38a3322d6a501769952d7afc74c2aabb.ppt
- Количество слайдов: 34
 The 63 rd International Symposium on Molecular Spectroscopy, WG 11 Columbus, Ohio, June 16 -20, 2008 Spectroscopic Identification of New Aromatic Molecular Radicals in Corona Discharge: α-Methylbenzyl Radical Chan Ho Park, Gi Woo Lee, Hyeon Geun Ahn, Sang Kuk Lee Department of Chemistry Pusan National University Pusan 609 -735, Korea Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
The 63 rd International Symposium on Molecular Spectroscopy, WG 11 Columbus, Ohio, June 16 -20, 2008 Spectroscopic Identification of New Aromatic Molecular Radicals in Corona Discharge: α-Methylbenzyl Radical Chan Ho Park, Gi Woo Lee, Hyeon Geun Ahn, Sang Kuk Lee Department of Chemistry Pusan National University Pusan 609 -735, Korea Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
 Previous works Mono-substituted benzyl radicals X : F, Cl, CH 3, CN Multi-substituted benzyl radicals X : F, Cl, CH 3 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Previous works Mono-substituted benzyl radicals X : F, Cl, CH 3, CN Multi-substituted benzyl radicals X : F, Cl, CH 3 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
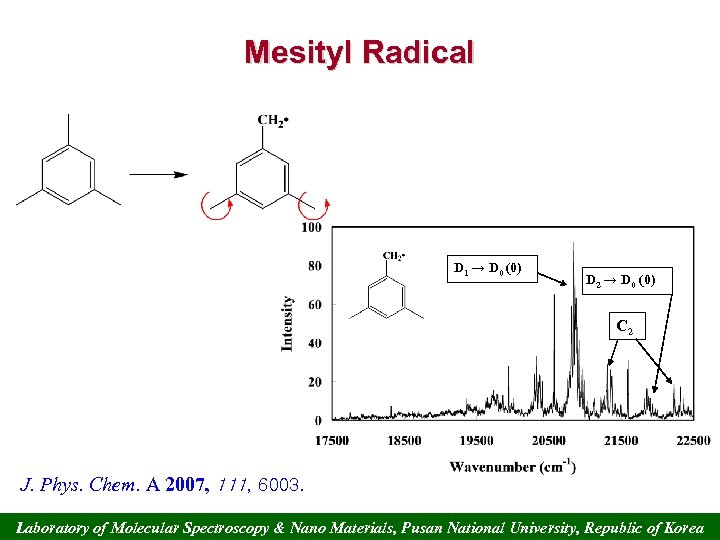 Mesityl Radical D 1 → D 0 (0) D 2 → D 0 (0) C 2 J. Phys. Chem. A 2007, 111, 6003. Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Mesityl Radical D 1 → D 0 (0) D 2 → D 0 (0) C 2 J. Phys. Chem. A 2007, 111, 6003. Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
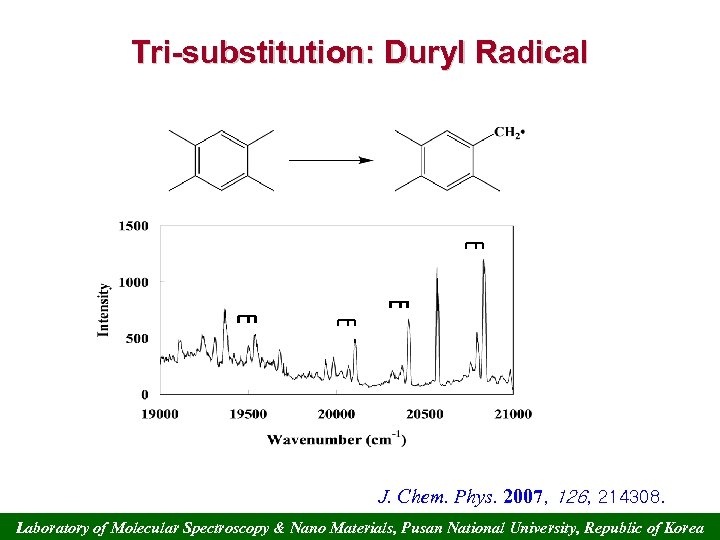 Tri-substitution: Duryl Radical 6 b J. Chem. Phys. 2007, 126, 214308. Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Tri-substitution: Duryl Radical 6 b J. Chem. Phys. 2007, 126, 214308. Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
