1e50116aa3d279dc674dcde12dbdc9a7.ppt
- Количество слайдов: 33

TB/HIV Update Central TB Division
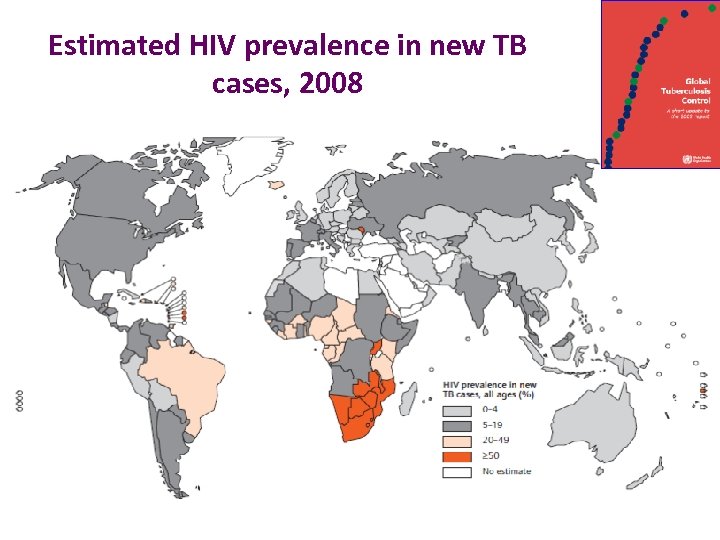
Estimated HIV prevalence in new TB cases, 2008
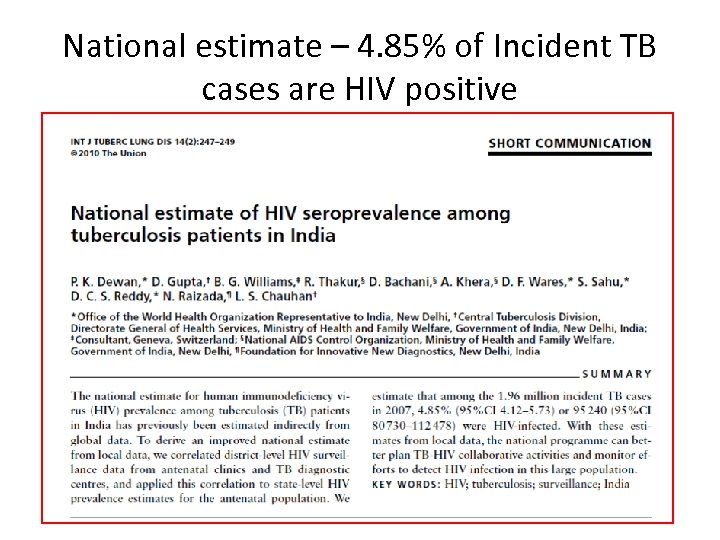
National estimate – 4. 85% of Incident TB cases are HIV positive
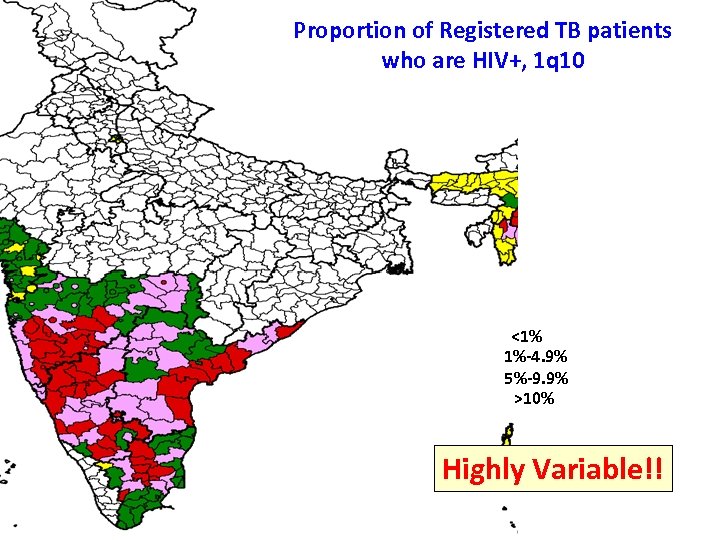
Proportion of Registered TB patients who are HIV+, 1 q 10 <1% 1%-4. 9% 5%-9. 9% >10% Highly Variable!!
