60a0024bce008c80240aa5d1885157f5.ppt
- Количество слайдов: 45
 TAVR: Emerging Technologies, New Valves and Adjunctive Equipment Jeffrey J. Popma, MD Director, Interventional Cardiology Clinical Services Beth Israel Deaconess Medical Center Associate Professor of Medicine Harvard Medical School Boston, MA 1
TAVR: Emerging Technologies, New Valves and Adjunctive Equipment Jeffrey J. Popma, MD Director, Interventional Cardiology Clinical Services Beth Israel Deaconess Medical Center Associate Professor of Medicine Harvard Medical School Boston, MA 1
 Jeffrey J. Popma, MD §Contracted Research / Grant Support: §Abbott Vascular §Abiomed, Inc. §Atrium Medical Corporation §Boston Scientific Corporation §Cordis Corporation §IDEV Technologies, Inc. §Medtronic, Inc.
Jeffrey J. Popma, MD §Contracted Research / Grant Support: §Abbott Vascular §Abiomed, Inc. §Atrium Medical Corporation §Boston Scientific Corporation §Cordis Corporation §IDEV Technologies, Inc. §Medtronic, Inc.
 §Consulting Fees: §Abbott Vascular §Boston Scientific Corporation My presentation will include off label discussions: Percutaneous aortic valves
§Consulting Fees: §Abbott Vascular §Boston Scientific Corporation My presentation will include off label discussions: Percutaneous aortic valves
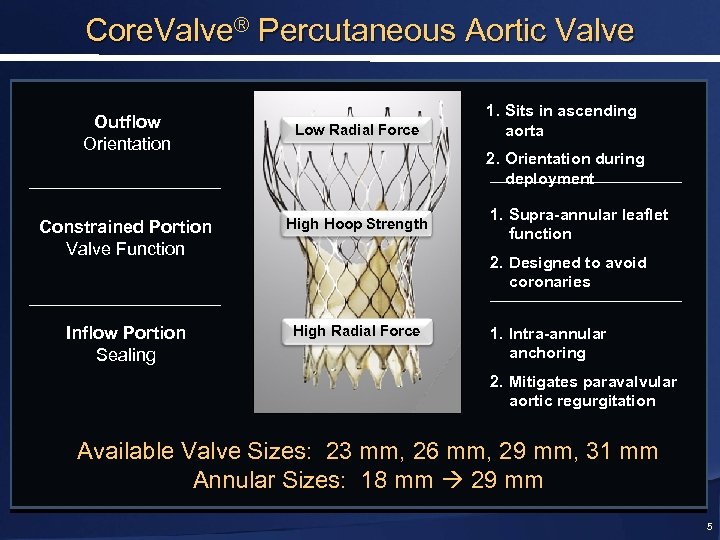 Core. Valve® Percutaneous Aortic Valve Outflow Orientation Low Radial Force 1. Sits in ascending aorta 2. Orientation during deployment Constrained Portion Valve Function High Hoop Strength Inflow Portion Sealing High Radial Force 1. Supra-annular leaflet function 2. Designed to avoid coronaries 1. Intra-annular anchoring 2. Mitigates paravalvular aortic regurgitation Available Valve Sizes: 23 mm, 26 mm, 29 mm, 31 mm Annular Sizes: 18 mm 29 mm 5
Core. Valve® Percutaneous Aortic Valve Outflow Orientation Low Radial Force 1. Sits in ascending aorta 2. Orientation during deployment Constrained Portion Valve Function High Hoop Strength Inflow Portion Sealing High Radial Force 1. Supra-annular leaflet function 2. Designed to avoid coronaries 1. Intra-annular anchoring 2. Mitigates paravalvular aortic regurgitation Available Valve Sizes: 23 mm, 26 mm, 29 mm, 31 mm Annular Sizes: 18 mm 29 mm 5
