0ab9970f07812c7e98dc98476e4eaf68.ppt
- Количество слайдов: 25
 THE 6 TH NATIONAL SCIENTIFIC CONFERENCE ON HIV/AIDS EVALUATION THE EFFECTIVENESS OF TESTING VIRAL LOAD COBAS® Ampli. Prep / COBAS® Taq. Man® HIV-1 Test v 2. 0 Phar. Nhat Mang/ Roche Vietnam
THE 6 TH NATIONAL SCIENTIFIC CONFERENCE ON HIV/AIDS EVALUATION THE EFFECTIVENESS OF TESTING VIRAL LOAD COBAS® Ampli. Prep / COBAS® Taq. Man® HIV-1 Test v 2. 0 Phar. Nhat Mang/ Roche Vietnam
 Outline 1. Background 2. Objectives 3. Methods 4. Results & Discussions 5. Conclusions 6. Recommendations The 6 th National Scientific Conference on HIV/AIDS
Outline 1. Background 2. Objectives 3. Methods 4. Results & Discussions 5. Conclusions 6. Recommendations The 6 th National Scientific Conference on HIV/AIDS
 Outline 1. Background 2. Objectives 3. Methods 4. Results & Discussions 5. Conclusions 6. Recommendations The 6 th National Scientific Conference on HIV/AIDS
Outline 1. Background 2. Objectives 3. Methods 4. Results & Discussions 5. Conclusions 6. Recommendations The 6 th National Scientific Conference on HIV/AIDS
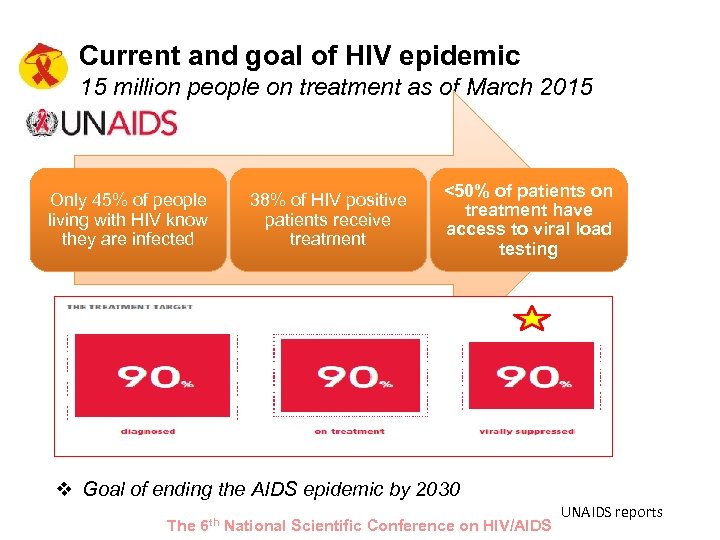 Current and goal of HIV epidemic 15 million people on treatment as of March 2015 Only 45% of people living with HIV know they are infected 38% of HIV positive patients receive treatment <50% of patients on treatment have access to viral load testing v Goal of ending the AIDS epidemic by 2030 The 6 th National Scientific Conference on HIV/AIDS UNAIDS reports
Current and goal of HIV epidemic 15 million people on treatment as of March 2015 Only 45% of people living with HIV know they are infected 38% of HIV positive patients receive treatment <50% of patients on treatment have access to viral load testing v Goal of ending the AIDS epidemic by 2030 The 6 th National Scientific Conference on HIV/AIDS UNAIDS reports
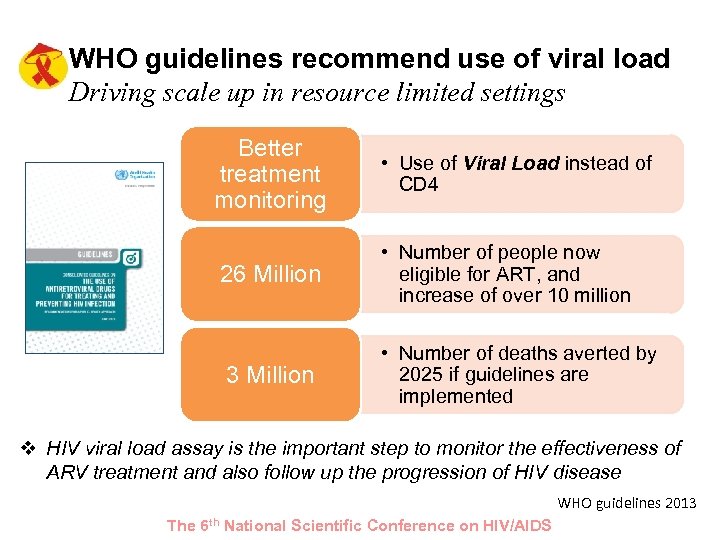 WHO guidelines recommend use of viral load Driving scale up in resource limited settings Better treatment monitoring • Use of Viral Load instead of CD 4 26 Million • Number of people now eligible for ART, and increase of over 10 million 3 Million • Number of deaths averted by 2025 if guidelines are implemented v HIV viral load assay is the important step to monitor the effectiveness of ARV treatment and also follow up the progression of HIV disease WHO guidelines 2013 The 6 th National Scientific Conference on HIV/AIDS
