * 2, 4 -isomer Mixed spectrum of 3 isomers Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 18
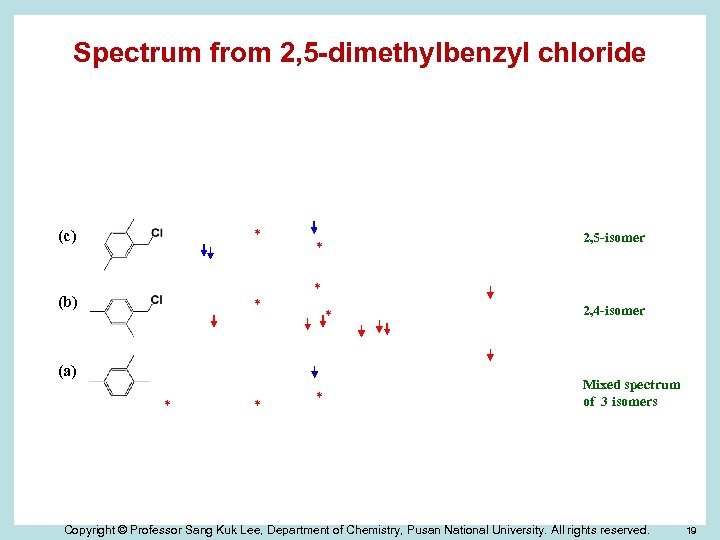 Spectrum from 2, 5 -dimethylbenzyl chloride (d) * * (c) (b) * * (a) * * 2, 5 -isomer * * 2, 4 -isomer Mixed spectrum of 3 isomers Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 19
Spectrum from 2, 5 -dimethylbenzyl chloride (d) * * (c) (b) * * (a) * * 2, 5 -isomer * * 2, 4 -isomer Mixed spectrum of 3 isomers Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 19
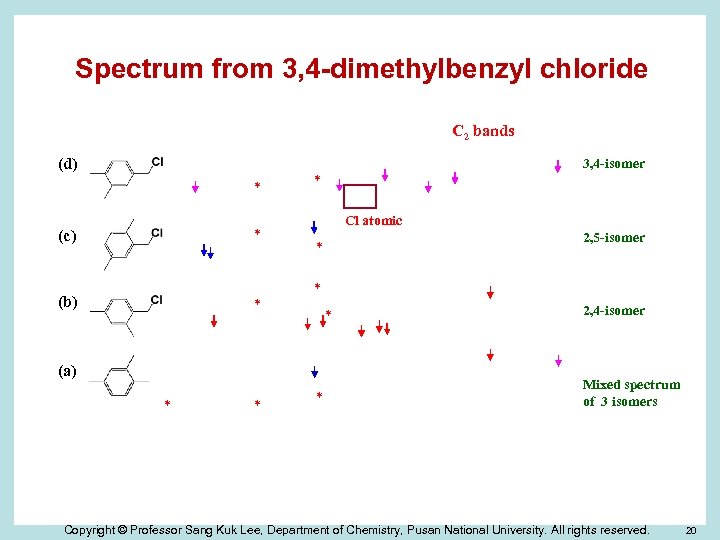 Spectrum from 3, 4 -dimethylbenzyl chloride C 2 bands (d) 3, 4 -isomer * * (c) (b) * * Cl atomic * * (a) * * 2, 5 -isomer * * 2, 4 -isomer Mixed spectrum of 3 isomers Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 20
Spectrum from 3, 4 -dimethylbenzyl chloride C 2 bands (d) 3, 4 -isomer * * (c) (b) * * Cl atomic * * (a) * * 2, 5 -isomer * * 2, 4 -isomer Mixed spectrum of 3 isomers Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 20
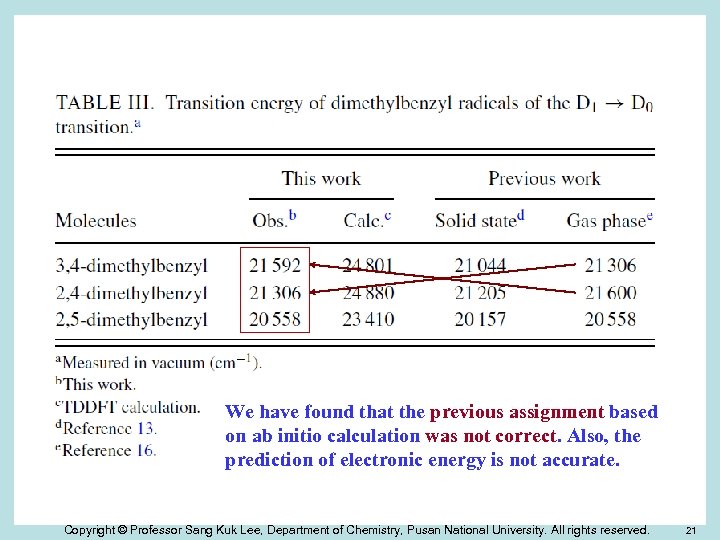 We have found that the previous assignment based on ab initio calculation was not correct. Also, the prediction of electronic energy is not accurate. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 21
We have found that the previous assignment based on ab initio calculation was not correct. Also, the prediction of electronic energy is not accurate. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 21
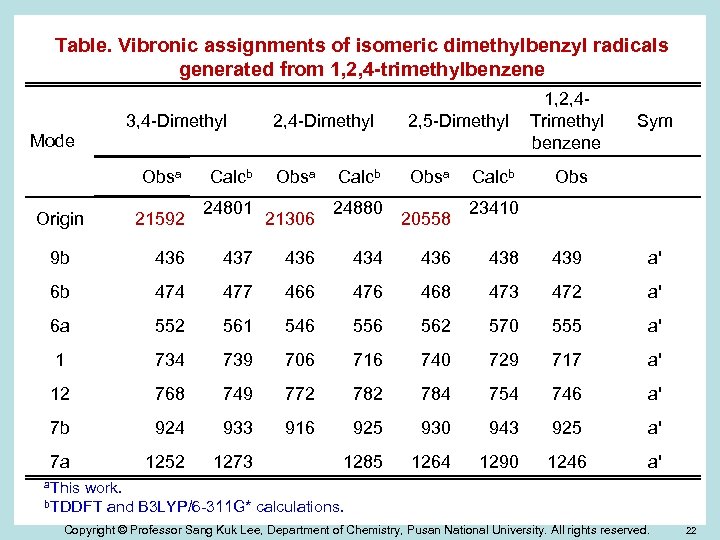 Table. Vibronic assignments of isomeric dimethylbenzyl radicals generated from 1, 2, 4 -trimethylbenzene 3, 4 -Dimethyl 2, 5 -Dimethyl Obsa Mode Obsa Origin 21592 Calcb 24801 21306 Calcb 24880 23410 20558 1, 2, 4 Trimethyl benzene Sym Obs 9 b 436 437 