6e9f1d8c7bb82a8a772e7500ce801b2f.ppt
- Количество слайдов: 32

Texas’ Newborn Screening Program House Committee on Public Health May 17, 2010

The core mission of Texas’ Newborn Screening Program is to save children’s lives through the early detection of life-threatening disorders. 2

Newborn Screening in Texas • DSHS performs all the Newborn Screening (NBS) tests for Texas • NBS program screens for congenital and heritable disorders • NBS consists of specimen collection, testing, reporting results, case management, diagnosis and treatment • NBS in Texas began in 1963 as a pilot program for the screening of phenylketonuria (PKU) 3

Newborn Screening in Texas currently screens for all 29 core panel disorders recommended by the American College of Medical Genetics (ACMG) • The program expanded from 7 to 27 disorders with passage of HB 790 in 2005 Ø Required expansion of NBS program using the ACMG recommended panel as funds allowed Ø Expanded screening began in December 2006 • Babies born in hospitals or birthing centers receive a hearing screen (or are referred for screening) Ø ACMG Core Panel includes hearing screen • Screening for Cystic Fibrosis was funded by the 81 st Legislature Ø Screening began December 1, 2009 4

Newborn Screening in Texas • For each of the core diseases, an accurate test is currently available • Early detection and treatment can lead to improved growth and development, increased life expectancy, and reduced medication, hospitalizations and mortality in children with these disorders 5

Newborn Screening in Texas • Each baby born in Texas is required to be screened twice Ø 24 – 48 hours of age or before leaving hospital, in order to detect some disorders at the earliest possible opportunity Ø The second screen at 1 – 2 weeks of age is necessary because some cases may only be detected on the second screen • There approximately 400, 000 births in Texas each year • Two screens for each child means ~ 800, 000 specimens collected each year 6

Newborn Screening in Texas • In FY 09, the NBS program followed-up on approximately 27, 000 abnormal screens a year • ~700 disorders diagnosed each year • Because every baby is tested soon after birth, any child who may have a screened disorder is identified early and can get early care 7
Blood Spot Uses • Newborn Screening Testing and Quality Assurance/Quality Control • Other beneficial uses – QA Required for Manufacturers of Public Health-Related Products – Research 8
QA/QC for Texas’ Newborn Screening Program In addition to the actual newborn screens, DSHS uses blood spots for QA/QC to: – Ensure that new tests, chemicals, and instruments meet the standards set by the manufacturer and are operating correctly and producing accurate test results – Monitor for continuous accuracy and reliability of test systems – Conduct comparison studies to ensure all instruments performing the same test produce the same result – Refine testing procedures to ensure the most accurate and reliable results possible 9

QA/QC for Texas’ Newborn Screening Program • Newborn Screening test kits, chemicals, and instruments – DSHS has a five-year contract with Perkin. Elmer for newborn screening equipment maintenance, reagents and other consumables. • As part of the contract, DSHS provides de-identified blood spots to Perkin. Elmer for quality assurance and control purposes. • DSHS provides 3, 800 de-identified spots per month, a contract value of $2 per spot, in exchange for consumables needed to maintain the equipment. • This allows for improvements to testing kits and equipment, which directly benefits the newborn screening program. 10

QA/QC for Texas’ Newborn Screening Program • Inter-laboratory comparisons ensure the DSHS Laboratory is producing results similar to other state newborn screening laboratories – DSHS shares de-identified specimens with other state public health laboratories to make sure new methods or tests are working correctly – Likewise, DSHS receives de-identified blood spots from other state newborn screening laboratories – Required for federal CLIA lab certification for the DSHS lab and other state labs – A similar process is used for many other laboratory tests 11

QA Related to Federal FDA Licensing Requirements for Manufacturing Certain Public Health-Related Products • Reagents and test kits for newborn screening and other diseases – Benefits public health by bringing improved products to market and ensures accuracy and validity of kits being used by DSHS and other state health departments – In 2006 and 2007, bio. Merieux provided HIV rapid testing kits to DSHS in exchange for de-identified bloodspots used to perform QA for those test kits. • DSHS used the HIV test kits in its HIV screening program. – In October 2008, DSHS provided 600 de-identified bloodspots to Avioq for HIV test kit analytical validation for submission to the FDA. • DSHS charged Avioq a $4 per spot handling fee to cover the costs of providing the specimens. The total amount received was $2, 400. 12