WHO guidelines recommend use of viral load Driving scale up in resource limited settings Better treatment monitoring • Use of Viral Load instead of CD 4 26 Million • Number of people now eligible for ART, and increase of over 10 million 3 Million • Number of deaths averted by 2025 if guidelines are implemented v HIV viral load assay is the important step to monitor the effectiveness of ARV treatment and also follow up the progression of HIV disease WHO guidelines 2013 The 6 th National Scientific Conference on HIV/AIDS
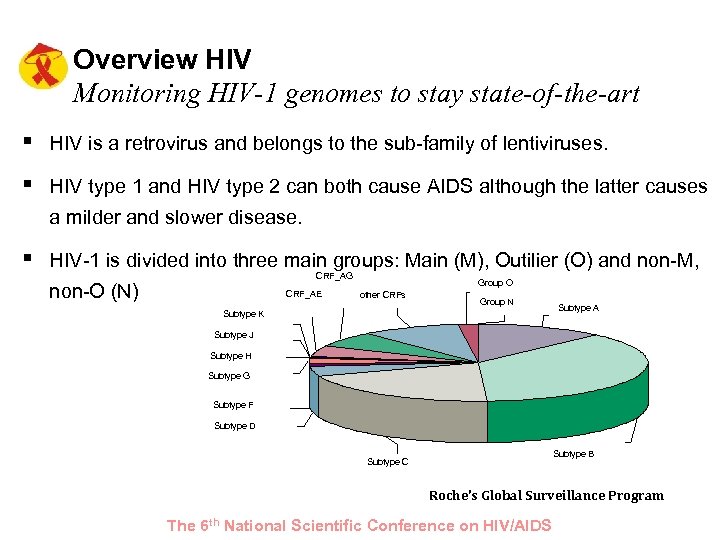 Overview HIV Monitoring HIV-1 genomes to stay state-of-the-art § HIV is a retrovirus and belongs to the sub-family of lentiviruses. § HIV type 1 and HIV type 2 can both cause AIDS although the latter causes a milder and slower disease. § HIV-1 is divided into three main groups: Main (M), Outilier (O) and non-M, CRF_AG non-O (N) CRF_AE Group O other CRFs Group N Subtype K Subtype A Subtype J Subtype H Subtype G Subtype F Subtype D Subtype B Subtype C Roche’s Global Surveillance Program The 6 th National Scientific Conference on HIV/AIDS
Overview HIV Monitoring HIV-1 genomes to stay state-of-the-art § HIV is a retrovirus and belongs to the sub-family of lentiviruses. § HIV type 1 and HIV type 2 can both cause AIDS although the latter causes a milder and slower disease. § HIV-1 is divided into three main groups: Main (M), Outilier (O) and non-M, CRF_AG non-O (N) CRF_AE Group O other CRFs Group N Subtype K Subtype A Subtype J Subtype H Subtype G Subtype F Subtype D Subtype B Subtype C Roche’s Global Surveillance Program The 6 th National Scientific Conference on HIV/AIDS
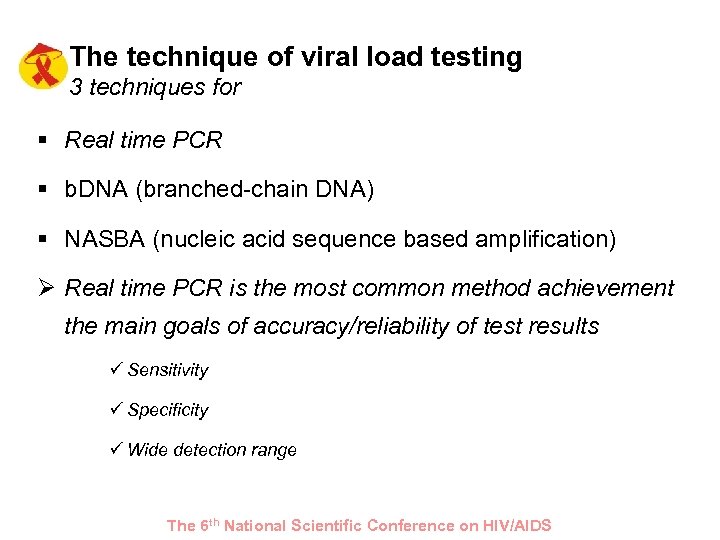 The technique of viral load testing 3 techniques for § Real time PCR § b. DNA (branched-chain DNA) § NASBA (nucleic acid sequence based amplification) Ø Real time PCR is the most common method achievement the main goals of accuracy/reliability of test results ü Sensitivity ü Specificity ü Wide detection range The 6 th National Scientific Conference on HIV/AIDS
The technique of viral load testing 3 techniques for § Real time PCR § b. DNA (branched-chain DNA) § NASBA (nucleic acid sequence based amplification) Ø Real time PCR is the most common method achievement the main goals of accuracy/reliability of test results ü Sensitivity ü Specificity ü Wide detection range The 6 th National Scientific Conference on HIV/AIDS