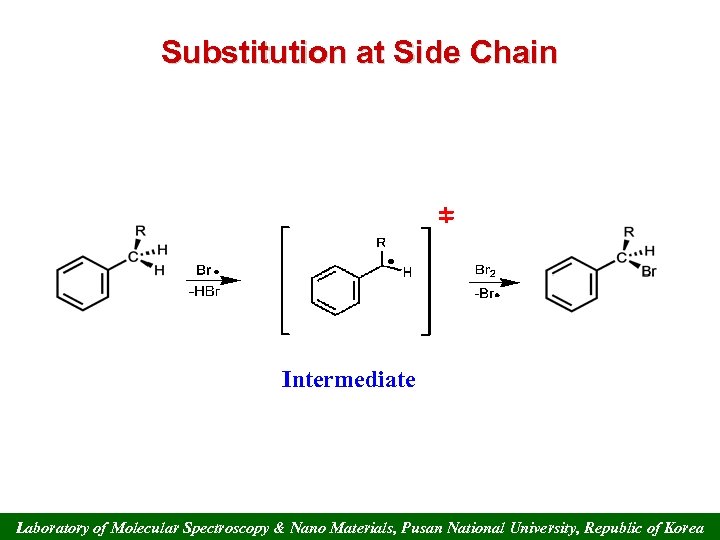 Substitution at Side Chain Intermediate Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Substitution at Side Chain Intermediate Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
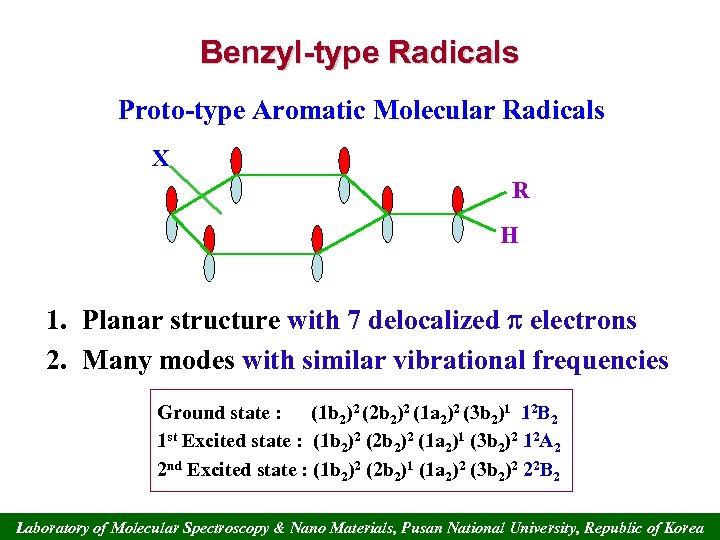 Benzyl-type Radicals Proto-type Aromatic Molecular Radicals X R H 1. Planar structure with 7 delocalized electrons 2. Many modes with similar vibrational frequencies Ground state : (1 b 2)2 (2 b 2)2 (1 a 2)2 (3 b 2)1 12 B 2 1 st Excited state : (1 b 2)2 (2 b 2)2 (1 a 2)1 (3 b 2)2 12 A 2 2 nd Excited state : (1 b 2)2 (2 b 2)1 (1 a 2)2 (3 b 2)2 22 B 2 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Benzyl-type Radicals Proto-type Aromatic Molecular Radicals X R H 1. Planar structure with 7 delocalized electrons 2. Many modes with similar vibrational frequencies Ground state : (1 b 2)2 (2 b 2)2 (1 a 2)2 (3 b 2)1 12 B 2 1 st Excited state : (1 b 2)2 (2 b 2)2 (1 a 2)1 (3 b 2)2 12 A 2 2 nd Excited state : (1 b 2)2 (2 b 2)1 (1 a 2)2 (3 b 2)2 22 B 2 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
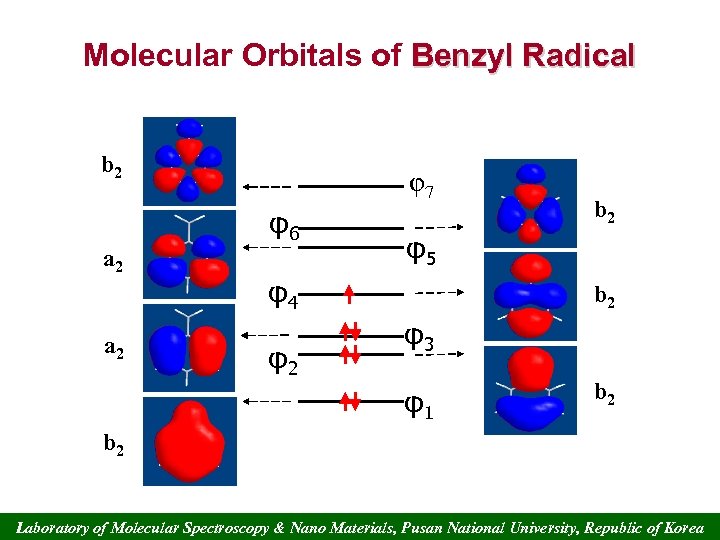 Molecular Orbitals of Benzyl Radical b 2 φ7 φ6 a 2 φ5 φ4 φ2 b 2 φ3 φ1 b 2 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Molecular Orbitals of Benzyl Radical b 2 φ7 φ6 a 2 φ5 φ4 φ2 b 2 φ3 φ1 b 2 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
