d245571a371fe7475a92cea5f166833e.ppt
- Количество слайдов: 32

TB Methodologies Dr. John G. Magee Regional Reference Centre for Mycobacteriology Health Protection Agency Regional Laboratory, Newcastle upon Tyne

Annual report, tuberculosis cases reported in 2000, England, Wales and Northern Ireland Of 6323 cases ONLY 3350 (53%) were culture confirmed Of 3729 with pulmonary disease: ONLY 2249 (60%) were culture confirmed ONLY 2513 had a smear result! ONLY 1406 (56%) were smear positive

Do. H - Getting ahead of the curve “Tuberculosis Action Plan” The HPA will work with reference laboratories and NHS microbiologists to improve the speed and consistency of laboratory diagnosis by: providing high quality diagnostic services through a network of suitably equipped and experienced laboratories l l standardising methods l establishing quality assurance/performance monitoring programmes covering. . liquid culture for all specimens. . molecular confirmation. . unique typing designation

Do. H - Getting ahead of the curve “Tuberculosis Action Plan” STANDARDS EXPECTED l smear turnaround time - 1 working day l all clinical samples to have access to automated liquid culture performed in experienced centres with large throughput and dedicated facilities and staff l all isolates referred to regional mycobacteriology centre for identification & susceptibility testing