436 434 436 438 439 a' 6 b 474 477 466 476 468 473 472 a' 6 a 552 561 546 556 562 570 555 a' 1 734 739 706 716 740 729 717 a' 12 768 749 772 784 754 746 a' 7 b 924 933 916 925 930 943 925 a' 7 a 1252 1273 1285 1264 1290 1246 a' a. This work. and B 3 LYP/6 -311 G* calculations. b. TDDFT Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 22
Table. Vibronic assignments of isomeric dimethylbenzyl radicals generated from 1, 2, 4 -trimethylbenzene 3, 4 -Dimethyl 2, 5 -Dimethyl Obsa Mode Obsa Origin 21592 Calcb 24801 21306 Calcb 24880 23410 20558 1, 2, 4 Trimethyl benzene Sym Obs 9 b 436 437 436 434 436 438 439 a' 6 b 474 477 466 476 468 473 472 a' 6 a 552 561 546 556 562 570 555 a' 1 734 739 706 716 740 729 717 a' 12 768 749 772 784 754 746 a' 7 b 924 933 916 925 930 943 925 a' 7 a 1252 1273 1285 1264 1290 1246 a' a. This work. and B 3 LYP/6 -311 G* calculations. b. TDDFT Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 22
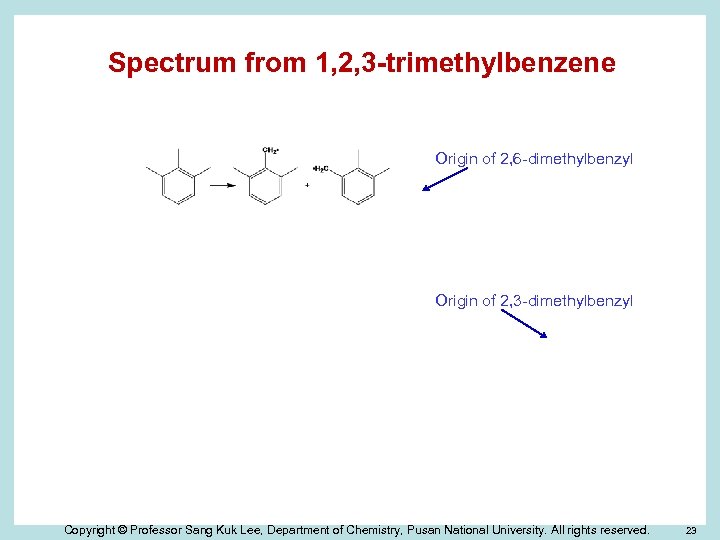 Spectrum from 1, 2, 3 -trimethylbenzene Origin of 2, 6 -dimethylbenzyl Origin of 2, 3 -dimethylbenzyl Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 23
Spectrum from 1, 2, 3 -trimethylbenzene Origin of 2, 6 -dimethylbenzyl Origin of 2, 3 -dimethylbenzyl Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 23
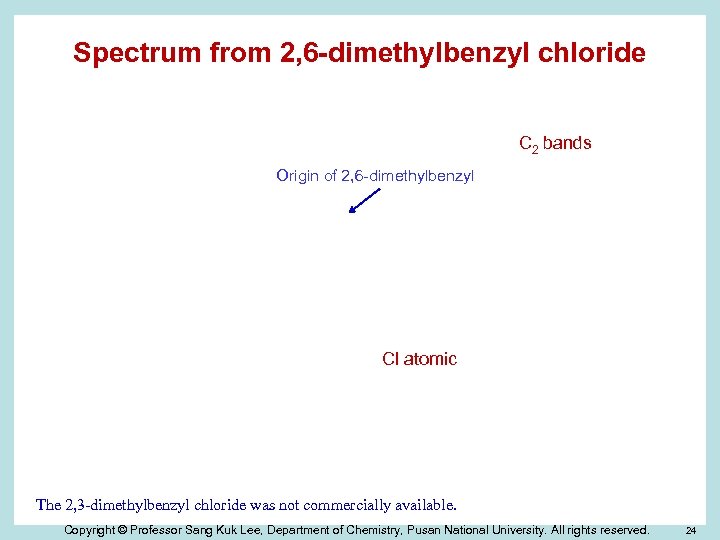 Spectrum from 2, 6 -dimethylbenzyl chloride C 2 bands Origin of 2, 6 -dimethylbenzyl Cl atomic The 2, 3 -dimethylbenzyl chloride was not commercially available. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 24
Spectrum from 2, 6 -dimethylbenzyl chloride C 2 bands Origin of 2, 6 -dimethylbenzyl Cl atomic The 2, 3 -dimethylbenzyl chloride was not commercially available. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 24