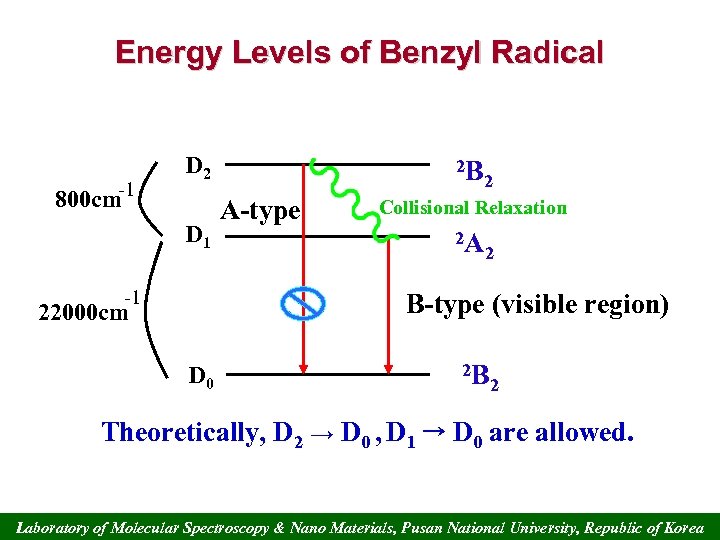 Energy Levels of Benzyl Radical -1 D 2 800 cm D 1 -1 2 B A-type 2 Collisional Relaxation 2 A 2 B-type (visible region) 22000 cm D 0 2 B 2 Theoretically, D 2 → D 0 , D 1 → D 0 are allowed. Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Energy Levels of Benzyl Radical -1 D 2 800 cm D 1 -1 2 B A-type 2 Collisional Relaxation 2 A 2 B-type (visible region) 22000 cm D 0 2 B 2 Theoretically, D 2 → D 0 , D 1 → D 0 are allowed. Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
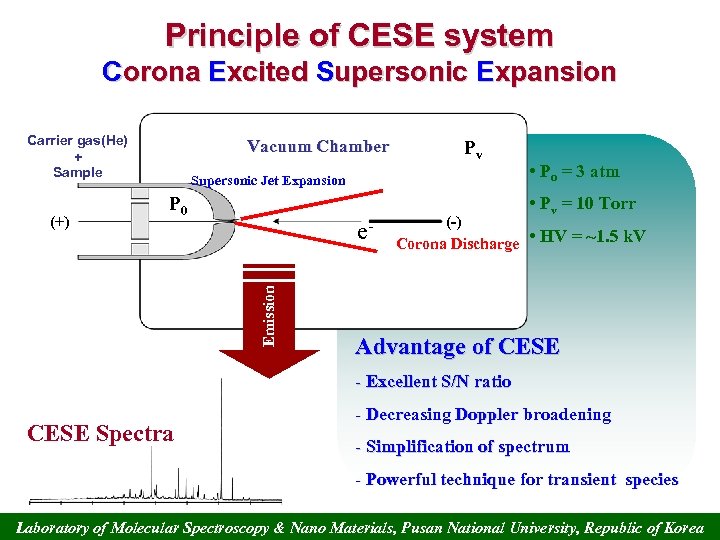 Principle of CESE system Corona Excited Supersonic Expansion Carrier gas(He) + Sample Pv Supersonic Jet Expansion P 0 • Po = 3 atm • Pv = 10 Torr e. Emission (+) Vacuum Chamber (-) Corona Discharge • HV = ~1. 5 k. V Advantage of CESE - Excellent S/N ratio CESE Spectra - Decreasing Doppler broadening - Simplification of spectrum - Powerful technique for transient species Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Principle of CESE system Corona Excited Supersonic Expansion Carrier gas(He) + Sample Pv Supersonic Jet Expansion P 0 • Po = 3 atm • Pv = 10 Torr e. Emission (+) Vacuum Chamber (-) Corona Discharge • HV = ~1. 5 k. V Advantage of CESE - Excellent S/N ratio CESE Spectra - Decreasing Doppler broadening - Simplification of spectrum - Powerful technique for transient species Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
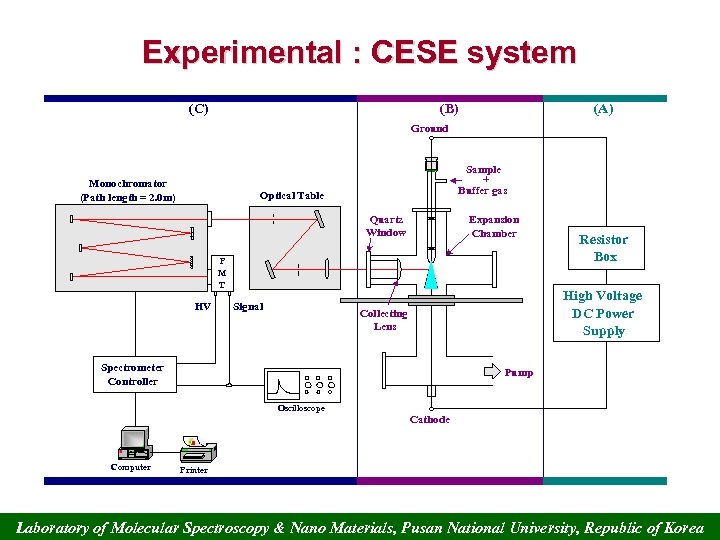 Experimental : CESE system (C) (B) (A) Ground Monochromator (Path length = 2. 0 m) Sample + Buffer gas Optical Table Quartz Window Expansion Chamber P M T HV Signal Resistor Box High Voltage DC Power Supply Collecting Lens Spectrometer Controller Pump Oscilloscope Cathode Computer Printer Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Experimental : CESE system (C) (B) (A) Ground Monochromator (Path length = 2. 