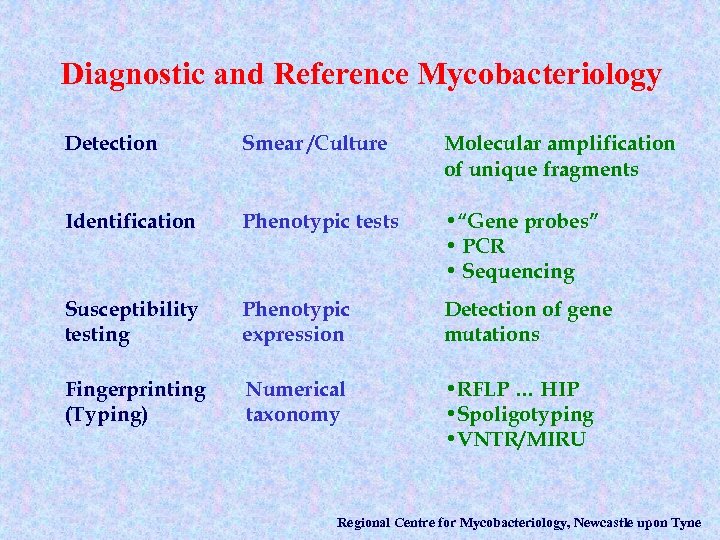
Diagnostic and Reference Mycobacteriology Detection Smear /Culture Molecular amplification of unique fragments Identification Phenotypic tests • “Gene probes” • PCR • Sequencing Susceptibility testing Phenotypic expression Detection of gene mutations Fingerprinting (Typing) Numerical taxonomy • RFLP … HIP • Spoligotyping • VNTR/MIRU Regional Centre for Mycobacteriology, Newcastle upon Tyne
Direct detection using molecular biological tests The problems are have something to do with low organism numbers and much to do with extraction of DNA from clinical samples
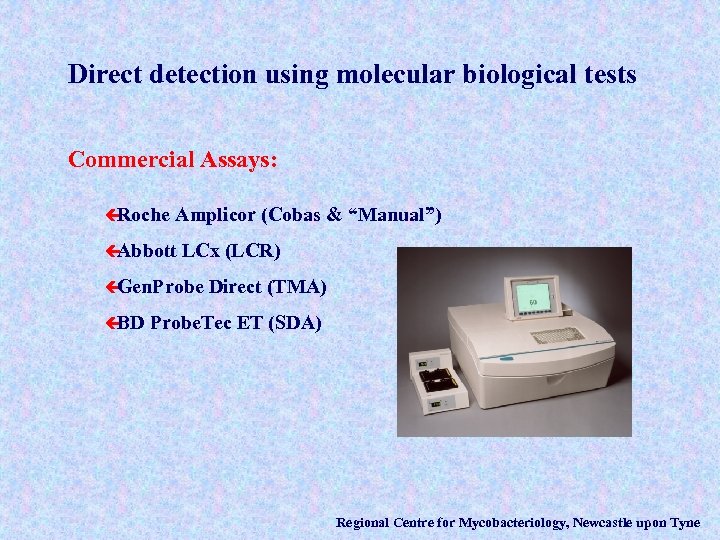
Direct detection using molecular biological tests Commercial Assays: ç Roche Amplicor (Cobas & “Manual”) ç Abbott LCx (LCR) ç Gen. Probe ç BD Direct (TMA) Probe. Tec ET (SDA) Regional Centre for Mycobacteriology, Newcastle upon Tyne
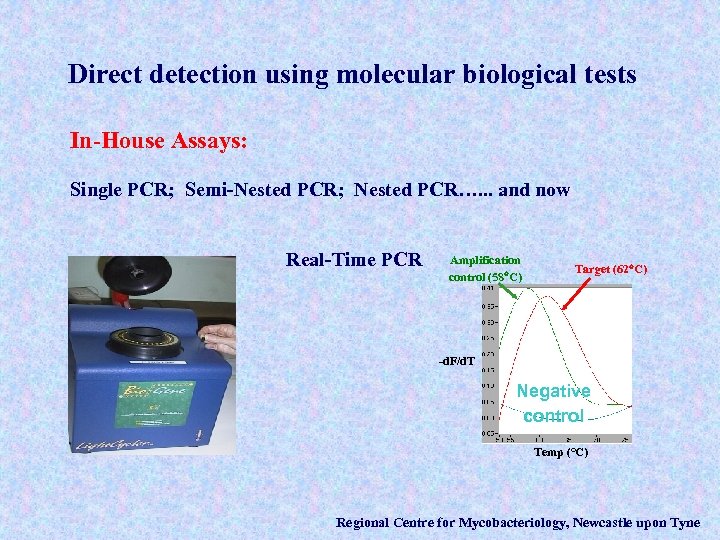
Direct detection using molecular biological tests In-House Assays: Single PCR; Semi-Nested PCR; Nested PCR…. . . and now Real-Time PCR Amplification control (58 C) Target (62 C) -d. F/d. T Negative control Temp (°C) Regional Centre for Mycobacteriology, Newcastle upon Tyne
