8880c40dba24d3ab239489b5c4ade22a.ppt
- Количество слайдов: 30
 Technology Update and Status of Current Clinical Trials Duane S. Pinto, MD MPH Jeffrey J. Popma, MD Interventional Cardiology Section Beth Israel Deaconess Medical Center Boston, MA Harvard Medical School Boston, MA
Technology Update and Status of Current Clinical Trials Duane S. Pinto, MD MPH Jeffrey J. Popma, MD Interventional Cardiology Section Beth Israel Deaconess Medical Center Boston, MA Harvard Medical School Boston, MA
 DISCLOSURES Duane S. Pinto, MD, MPH, FACC I have no conflicts to disclose regarding this presentation
DISCLOSURES Duane S. Pinto, MD, MPH, FACC I have no conflicts to disclose regarding this presentation
 Transcatheter Mitral Interventions • Leaflet repair – Edge-to-edge repair (Mitraclip) • Annular repair – Sinoplasty (Monarc, Carillon, Viacor PTMA) – Direct reshaping (Mitralign, GDS) • Other • Hybrid devices (Micardia) • Transcatheter MVR • Perivalvular leak closure • Mitral valvuloplasty
Transcatheter Mitral Interventions • Leaflet repair – Edge-to-edge repair (Mitraclip) • Annular repair – Sinoplasty (Monarc, Carillon, Viacor PTMA) – Direct reshaping (Mitralign, GDS) • Other • Hybrid devices (Micardia) • Transcatheter MVR • Perivalvular leak closure • Mitral valvuloplasty
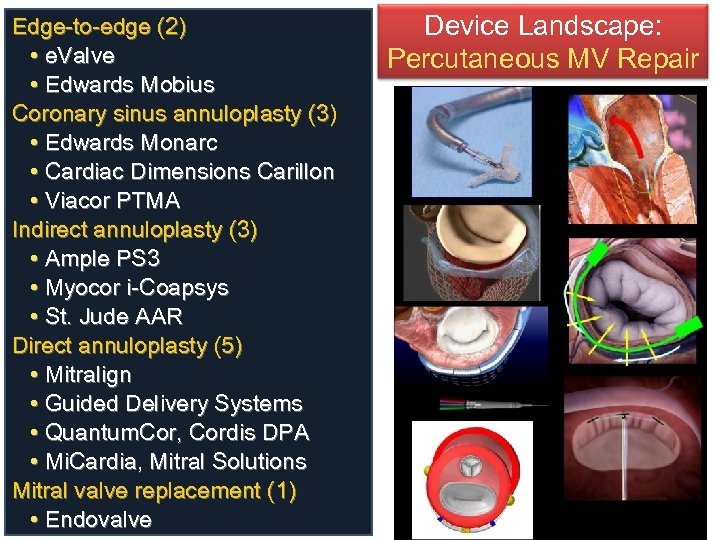 Edge-to-edge (2) • e. Valve • Edwards Mobius Coronary sinus annuloplasty (3) • Edwards Monarc • Cardiac Dimensions Carillon • Viacor PTMA Indirect annuloplasty (3) • Ample PS 3 • Myocor i-Coapsys • St. Jude AAR Direct annuloplasty (5) • Mitralign • Guided Delivery Systems • Quantum. Cor, Cordis DPA • Mi. Cardia, Mitral Solutions Mitral valve replacement (1) • Endovalve Device Landscape: Percutaneous MV Repair
Edge-to-edge (2) • e. Valve • Edwards Mobius Coronary sinus annuloplasty (3) • Edwards Monarc • Cardiac Dimensions Carillon • Viacor PTMA Indirect annuloplasty (3) • Ample PS 3 • Myocor i-Coapsys • St. Jude AAR Direct annuloplasty (5) • Mitralign • Guided Delivery Systems • Quantum. Cor, Cordis DPA • Mi. Cardia, Mitral Solutions Mitral valve replacement (1) • Endovalve Device Landscape: Percutaneous MV Repair
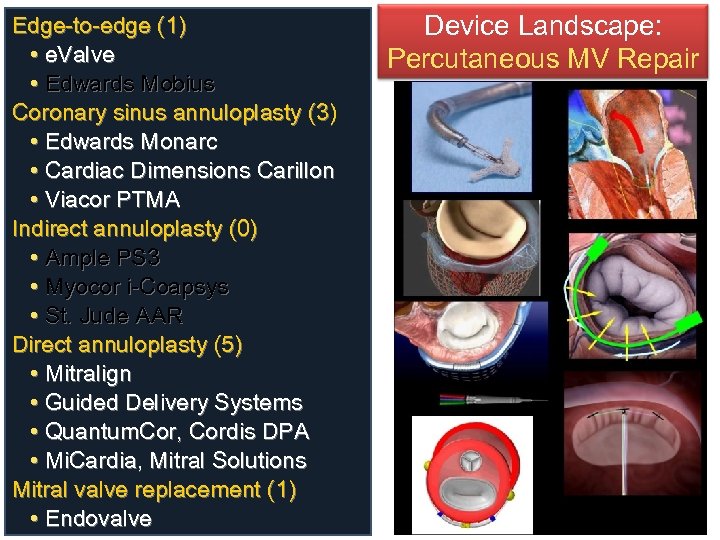 Edge-to-edge (1) • e. Valve • Edwards Mobius Coronary sinus annuloplasty (3) • Edwards Monarc • Cardiac Dimensions Carillon • Viacor PTMA Indirect annuloplasty (0) • Ample PS 3 • Myocor i-Coapsys • St. Jude AAR Direct annuloplasty (5) • Mitralign • Guided Delivery Systems • Quantum. Cor, Cordis DPA • Mi. Cardia, Mitral Solutions Mitral valve replacement (1) • Endovalve Device Landscape: Percutaneous MV Repair