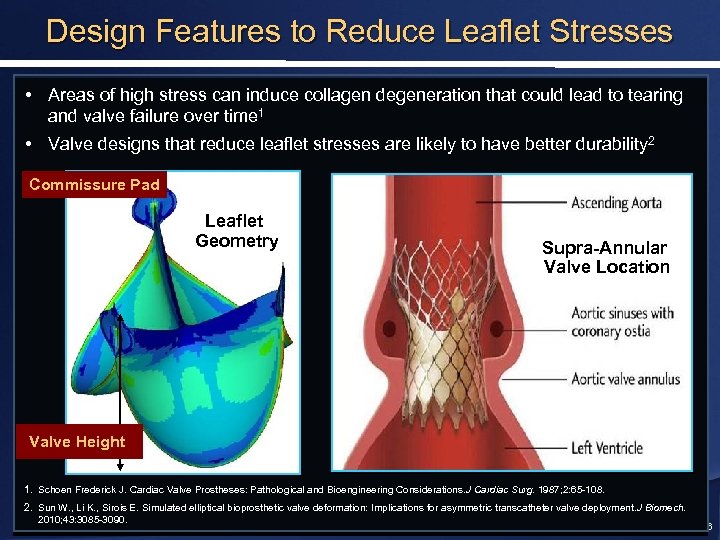 Design Features to Reduce Leaflet Stresses • Areas of high stress can induce collagen degeneration that could lead to tearing and valve failure over time 1 • Valve designs that reduce leaflet stresses are likely to have better durability 2 Commissure Pad Leaflet Geometry Supra-Annular Valve Location Valve Height 1. Schoen Frederick J. Cardiac Valve Prostheses: Pathological and Bioengineering Considerations. J Cardiac Surg. 1987; 2: 65 -108. 2. Sun W. , Li K. , Sirois E. Simulated elliptical bioprosthetic valve deformation: Implications for asymmetric transcatheter valve deployment. J Biomech. 2010; 43: 3085 -3090. 6
Design Features to Reduce Leaflet Stresses • Areas of high stress can induce collagen degeneration that could lead to tearing and valve failure over time 1 • Valve designs that reduce leaflet stresses are likely to have better durability 2 Commissure Pad Leaflet Geometry Supra-Annular Valve Location Valve Height 1. Schoen Frederick J. Cardiac Valve Prostheses: Pathological and Bioengineering Considerations. J Cardiac Surg. 1987; 2: 65 -108. 2. Sun W. , Li K. , Sirois E. Simulated elliptical bioprosthetic valve deformation: Implications for asymmetric transcatheter valve deployment. J Biomech. 2010; 43: 3085 -3090. 6
 Majority of US Core. Valve Implants in Hybrid ORs 7
Majority of US Core. Valve Implants in Hybrid ORs 7
 Core. Valve US Trial: Current Status Core. Valve U. S. Pivotal Trial d te t le en p m llm 12 o o 0 C nr 2 E an J “Extreme Risk” (Up to 687) “High Risk” N=790 Iliofemoral access ? No Core. Valve Observational Up to 200 Randomization 1: 1* Yes Core. Valve Single Arm N=487 Core. Valve N=395 SAVR N=395 8
Core. Valve US Trial: Current Status Core. Valve U. S. Pivotal Trial d te t le en p m llm 12 o o 0 C nr 2 E an J “Extreme Risk” (Up to 687) “High Risk” N=790 Iliofemoral access ? No Core. Valve Observational Up to 200 Randomization 1: 1* Yes Core. Valve Single Arm N=487 Core. Valve N=395 SAVR N=395 8