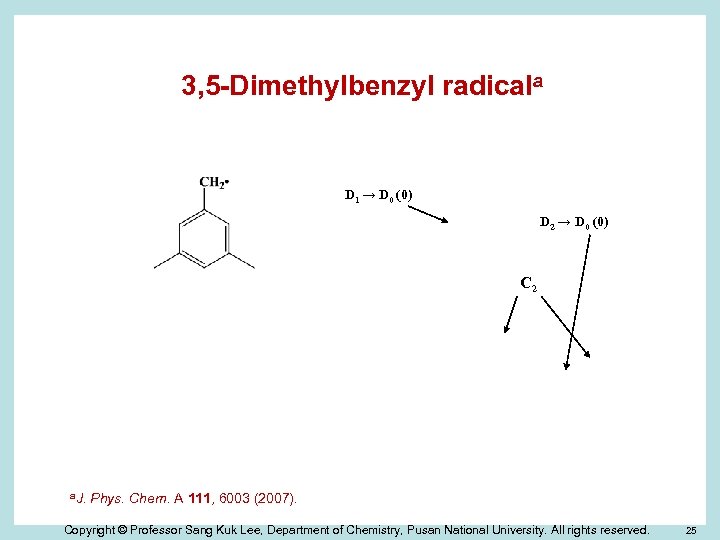 3, 5 -Dimethylbenzyl radicala D 1 → D 0 (0) D 2 → D 0 (0) C 2 a. J. Phys. Chem. A 111, 6003 (2007). Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 25
3, 5 -Dimethylbenzyl radicala D 1 → D 0 (0) D 2 → D 0 (0) C 2 a. J. Phys. Chem. A 111, 6003 (2007). Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 25
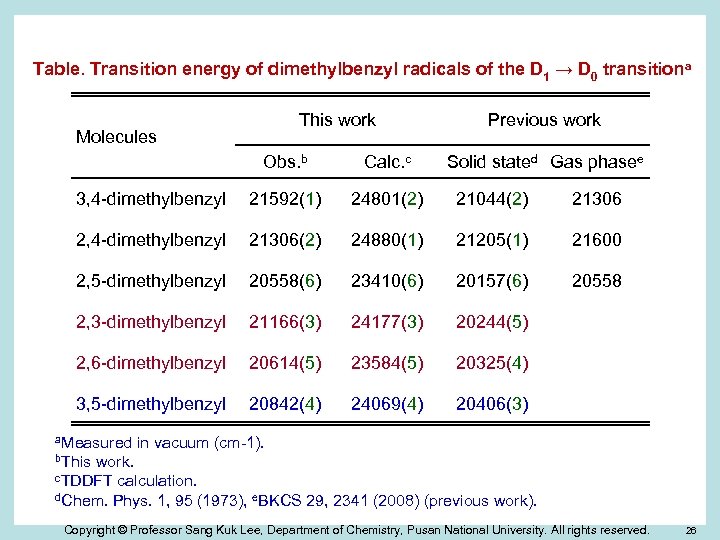 Table. Transition energy of dimethylbenzyl radicals of the D 1 → D 0 transitiona This work Molecules Previous work Obs. b Calc. c 3, 4 -dimethylbenzyl 21592(1) 24801(2) 21044(2) 21306 2, 4 -dimethylbenzyl 21306(2) 24880(1) 21205(1) 21600 2, 5 -dimethylbenzyl 20558(6) 23410(6) 20157(6) 20558 2, 3 -dimethylbenzyl 21166(3) 24177(3) 20244(5) 2, 6 -dimethylbenzyl 20614(5) 23584(5) 20325(4) 3, 5 -dimethylbenzyl 20842(4) 24069(4) 20406(3) a. Measured Solid stated Gas phasee in vacuum (cm-1). b. This work. c. TDDFT calculation. d. Chem. Phys. 1, 95 (1973), e. BKCS 29, 2341 (2008) (previous work). Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 26
Table. Transition energy of dimethylbenzyl radicals of the D 1 → D 0 transitiona This work Molecules Previous work Obs. b Calc. c 3, 4 -dimethylbenzyl 21592(1) 24801(2) 21044(2) 21306 2, 4 -dimethylbenzyl 21306(2) 24880(1) 21205(1) 21600 2, 5 -dimethylbenzyl 20558(6) 23410(6) 20157(6) 20558 2, 3 -dimethylbenzyl 21166(3) 24177(3) 20244(5) 2, 6 -dimethylbenzyl 20614(5) 23584(5) 20325(4) 3, 5 -dimethylbenzyl 20842(4) 24069(4) 20406(3) a. Measured Solid stated Gas phasee in vacuum (cm-1). b. This work. c. TDDFT calculation. d. Chem. Phys. 1, 95 (1973), e. BKCS 29, 2341 (2008) (previous work). Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 26