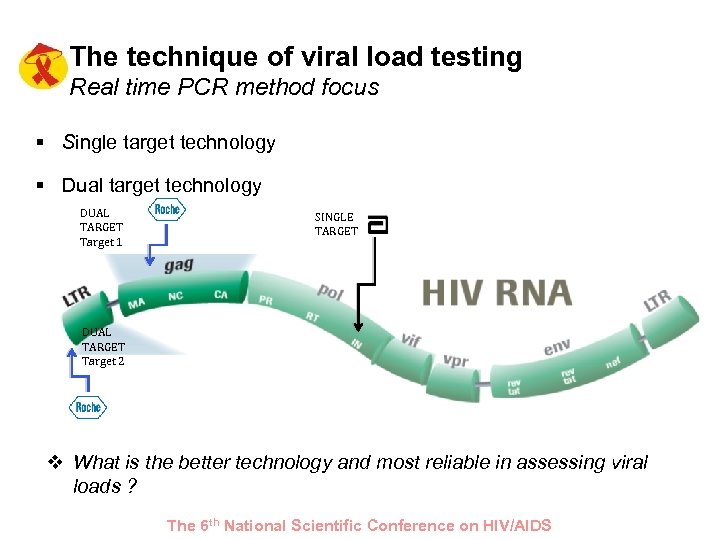 The technique of viral load testing Real time PCR method focus § Single target technology § Dual target technology DUAL TARGET Target 1 SINGLE TARGET DUAL TARGET Target 2 v What is the better technology and most reliable in assessing viral loads ? The 6 th National Scientific Conference on HIV/AIDS
The technique of viral load testing Real time PCR method focus § Single target technology § Dual target technology DUAL TARGET Target 1 SINGLE TARGET DUAL TARGET Target 2 v What is the better technology and most reliable in assessing viral loads ? The 6 th National Scientific Conference on HIV/AIDS
 Outline 1. Background 2. Objectives 3. Methods 4. Results & Discussions 5. Conclusions 6. Recommendations The 6 th National Scientific Conference on HIV/AIDS
Outline 1. Background 2. Objectives 3. Methods 4. Results & Discussions 5. Conclusions 6. Recommendations The 6 th National Scientific Conference on HIV/AIDS
 Comparative RNA Quantification of HIV-1 CAP/CTM HIV-1 v 2. 0 and Real-Time HIV-1 PCR Assays § Objective 1: To compare the effectiveness of detection of Roche CAP/CTM HIV-1 v 2. 0 and Real-Time HIV-1 PCR Assays for the detection and quantification of HIV-1 RNA in plasma sample containing different HIV-1 groups and subtypes § Objective 2: Reinforce the DUAL target technology to enhances reliability and minimizes the effects against natural mutations than SINGLE Target The 6 th National Scientific Conference on HIV/AIDS
Comparative RNA Quantification of HIV-1 CAP/CTM HIV-1 v 2. 0 and Real-Time HIV-1 PCR Assays § Objective 1: To compare the effectiveness of detection of Roche CAP/CTM HIV-1 v 2. 0 and Real-Time HIV-1 PCR Assays for the detection and quantification of HIV-1 RNA in plasma sample containing different HIV-1 groups and subtypes § Objective 2: Reinforce the DUAL target technology to enhances reliability and minimizes the effects against natural mutations than SINGLE Target The 6 th National Scientific Conference on HIV/AIDS
 Outline 1. Background 2. Objectives 3. Methods 4. Results & Discussions 5. Conclusions 6. Recommendations The 6 th National Scientific Conference on HIV/AIDS
Outline 1. Background 2. Objectives 3. Methods 4. Results & Discussions 5. Conclusions 6. Recommendations The 6 th National Scientific Conference on HIV/AIDS