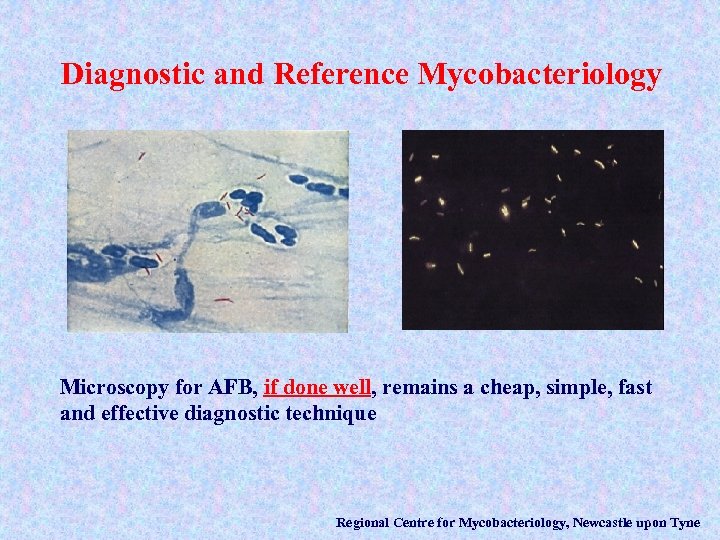
Diagnostic and Reference Mycobacteriology Microscopy for AFB, if done well, remains a cheap, simple, fast and effective diagnostic technique Regional Centre for Mycobacteriology, Newcastle upon Tyne
Isolation Regional Centre for Mycobacteriology, Newcastle upon Tyne
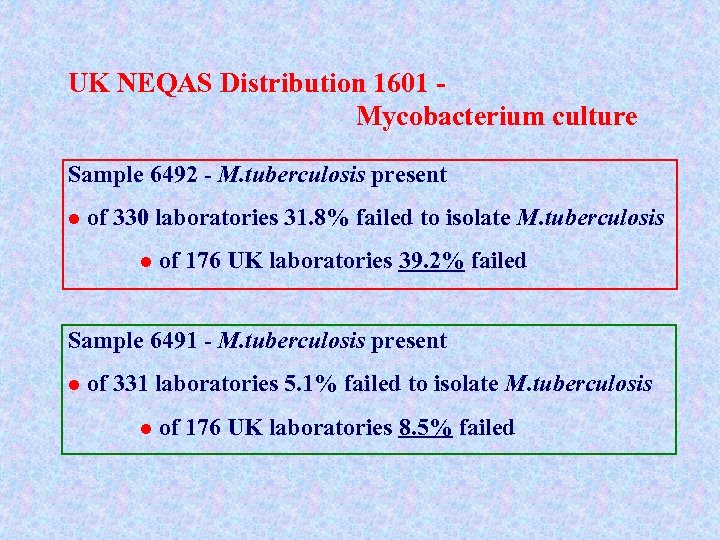
UK NEQAS Distribution 1601 Mycobacterium culture Sample 6492 - M. tuberculosis present l of 330 laboratories 31. 8% failed to isolate M. tuberculosis l of 176 UK laboratories 39. 2% failed Sample 6491 - M. tuberculosis present l of 331 laboratories 5. 1% failed to isolate M. tuberculosis l of 176 UK laboratories 8. 5% failed

Isolation: Liquid Media circa 1983 Regional Centre for Mycobacteriology, Newcastle upon Tyne

Isolation: Liquid Media circa 1993 Regional Centre for Mycobacteriology, Newcastle upon Tyne

Continuous Automated Mycobacterial Liquid Culture systems Regional Centre for Mycobacteriology, Newcastle upon Tyne
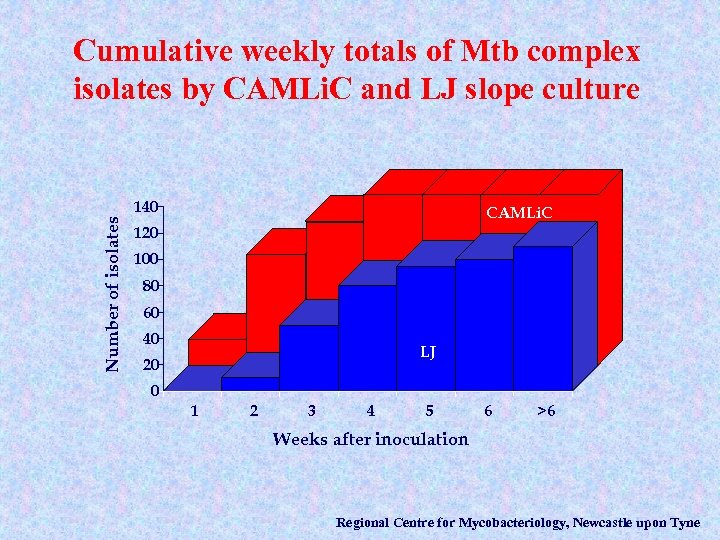
Cumulative weekly totals of Mtb complex isolates by CAMLi. C and LJ slope culture Number of isolates 140 CAMLi. C 120 100 80 60 40 LJ 20 0 1 2 3 4 5 6 >6 Weeks after inoculation Regional Centre for Mycobacteriology, Newcastle upon Tyne