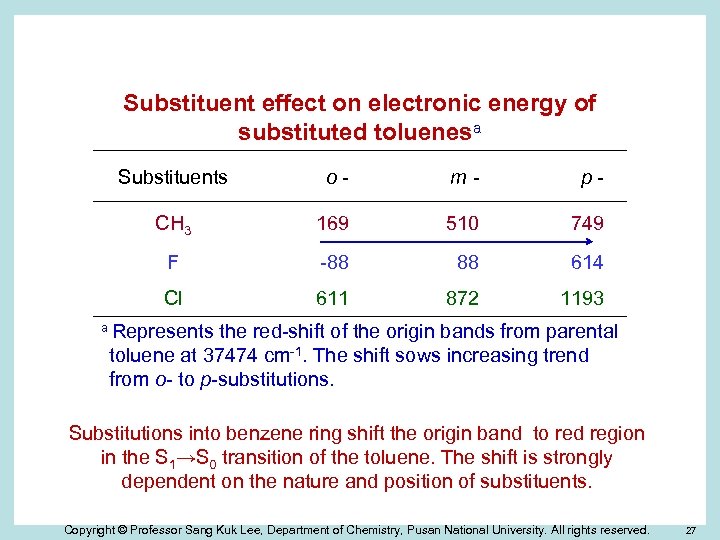 Substituent effect on electronic energy of substituted toluenesa Substituents o- m- p- CH 3 169 510 749 F -88 88 614 Cl 611 872 1193 a Represents the red-shift of the origin bands from parental toluene at 37474 cm-1. The shift sows increasing trend from o- to p-substitutions. Substitutions into benzene ring shift the origin band to red region in the S 1→S 0 transition of the toluene. The shift is strongly dependent on the nature and position of substituents. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 27
Substituent effect on electronic energy of substituted toluenesa Substituents o- m- p- CH 3 169 510 749 F -88 88 614 Cl 611 872 1193 a Represents the red-shift of the origin bands from parental toluene at 37474 cm-1. The shift sows increasing trend from o- to p-substitutions. Substitutions into benzene ring shift the origin band to red region in the S 1→S 0 transition of the toluene. The shift is strongly dependent on the nature and position of substituents. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 27
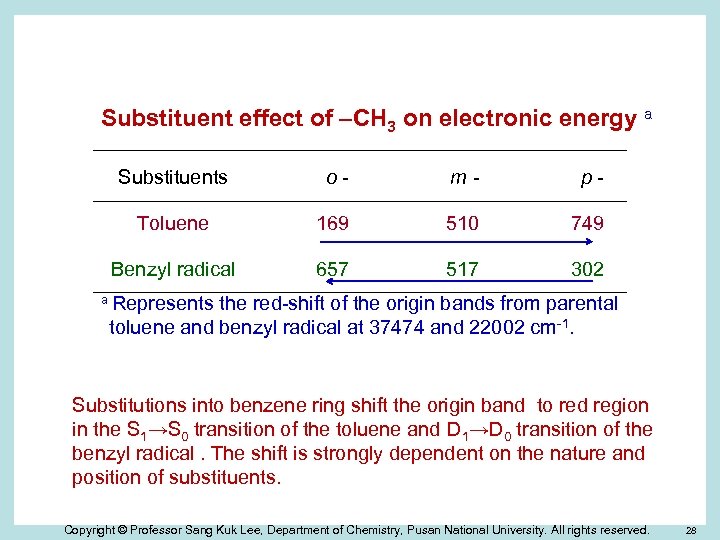 Substituent effect of –CH 3 on electronic energy a Substituents o- m- p- Toluene 169 510 749 Benzyl radical 657 517 302 a Represents the red-shift of the origin bands from parental toluene and benzyl radical at 37474 and 22002 cm-1. Substitutions into benzene ring shift the origin band to red region in the S 1→S 0 transition of the toluene and D 1→D 0 transition of the benzyl radical. The shift is strongly dependent on the nature and position of substituents. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 28