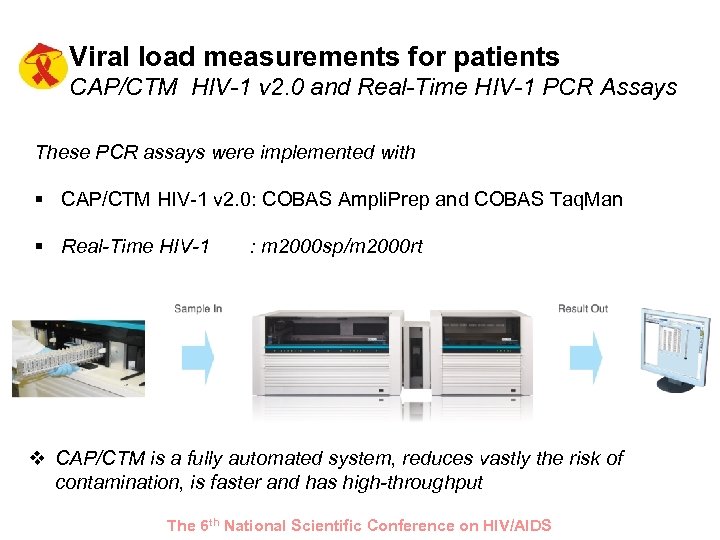 Viral load measurements for patients CAP/CTM HIV-1 v 2. 0 and Real-Time HIV-1 PCR Assays These PCR assays were implemented with § CAP/CTM HIV-1 v 2. 0: COBAS Ampli. Prep and COBAS Taq. Man § Real-Time HIV-1 : m 2000 sp/m 2000 rt v CAP/CTM is a fully automated system, reduces vastly the risk of contamination, is faster and has high-throughput The 6 th National Scientific Conference on HIV/AIDS
Viral load measurements for patients CAP/CTM HIV-1 v 2. 0 and Real-Time HIV-1 PCR Assays These PCR assays were implemented with § CAP/CTM HIV-1 v 2. 0: COBAS Ampli. Prep and COBAS Taq. Man § Real-Time HIV-1 : m 2000 sp/m 2000 rt v CAP/CTM is a fully automated system, reduces vastly the risk of contamination, is faster and has high-throughput The 6 th National Scientific Conference on HIV/AIDS
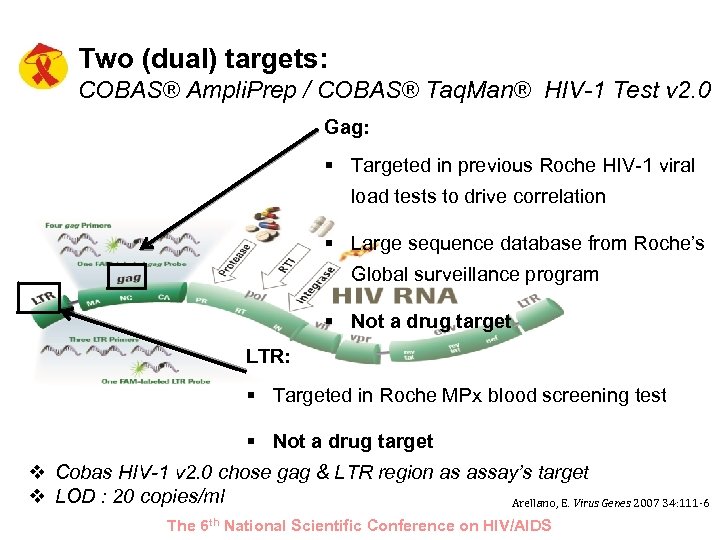 Two (dual) targets: COBAS® Ampli. Prep / COBAS® Taq. Man® HIV-1 Test v 2. 0 Gag: § Targeted in previous Roche HIV-1 viral load tests to drive correlation § Large sequence database from Roche’s Global surveillance program § Not a drug target LTR: § Targeted in Roche MPx blood screening test § Not a drug target v Cobas HIV-1 v 2. 0 chose gag & LTR region as assay’s target v LOD : 20 copies/ml Arellano, E. Virus Genes 2007 34: 111 -6 The 6 th National Scientific Conference on HIV/AIDS
Two (dual) targets: COBAS® Ampli. Prep / COBAS® Taq. Man® HIV-1 Test v 2. 0 Gag: § Targeted in previous Roche HIV-1 viral load tests to drive correlation § Large sequence database from Roche’s Global surveillance program § Not a drug target LTR: § Targeted in Roche MPx blood screening test § Not a drug target v Cobas HIV-1 v 2. 0 chose gag & LTR region as assay’s target v LOD : 20 copies/ml Arellano, E. Virus Genes 2007 34: 111 -6 The 6 th National Scientific Conference on HIV/AIDS
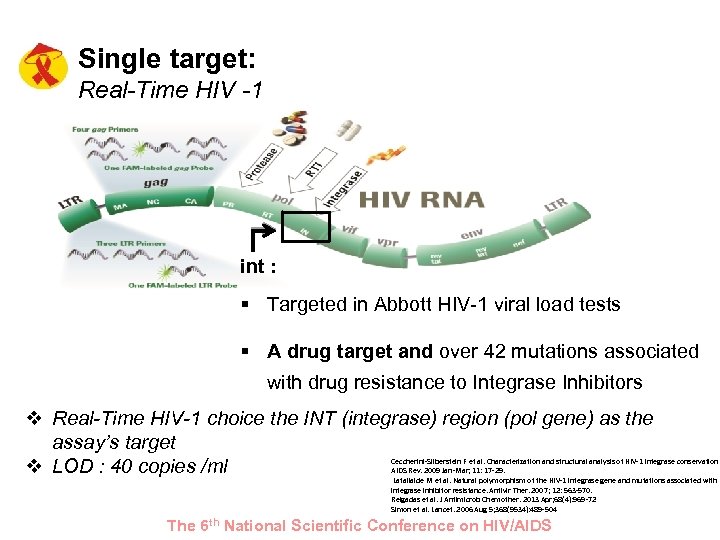 Single target: Real-Time HIV -1 int : § Targeted in Abbott HIV-1 viral load tests § A drug target and over 42 mutations associated with drug resistance to Integrase Inhibitors v Real-Time HIV-1 choice the INT (integrase) region (pol gene) as the assay’s target v LOD : 40 copies /ml Ceccherini-Silberstein F et al. Characterization and structural analysis of HIV-1 integrase conservation. AIDS Rev. 2009 Jan-Mar; 11: 17 -29. Latallaide M et al. Natural polymorphism of the HIV-1 integrase gene and mutations associated with integrase inhibitor resistance. Antivir Ther. 2007; 12: 563 -570. Reigadas et al. J Antimicrob Chemother. 2013 Apr; 68(4): 969 -72 Simon et al. Lancet. 2006 Aug 5; 368(9534): 489 -504 The 6 th National Scientific Conference on HIV/AIDS