0 m) Sample + Buffer gas Optical Table Quartz Window Expansion Chamber P M T HV Signal Resistor Box High Voltage DC Power Supply Collecting Lens Spectrometer Controller Pump Oscilloscope Cathode Computer Printer Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
 Overview of CESE system Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Overview of CESE system Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
 Jet Emission in CESE system Discharge in CESE Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Jet Emission in CESE system Discharge in CESE Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
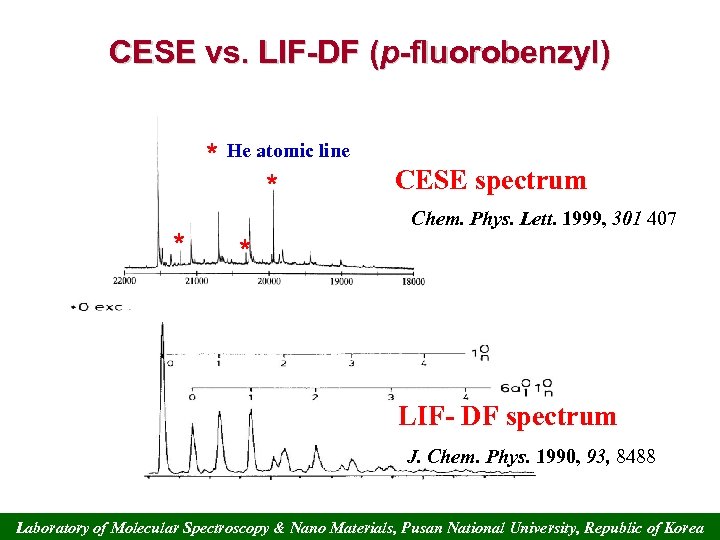 CESE vs. LIF-DF (p-fluorobenzyl) * He atomic line * * CESE spectrum Chem. Phys. Lett. 1999, 301 407 * LIF- DF spectrum J. Chem. Phys. 1990, 93, 8488 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
CESE vs. LIF-DF (p-fluorobenzyl) * He atomic line * * CESE spectrum Chem. Phys. Lett. 1999, 301 407 * LIF- DF spectrum J. Chem. Phys. 1990, 93, 8488 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
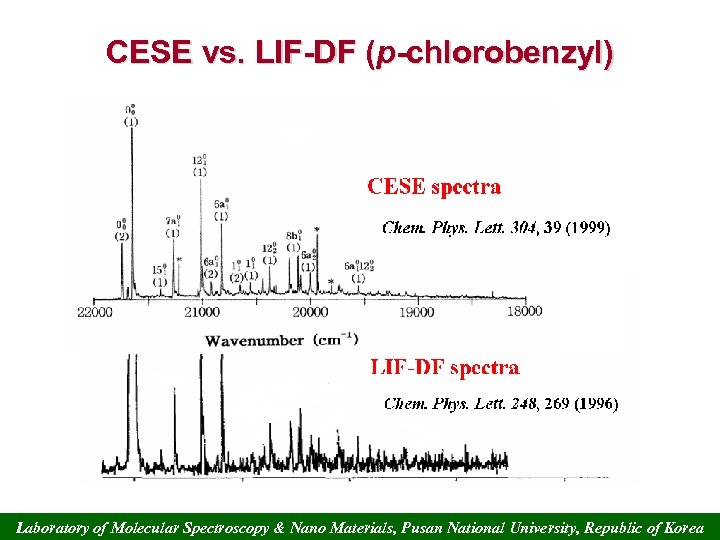 CESE vs. LIF-DF (p-chlorobenzyl) Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
CESE vs. LIF-DF (p-chlorobenzyl) Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
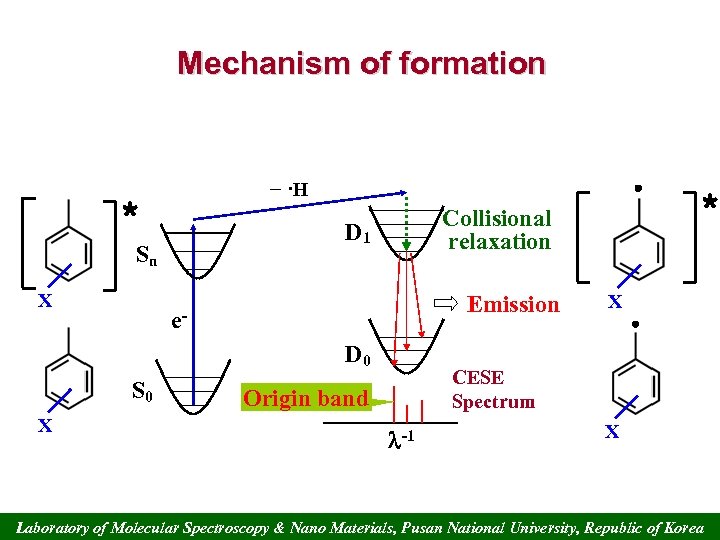 Mechanism of formation − ·H * D 1 Sn X Emission e. D 0 S 0 X * Collisional relaxation X CESE Spectrum Origin band -1 X Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Mechanism of formation − ·H * D 1 Sn X Emission e. D 0 S 0 X * Collisional relaxation X CESE Spectrum Origin band -1 X Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