Substituent effect of –CH 3 on electronic energy a Substituents o- m- p- Toluene 169 510 749 Benzyl radical 657 517 302 a Represents the red-shift of the origin bands from parental toluene and benzyl radical at 37474 and 22002 cm-1. Substitutions into benzene ring shift the origin band to red region in the S 1→S 0 transition of the toluene and D 1→D 0 transition of the benzyl radical. The shift is strongly dependent on the nature and position of substituents. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 28
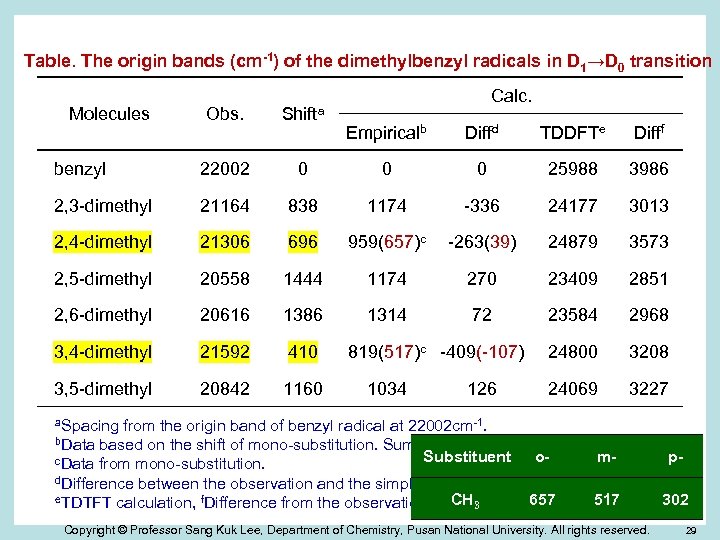 Table. The origin bands (cm-1) of the dimethylbenzyl radicals in D 1→D 0 transition Obs. Shifta Molecules benzyl 22002 2, 3 -dimethyl Calc. Empiricalb Diffd TDDFTe Difff 0 0 0 25988 3986 21164 838 1174 -336 24177 3013 2, 4 -dimethyl 21306 696 959(657)c -263(39) 24879 3573 2, 5 -dimethyl 20558 1444 1174 270 23409 2851 2, 6 -dimethyl 20616 1386 1314 72 23584 2968 3, 4 -dimethyl 21592 410 24800 3208 3, 5 -dimethyl 20842 1160 24069 3227 819(517)c -409(-107) 1034 126 a. Spacing from the origin band of benzyl radical at 22002 cm-1. b. Data based on the shift of mono-substitution. Summation of two mono-substitutions. Substituent ompc. Data from mono-substitution. d. Difference between the observation and the simple summation. e. TDTFT calculation, f. Difference from the observation. CH 3 657 517 302 Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 29
Table. The origin bands (cm-1) of the dimethylbenzyl radicals in D 1→D 0 transition Obs. Shifta Molecules benzyl 22002 2, 3 -dimethyl Calc. Empiricalb Diffd TDDFTe Difff 0 0 0 25988 3986 21164 838 1174 -336 24177 3013 2, 4 -dimethyl 21306 696 959(657)c -263(39) 24879 3573 2, 5 -dimethyl 20558 1444 1174 270 23409 2851 2, 6 -dimethyl 20616 1386 1314 72 23584 2968 3, 4 -dimethyl 21592 410 24800 3208 3, 5 -dimethyl 20842 1160 24069 3227 819(517)c -409(-107) 1034 126 a. Spacing from the origin band of benzyl radical at 22002 cm-1. b. Data based on the shift of mono-substitution. Summation of two mono-substitutions. Substituent ompc. Data from mono-substitution. d. Difference between the observation and the simple summation. e. TDTFT calculation, f. Difference from the observation. CH 3 657 517 302 Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 29
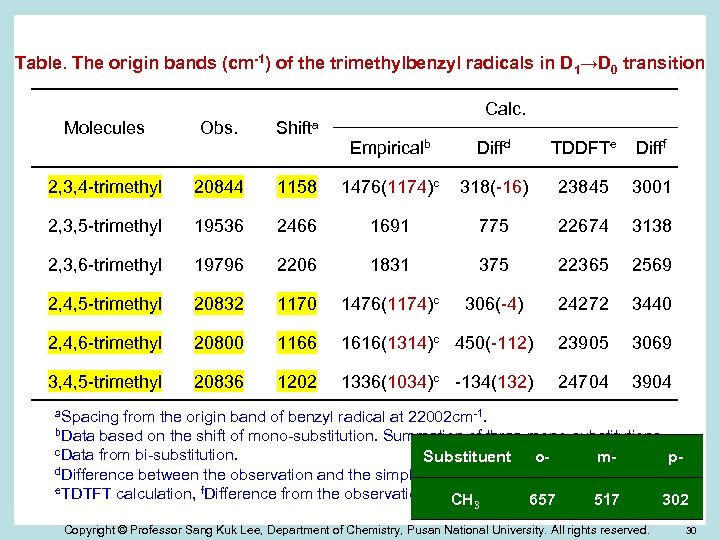 Table. The origin bands (cm-1) of the trimethylbenzyl radicals in D 1→D 0 transition Molecules Obs. Shifta 2, 3, 4 -trimethyl 20844 2, 3, 5 -trimethyl Calc. Empiricalb Diffd TDDFTe Difff 1158 1476(1174)c 318(-16) 23845 3001 19536 2466 1691 775 22674 3138 2, 3, 6 -trimethyl 19796 2206 1831 375 22365 2569 2, 4, 5 -trimethyl 20832 1170 1476(1174)c 306(-4) 24272 3440 2, 4, 6 -trimethyl 20800 1166 1616(1314)c 450(-112) 23905 3069 3, 4, 5 -trimethyl 20836 1202 1336(1034)c -134(132) 24704 3904 a. Spacing from the origin band of benzyl radical at 22002 cm-1. b. Data based on the shift of mono-substitution. Summation of three mono-substitutions. c. Data from bi-substitution. Substituent ompd. Difference between the observation and the simple summation. e. TDTFT calculation, f. Difference from the observation. CH 657 517 302 3 Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 30
Table. The origin bands (cm-1) of the trimethylbenzyl radicals in D 1→D 0 transition Molecules Obs. Shifta 2, 3, 4 -trimethyl 20844 2, 3, 5 -trimethyl Calc. Empiricalb Diffd TDDFTe Difff 1158 1476(1174)c 318(-16) 23845 3001 19536 2466 1691 775 22674 3138 2, 3, 6 -trimethyl 19796 2206 1831 375 22365 2569 2, 4, 5 -trimethyl 20832 1170 1476(1174)c 306(-4) 24272 3440 2, 4, 6 -trimethyl 20800 1166 1616(1314)c 