Using Blood Spots for Research • Prior to 2009, a small percentage of newborn screening blood spots were been used in research projects • A procedure was in place to review the significance, scientific merit and ethical integrity of all requests for blood spots for research • As appropriate, the researcher would then submit the study to the DSHS Institutional Review Board (IRB) – In the past, de-identified blood spots were exempt from IRB review 13

Using Blood Spots for Research • The main purpose of the DSHS IRB review is to ensure the safety, rights and welfare of human participants involved in the proposed research • The DSHS IRB that reviews social, behavioral, educational and health science research has 13 members— 12 affiliated with DSHS and 1 non-DSHS member • The committee meets monthly to review applications that have been submitted for research studies, including those requesting the use of NBS blood spots • If the study is approved, the researcher may then receive and use de-identified blood spots only as provided in the final IRB approval of the study • No identifying information has been released outside of the department to a researcher except when advance parental consent was obtained 14
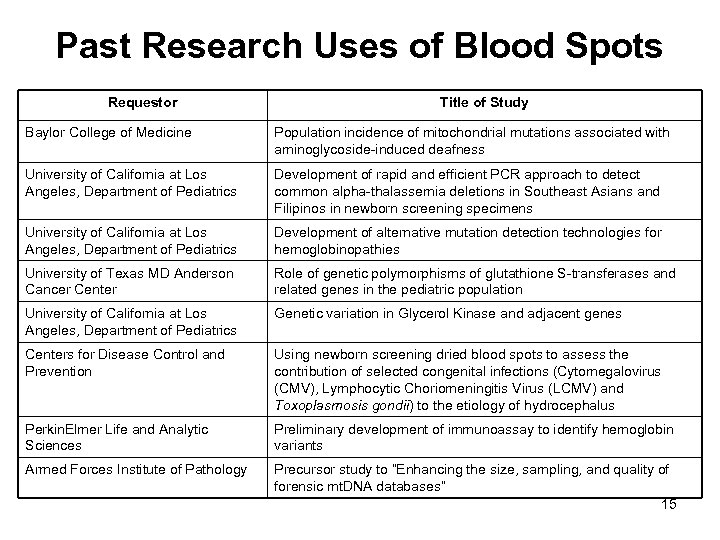
Past Research Uses of Blood Spots Requestor Title of Study Baylor College of Medicine Population incidence of mitochondrial mutations associated with aminoglycoside-induced deafness University of California at Los Angeles, Department of Pediatrics Development of rapid and efficient PCR approach to detect common alpha-thalassemia deletions in Southeast Asians and Filipinos in newborn screening specimens University of California at Los Angeles, Department of Pediatrics Development of alternative mutation detection technologies for hemoglobinopathies University of Texas MD Anderson Cancer Center Role of genetic polymorphisms of glutathione S-transferases and related genes in the pediatric population University of California at Los Angeles, Department of Pediatrics Genetic variation in Glycerol Kinase and adjacent genes Centers for Disease Control and Prevention Using newborn screening dried blood spots to assess the contribution of selected congenital infections (Cytomegalovirus (CMV), Lymphocytic Choriomeningitis Virus (LCMV) and Toxoplasmosis gondii) to the etiology of hydrocephalus Perkin. Elmer Life and Analytic Sciences Preliminary development of immunoassay to identify hemoglobin variants Armed Forces Institute of Pathology Precursor study to “Enhancing the size, sampling, and quality of forensic mt. DNA databases” 15
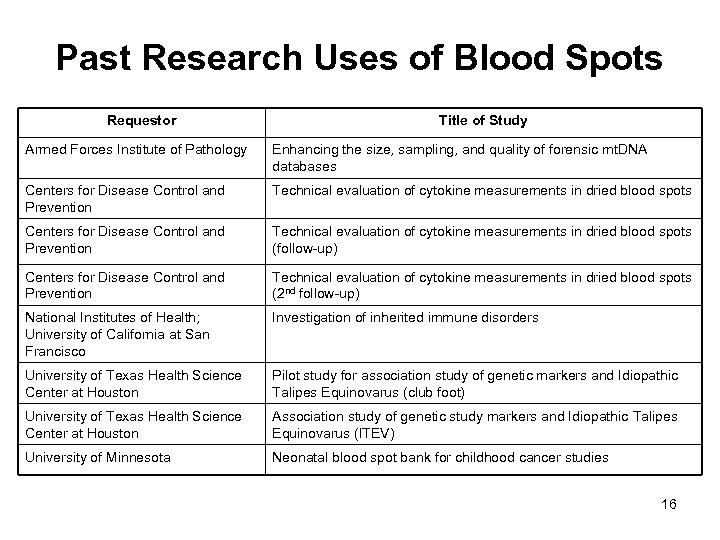
Past Research Uses of Blood Spots Requestor Title of Study Armed Forces Institute of Pathology Enhancing the size, sampling, and quality of forensic mt. DNA databases Centers for Disease Control and Prevention Technical evaluation of cytokine measurements in dried blood spots (follow-up) Centers for Disease Control and Prevention Technical evaluation of cytokine measurements in dried blood spots (2 nd follow-up) National Institutes of Health; University of California at San Francisco Investigation of inherited immune disorders University of Texas Health Science Center at Houston Pilot study for association study of genetic markers and Idiopathic Talipes Equinovarus (club foot) University of Texas Health Science Center at Houston Association study of genetic study markers and Idiopathic Talipes Equinovarus (ITEV) University of Minnesota Neonatal blood spot bank for childhood cancer studies 16
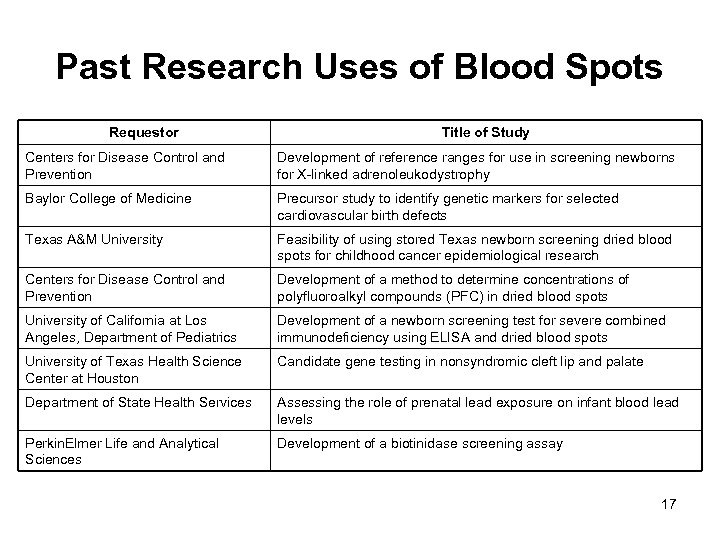
Past Research Uses of Blood Spots Requestor Title of Study Centers for Disease Control and Prevention Development of reference ranges for use in screening newborns for X-linked adrenoleukodystrophy Baylor College of Medicine Precursor study to identify genetic markers for selected cardiovascular birth defects Texas A&M University Feasibility of using stored Texas newborn screening dried blood spots for childhood cancer epidemiological research Centers for Disease Control and Prevention Development of a method to determine concentrations of polyfluoroalkyl compounds (PFC) in dried blood spots University of California at Los Angeles, Department of Pediatrics Development of a newborn screening test for severe combined immunodeficiency using ELISA and dried blood spots University of Texas Health Science Center at Houston Candidate gene testing in nonsyndromic cleft lip and palate Department of State Health Services Assessing the role of prenatal lead exposure on infant blood lead levels Perkin. Elmer Life and Analytical Sciences Development of a biotinidase screening assay 17