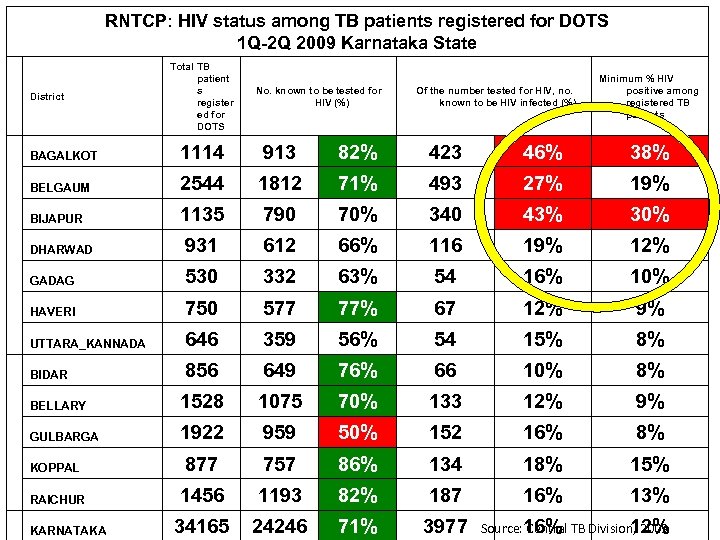
RNTCP: HIV status among TB patients registered for DOTS 1 Q-2 Q 2009 Karnataka State District Total TB patient s register ed for DOTS No. known to be tested for HIV (%) Of the number tested for HIV, no. known to be HIV infected (%) Minimum % HIV positive among registered TB patients BAGALKOT 1114 913 82% 423 46% 38% BELGAUM 2544 1812 71% 493 27% 19% BIJAPUR 1135 790 70% 340 43% 30% DHARWAD 931 612 66% 116 19% 12% GADAG 530 332 63% 54 16% 10% HAVERI 750 577 77% 67 12% 9% UTTARA_KANNADA 646 359 56% 54 15% 8% BIDAR 856 649 76% 66 10% 8% BELLARY 1528 1075 70% 133 12% 9% GULBARGA 1922 959 50% 152 16% 8% KOPPAL 877 757 86% 134 18% 15% RAICHUR 1456 1193 82% 187 16% 13% 34165 24246 71% 3977 KARNATAKA Source: 16% TB Division, 2009 Central 12%
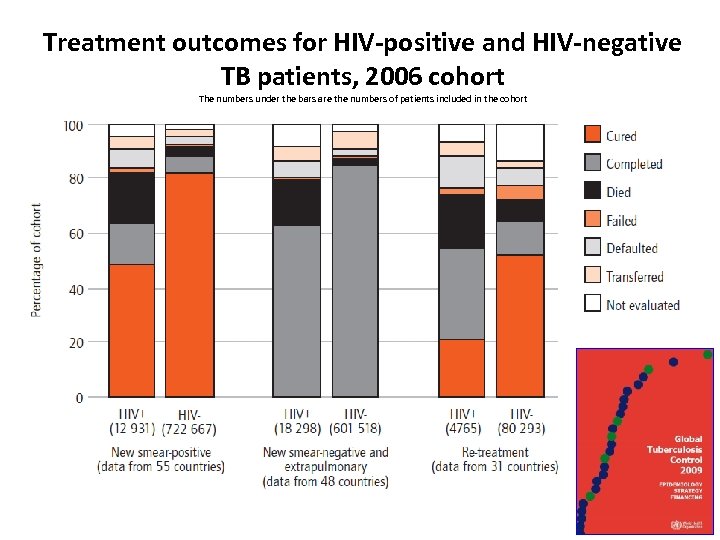
Treatment outcomes for HIV-positive and HIV-negative TB patients, 2006 cohort The numbers under the bars are the numbers of patients included in the cohort
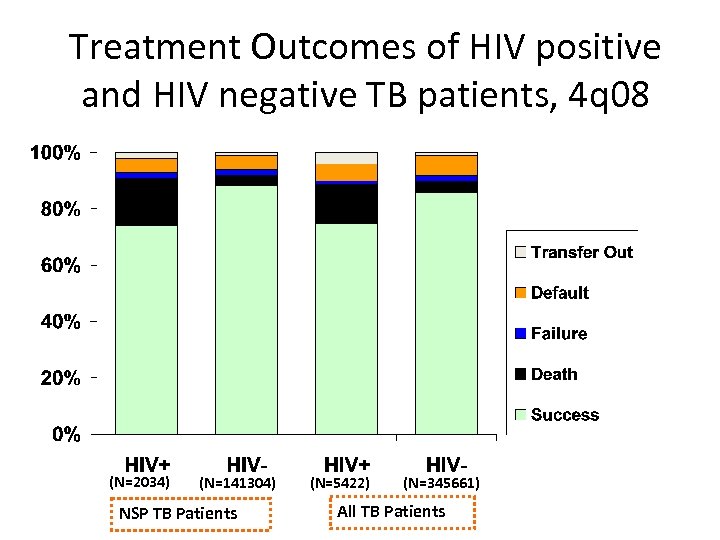
Treatment Outcomes of HIV positive and HIV negative TB patients, 4 q 08 (N=2034) (N=141304) NSP TB Patients (N=5422) (N=345661) All TB Patients
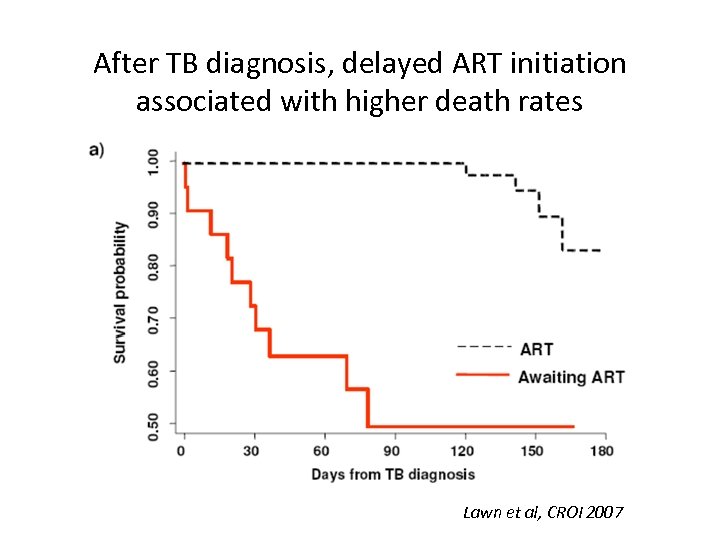
After TB diagnosis, delayed ART initiation associated with higher death rates Lawn et al, CROI 2007
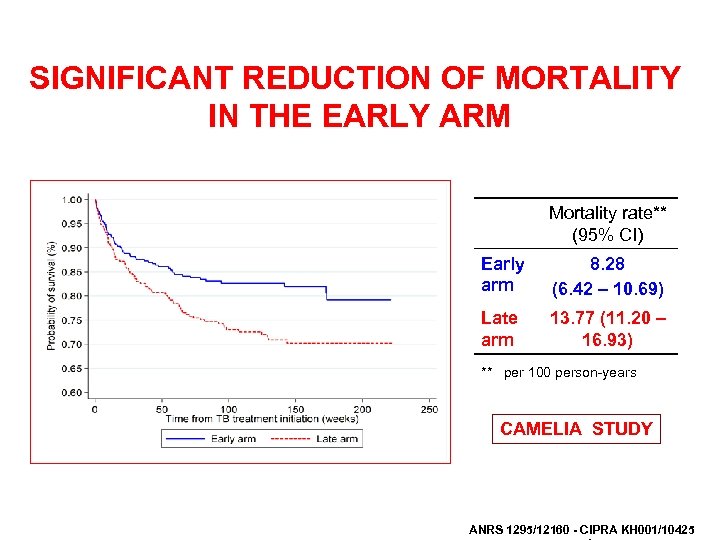