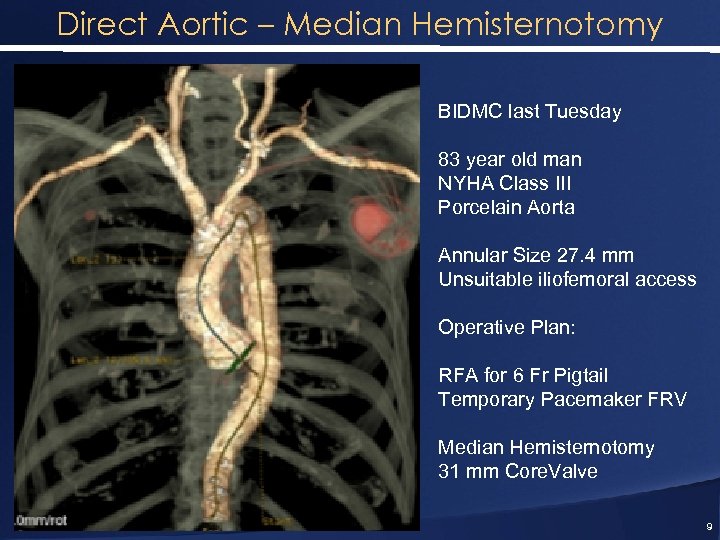 Direct Aortic – Median Hemisternotomy BIDMC last Tuesday 83 year old man NYHA Class III Porcelain Aorta Annular Size 27. 4 mm Unsuitable iliofemoral access Operative Plan: RFA for 6 Fr Pigtail Temporary Pacemaker FRV Median Hemisternotomy 31 mm Core. Valve 9
Direct Aortic – Median Hemisternotomy BIDMC last Tuesday 83 year old man NYHA Class III Porcelain Aorta Annular Size 27. 4 mm Unsuitable iliofemoral access Operative Plan: RFA for 6 Fr Pigtail Temporary Pacemaker FRV Median Hemisternotomy 31 mm Core. Valve 9
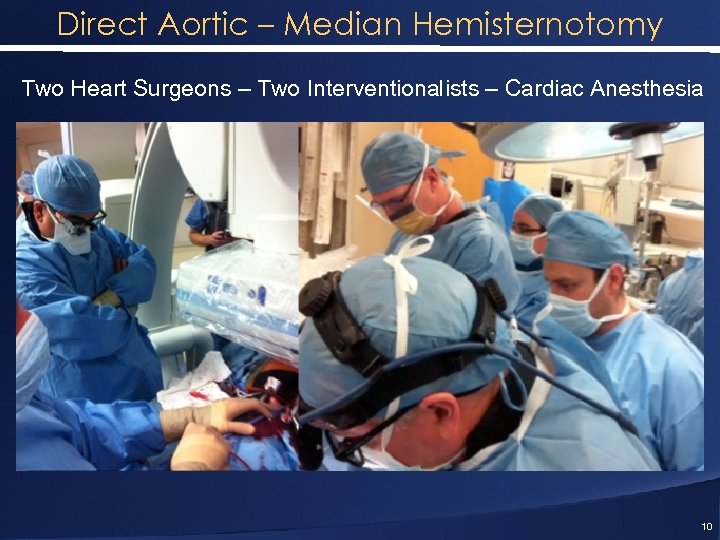 Direct Aortic – Median Hemisternotomy Two Heart Surgeons – Two Interventionalists – Cardiac Anesthesia 10
Direct Aortic – Median Hemisternotomy Two Heart Surgeons – Two Interventionalists – Cardiac Anesthesia 10
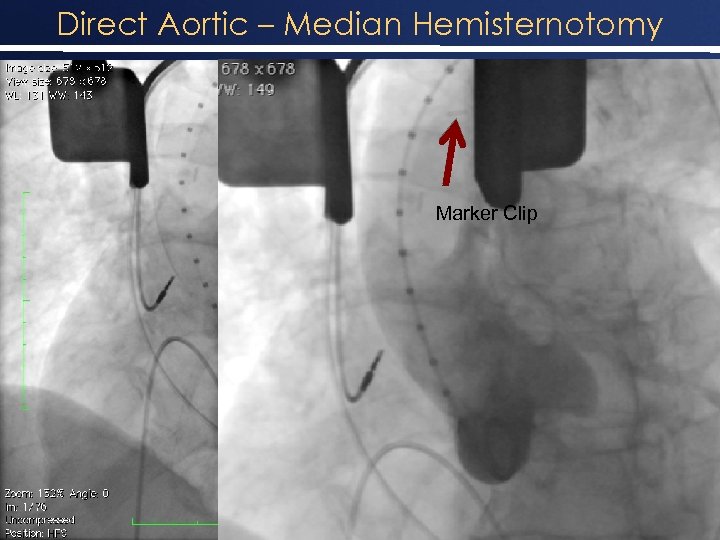 Direct Aortic – Median Hemisternotomy Marker Clip 11
Direct Aortic – Median Hemisternotomy Marker Clip 11
 Direct Aortic – Median Hemisternotomy 12
Direct Aortic – Median Hemisternotomy 12
 Direct Aortic – Median Hemisternotomy 13
Direct Aortic – Median Hemisternotomy 13
 Direct Aortic – Median Hemisternotomy 14
Direct Aortic – Median Hemisternotomy 14
 Direct Aortic – Median Hemisternotomy Overnight CV-ICU Ambulating Next Day Home on POD #4 15
Direct Aortic – Median Hemisternotomy Overnight CV-ICU Ambulating Next Day Home on POD #4 15
 TAVR – What We Have Learned • Embolic strokes have become a major concern following TAVR --- new technology will focus on reducing the occurrence of neurologic events after the procedure • Vascular complications and closure devices • New TAVR devices - Repositionable and Retrieval - Reducing peri-valvular regurgitation - New Transapical devices 16
TAVR – What We Have Learned • Embolic strokes have become a major concern following TAVR --- new technology will focus on reducing the occurrence of neurologic events after the procedure • Vascular complications and closure devices • New TAVR devices - Repositionable and Retrieval - Reducing peri-valvular regurgitation - New Transapical devices 16