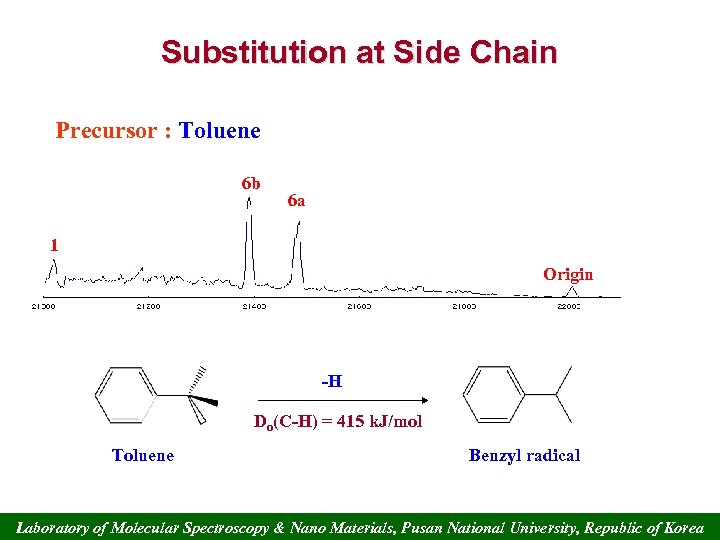 Substitution at Side Chain Precursor : Toluene 6 b 6 a 1 Origin -H Do(C-H) = 415 k. J/mol Toluene Benzyl radical Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Substitution at Side Chain Precursor : Toluene 6 b 6 a 1 Origin -H Do(C-H) = 415 k. J/mol Toluene Benzyl radical Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
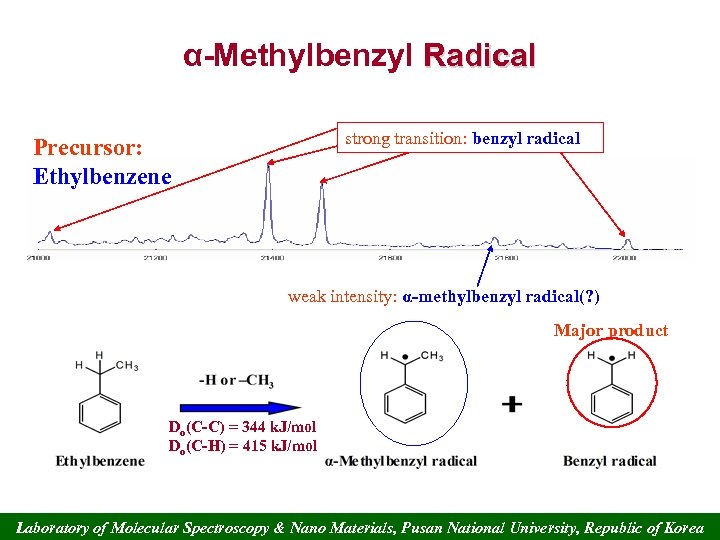 α-Methylbenzyl Radical strong transition: benzyl radical Precursor: Ethylbenzene weak intensity: α-methylbenzyl radical(? ) Major product Do(C-C) = 344 k. J/mol Do(C-H) = 415 k. J/mol Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
α-Methylbenzyl Radical strong transition: benzyl radical Precursor: Ethylbenzene weak intensity: α-methylbenzyl radical(? ) Major product Do(C-C) = 344 k. J/mol Do(C-H) = 415 k. J/mol Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
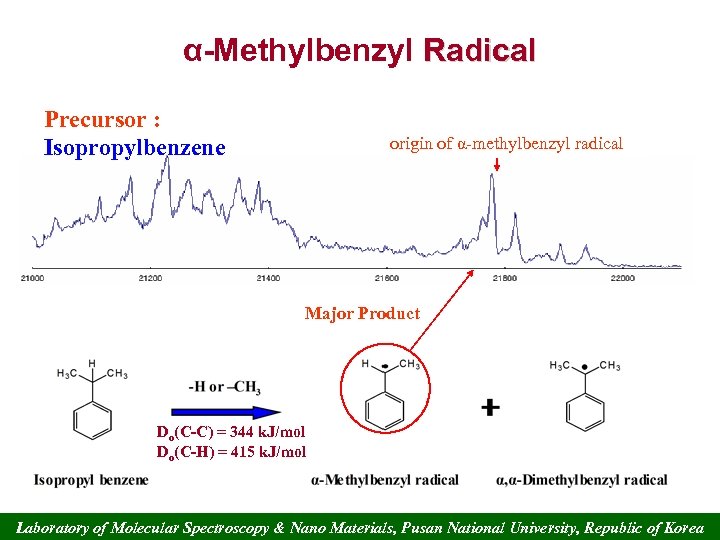 α-Methylbenzyl Radical Precursor : Isopropylbenzene origin of α-methylbenzyl radical Major Product Do(C-C) = 344 k. J/mol Do(C-H) = 415 k. J/mol Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
α-Methylbenzyl Radical Precursor : Isopropylbenzene origin of α-methylbenzyl radical Major Product Do(C-C) = 344 k. J/mol Do(C-H) = 415 k. J/mol Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
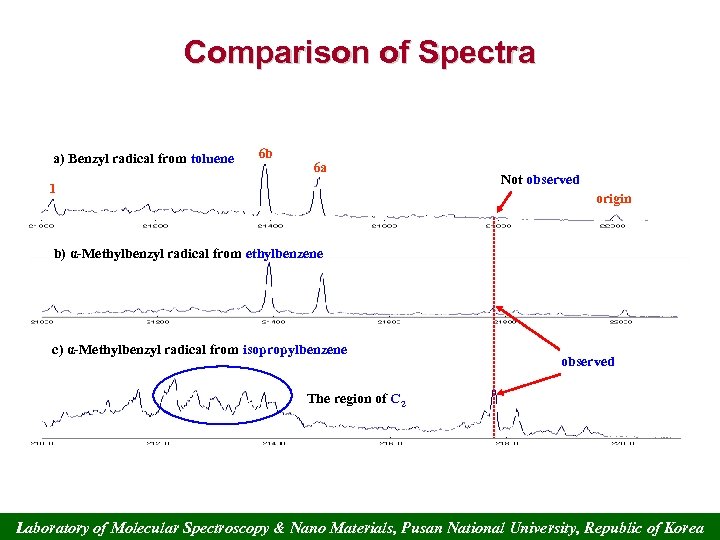 Comparison of Spectra a) Benzyl radical from toluene 6 b 6 a 1 Not observed origin b) α-Methylbenzyl radical from ethylbenzene c) α-Methylbenzyl radical from isopropylbenzene observed The region of C 2 