450(-112) 23905 3069 3, 4, 5 -trimethyl 20836 1202 1336(1034)c -134(132) 24704 3904 a. Spacing from the origin band of benzyl radical at 22002 cm-1. b. Data based on the shift of mono-substitution. Summation of three mono-substitutions. c. Data from bi-substitution. Substituent ompd. Difference between the observation and the simple summation. e. TDTFT calculation, f. Difference from the observation. CH 657 517 302 3 Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 30
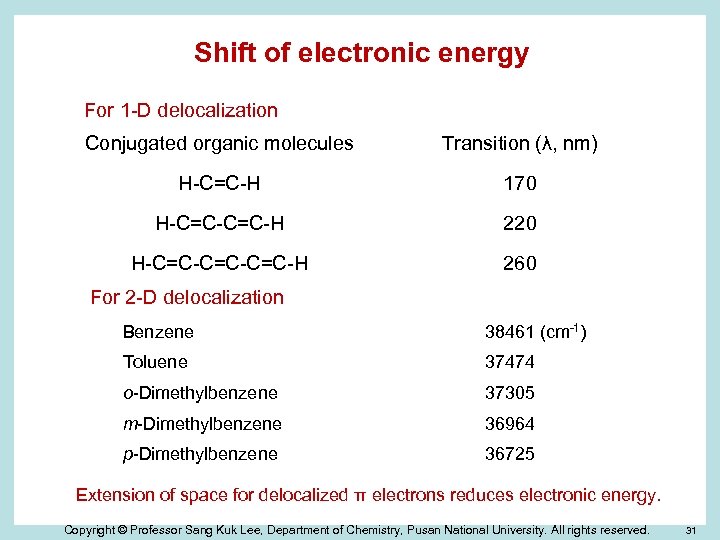 Shift of electronic energy For 1 -D delocalization Conjugated organic molecules Transition (λ, nm) H-C=C-H 170 H-C=C-H 220 H-C=C-C=C-H 260 For 2 -D delocalization Benzene 38461 (cm-1) Toluene 37474 o-Dimethylbenzene 37305 m-Dimethylbenzene 36964 p-Dimethylbenzene 36725 Extension of space for delocalized π electrons reduces electronic energy. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 31
Shift of electronic energy For 1 -D delocalization Conjugated organic molecules Transition (λ, nm) H-C=C-H 170 H-C=C-H 220 H-C=C-C=C-H 260 For 2 -D delocalization Benzene 38461 (cm-1) Toluene 37474 o-Dimethylbenzene 37305 m-Dimethylbenzene 36964 p-Dimethylbenzene 36725 Extension of space for delocalized π electrons reduces electronic energy. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 31
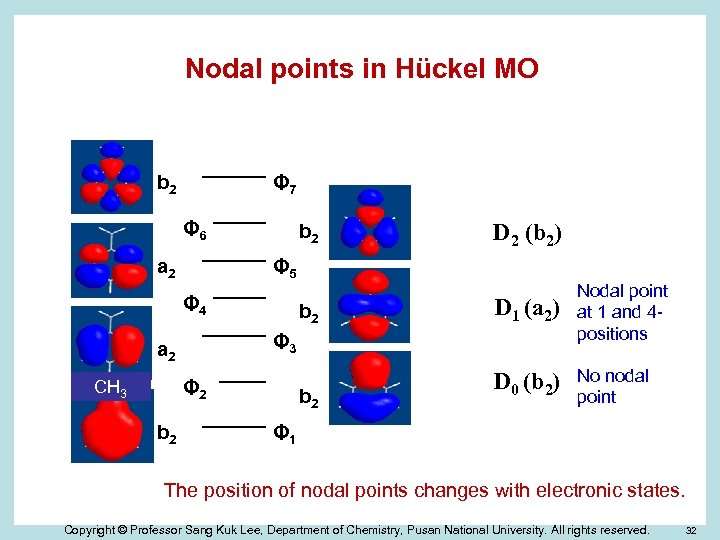 Nodal points in Hückel MO b 2 Φ 7 Φ 6 a 2 b 2 Φ 5 Φ 4 b 2 Φ 2 b 2 D 1 (a 2) Nodal point at 1 and 4 positions D 0 (b 2) No nodal point Φ 3 a 2 CH 3 D 2 (b 2) b 2 Φ 1 The position of nodal points changes with electronic states. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 32