 Stroke Following s. AVR and TAVR Etiology of Strokes • During TAVR: TCD has shown that the majority of procedural embolic events occurred during BAV, manipulation of catheters across the aortic valve, and valve implantation. • During AVR, TCD evidence of emboli during insertion of an aortic cannula at the start of CPB and after declamping the aorta • Late embolic events post-AVR are presumably caused by debris from the prosthesis and atrial fibrillation Daneault J Am Coll Cardiol 2011; 58: 2143– 50 17
Stroke Following s. AVR and TAVR Etiology of Strokes • During TAVR: TCD has shown that the majority of procedural embolic events occurred during BAV, manipulation of catheters across the aortic valve, and valve implantation. • During AVR, TCD evidence of emboli during insertion of an aortic cannula at the start of CPB and after declamping the aorta • Late embolic events post-AVR are presumably caused by debris from the prosthesis and atrial fibrillation Daneault J Am Coll Cardiol 2011; 58: 2143– 50 17
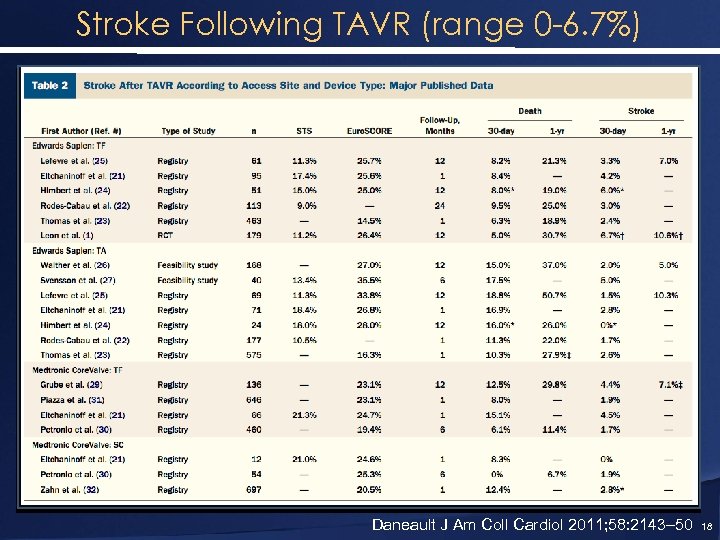 Stroke Following TAVR (range 0 -6. 7%) Daneault J Am Coll Cardiol 2011; 58: 2143– 50 18
Stroke Following TAVR (range 0 -6. 7%) Daneault J Am Coll Cardiol 2011; 58: 2143– 50 18
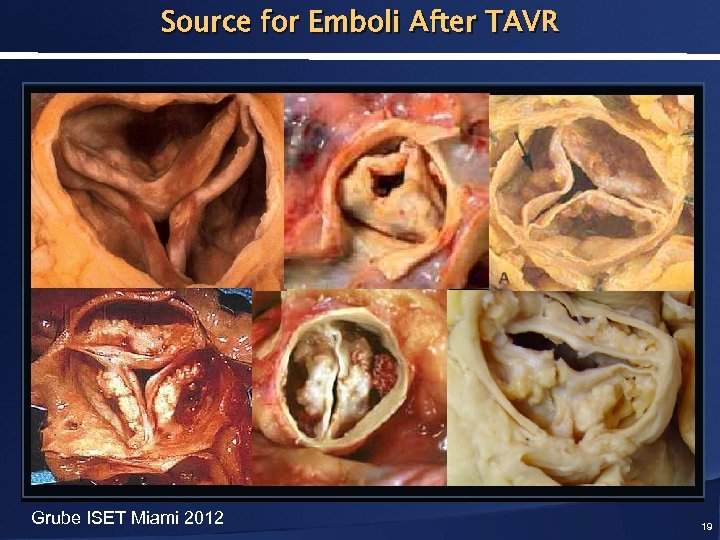 Source for Emboli After TAVR Grube ISET Miami 2012 19
Source for Emboli After TAVR Grube ISET Miami 2012 19
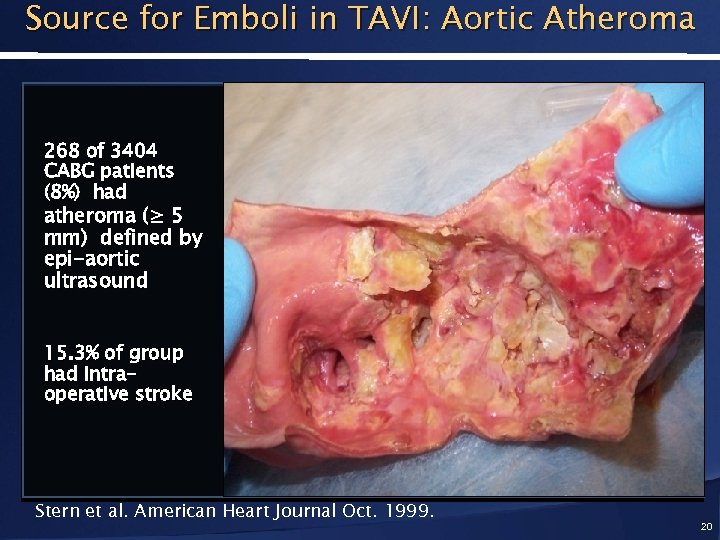 Source for Emboli in TAVI: Aortic Atheroma 268 of 3404 CABG patients (8%) had atheroma (≥ 5 mm) defined by epi-aortic ultrasound 15. 3% of group had intraoperative stroke Stern et al. American Heart Journal Oct. 1999. 20
Source for Emboli in TAVI: Aortic Atheroma 268 of 3404 CABG patients (8%) had atheroma (≥ 5 mm) defined by epi-aortic ultrasound 15. 3% of group had intraoperative stroke Stern et al. American Heart Journal Oct. 1999. 20
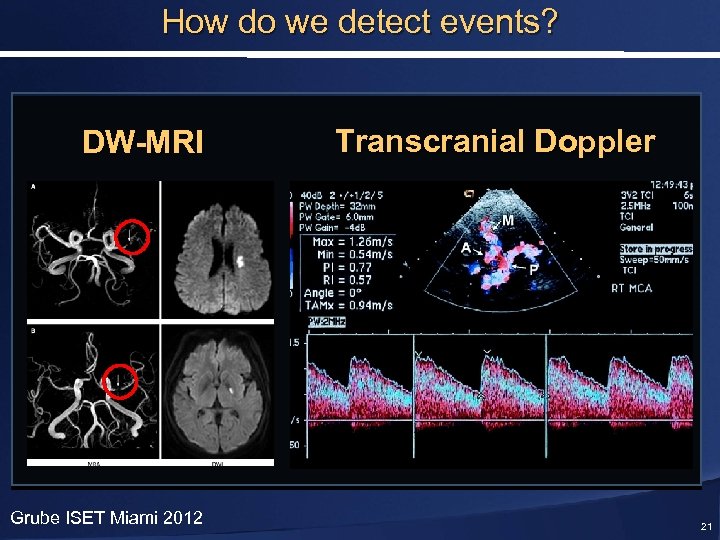 How do we detect events? DW-MRI Grube ISET Miami 2012 Transcranial Doppler 21