Edge-to-edge (1) • e. Valve • Edwards Mobius Coronary sinus annuloplasty (3) • Edwards Monarc • Cardiac Dimensions Carillon • Viacor PTMA Indirect annuloplasty (0) • Ample PS 3 • Myocor i-Coapsys • St. Jude AAR Direct annuloplasty (5) • Mitralign • Guided Delivery Systems • Quantum. Cor, Cordis DPA • Mi. Cardia, Mitral Solutions Mitral valve replacement (1) • Endovalve Device Landscape: Percutaneous MV Repair
 Edge to Edge (Evalve)
Edge to Edge (Evalve)
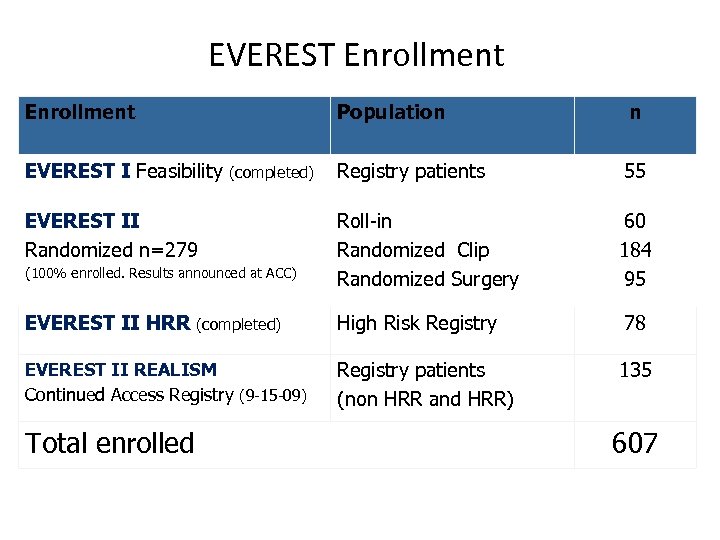 EVEREST Enrollment Population n Registry patients 55 Roll-in Randomized Clip Randomized Surgery 60 184 95 EVEREST II HRR (completed) High Risk Registry 78 EVEREST II REALISM Continued Access Registry (9 -15 -09) Registry patients (non HRR and HRR) 135 EVEREST I Feasibility (completed) EVEREST II Randomized n=279 (100% enrolled. Results announced at ACC) Total enrolled 607
EVEREST Enrollment Population n Registry patients 55 Roll-in Randomized Clip Randomized Surgery 60 184 95 EVEREST II HRR (completed) High Risk Registry 78 EVEREST II REALISM Continued Access Registry (9 -15 -09) Registry patients (non HRR and HRR) 135 EVEREST I Feasibility (completed) EVEREST II Randomized n=279 (100% enrolled. Results announced at ACC) Total enrolled 607
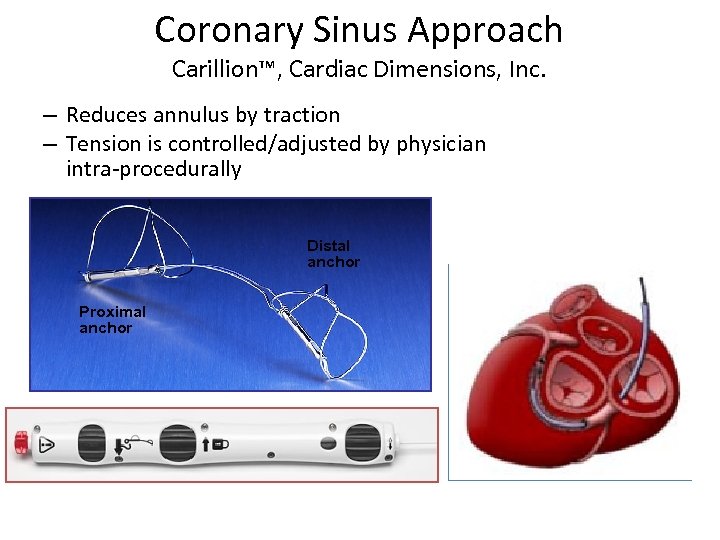 Coronary Sinus Approach Carillion™, Cardiac Dimensions, Inc. – Reduces annulus by traction – Tension is controlled/adjusted by physician intra-procedurally Distal anchor Proximal anchor
Coronary Sinus Approach Carillion™, Cardiac Dimensions, Inc. – Reduces annulus by traction – Tension is controlled/adjusted by physician intra-procedurally Distal anchor Proximal anchor
 AMADEUS™ & TITAN™ Patient Enrollment • CAD requiring intervention • Inadequate Vein Size • Dissection / Perforation on Access • Insufficient MR Reduction • Coronary Compromise • 1 st Gen Device Anchor Slip
AMADEUS™ & TITAN™ Patient Enrollment • CAD requiring intervention • Inadequate Vein Size • Dissection / Perforation on Access • Insufficient MR Reduction • Coronary Compromise • 1 st Gen Device Anchor Slip
 Summary of TITAN • In 53 pts enrolled, with 36 implants, Reduction of FMR with the coronary sinus based CARILLON Mitral Contour System is feasible and has a 1. 7% MAE at 30 days • Mean reduction in MR of 35% by independent echo parameters • Significant and sustained improvement of 1 NYHA class, 6 minute walk test and quality of life assessments at 1 and 6 months in the intervention group