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Comparison of Spectra a) Benzyl radical from toluene 6 b 6 a 1 Not observed origin b) α-Methylbenzyl radical from ethylbenzene c) α-Methylbenzyl radical from isopropylbenzene observed The region of C 2 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
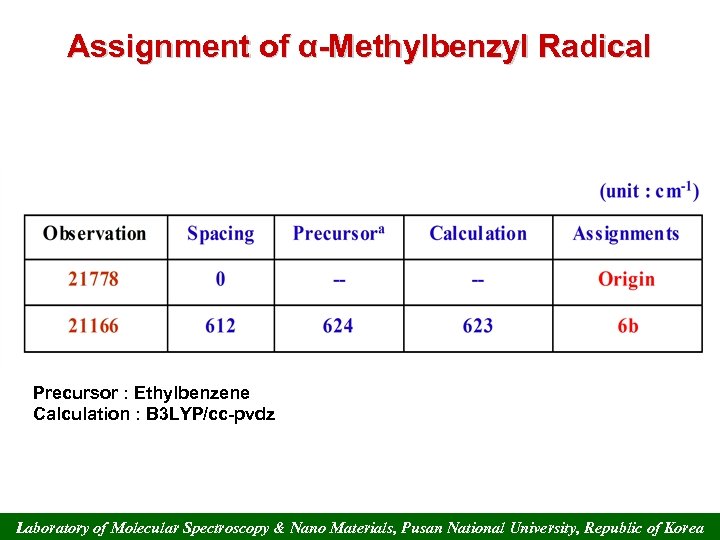 Assignment of α-Methylbenzyl Radical Precursor : Ethylbenzene Calculation : B 3 LYP/cc-pvdz Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Assignment of α-Methylbenzyl Radical Precursor : Ethylbenzene Calculation : B 3 LYP/cc-pvdz Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
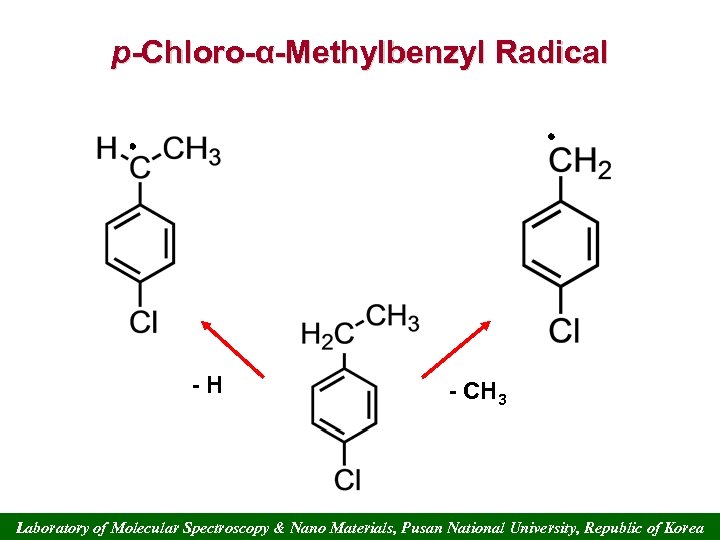 p-Chloro-α-Methylbenzyl Radical -H - CH 3 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
p-Chloro-α-Methylbenzyl Radical -H - CH 3 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
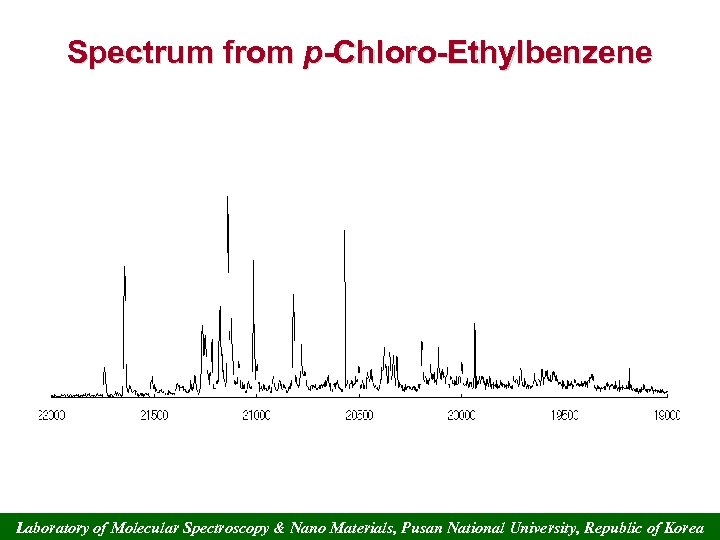 Spectrum from p-Chloro-Ethylbenzene Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Spectrum from p-Chloro-Ethylbenzene Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
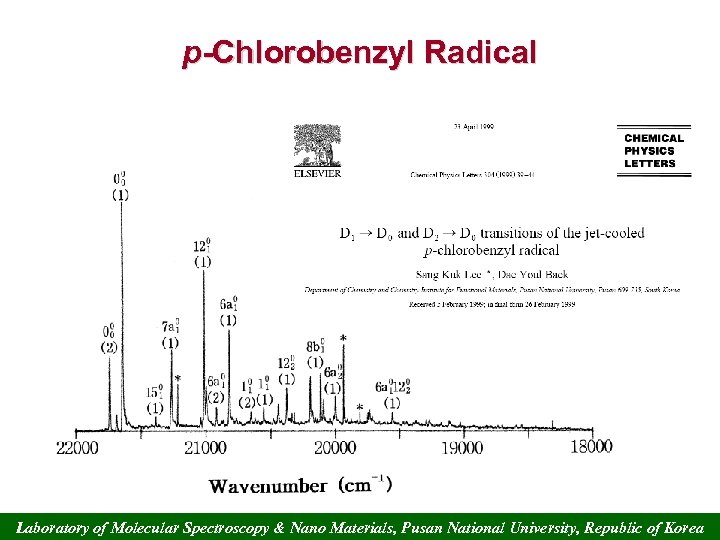 p-Chlorobenzyl Radical Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
p-Chlorobenzyl Radical Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
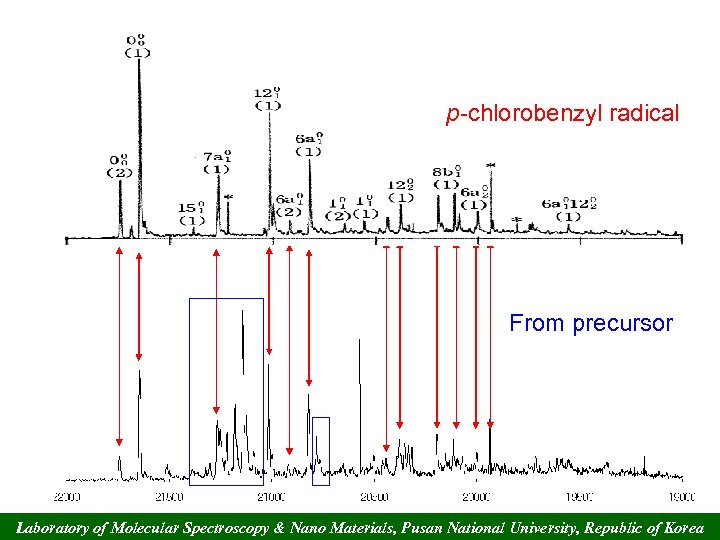 p-chlorobenzyl radical From precursor Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
p-chlorobenzyl radical From precursor Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