SIGNIFICANT REDUCTION OF MORTALITY IN THE EARLY ARM Mortality rate** (95% CI) Early arm 8. 28 (6. 42 – 10. 69) Late arm 13. 77 (11. 20 – 16. 93) ** per 100 person-years CAMELIA STUDY ANRS 1295/12160 - CIPRA KH 001/10425
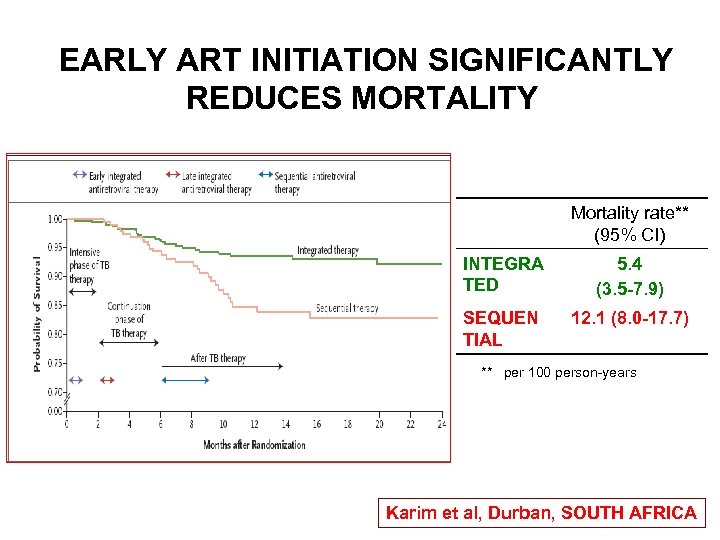
EARLY ART INITIATION SIGNIFICANTLY REDUCES MORTALITY Mortality rate** (95% CI) INTEGRA TED 5. 4 (3. 5 -7. 9) SEQUEN TIAL 12. 1 (8. 0 -17. 7) ** per 100 person-years Karim et al, Durban, SOUTH AFRICA

“Nationally, RNTCP should be able to reverse the increases in TB burden due to HIV but, to ensure that TB mortality is reduced by 50% or more by 2015, HIVinfected TB patients should be provided with antiretroviral therapy in addition to the recommended treatment for TB. ”
Summary: TB-HIV Interaction in India • India has the highest burden of TB, and a high burden of HIV in the world • Most TB is among persons without HIV; magnitude variable • HIV may slow down TB control efforts in India – Particularly efforts to reduce mortality • Enormous need for improved TB-HIV programme collaboration

Response to TB-HIV

The STOP TB Strategy, 2009 Updated language underlined 1. 2. Pursue high-quality DOTS expansion and enhancement a. b. c. d. e. Secure political commitment, with adequate and sustained financing Ensure early case detection, and diagnosis through quality-assured bacteriology Provide standardised treatment with supervision, and patient