Summary of TITAN • In 53 pts enrolled, with 36 implants, Reduction of FMR with the coronary sinus based CARILLON Mitral Contour System is feasible and has a 1. 7% MAE at 30 days • Mean reduction in MR of 35% by independent echo parameters • Significant and sustained improvement of 1 NYHA class, 6 minute walk test and quality of life assessments at 1 and 6 months in the intervention group
 Status of U. S. Pivotal Trial • The improvements in echo and clinical parameters seen in AMADEUS and TITAN supported the role for further study • Based upon the results from the TITAN study, an application for a US pivotal study (IDE) was submitted to the FDA in 2009. • During the review process of the pivotal study application (IDE), however, the company identified potential design improvements. These design improvements are currently under evaluation.
Status of U. S. Pivotal Trial • The improvements in echo and clinical parameters seen in AMADEUS and TITAN supported the role for further study • Based upon the results from the TITAN study, an application for a US pivotal study (IDE) was submitted to the FDA in 2009. • During the review process of the pivotal study application (IDE), however, the company identified potential design improvements. These design improvements are currently under evaluation.
 Coronary Sinus Approach Edwards MONARC™ System Sliding Knob 12 F guiding catheter 9 F delivery system Handle Location of Implant (Internal) Bridge Proximal Anchor Elongated bridge at implant Distal Anchor Foreshortened state at ~4 -6 weeks
Coronary Sinus Approach Edwards MONARC™ System Sliding Knob 12 F guiding catheter 9 F delivery system Handle Location of Implant (Internal) Bridge Proximal Anchor Elongated bridge at implant Distal Anchor Foreshortened state at ~4 -6 weeks
 Conclusions • EVOLUTION interim data with the MONARC™ system suggests: • Implantation in the coronary sinus is feasible • At 24 -months, 72% of patients are event-free as defined by the protocol • Encouraging 24 -months results in terms of MR reduction and physiological parameters – Efficacy is preserved from 1 year to 2 years • EVOLUTION II will assess functional and clinical outcomes of the MONARC™ system for patients with functional mitral regurgitation