Single target: Real-Time HIV -1 int : § Targeted in Abbott HIV-1 viral load tests § A drug target and over 42 mutations associated with drug resistance to Integrase Inhibitors v Real-Time HIV-1 choice the INT (integrase) region (pol gene) as the assay’s target v LOD : 40 copies /ml Ceccherini-Silberstein F et al. Characterization and structural analysis of HIV-1 integrase conservation. AIDS Rev. 2009 Jan-Mar; 11: 17 -29. Latallaide M et al. Natural polymorphism of the HIV-1 integrase gene and mutations associated with integrase inhibitor resistance. Antivir Ther. 2007; 12: 563 -570. Reigadas et al. J Antimicrob Chemother. 2013 Apr; 68(4): 969 -72 Simon et al. Lancet. 2006 Aug 5; 368(9534): 489 -504 The 6 th National Scientific Conference on HIV/AIDS
 Outline 1. Background 2. Objectives 3. Methods 4. Results & Discussions 5. Conclusions 6. Recommendations The 6 th National Scientific Conference on HIV/AIDS
Outline 1. Background 2. Objectives 3. Methods 4. Results & Discussions 5. Conclusions 6. Recommendations The 6 th National Scientific Conference on HIV/AIDS
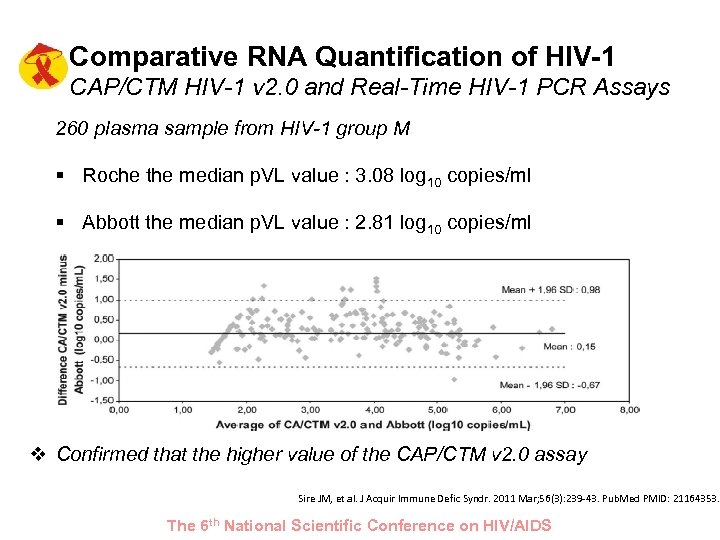 Comparative RNA Quantification of HIV-1 CAP/CTM HIV-1 v 2. 0 and Real-Time HIV-1 PCR Assays 260 plasma sample from HIV-1 group M § Roche the median p. VL value : 3. 08 log 10 copies/ml § Abbott the median p. VL value : 2. 81 log 10 copies/ml v Confirmed that the higher value of the CAP/CTM v 2. 0 assay Sire JM, et al. J Acquir Immune Defic Syndr. 2011 Mar; 56(3): 239 -43. Pub. Med PMID: 21164353. The 6 th National Scientific Conference on HIV/AIDS
Comparative RNA Quantification of HIV-1 CAP/CTM HIV-1 v 2. 0 and Real-Time HIV-1 PCR Assays 260 plasma sample from HIV-1 group M § Roche the median p. VL value : 3. 08 log 10 copies/ml § Abbott the median p. VL value : 2. 81 log 10 copies/ml v Confirmed that the higher value of the CAP/CTM v 2. 0 assay Sire JM, et al. J Acquir Immune Defic Syndr. 2011 Mar; 56(3): 239 -43. Pub. Med PMID: 21164353. The 6 th National Scientific Conference on HIV/AIDS