support Ensure effective drug supply and management Monitor and evaluate performance and impact Address TB-HIV, MDR-TB, and the needs of poor and vulnerable populations a. “Scale–up” collaborative TB/HIV activities 3. b. c. Scale-up prevention and management of multidrug-resistant TB (MDR-TB) Address the needs of TB contacts, and poor and vulnerable populations a. b. c. d. Help improve health policies, human resources development, financing, supplies, service delivery and information Strengthen infection control in health services, other congregate settings and households Upgrade laboratory networks, and implement the Practical Approach to Lung Health (PAL) Adapt approaches from other fields and sectors, and foster action on the social determinants of health a. b. Involve all public, voluntary, corporate and private providers through Public-Private Mix (PPM) approaches Promote use of the International Standards for Tuberculosis Care (ISTC) a. b. c. Pursue advocacy, communication and social mobilization Foster community participation in TB care, prevention and health promotion Promote use of the Patients' Charter for Tuberculosis Care a. b. Conduct programme-based operational research, and introduce new tools into practice Advocate for and participate in research to develop new diagnostics, drugs and vaccines Contribute to health system strengthening based on primary health care 4. Engage all care providers 5. Empower people with TB, and communities through partnership 6. Enable and promote research 2006/rev. 2009

Evolution of TB-HIV collaborative activities in India • 2001–First TBHIV “Joint Action Plan” developed; Basic activities in 6 high-HIV burden states • 2003 - Cross referral piloted in MH and initiated in 6 states • 2004–Expanded to 8 additional States • 2005–Joint training modules, surveillance • 2007–Expanded surveillance, CPT/Routine referral pilot, National Framework for TB/HIV • 2008–National Framework revised, all-India implementation begins with Intensified package in 9 states • 2009 – National Framework revised, Intensified package scaled up to include 8 more states • 2010 – Intensified package launched in 11 states
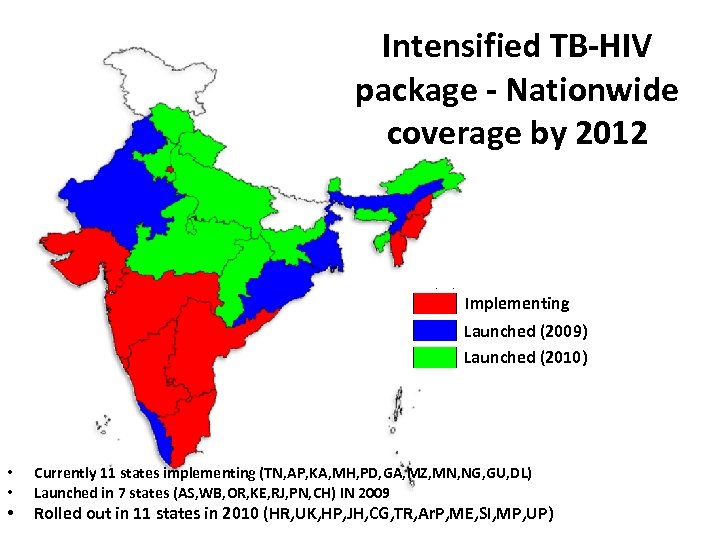
Intensified TB-HIV package - Nationwide coverage by 2012 Implementing Launched (2009) Launched (2010) • • • Currently 11 states implementing (TN, AP, KA, MH, PD, GA, MZ, MN, NG, GU, DL) Launched in 7 states (AS, WB, OR, KE, RJ, PN, CH) IN 2009 Rolled out in 11 states in 2010 (HR, UK, HP, JH, CG, TR, Ar. P, ME, SI, MP, UP)
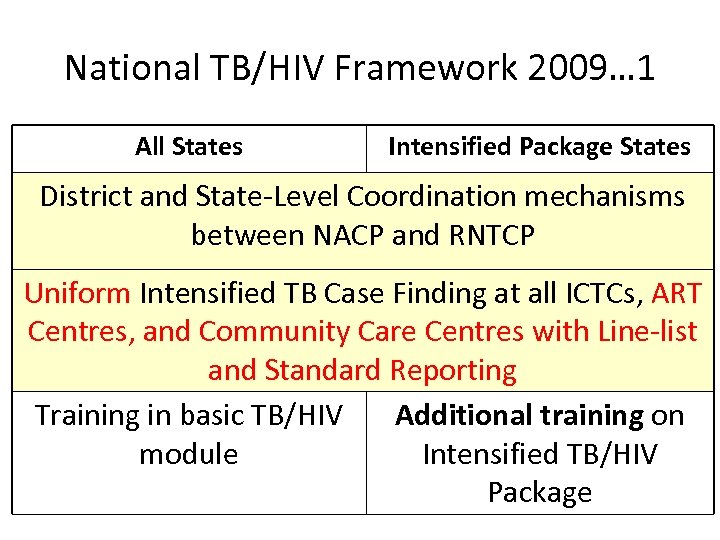
National TB/HIV Framework 2009… 1 All States Intensified Package States District and State-Level Coordination mechanisms between NACP and RNTCP Uniform Intensified TB Case Finding at all ICTCs, ART Centres, and Community Care Centres with Line-list and Standard Reporting Training in basic TB/HIV Additional training on module Intensified TB/HIV Package