Surety of Competence Detection of Acid-Fast Bacilli: 20 smears per week 1000 per year Mycobacterial Culture: 25 samples per week 1250 per year Susceptibility testing: 20 isolates per week 1000 per year Regional Centre for Mycobacteriology, Newcastle upon Tyne

UK NEQAS Distribution 1601 Mycobacterium culture Sample 6490 - M. tuberculosis NOT present 8/331 laboratories (2. 4%) isolated a mycobacterium! In the last 4 negative samples there were 11, 4, 2 & 10 false positives False positivity is probably due to laboratory cross contamination. NEQAS refer us to Breese at al. Arch Pathol Lab Med 2001, 125(9): 1213 BUT see alsode Boer et al. J Clin Microbiol 2002; 4004 They found that labs processing <3000 samples per annum showed a greater risk of cross-contamination
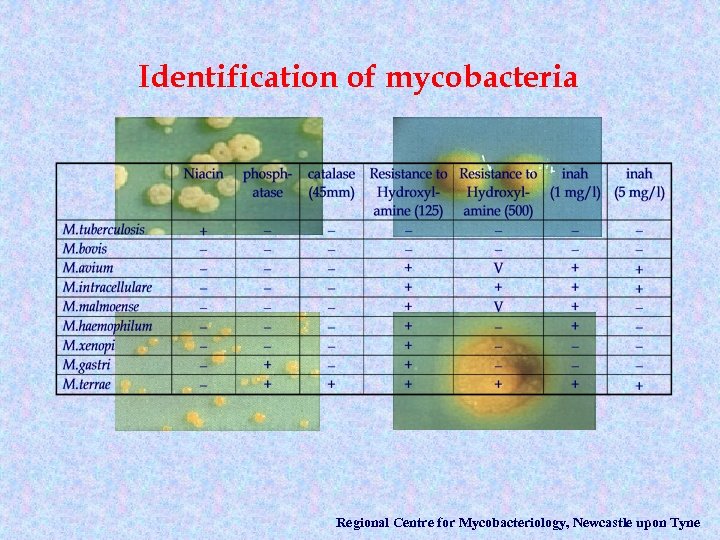
Identification of mycobacteria Regional Centre for Mycobacteriology, Newcastle upon Tyne
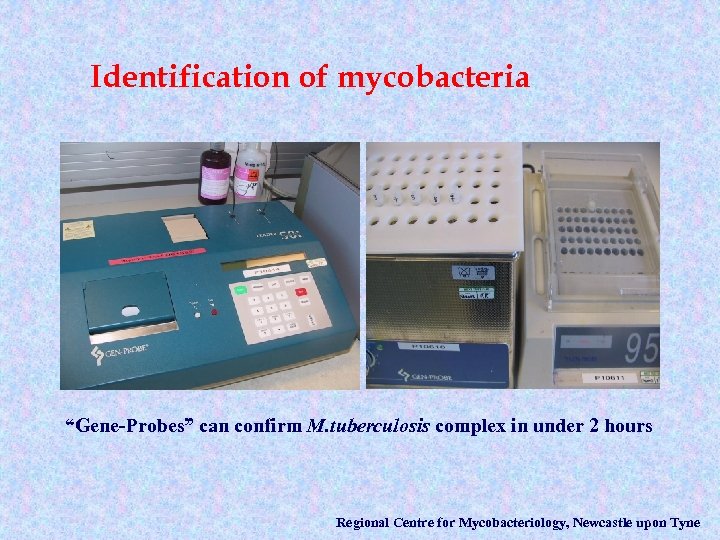
Identification of mycobacteria “Gene-Probes” can confirm M. tuberculosis complex in under 2 hours Regional Centre for Mycobacteriology, Newcastle upon Tyne