Conclusions • EVOLUTION interim data with the MONARC™ system suggests: • Implantation in the coronary sinus is feasible • At 24 -months, 72% of patients are event-free as defined by the protocol • Encouraging 24 -months results in terms of MR reduction and physiological parameters – Efficacy is preserved from 1 year to 2 years • EVOLUTION II will assess functional and clinical outcomes of the MONARC™ system for patients with functional mitral regurgitation
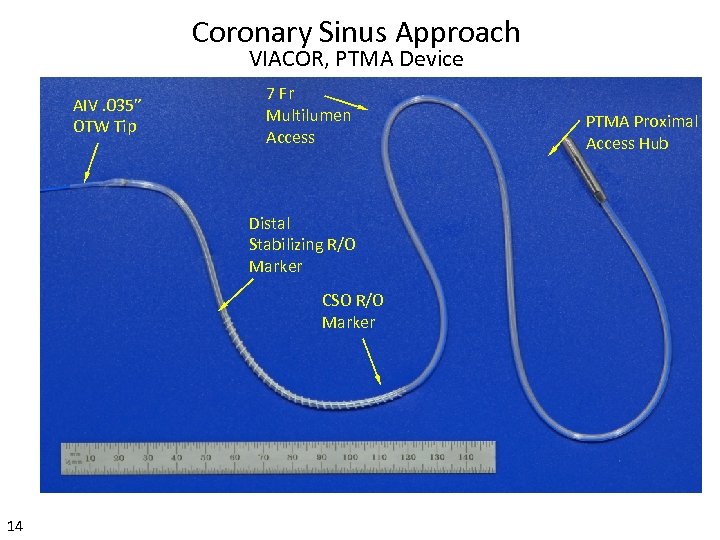 Coronary Sinus Approach VIACOR, PTMA Device AIV. 035” OTW Tip 7 Fr Multilumen Access Distal Stabilizing R/O Marker CSO R/O Marker 14 PTMA Proximal Access Hub
Coronary Sinus Approach VIACOR, PTMA Device AIV. 035” OTW Tip 7 Fr Multilumen Access Distal Stabilizing R/O Marker CSO R/O Marker 14 PTMA Proximal Access Hub
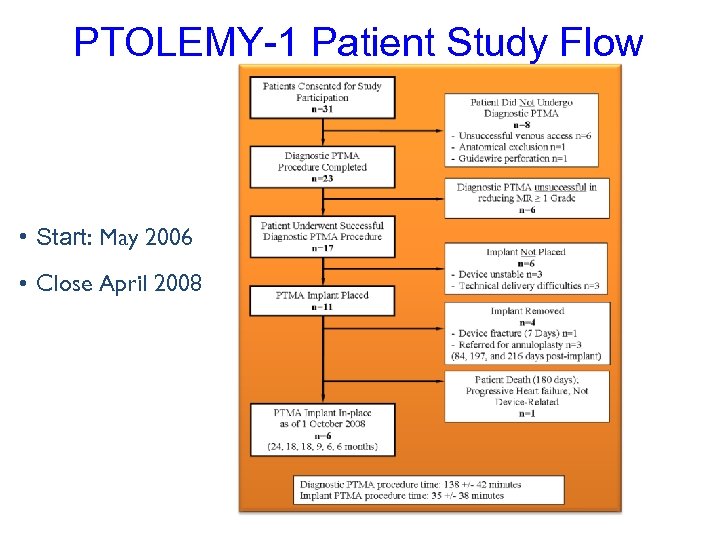 PTOLEMY-1 Patient Study Flow • Start: May 2006 • Close April 2008
PTOLEMY-1 Patient Study Flow • Start: May 2006 • Close April 2008
 PTOLEMY 2 Trial – Europe & Canada Indications: II + III NYHA Heart Failure quantitative echo criteria for moderate to severe MR Number of Subjects: 50 enrolled, 30 implants – Europe 30 enrolled, 20 implants - Canada Follow-up periods: Discharge, 1, 3, 6, & 12 mo 2 – 5 years implanted patients Design: Non-randomized, parallel follow-up 8 Centers Europe 3 Centers Canada Enrollment rate = 1 pt /mo /ctr Endpoints: Safety – 30 days 1° Device / Procedure-Related Major Cardiac Adverse Events Efficacy – 6 months & 12 months 1° MR reduction – echo core lab 2° Quality of life: - Minnesota QOL - 6 minute walk distance Obs: EQ-5 D Obs: Left ventricular size Obs: Rehospitalization rate Obs: Mortality and morbidity
PTOLEMY 2 Trial – Europe & Canada Indications: II + III NYHA Heart Failure quantitative echo criteria for moderate to severe MR Number of Subjects: 50 enrolled, 30 implants – Europe 30 enrolled, 20 implants - Canada Follow-up periods: Discharge, 1, 3, 6, & 12 mo 2 – 5 years implanted patients Design: Non-randomized, parallel follow-up 8 Centers Europe 3 Centers Canada Enrollment rate = 1 pt /mo /ctr Endpoints: Safety – 30 days 1° Device / Procedure-Related Major Cardiac Adverse Events Efficacy – 6 months & 12 months 1° MR reduction – echo core lab 2° Quality of life: - Minnesota QOL - 6 minute walk distance Obs: EQ-5 D Obs: Left ventricular size Obs: Rehospitalization rate Obs: Mortality and morbidity