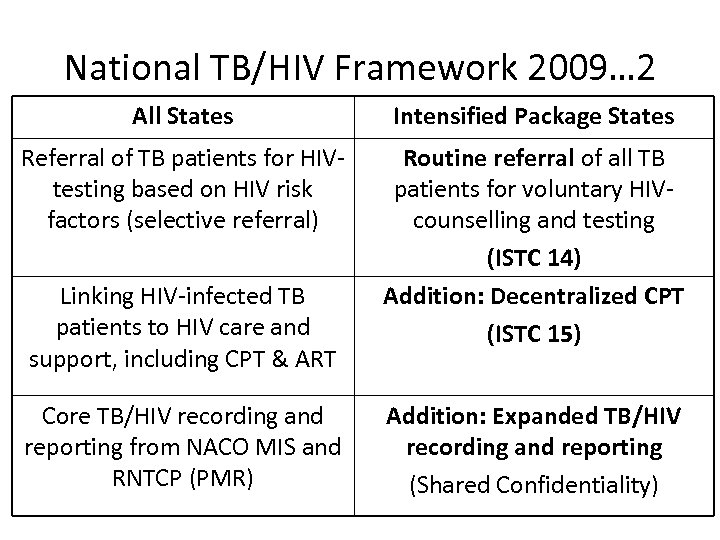
National TB/HIV Framework 2009… 2 All States Intensified Package States Referral of TB patients for HIVtesting based on HIV risk factors (selective referral) Routine referral of all TB patients for voluntary HIVcounselling and testing (ISTC 14) Addition: Decentralized CPT (ISTC 15) Linking HIV-infected TB patients to HIV care and support, including CPT & ART Core TB/HIV recording and reporting from NACO MIS and RNTCP (PMR) Addition: Expanded TB/HIV recording and reporting (Shared Confidentiality)

All TBHIV Training Modules revised
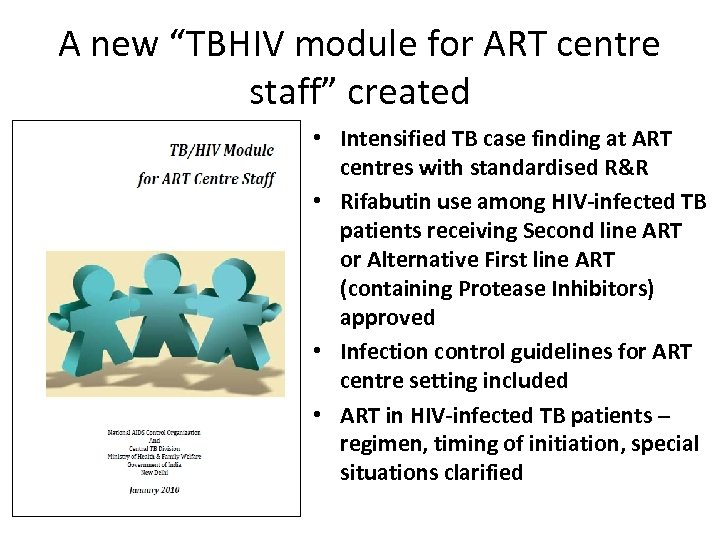
A new “TBHIV module for ART centre staff” created • Intensified TB case finding at ART centres with standardised R&R • Rifabutin use among HIV-infected TB patients receiving Second line ART or Alternative First line ART (containing Protease Inhibitors) approved • Infection control guidelines for ART centre setting included • ART in HIV-infected TB patients – regimen, timing of initiation, special situations clarified
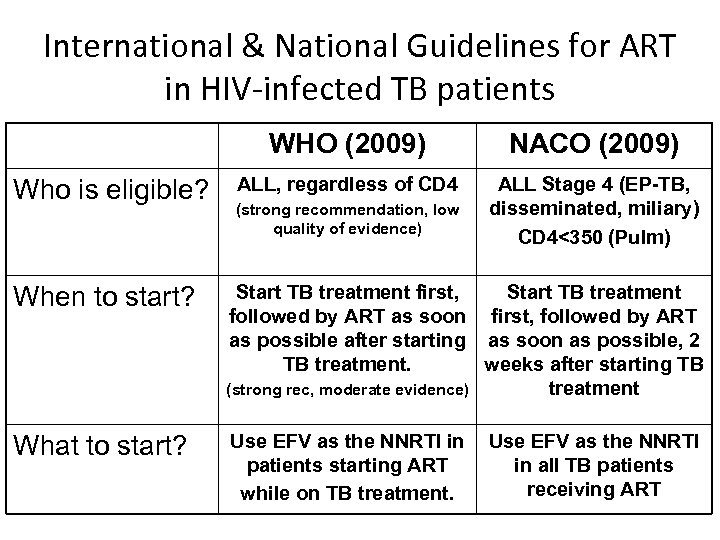