Identification of mycobacteria l l “Genetic probes” are NOT amplification procedures They can identify a limited number of common species: M. tuberculosis complex M. avium M. intracellulare M. kansasii M. gordonae Regional Centre for Mycobacteriology, Newcastle upon Tyne
Identification of Mycobacteria l Characterisation of mycolic acids by HPLC l Detection of unique fragments of genomic DNA l 16 S r. RNA sequencing [ equipment database variances ] Regional Centre for Mycobacteriology, Newcastle upon Tyne
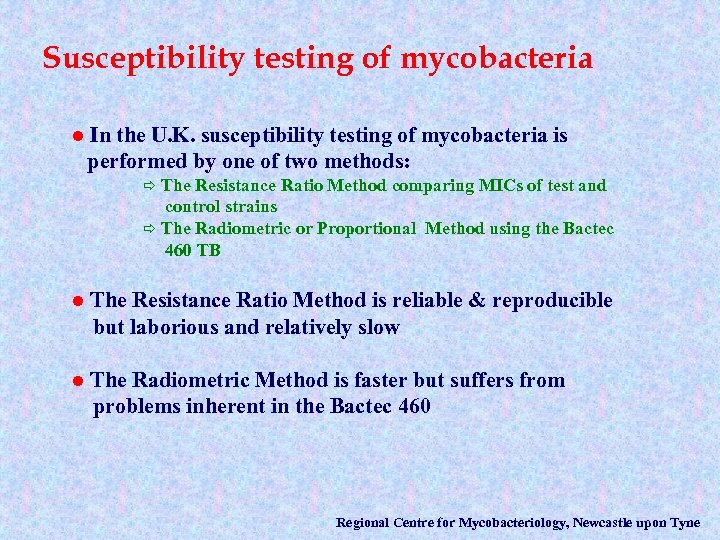
Susceptibility testing of mycobacteria l In the U. K. susceptibility testing of mycobacteria is performed by one of two methods: The Resistance Ratio Method comparing MICs of test and control strains ð The Radiometric or Proportional Method using the Bactec 460 TB ð l The Resistance Ratio Method is reliable & reproducible but laborious and relatively slow l The Radiometric Method is faster but suffers from problems inherent in the Bactec 460 Regional Centre for Mycobacteriology, Newcastle upon Tyne

Continuous Automated Mycobacterial Liquid Culture Systems Regional Centre for Mycobacteriology, Newcastle upon Tyne
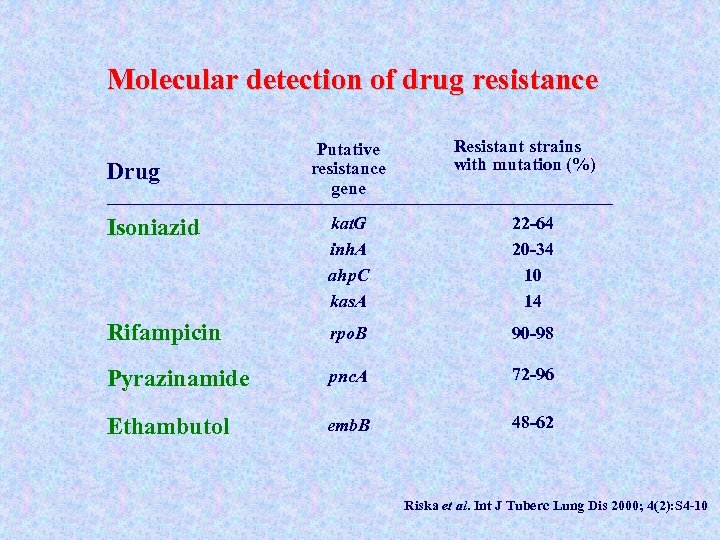