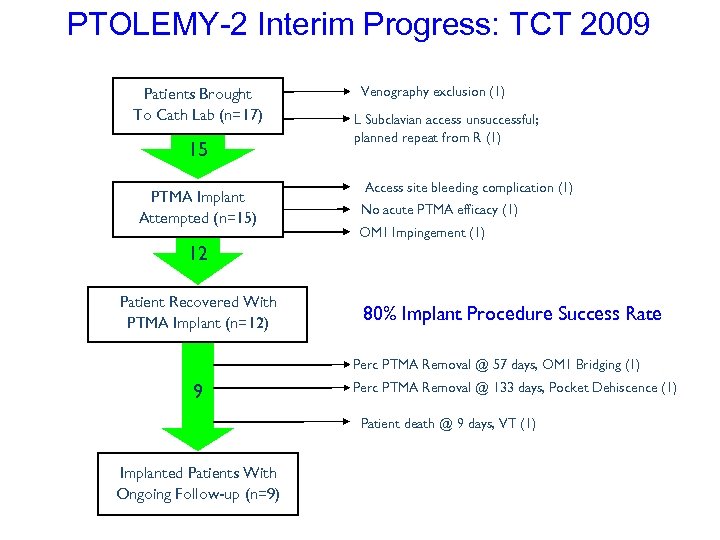 PTOLEMY-2 Interim Progress: TCT 2009 Patients Brought To Cath Lab (n=17) 15 PTMA Implant Attempted (n=15) Venography exclusion (1) L Subclavian access unsuccessful; planned repeat from R (1) Access site bleeding complication (1) No acute PTMA efficacy (1) OM 1 Impingement (1) 12 Patient Recovered With PTMA Implant (n=12) 80% Implant Procedure Success Rate Perc PTMA Removal @ 57 days, OM 1 Bridging (1) 9 Perc PTMA Removal @ 133 days, Pocket Dehiscence (1) Patient death @ 9 days, VT (1) Implanted Patients With Ongoing Follow-up (n=9)
PTOLEMY-2 Interim Progress: TCT 2009 Patients Brought To Cath Lab (n=17) 15 PTMA Implant Attempted (n=15) Venography exclusion (1) L Subclavian access unsuccessful; planned repeat from R (1) Access site bleeding complication (1) No acute PTMA efficacy (1) OM 1 Impingement (1) 12 Patient Recovered With PTMA Implant (n=12) 80% Implant Procedure Success Rate Perc PTMA Removal @ 57 days, OM 1 Bridging (1) 9 Perc PTMA Removal @ 133 days, Pocket Dehiscence (1) Patient death @ 9 days, VT (1) Implanted Patients With Ongoing Follow-up (n=9)
 Direct Suture Mediated Annuloplasty Mitralign, Accelerated Technologies Mitralign FIM Studies in Europe, Paraguay and Brazil Phase I Trident • FIM trident experience in Paraguay, Germany and Czech Republic • 17 pts enrolled, 8 implanted, with long-term FU • Safety and efficacy issues • Significant changes in the system to simplify few
Direct Suture Mediated Annuloplasty Mitralign, Accelerated Technologies Mitralign FIM Studies in Europe, Paraguay and Brazil Phase I Trident • FIM trident experience in Paraguay, Germany and Czech Republic • 17 pts enrolled, 8 implanted, with long-term FU • Safety and efficacy issues • Significant changes in the system to simplify few
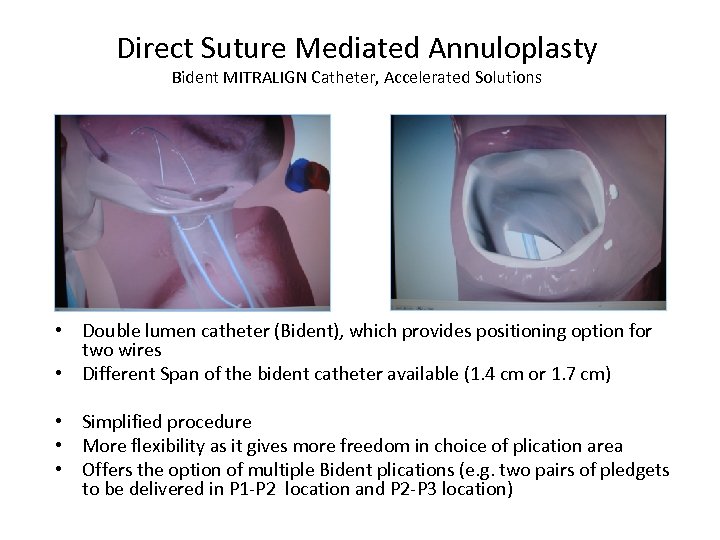 Direct Suture Mediated Annuloplasty Bident MITRALIGN Catheter, Accelerated Solutions • Double lumen catheter (Bident), which provides positioning option for two wires • Different Span of the bident catheter available (1. 4 cm or 1. 7 cm) • Simplified procedure • More flexibility as it gives more freedom in choice of plication area • Offers the option of multiple Bident plications (e. g. two pairs of pledgets to be delivered in P 1 -P 2 location and P 2 -P 3 location)
Direct Suture Mediated Annuloplasty Bident MITRALIGN Catheter, Accelerated Solutions • Double lumen catheter (Bident), which provides positioning option for two wires • Different Span of the bident catheter available (1. 4 cm or 1. 7 cm) • Simplified procedure • More flexibility as it gives more freedom in choice of plication area • Offers the option of multiple Bident plications (e. g. two pairs of pledgets to be delivered in P 1 -P 2 location and P 2 -P 3 location)