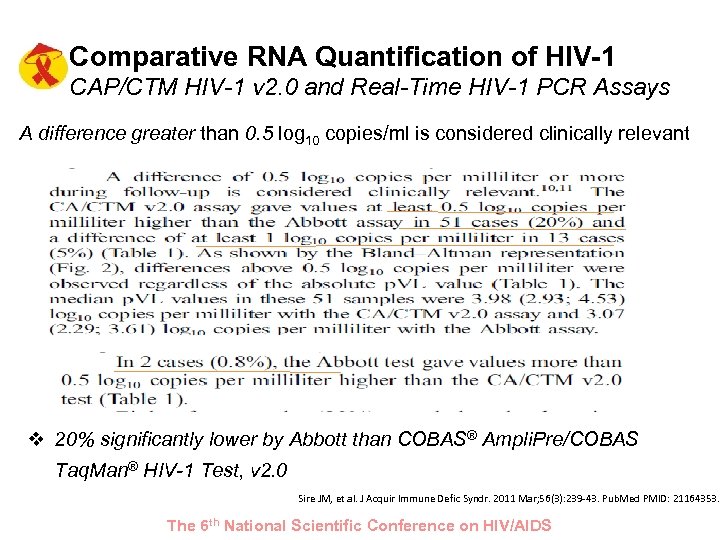 Comparative RNA Quantification of HIV-1 CAP/CTM HIV-1 v 2. 0 and Real-Time HIV-1 PCR Assays A difference greater than 0. 5 log 10 copies/ml is considered clinically relevant v 20% significantly lower by Abbott than COBAS® Ampli. Pre/COBAS Taq. Man® HIV-1 Test, v 2. 0 Sire JM, et al. J Acquir Immune Defic Syndr. 2011 Mar; 56(3): 239 -43. Pub. Med PMID: 21164353. The 6 th National Scientific Conference on HIV/AIDS
Comparative RNA Quantification of HIV-1 CAP/CTM HIV-1 v 2. 0 and Real-Time HIV-1 PCR Assays A difference greater than 0. 5 log 10 copies/ml is considered clinically relevant v 20% significantly lower by Abbott than COBAS® Ampli. Pre/COBAS Taq. Man® HIV-1 Test, v 2. 0 Sire JM, et al. J Acquir Immune Defic Syndr. 2011 Mar; 56(3): 239 -43. Pub. Med PMID: 21164353. The 6 th National Scientific Conference on HIV/AIDS
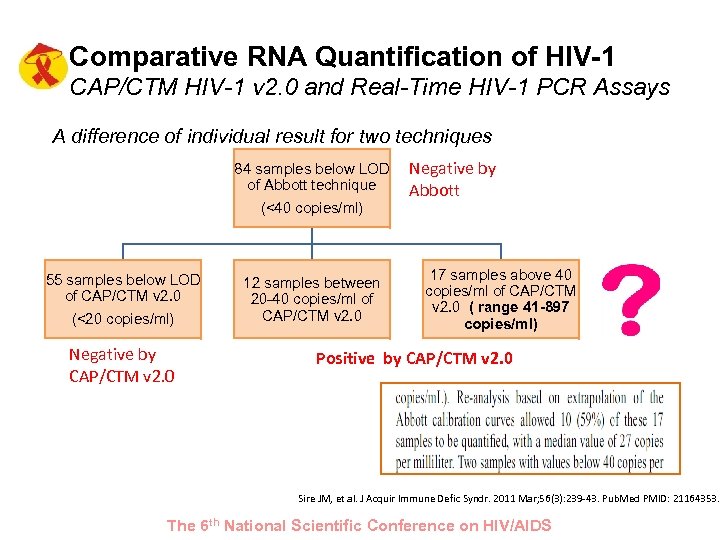 Comparative RNA Quantification of HIV-1 CAP/CTM HIV-1 v 2. 0 and Real-Time HIV-1 PCR Assays A difference of individual result for two techniques 84 samples below LOD of Abbott technique (<40 copies/ml) 55 samples below LOD of CAP/CTM v 2. 0 (<20 copies/ml) Negative by CAP/CTM v 2. 0 12 samples between 20 -40 copies/ml of CAP/CTM v 2. 0 Negative by Abbott 17 samples above 40 copies/ml of CAP/CTM v 2. 0 ( range 41 -897 copies/ml) Positive by CAP/CTM v 2. 0 Sire JM, et al. J Acquir Immune Defic Syndr. 2011 Mar; 56(3): 239 -43. Pub. Med PMID: 21164353. The 6 th National Scientific Conference on HIV/AIDS
Comparative RNA Quantification of HIV-1 CAP/CTM HIV-1 v 2. 0 and Real-Time HIV-1 PCR Assays A difference of individual result for two techniques 84 samples below LOD of Abbott technique (<40 copies/ml) 55 samples below LOD of CAP/CTM v 2. 0 (<20 copies/ml) Negative by CAP/CTM v 2. 0 12 samples between 20 -40 copies/ml of CAP/CTM v 2. 0 Negative by Abbott 17 samples above 40 copies/ml of CAP/CTM v 2. 0 ( range 41 -897 copies/ml) Positive by CAP/CTM v 2. 0 Sire JM, et al. J Acquir Immune Defic Syndr. 2011 Mar; 56(3): 239 -43. Pub. Med PMID: 21164353. The 6 th National Scientific Conference on HIV/AIDS
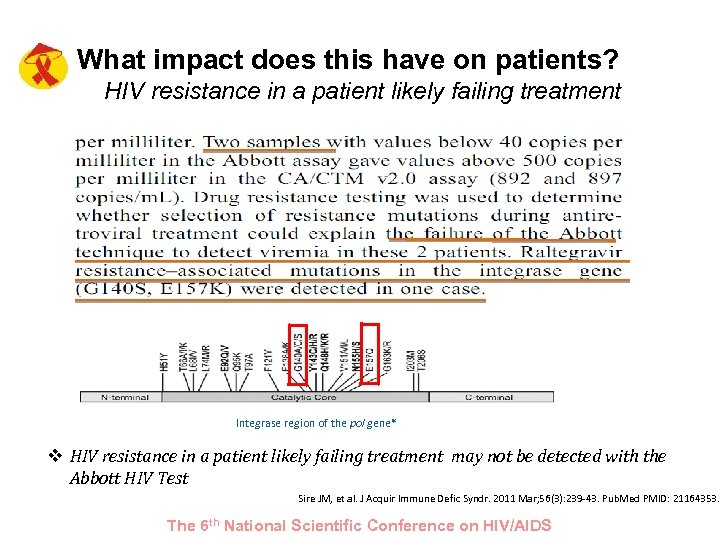 What impact does this have on patients? HIV resistance in a patient likely failing treatment Integrase region of the pol gene* v HIV resistance in a patient likely failing treatment may not be detected with the Abbott HIV Test Sire JM, et al. J Acquir Immune Defic Syndr. 2011 Mar; 56(3): 239 -43. Pub. Med PMID: 21164353. The 6 th National Scientific Conference on HIV/AIDS