Molecular detection of drug resistance Drug Putative resistance gene Resistant strains with mutation (%) Isoniazid kat. G inh. A ahp. C kas. A 22 -64 20 -34 10 14 Rifampicin rpo. B 90 -98 Pyrazinamide pnc. A 72 -96 Ethambutol emb. B 48 -62 Riska et al. Int J Tuberc Lung Dis 2000; 4(2): S 4 -10
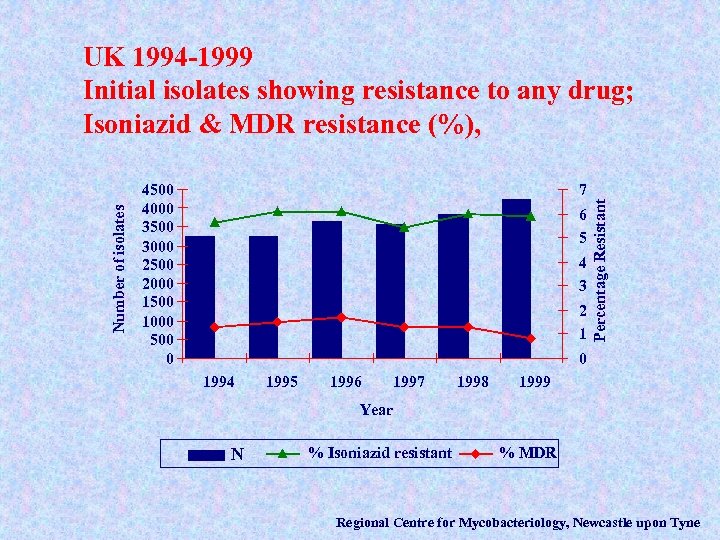
4500 4000 3500 3000 2500 2000 1500 1000 500 0 7 6 5 4 3 2 1 Percentage Resistant Number of isolates UK 1994 -1999 Initial isolates showing resistance to any drug; Isoniazid & MDR resistance (%), 0 1994 1995 1996 1997 1998 1999 Year N N % Isoniazid resistant % MDR Regional Centre for Mycobacteriology, Newcastle upon Tyne

Do. H - Getting ahead of the curve “Tuberculosis Action Plan” Develop and implement protocols for DNA fingerprinting taking customers needs into account. Establish a central database … linking fingerprinting and epidemiological data
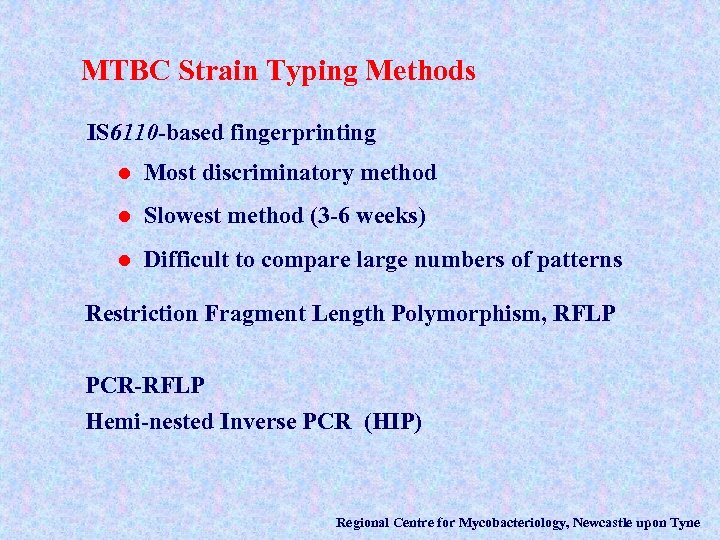
MTBC Strain Typing Methods IS 6110 -based fingerprinting l Most discriminatory method l Slowest method (3 -6 weeks) l Difficult to compare large numbers of patterns Restriction Fragment Length Polymorphism, RFLP PCR-RFLP Hemi-nested Inverse PCR (HIP) Regional Centre for Mycobacteriology, Newcastle upon Tyne
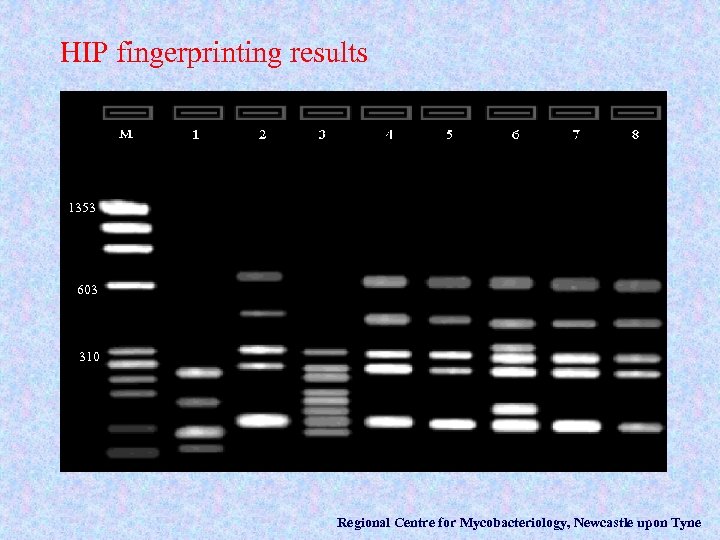
HIP fingerprinting results 1353 603 310 Regional Centre for Mycobacteriology, Newcastle upon Tyne