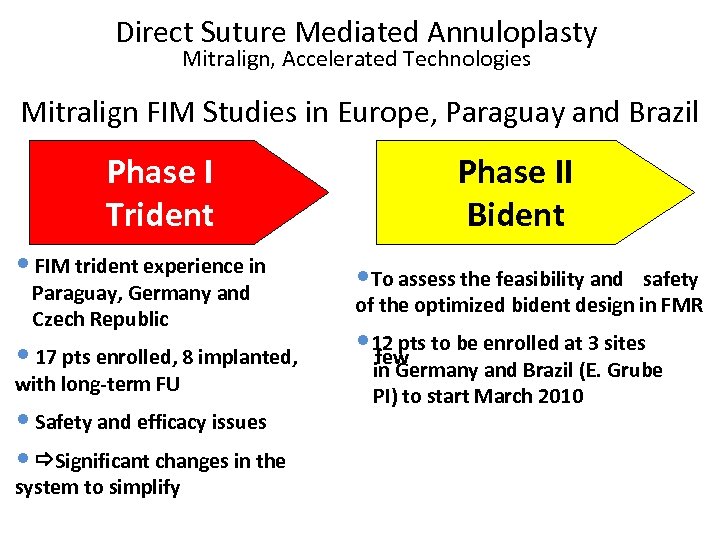 Direct Suture Mediated Annuloplasty Mitralign, Accelerated Technologies Mitralign FIM Studies in Europe, Paraguay and Brazil Phase I Trident Phase II Bident • FIM trident experience in • To assess the feasibility and • 17 pts enrolled, 8 implanted, • 12 pts to be enrolled at 3 sites few Paraguay, Germany and Czech Republic with long-term FU • Safety and efficacy issues • Significant changes in the system to simplify safety of the optimized bident design in FMR in Germany and Brazil (E. Grube PI) to start March 2010
Direct Suture Mediated Annuloplasty Mitralign, Accelerated Technologies Mitralign FIM Studies in Europe, Paraguay and Brazil Phase I Trident Phase II Bident • FIM trident experience in • To assess the feasibility and • 17 pts enrolled, 8 implanted, • 12 pts to be enrolled at 3 sites few Paraguay, Germany and Czech Republic with long-term FU • Safety and efficacy issues • Significant changes in the system to simplify safety of the optimized bident design in FMR in Germany and Brazil (E. Grube PI) to start March 2010
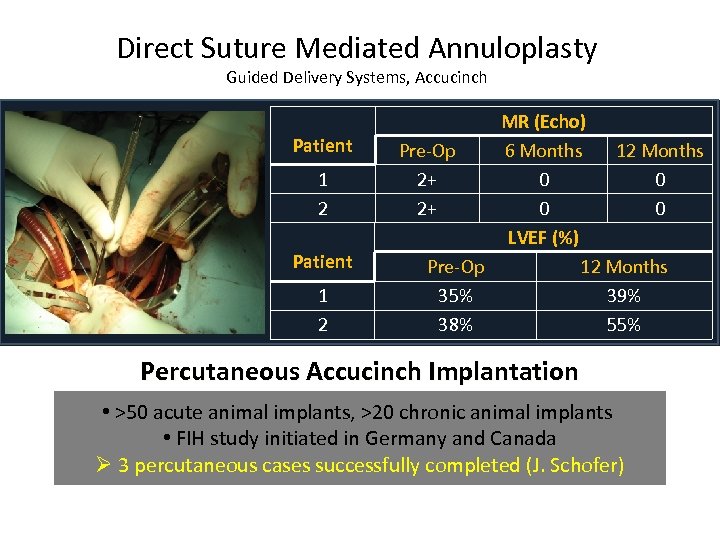 Direct Suture Mediated Annuloplasty Guided Delivery Systems, Accucinch Patient 1 2 MR (Echo) Pre-Op 6 Months 12 Months 2+ 0 0 LVEF (%) Pre-Op 12 Months 35% 39% 38% 55% Percutaneous Accucinch Implantation • >50 acute animal implants, >20 chronic animal implants • FIH study initiated in Germany and Canada Ø 3 percutaneous cases successfully completed (J. Schofer)
Direct Suture Mediated Annuloplasty Guided Delivery Systems, Accucinch Patient 1 2 MR (Echo) Pre-Op 6 Months 12 Months 2+ 0 0 LVEF (%) Pre-Op 12 Months 35% 39% 38% 55% Percutaneous Accucinch Implantation • >50 acute animal implants, >20 chronic animal implants • FIH study initiated in Germany and Canada Ø 3 percutaneous cases successfully completed (J. Schofer)
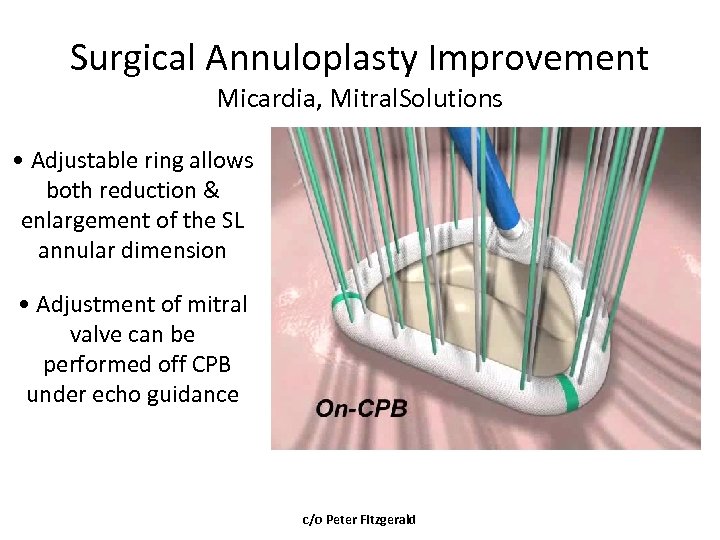 Surgical Annuloplasty Improvement Micardia, Mitral. Solutions • Adjustable ring allows both reduction & enlargement of the SL annular dimension • Adjustment of mitral valve can be performed off CPB under echo guidance c/o Peter Fitzgerald
Surgical Annuloplasty Improvement Micardia, Mitral. Solutions • Adjustable ring allows both reduction & enlargement of the SL annular dimension • Adjustment of mitral valve can be performed off CPB under echo guidance c/o Peter Fitzgerald
 MARS Trial (n=68) 5 EU centers N=45 N=32 * Mc. Gee et al. J Th and CV Surg. December 2004 23