International & National Guidelines for ART in HIV-infected TB patients WHO (2009) Who is eligible? NACO (2009) ALL, regardless of CD 4 ALL Stage 4 (EP-TB, disseminated, miliary) CD 4<350 (Pulm) (strong recommendation, low quality of evidence) When to start? Start TB treatment first, Start TB treatment followed by ART as soon first, followed by ART as possible after starting as soon as possible, 2 TB treatment. weeks after starting TB (strong rec, moderate evidence) treatment What to start? Use EFV as the NNRTI in patients starting ART while on TB treatment. Use EFV as the NNRTI in all TB patients receiving ART

TB/HIV Performance
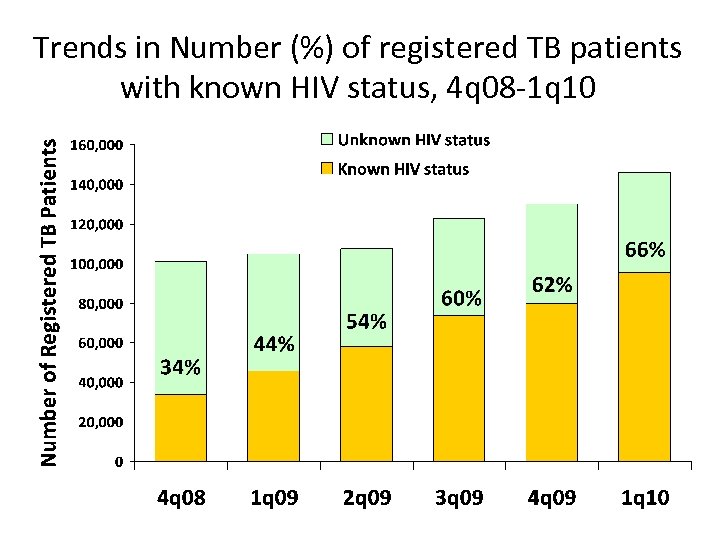
Trends in Number (%) of registered TB patients with known HIV status, 4 q 08 -1 q 10
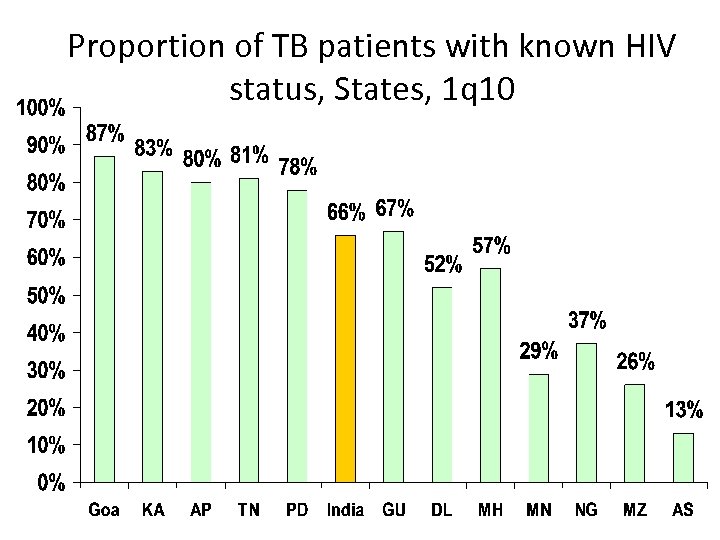
Proportion of TB patients with known HIV status, States, 1 q 10
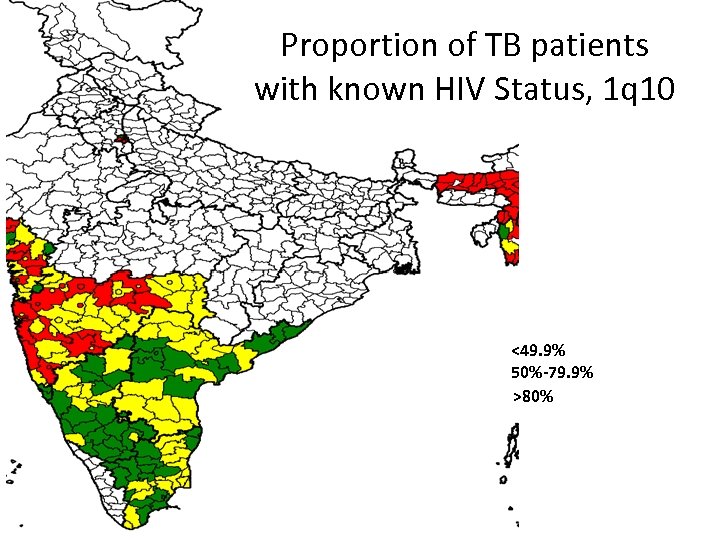
Proportion of TB patients with known HIV Status, 1 q 10 <49. 9% 50%-79. 9% >80%
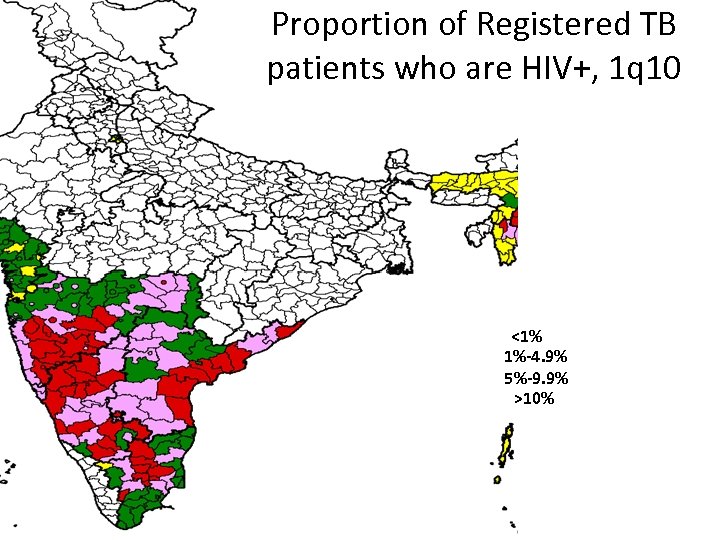
Proportion of Registered TB patients who are HIV+, 1 q 10 <1% 1%-4. 9% 5%-9. 9% >10%
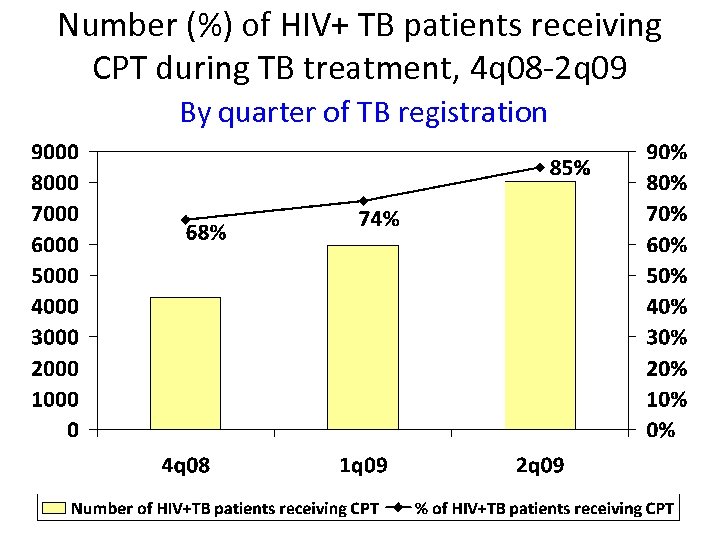
Number (%) of HIV+ TB patients receiving CPT during TB treatment, 4 q 08 -2 q 09 By quarter of TB registration
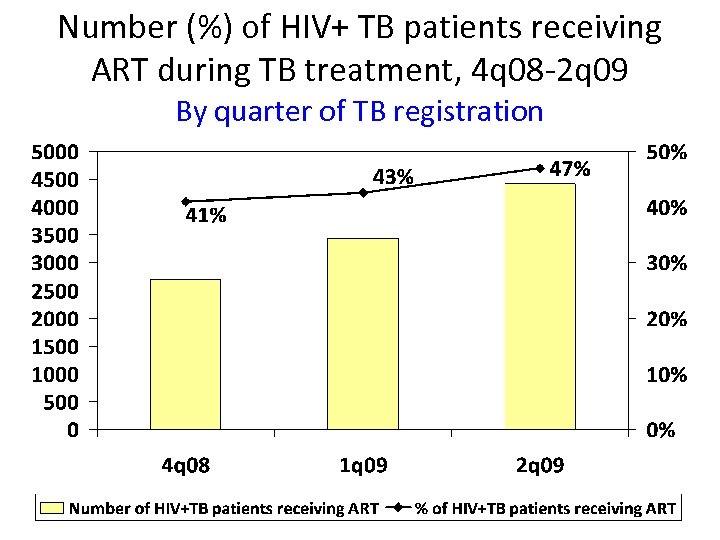
Number (%) of HIV+ TB patients receiving ART during TB treatment, 4 q 08 -2 q 09 By quarter of TB registration
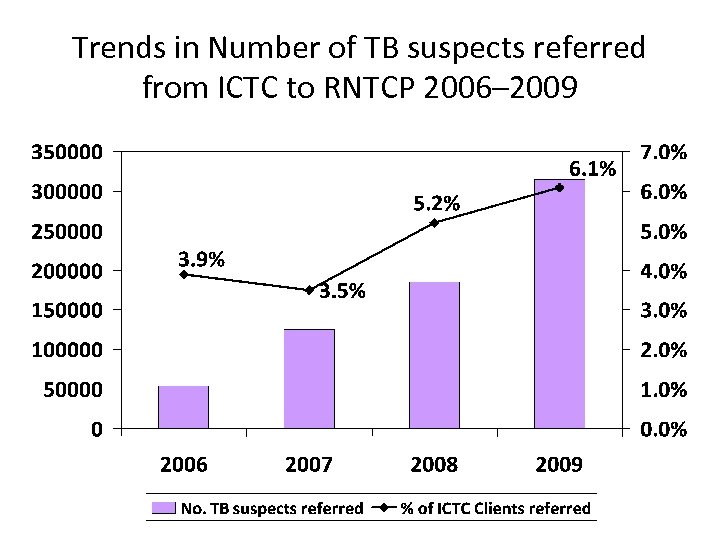
Trends in Number of TB suspects referred from ICTC to RNTCP 2006– 2009