Spoligotyping Spacer Oligonucleotide Typing l Less discriminatory than IS 6110 typing … BUT. . . l Faster turnaround time l Digital results, facilitating comparisons l Does not require viable cultures Regional Centre for Mycobacteriology, Newcastle upon Tyne
VNTR - MIRU Variable Number of Tandem Repeats ð Measures variability in 6 loci Mycobacterial Interspersed Repetitive Units ð Measures variability in 12 loci PCR-based = rapid turnaround l Digital results, facilitates comparisons l Highly discriminatory l Does not require viable cultures l High throughput (automated sequence analysers) l Regional Centre for Mycobacteriology, Newcastle upon Tyne
Future Genotyping Strategy l Primary typing will be high throughput, automated, PCR-based i. e. MIRU/VNTR l Secondary typing by IS 6110 RFLP when needed for discrimination Regional Centre for Mycobacteriology, Newcastle upon Tyne
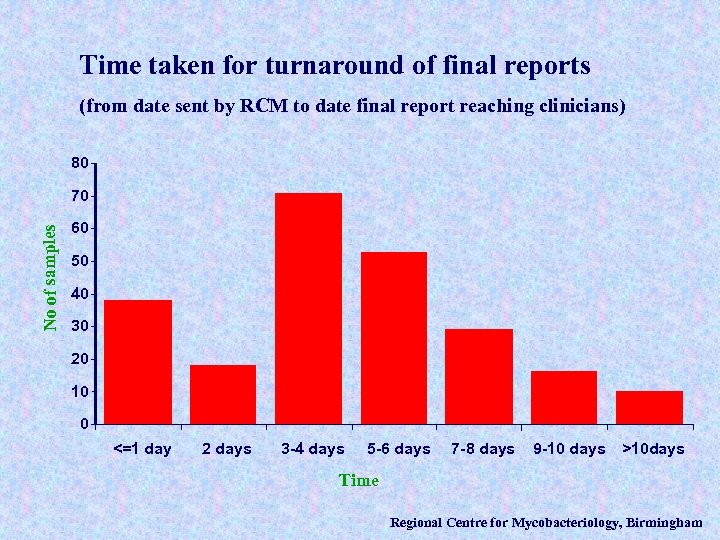
Time taken for turnaround of final reports (from date sent by RCM to date final report reaching clinicians) 80 No of samples 70 60 50 40 30 20 10 0 <=1 day 2 days 3 -4 days 5 -6 days 7 -8 days 9 -10 days >10 days Time Regional Centre for Mycobacteriology, Birmingham
d245571a371fe7475a92cea5f166833e.ppt