MARS Trial (n=68) 5 EU centers N=45 N=32 * Mc. Gee et al. J Th and CV Surg. December 2004 23
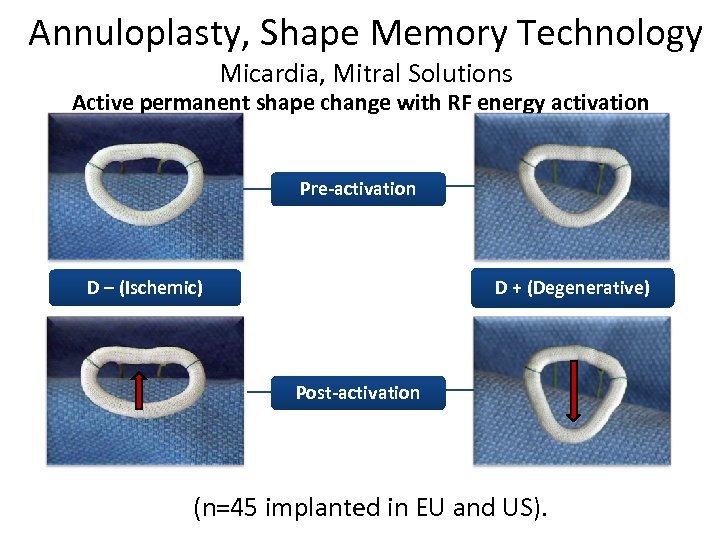 Annuloplasty, Shape Memory Technology Micardia, Mitral Solutions Active permanent shape change with RF energy activation Pre-activation D – (Ischemic) D + (Degenerative) Post-activation (n=45 implanted in EU and US).
Annuloplasty, Shape Memory Technology Micardia, Mitral Solutions Active permanent shape change with RF energy activation Pre-activation D – (Ischemic) D + (Degenerative) Post-activation (n=45 implanted in EU and US).
 Percutaneous Reshaping • Percutaneous implantation of device is feasible • The implanted ring is accessed via transeptal approach. • Thru a Deflectable guide catheter system for optimal positioning Maurice Buchbinder, MD Foundation for Cardiovascular Medicine
Percutaneous Reshaping • Percutaneous implantation of device is feasible • The implanted ring is accessed via transeptal approach. • Thru a Deflectable guide catheter system for optimal positioning Maurice Buchbinder, MD Foundation for Cardiovascular Medicine
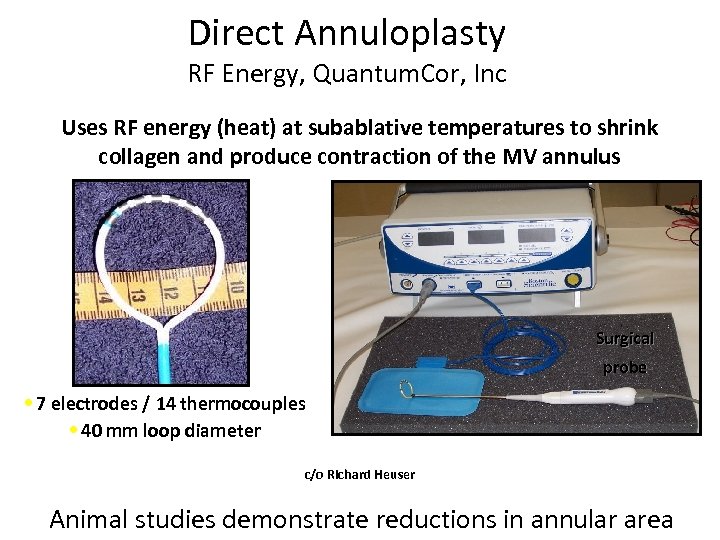 Direct Annuloplasty RF Energy, Quantum. Cor, Inc Uses RF energy (heat) at subablative temperatures to shrink collagen and produce contraction of the MV annulus Surgical probe • 7 electrodes / 14 thermocouples • 40 mm loop diameter c/o Richard Heuser Animal studies demonstrate reductions in annular area
Direct Annuloplasty RF Energy, Quantum. Cor, Inc Uses RF energy (heat) at subablative temperatures to shrink collagen and produce contraction of the MV annulus Surgical probe • 7 electrodes / 14 thermocouples • 40 mm loop diameter c/o Richard Heuser Animal studies demonstrate reductions in annular area
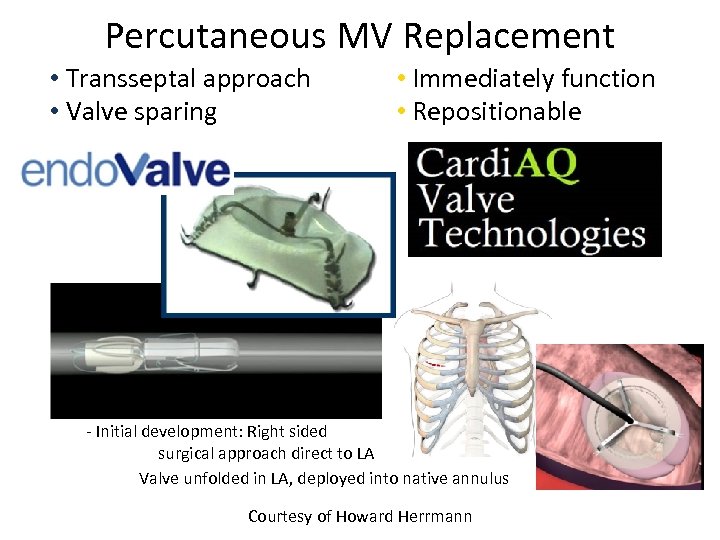 Percutaneous MV Replacement • Transseptal approach • Valve sparing (like repair) • Immediately function • Repositionable - Valve folded in catheter - Initial development: Right sided surgical approach direct to LA Valve unfolded in LA, deployed into native annulus Courtesy of Howard Herrmann
Percutaneous MV Replacement • Transseptal approach • Valve sparing (like repair) • Immediately function • Repositionable - Valve folded in catheter - Initial development: Right sided surgical approach direct to LA Valve unfolded in LA, deployed into native annulus Courtesy of Howard Herrmann