HB 1672 made five changes to Chapter 33, Texas Health and Safety Code: • Requires disclosure to parents, managing conservators or guardians that newborn screening specimens will be retained and may be used for other purposes • Allows parents or adults to request destruction of their child’s or their own specimen(s) 18
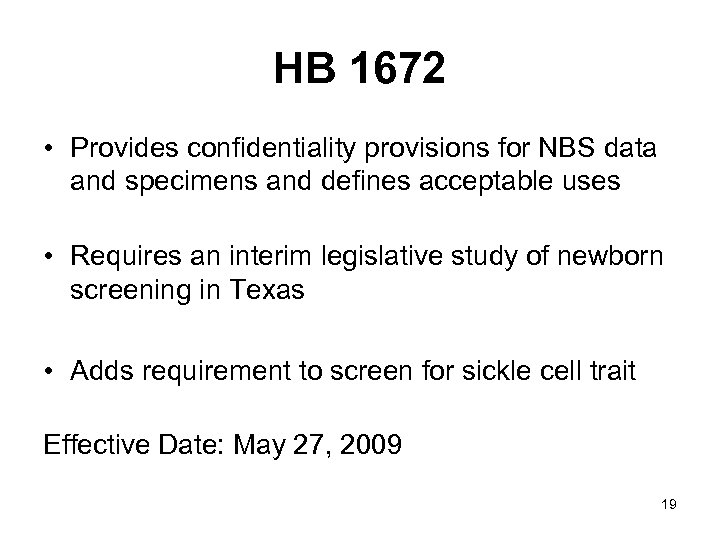
HB 1672 • Provides confidentiality provisions for NBS data and specimens and defines acceptable uses • Requires an interim legislative study of newborn screening in Texas • Adds requirement to screen for sickle cell trait Effective Date: May 27, 2009 19