What impact does this have on patients? HIV resistance in a patient likely failing treatment Integrase region of the pol gene* v HIV resistance in a patient likely failing treatment may not be detected with the Abbott HIV Test Sire JM, et al. J Acquir Immune Defic Syndr. 2011 Mar; 56(3): 239 -43. Pub. Med PMID: 21164353. The 6 th National Scientific Conference on HIV/AIDS
 More Literature demonstrates…underquantitation! Janse van Rensburg Paba Pas Church The Abbott assay appeared to underestimate viral load 40 -50% of the time compared to 2 nd WHO Standard 30 samples not quantified by the Abbott Real. Time HIV-1 assay were quantitated by COBAS® Ampli. Prep/COBAS Taq. Man® HIV -1 Test, v 2. 0 Three samples (1%) underquantified by Abbott assay by more than 1 log 10 relative to COBAS® Ampli. Prep/COBAS Taq. Man® HIV-1 Test, v 2. 0 Abbott underquants 10% of the samples (n=50, >1 log 10 (cp/m. L) = 5) The 6 th National Scientific Conference on HIV/AIDS
More Literature demonstrates…underquantitation! Janse van Rensburg Paba Pas Church The Abbott assay appeared to underestimate viral load 40 -50% of the time compared to 2 nd WHO Standard 30 samples not quantified by the Abbott Real. Time HIV-1 assay were quantitated by COBAS® Ampli. Prep/COBAS Taq. Man® HIV -1 Test, v 2. 0 Three samples (1%) underquantified by Abbott assay by more than 1 log 10 relative to COBAS® Ampli. Prep/COBAS Taq. Man® HIV-1 Test, v 2. 0 Abbott underquants 10% of the samples (n=50, >1 log 10 (cp/m. L) = 5) The 6 th National Scientific Conference on HIV/AIDS
 Outline 1. Background 2. Objectives 3. Methods 4. Results & Discussions 5. Conclusions 6. Recommendations The 6 th National Scientific Conference on HIV/AIDS
Outline 1. Background 2. Objectives 3. Methods 4. Results & Discussions 5. Conclusions 6. Recommendations The 6 th National Scientific Conference on HIV/AIDS
 Comparative RNA Quantification of HIV-1 Stay One Step Ahead with the HIV-1 dual target assay v Objective 1: § Higher values obtained with CAP/CTM test v 2. 0 test relative to the Abbott test § Higher sensitivity of CAP/CTM test v 2. 0 assay (20 copies) compared with the Abbott test § The higher meaningful for clinicians and patient because the CAP/CTM test v 2. 0 test may show low-level viral load in patients whose viral load is undetectable with other methods v Objective 2: § Abbott – Single target might be impaired by mutations in the integrase target region. Beside that Roche CAP/CTM dual target builds redundancy into the assay, potentially avoids underquantification The 6 th National Scientific Conference on HIV/AIDS