How do we detect events? DW-MRI Grube ISET Miami 2012 Transcranial Doppler 21
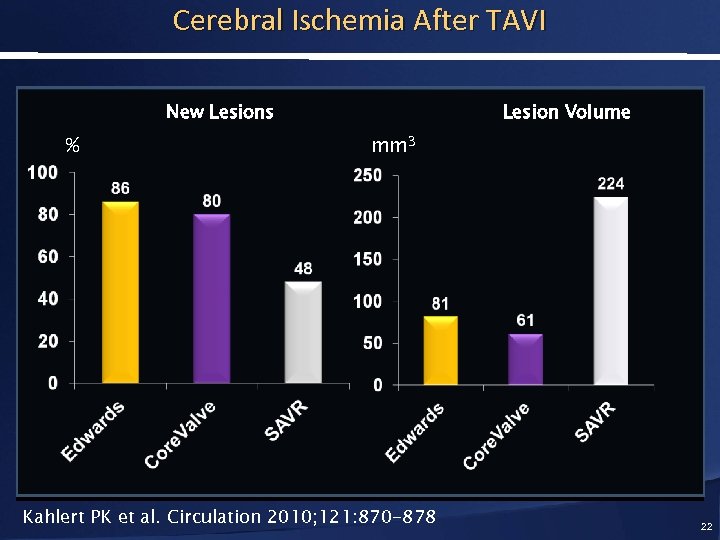 Cerebral Ischemia After TAVI New Lesions % Lesion Volume mm 3 Kahlert PK et al. Circulation 2010; 121: 870 -878 22
Cerebral Ischemia After TAVI New Lesions % Lesion Volume mm 3 Kahlert PK et al. Circulation 2010; 121: 870 -878 22
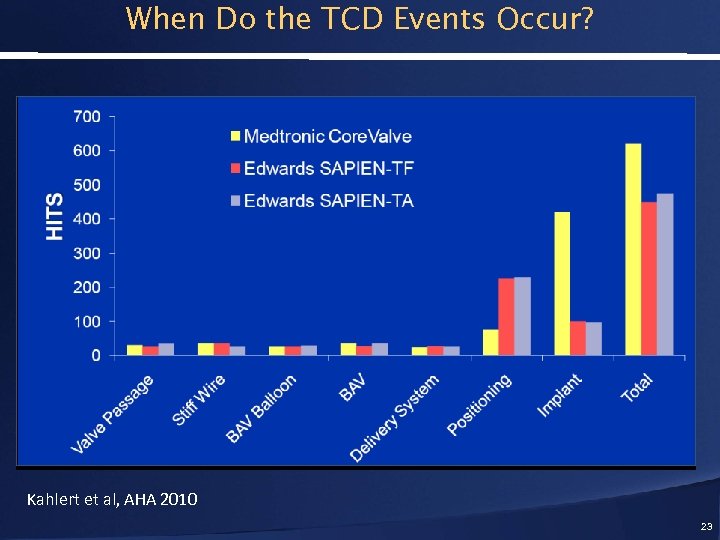 When Do the TCD Events Occur? Kahlert et al, AHA 2010 23
When Do the TCD Events Occur? Kahlert et al, AHA 2010 23
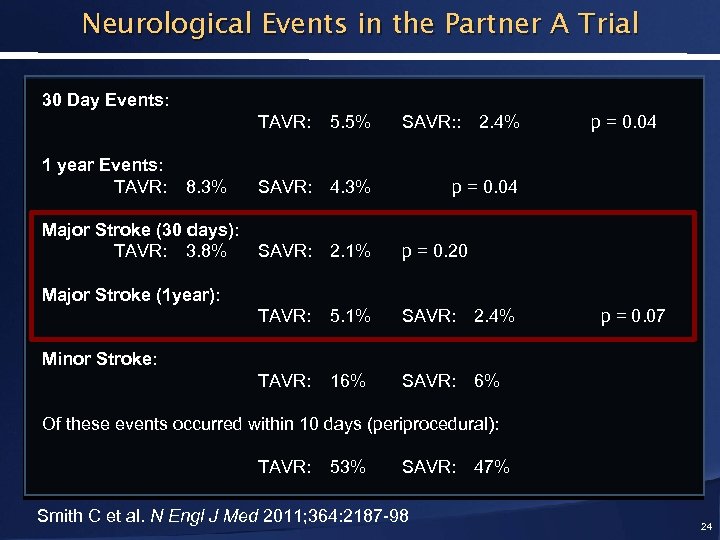 Neurological Events in the Partner A Trial 30 Day Events: TAVR: 1 year Events: TAVR: 8. 3% Major Stroke (30 days): TAVR: 3. 8% 5. 5% SAVR: : 2. 4% SAVR: 4. 3% p = 0. 04 SAVR: 2. 1% p = 0. 20 TAVR: 5. 1% SAVR: 2. 4% TAVR: 16% p = 0. 04 SAVR: 6% Major Stroke (1 year): p = 0. 07 Minor Stroke: Of these events occurred within 10 days (periprocedural): TAVR: 53% SAVR: Smith C et al. N Engl J Med 2011; 364: 2187 -98 47% 24
Neurological Events in the Partner A Trial 30 Day Events: TAVR: 1 year Events: TAVR: 8. 3% Major Stroke (30 days): TAVR: 3. 8% 5. 5% SAVR: : 2. 4% SAVR: 4. 3% p = 0. 04 SAVR: 2. 1% p = 0. 20 TAVR: 5. 1% SAVR: 2. 4% TAVR: 16% p = 0. 04 SAVR: 6% Major Stroke (1 year): p = 0. 07 Minor Stroke: Of these events occurred within 10 days (periprocedural): TAVR: 53% SAVR: Smith C et al. N Engl J Med 2011; 364: 2187 -98 47% 24
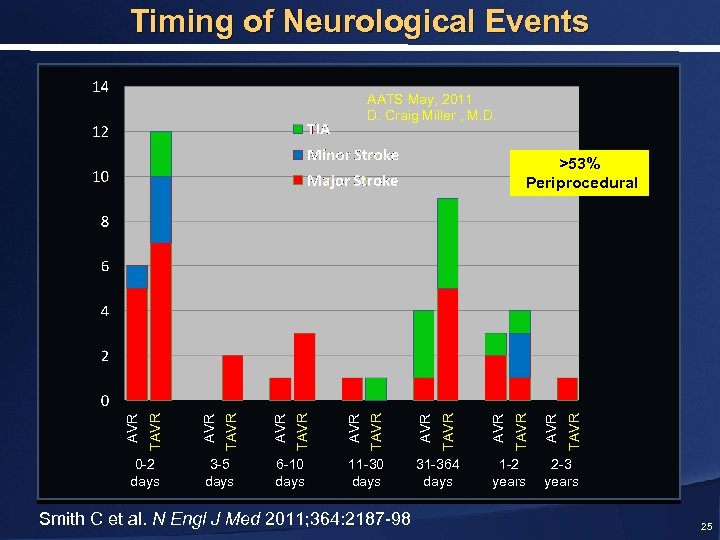 Timing of Neurological Events AATS May, 2011 D. Craig Miller , M. D. 0 -2 days 3 -5 days 6 -10 days 11 -30 days Smith C et al. N Engl J Med 2011; 364: 2187 -98 31 -364 days 1 -2 years TAVR AVR TAVR AVR TAVR >53% Periprocedural 2 -3 years 25
Timing of Neurological Events AATS May, 2011 D. Craig Miller , M. D. 0 -2 days 3 -5 days 6 -10 days 11 -30 days Smith C et al. N Engl J Med 2011; 364: 2187 -98 31 -364 days 1 -2 years TAVR AVR TAVR AVR TAVR >53% Periprocedural 2 -3 years 25
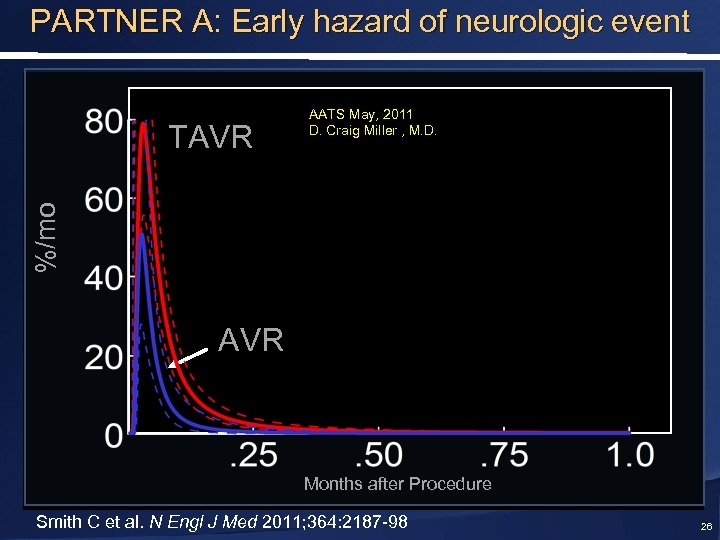 PARTNER A: Early hazard of neurologic event %/mo TAVR AATS May, 2011 D. Craig Miller , M. D. AVR Months after Procedure Smith C et al. N Engl J Med 2011; 364: 2187 -98 26