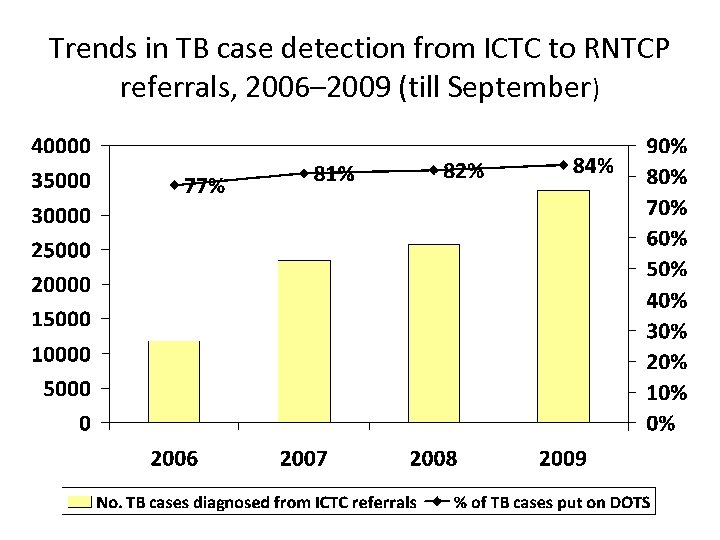
Trends in TB case detection from ICTC to RNTCP referrals, 2006– 2009 (till September)

Next Steps – 2010 -15 • Intensified TB/HIV package - Nationwide coverage by 2012 – Provider-initiated HIV testing for all TB patients – Immediate and accountable linkage of HIV-infected TB patients to NACP for HIV care and treatment • Intensified TB case finding and reporting – Consolidation in all HIV care settings • Completed clinical and operational research on IPT for TB/HIV with policy decisions • Implementation of airborne infection control measures • HIV Surveillance among TB suspects at some sentinel sites • RCT among HIV-infected TB patients comparing daily v/s intermittent regimens

Role of Medical College in TB/HIV collaborative activities • Academics – Frequent updates / CMEs for faculty and students – Demonstration of TB/HIV care settings to students • Patient Care – Implementation of ICF and IC at ICTCs and ART centres – Implementation of PITC for TB patients and Early ART initiation for HIV-infected TB patients • Research – Operational Research and PG Thesis – Funding available under RNTCP • Quality Assurance – Part of RNTCP Internal Evaluations and Joint TB/HIV Visits – Peer Pressure on professional colleagues to follow ISTC
Thanks. . A dedicated webpage for TB-HIV
1e50116aa3d279dc674dcde12dbdc9a7.ppt