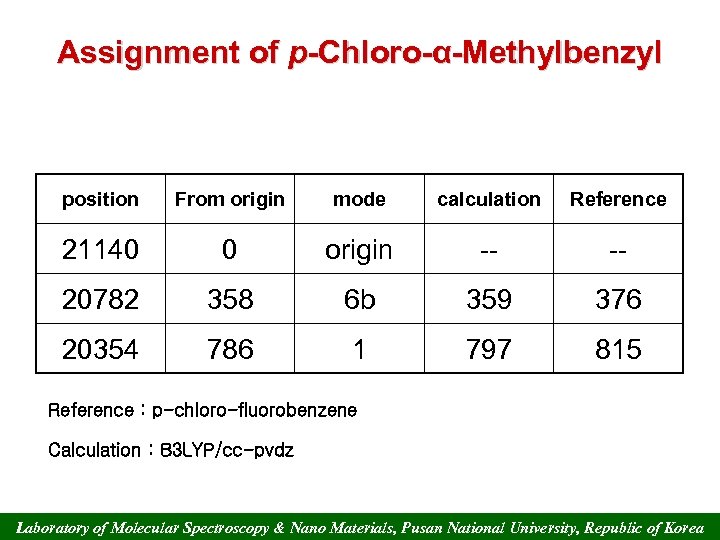 Assignment of p-Chloro-α-Methylbenzyl position From origin mode calculation Reference 21140 0 origin -- -- 20782 358 6 b 359 376 20354 786 1 797 815 Reference : p-chloro-fluorobenzene Calculation : B 3 LYP/cc-pvdz Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Assignment of p-Chloro-α-Methylbenzyl position From origin mode calculation Reference 21140 0 origin -- -- 20782 358 6 b 359 376 20354 786 1 797 815 Reference : p-chloro-fluorobenzene Calculation : B 3 LYP/cc-pvdz Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
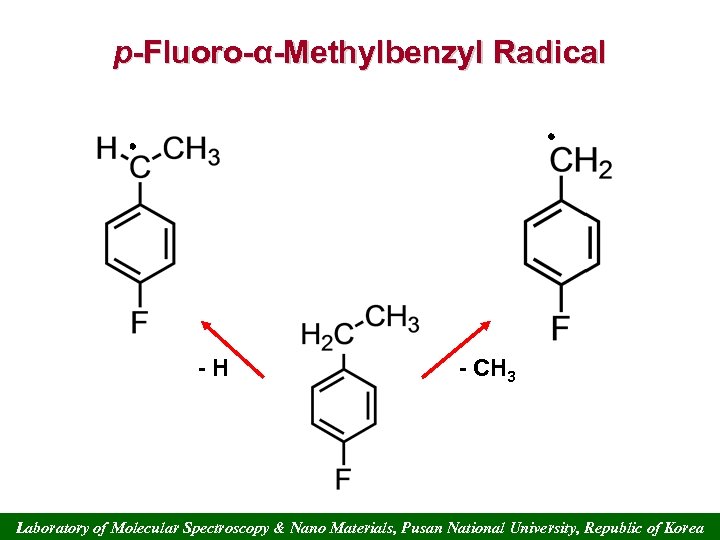 p-Fluoro-α-Methylbenzyl Radical -H - CH 3 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
p-Fluoro-α-Methylbenzyl Radical -H - CH 3 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
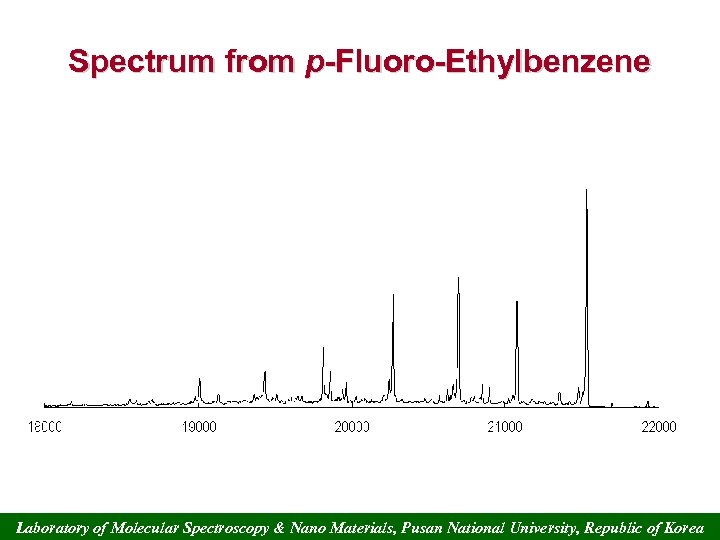 Spectrum from p-Fluoro-Ethylbenzene Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Spectrum from p-Fluoro-Ethylbenzene Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
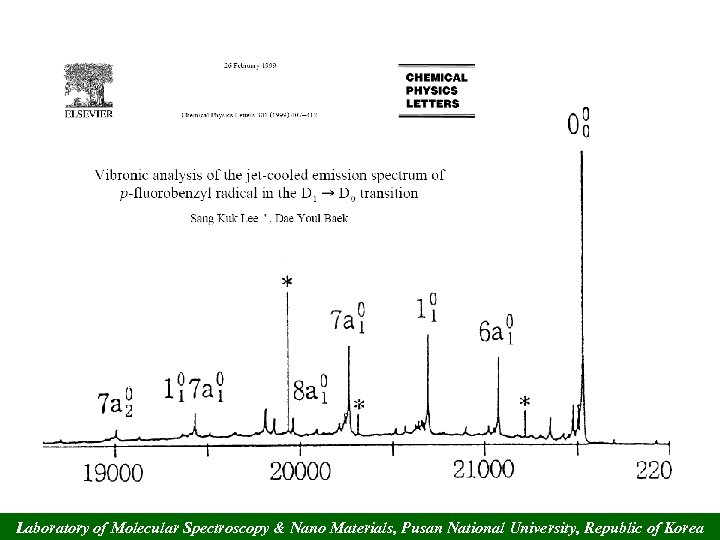 Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
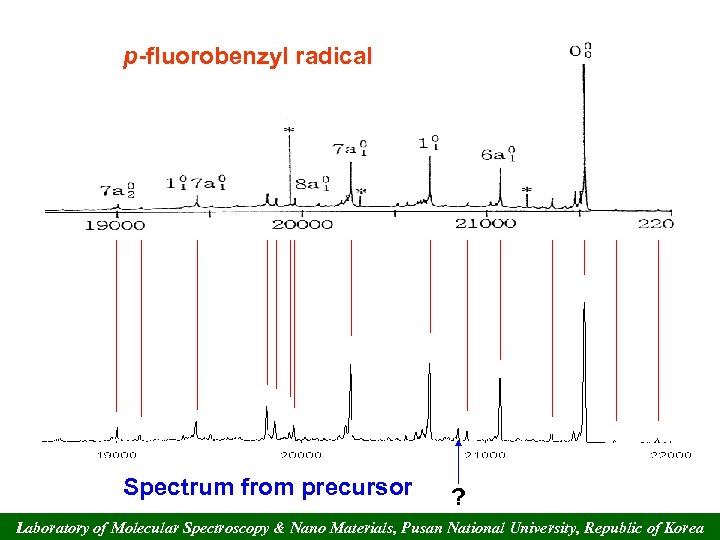 p-fluorobenzyl radical Spectrum from precursor ? Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
p-fluorobenzyl radical Spectrum from precursor ? Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
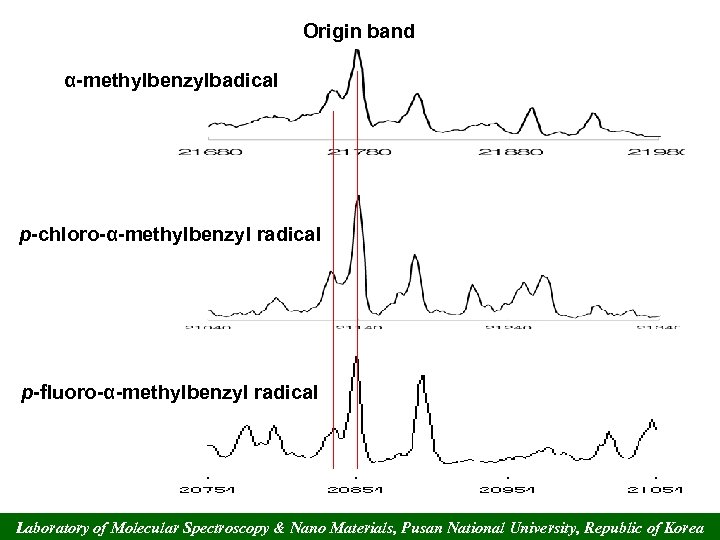 Origin band α-methylbenzylbadical p-chloro-α-methylbenzyl radical p-fluoro-α-methylbenzyl radical Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Origin band α-methylbenzylbadical p-chloro-α-methylbenzyl radical p-fluoro-α-methylbenzyl radical Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