PARTNER A: Early hazard of neurologic event %/mo TAVR AATS May, 2011 D. Craig Miller , M. D. AVR Months after Procedure Smith C et al. N Engl J Med 2011; 364: 2187 -98 26
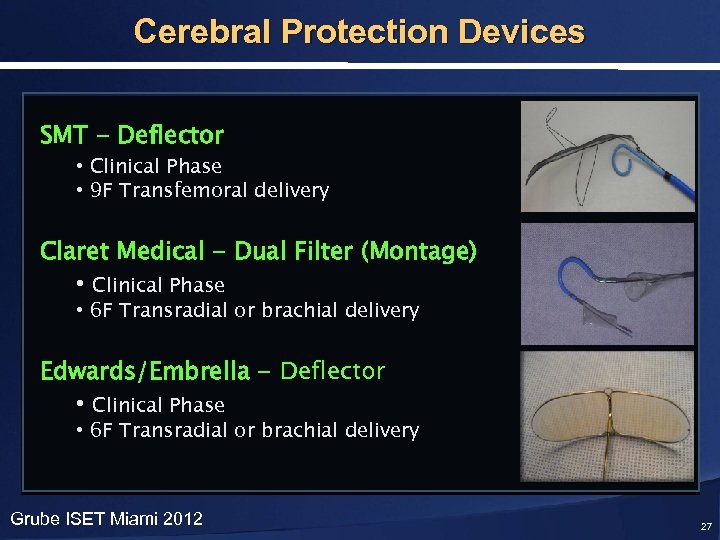 Cerebral Protection Devices SMT - Deflector • Clinical Phase • 9 F Transfemoral delivery Claret Medical - Dual Filter (Montage) • Clinical Phase • 6 F Transradial or brachial delivery Edwards/Embrella - Deflector • Clinical Phase • 6 F Transradial or brachial delivery Grube ISET Miami 2012 27
Cerebral Protection Devices SMT - Deflector • Clinical Phase • 9 F Transfemoral delivery Claret Medical - Dual Filter (Montage) • Clinical Phase • 6 F Transradial or brachial delivery Edwards/Embrella - Deflector • Clinical Phase • 6 F Transradial or brachial delivery Grube ISET Miami 2012 27
 Claret Dual Filter “Montage” 2 nd Generation Grube ISET Miami 2012 28
Claret Dual Filter “Montage” 2 nd Generation Grube ISET Miami 2012 28
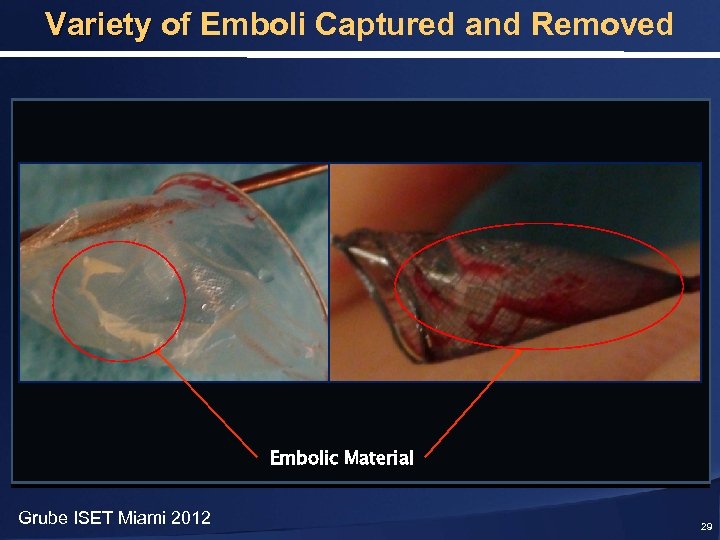 Variety of Emboli Captured and Removed Embolic Material Grube ISET Miami 2012 29
Variety of Emboli Captured and Removed Embolic Material Grube ISET Miami 2012 29
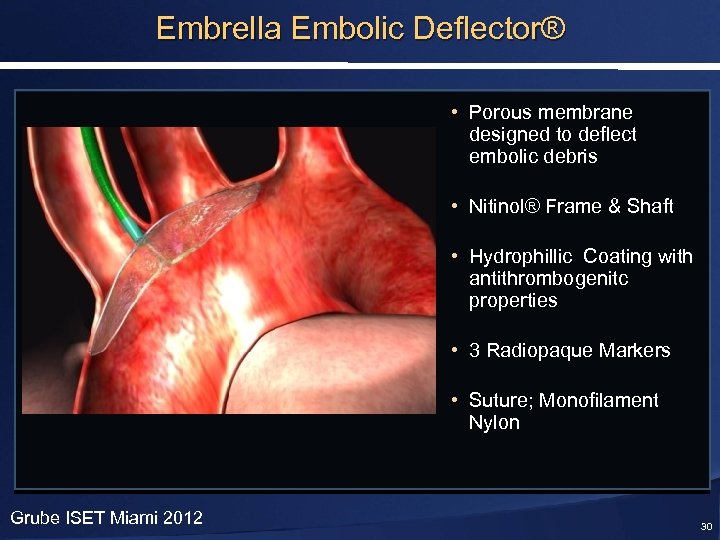 Embrella Embolic Deflector® • Porous membrane designed to deflect embolic debris • Nitinol® Frame & Shaft • Hydrophillic Coating with antithrombogenitc properties • 3 Radiopaque Markers • Suture; Monofilament Nylon Grube ISET Miami 2012 30
Embrella Embolic Deflector® • Porous membrane designed to deflect embolic debris • Nitinol® Frame & Shaft • Hydrophillic Coating with antithrombogenitc properties • 3 Radiopaque Markers • Suture; Monofilament Nylon Grube ISET Miami 2012 30
 Edwards-Embrella Deflector Grube ISET Miami 2012 31
Edwards-Embrella Deflector Grube ISET Miami 2012 31
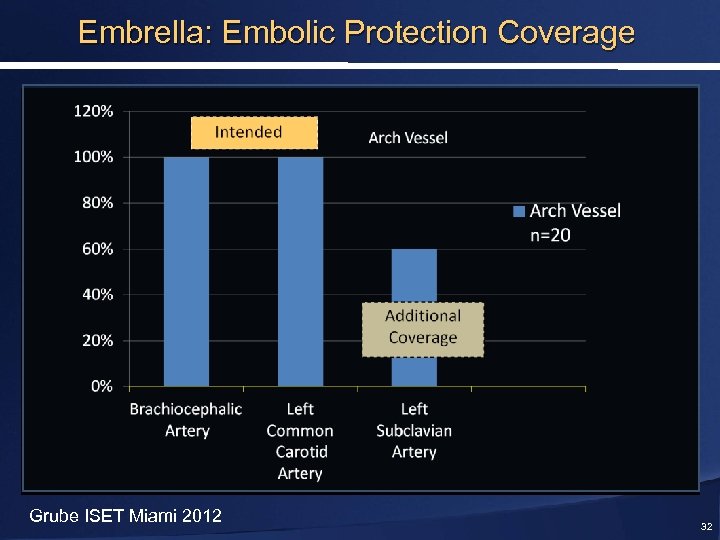 Embrella: Embolic Protection Coverage Grube ISET Miami 2012 32
Embrella: Embolic Protection Coverage Grube ISET Miami 2012 32