Implementation of HB 1672 • DSHS is complying with all the requirements of HB 1672 • Beginning August 1, 2009 – Disclosure/destruction request form was distributed to providers • Was already available on DSHS website – Labels added to existing NBS collection kits with checkbox for physician verification that disclosure form was given to parent of newborn • Starting Spring/Early Summer 2010 – Disclosure/destruction request form will also be part of the NBS collection form 20

NBS Collection Form Disclosure/Directive to Destroy 21

Disclosure to Parents and Destruction Directive • The disclosure/destruction directive form is called “Use and Storage of Newborn Screening Blood Spot Cards” • Form is given to parents each time a newborn screening blood spot specimen is collected • The form provides information on why the blood spot cards are collected and stored • If they wish to have their child’s blood spots destroyed, the parent, legal guardian, or managing conservator completes the form and sends it to DSHS 22

“Use and Storage of NBS Blood Spot Cards”—Disclosure PROVIDER: Give this form to the parent, managing conservator, or legal guardian for review. Use and Storage of Newborn Screening Blood Spot Cards PARENT / MANAGING CONSERVATOR / LEGAL GUARDIAN – PLEASE READ CAREFULLY: What is newborn screening? The Texas Newborn Screening Program checks Texas babies for a list of serious medical conditions. These conditions can cause death or severe disability. Finding a medical problem during newborn screening can help prevent problems and may save your baby’s life. How does your baby get screened? A small amount of your baby’s blood is placed on a special blood spot card. The blood spot card is sent to the state laboratory and tested. What happens after the blood is tested? After testing, blood spot cards are safely stored by the Texas Department of State Health Services (DSHS) because they still have important public health uses. The main uses are: 1) quality assurance/quality control, such as making sure that testing equipment continues to produce accurate newborn screening test results for Texas babies; and 2) medical research [see Texas Health and Safety Code Sec. 33. 017(b)-(c) for a complete list of uses allowed by law]. Specific information that could identify your child and connect him/her to a particular blood spot card is not allowed outside of DSHS without permission from the child’s parent, managing conservator, or legal guardian unless otherwise provided by law. You can have your baby’s blood sample destroyed if you do not want it to be used after the newborn screening tests are completed. If you are okay with the sample being stored and used, as described above, then there are no further steps for you to take. If you want your baby’s blood sample to be destroyed, YOU must fill out ALL of the information on this form and send it back to DSHS at the address given below (DSHS will also accept the form from your healthcare provider, once you have completed and signed the form). If the newborn screening blood spot card is destroyed, the blood sample will not be available for any future needs you may have for the sample. 23
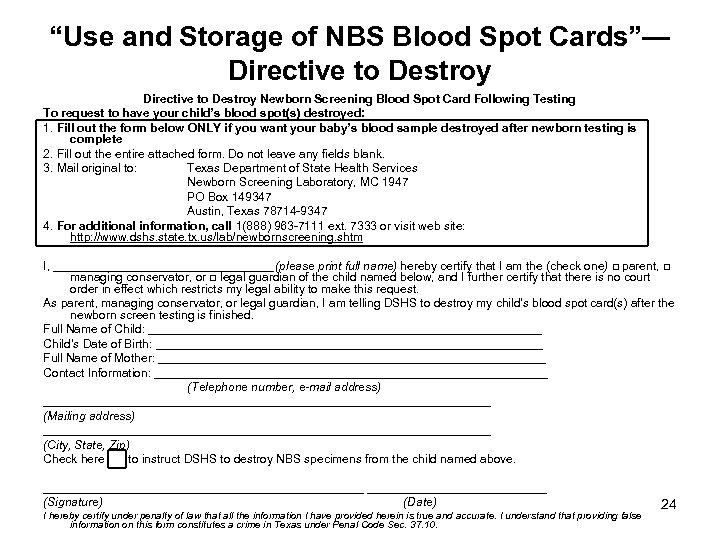
“Use and Storage of NBS Blood Spot Cards”— Directive to Destroy Newborn Screening Blood Spot Card Following Testing To request to have your child’s blood spot(s) destroyed: 1. Fill out the form below ONLY if you want your baby’s blood sample destroyed after newborn testing is complete 2. Fill out the entire attached form. Do not leave any fields blank. 3. Mail original to: Texas Department of State Health Services Newborn Screening Laboratory, MC 1947 PO Box 149347 Austin, Texas 78714 -9347 4. For additional information, call 1(888) 963 -7111 ext. 7333 or visit web site: http: //www. dshs. state. tx. us/lab/newbornscreening. shtm I, _________________ (please print full name) hereby certify that I am the (check one) □ parent, □ managing conservator, or □ legal guardian of the child named below, and I further certify that there is no court order in effect which restricts my legal ability to make this request. As parent, managing conservator, or legal guardian, I am telling DSHS to destroy my child’s blood spot card(s) after the newborn screen testing is finished. Full Name of Child: ______________________________ Child’s Date of Birth: _____________________________ Full Name of Mother: _____________________________ Contact Information: ______________________________ (Telephone number, e-mail address) __________________________________ (Mailing address) __________________________________ (City, State, Zip) Check here to instruct DSHS to destroy NBS specimens from the child named above. ________________________ (Signature) (Date) I hereby certify under penalty of law that all the information I have provided herein is true and accurate. I understand that providing false information on this form constitutes a crime in Texas under Penal Code Sec. 37. 10. 24