Nodal points in Hückel MO b 2 Φ 7 Φ 6 a 2 b 2 Φ 5 Φ 4 b 2 Φ 2 b 2 D 1 (a 2) Nodal point at 1 and 4 positions D 0 (b 2) No nodal point Φ 3 a 2 CH 3 D 2 (b 2) b 2 Φ 1 The position of nodal points changes with electronic states. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 32
 Summary Ø We successfully observed the vibronic emission spectra of dimethylbenzyl radicals using a technique of CESE. Ø We clearly identify the bands belonging to each isomer using dimethylbenzyl chlorides as a precursor. Ø We explain the smaller red-shift of the origin bands of benzyl-type radicals with substituent at 4 -position using Hückel MO theory for the first time. The smaller shift is observed from other multi-substituted benzyl radicals. Ø This observation may provides direct evidence of nodal points at a given electronic state. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 33
Summary Ø We successfully observed the vibronic emission spectra of dimethylbenzyl radicals using a technique of CESE. Ø We clearly identify the bands belonging to each isomer using dimethylbenzyl chlorides as a precursor. Ø We explain the smaller red-shift of the origin bands of benzyl-type radicals with substituent at 4 -position using Hückel MO theory for the first time. The smaller shift is observed from other multi-substituted benzyl radicals. Ø This observation may provides direct evidence of nodal points at a given electronic state. Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 33
 Acknowledgments Funding for Basic Sciences National Research Foundation of Korea (2011 -2014) Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 34
Acknowledgments Funding for Basic Sciences National Research Foundation of Korea (2011 -2014) Copyright © Professor Sang Kuk Lee, Department of Chemistry, Pusan National University. All rights reserved. 34