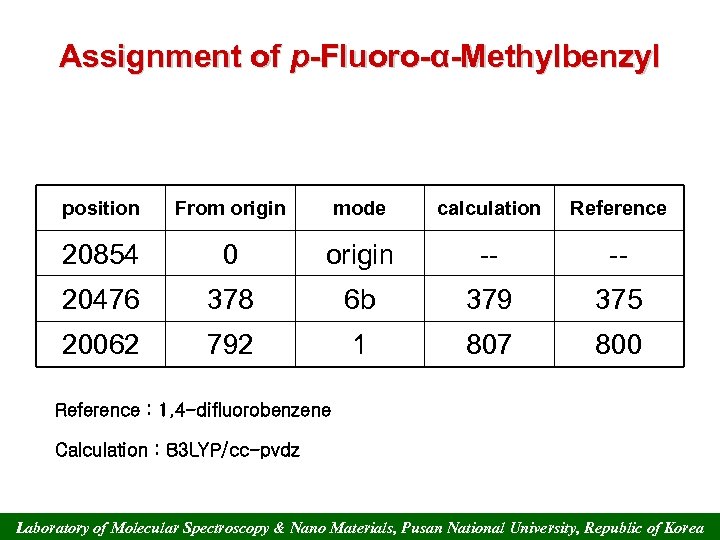 Assignment of p-Fluoro-α-Methylbenzyl position From origin mode calculation Reference 20854 0 origin -- -- 20476 378 6 b 379 375 20062 792 1 807 800 Reference : 1, 4 -difluorobenzene Calculation : B 3 LYP/cc-pvdz Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Assignment of p-Fluoro-α-Methylbenzyl position From origin mode calculation Reference 20854 0 origin -- -- 20476 378 6 b 379 375 20062 792 1 807 800 Reference : 1, 4 -difluorobenzene Calculation : B 3 LYP/cc-pvdz Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
 Summary 1. Developed CESE system for spectroscopy of molecular radicals 2. Observed many substituted benzyl radicals in the gas phase. 3. Detected new spectroscopic evidence of αmethylbenzyl radicals for the first time. Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Summary 1. Developed CESE system for spectroscopy of molecular radicals 2. Observed many substituted benzyl radicals in the gas phase. 3. Detected new spectroscopic evidence of αmethylbenzyl radicals for the first time. Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
 Acknowledgments Funding for Basic Sciences 1. Basic Research Program, Korea Research Foundation 2. Special Basic Research Program, Korea Science and Engineering Foundation Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Acknowledgments Funding for Basic Sciences 1. Basic Research Program, Korea Research Foundation 2. Special Basic Research Program, Korea Science and Engineering Foundation Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
 Thank you for your attention Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea
Thank you for your attention Laboratory of Molecular Spectroscopy & Nano Materials, Pusan National University, Republic of Korea