Disclosure to Parents and Destruction Directive • Form will continue to be available on website • Form is currently available in English, Spanish and Vietnamese • 21, 210 requests for blood spot destruction have been received as of 5/12/10 • Requestors are notified by letter that DSHS has destroyed the requested samples 25

Destruction Process • Requests are received in lab and verified to make sure information is complete • Specimens pulled and verified to ensure they match the request • Specimens delivered to Virology department and Autoclaved to destroy any DNA • Virology documents when sample was Autoclaved and returns documentation to NBS lab staff • Specimen cards treated as medical waste and incinerated • Requestor is sent notification letter that destruction of specimens completed 26

NBS Lawsuit • In March 2009, DSHS and Texas A&M University were sued in federal court regarding the State of Texas Newborn Screening Program • The lawsuit challenged the constitutionality of how the program operated under Texas law • At issue was the storage and use of the newborn screening blood spots after newborn screening was completed 27

NBS Lawsuit Settlement • In December 2009, the lawsuit against the Newborn Screening program was settled • DSHS believes settling the lawsuit was in the best interest of the State of Texas – Preserves the NBS program’s core mission to screen all newborn babies for life-threatening disorders – Avoids cost of class action lawsuit 28

NBS Lawsuit Settlement • As a result of the settlement, DSHS destroyed blood spot cards received by the department prior to May 27, 2009 – HB 1672, which expressly authorized the storage and specified uses of the samples, was signed into law on this date • Blood spot destruction was completed on March 2, 2010 – 5. 3 million samples were destroyed • DSHS continues to store samples received on May 27, 2009 or later if parents do not opt to have them destroyed, under provisions of HB 1672 29

Management of NBS Specimens and Data • Moving forward, DSHS is strengthening the agency’s process for reviewing and approving requests to use blood spots • The new process will be more defined and include more levels of review and approval based on the intended use • Some uses will require DSHS Commissioner approval • Only uses related to public health will be approved • Although certain QA/QC or research use is permissible under the statute, DSHS may not approve all such uses • DSHS IRB processes are also being reviewed and refined 30

Remaining Questions • Should the state recoup costs of providing blood spots? • Should specific consent be required for any use of blood spots in research (external, internal, etc. )? 31

Final Points • Newborn screening is a critical program for every child born in Texas • Blood spots can provide valuable information for advances in identification and treatment of human diseases and illnesses • We are revising our processes to ensure the right safeguards are in place and that there is more transparency • Our policies and approaches are evolving along with science and public attitudes toward the use of blood spots • DSHS continues to be very sensitive to the privacy concerns of parents and the confidentiality of all medical information 32
6e9f1d8c7bb82a8a772e7500ce801b2f.ppt