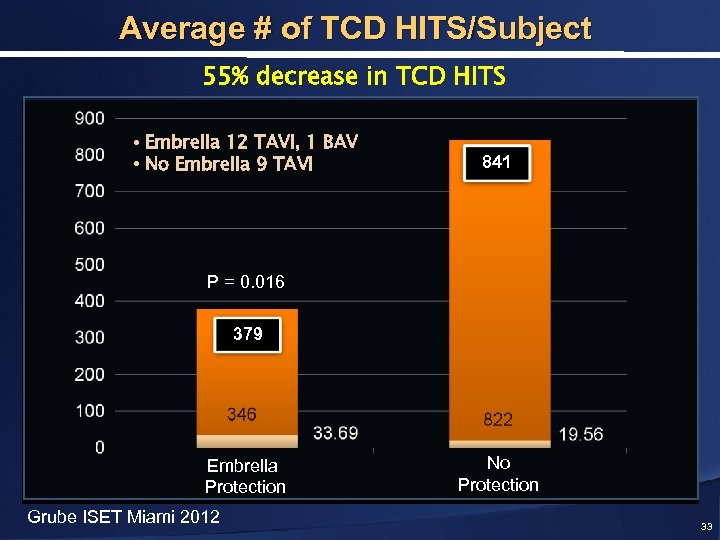 Average # of TCD HITS/Subject 55% decrease in TCD HITS • Embrella 12 TAVI, 1 BAV • No Embrella 9 TAVI 841 P = 0. 016 379 Embrella Protection Grube ISET Miami 2012 No Protection 33
Average # of TCD HITS/Subject 55% decrease in TCD HITS • Embrella 12 TAVI, 1 BAV • No Embrella 9 TAVI 841 P = 0. 016 379 Embrella Protection Grube ISET Miami 2012 No Protection 33
 TAVR – What We Have Learned • Embolic strokes have become a major concern following TAVR --- new technology will focus on reducing the occurrence of neurologic events after the procedure • Vascular complications and closure devices • New TAVR devices - Repositionable and Retrieval - Reducing peri-valvular regurgitation - New Transapical devices Grube ISET Miami 2012 34
TAVR – What We Have Learned • Embolic strokes have become a major concern following TAVR --- new technology will focus on reducing the occurrence of neurologic events after the procedure • Vascular complications and closure devices • New TAVR devices - Repositionable and Retrieval - Reducing peri-valvular regurgitation - New Transapical devices Grube ISET Miami 2012 34
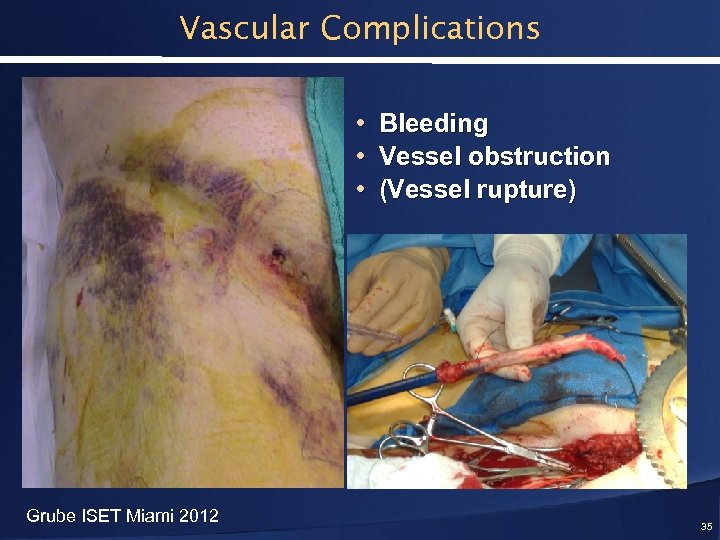 Vascular Complications • Bleeding • Vessel obstruction • (Vessel rupture) Grube ISET Miami 2012 35
Vascular Complications • Bleeding • Vessel obstruction • (Vessel rupture) Grube ISET Miami 2012 35
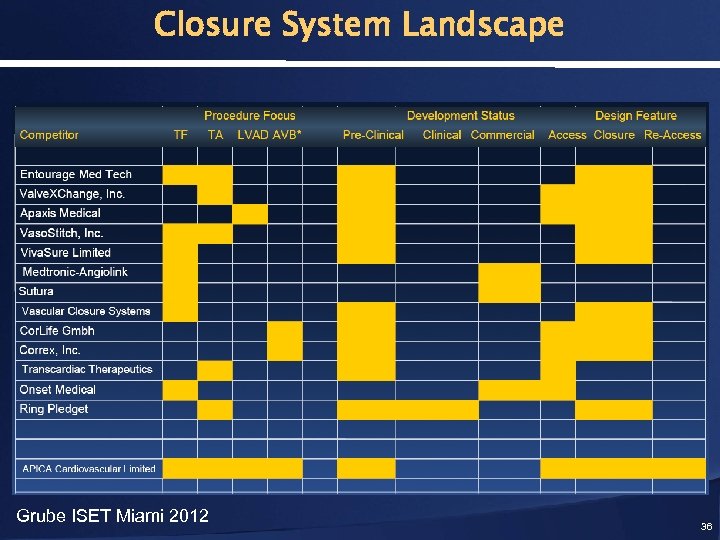 Closure System Landscape Grube ISET Miami 2012 36
Closure System Landscape Grube ISET Miami 2012 36
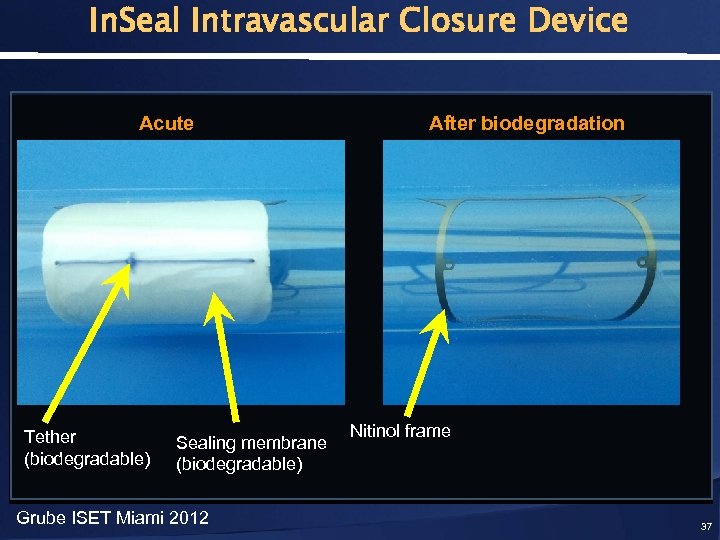 In. Seal Intravascular Closure Device Acute Tether (biodegradable) Sealing membrane (biodegradable) Grube ISET Miami 2012 After biodegradation Nitinol frame 37
In. Seal Intravascular Closure Device Acute Tether (biodegradable) Sealing membrane (biodegradable) Grube ISET Miami 2012 After biodegradation Nitinol frame 37
 TAVR – What We Have Learned • Embolic strokes have become a major concern following TAVR --- new technology will focus on reducing the occurrence of neurologic events after the procedure • Vascular complications and closure devices • New TAVR devices - Repositionable and Retrieval - Reducing peri-valvular regurgitation - New Transapical devices Grube ISET Miami 2012 38