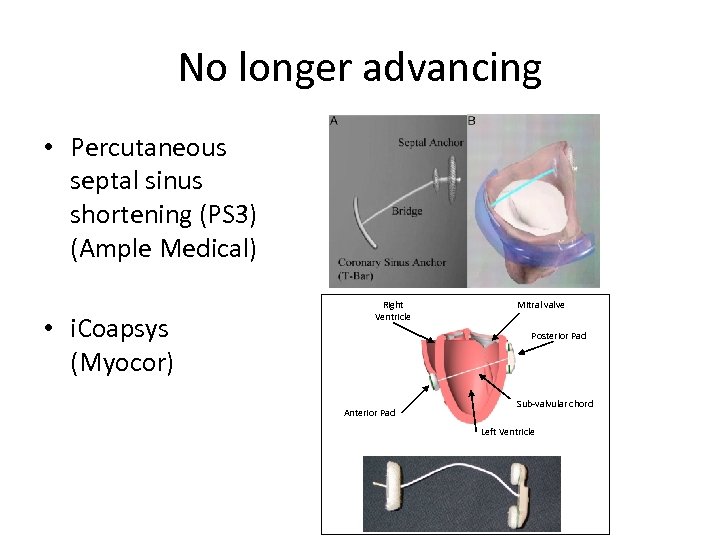 No longer advancing • Percutaneous septal sinus shortening (PS 3) (Ample Medical) • i. Coapsys (Myocor) Right Ventricle Mitral valve Posterior Pad Anterior Pad Sub-valvular chord Left Ventricle
No longer advancing • Percutaneous septal sinus shortening (PS 3) (Ample Medical) • i. Coapsys (Myocor) Right Ventricle Mitral valve Posterior Pad Anterior Pad Sub-valvular chord Left Ventricle
 Conclusion • The bar is high for repair in degenerative MR (Evalve, Mitralign) – Surgical results of repair in the low risk population are outstanding – Await Late Breaking Trial Results at ACC 2010 • Functional MR is a difficult disease to treat given the complex interplay of the various components of the MV apparatus and ventricle – Multiple technologies are utilizing various approaches to tackle a complex geometric problem which may not be best served by a single strategy • There are some patients who shouldn’t get MV surgery but should have better MV function. • Significant timeline to market approval and excessive requirement for capital investment is a barrier to entry – Outcome measurements for clinical trial design continue to challenge development of several of the technologies
Conclusion • The bar is high for repair in degenerative MR (Evalve, Mitralign) – Surgical results of repair in the low risk population are outstanding – Await Late Breaking Trial Results at ACC 2010 • Functional MR is a difficult disease to treat given the complex interplay of the various components of the MV apparatus and ventricle – Multiple technologies are utilizing various approaches to tackle a complex geometric problem which may not be best served by a single strategy • There are some patients who shouldn’t get MV surgery but should have better MV function. • Significant timeline to market approval and excessive requirement for capital investment is a barrier to entry – Outcome measurements for clinical trial design continue to challenge development of several of the technologies
 Will One Size Fit All? • Likely not • Lessons learned from surgical and first generation systems will guide progress regarding patient selection and multimodality/hybrid approaches will guide development and improvement in outcomes • Timeline for development will not be rapid
Will One Size Fit All? • Likely not • Lessons learned from surgical and first generation systems will guide progress regarding patient selection and multimodality/hybrid approaches will guide development and improvement in outcomes • Timeline for development will not be rapid