Comparative RNA Quantification of HIV-1 Stay One Step Ahead with the HIV-1 dual target assay v Objective 1: § Higher values obtained with CAP/CTM test v 2. 0 test relative to the Abbott test § Higher sensitivity of CAP/CTM test v 2. 0 assay (20 copies) compared with the Abbott test § The higher meaningful for clinicians and patient because the CAP/CTM test v 2. 0 test may show low-level viral load in patients whose viral load is undetectable with other methods v Objective 2: § Abbott – Single target might be impaired by mutations in the integrase target region. Beside that Roche CAP/CTM dual target builds redundancy into the assay, potentially avoids underquantification The 6 th National Scientific Conference on HIV/AIDS
 Outline 1. Background 2. Objectives 3. Methods 4. Results & Discussions 5. Conclusions 6. Recommendations The 6 th National Scientific Conference on HIV/AIDS
Outline 1. Background 2. Objectives 3. Methods 4. Results & Discussions 5. Conclusions 6. Recommendations The 6 th National Scientific Conference on HIV/AIDS
 Value of result …… COBAS® Ampli. Prep / COBAS® Taq. Man® HIV-1 Test v 2. 0 § Provides diagnostic accuracy of test results even if mutations occur in one of the two regions § Dual target minimizes the effects against MUTATIONS and also represents an important step forward § Is fully traceable to WHO international standards and CE-IVD certificated The 6 th National Scientific Conference on HIV/AIDS
Value of result …… COBAS® Ampli. Prep / COBAS® Taq. Man® HIV-1 Test v 2. 0 § Provides diagnostic accuracy of test results even if mutations occur in one of the two regions § Dual target minimizes the effects against MUTATIONS and also represents an important step forward § Is fully traceable to WHO international standards and CE-IVD certificated The 6 th National Scientific Conference on HIV/AIDS
 Thank you Doing now what patients need next The 6 th National Scientific Conference on HIV/AIDS
Thank you Doing now what patients need next The 6 th National Scientific Conference on HIV/AIDS