TAVR – What We Have Learned • Embolic strokes have become a major concern following TAVR --- new technology will focus on reducing the occurrence of neurologic events after the procedure • Vascular complications and closure devices • New TAVR devices - Repositionable and Retrieval - Reducing peri-valvular regurgitation - New Transapical devices Grube ISET Miami 2012 38
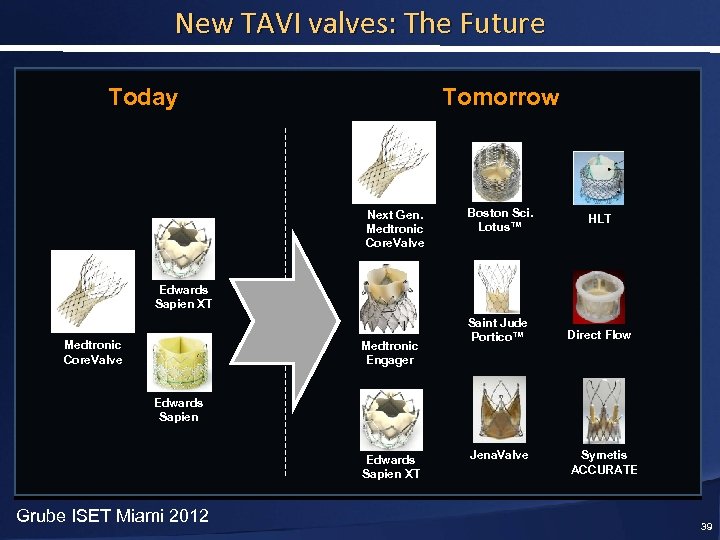 New TAVI valves: The Future Today Tomorrow Next Gen. Medtronic Core. Valve Boston Sci. Lotus™ HLT Saint Jude Portico™ Direct Flow Edwards Sapien XT Medtronic Core. Valve Medtronic Engager Edwards Sapien XT Grube ISET Miami 2012 Jena. Valve Symetis ACCURATE 39
New TAVI valves: The Future Today Tomorrow Next Gen. Medtronic Core. Valve Boston Sci. Lotus™ HLT Saint Jude Portico™ Direct Flow Edwards Sapien XT Medtronic Core. Valve Medtronic Engager Edwards Sapien XT Grube ISET Miami 2012 Jena. Valve Symetis ACCURATE 39
 Sadra Lotus™ Valve Concept • Braided nitinol stent structure • Radial expansion as it shortens § Enables a more flexible delivery system § Enables device repositioning or retrieval § Provides significant radial strength • Nitinol Frame designed for retrieval and repositioning • Adaptive Seal to conform to irregular anatomical surfaces, and to minimize perivalvula leaks • Locking Mechanism • Bovine Pericardium Proven material 40
Sadra Lotus™ Valve Concept • Braided nitinol stent structure • Radial expansion as it shortens § Enables a more flexible delivery system § Enables device repositioning or retrieval § Provides significant radial strength • Nitinol Frame designed for retrieval and repositioning • Adaptive Seal to conform to irregular anatomical surfaces, and to minimize perivalvula leaks • Locking Mechanism • Bovine Pericardium Proven material 40
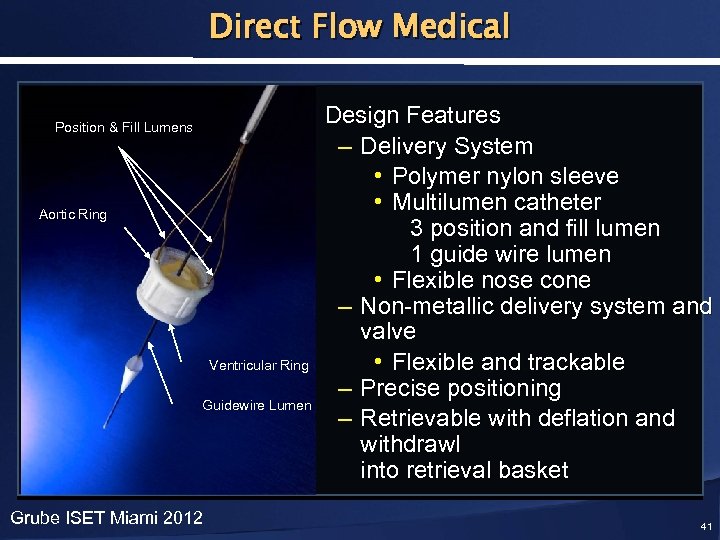 Direct Flow Medical Position & Fill Lumens Aortic Ring • Design Features – Delivery System • Polymer nylon sleeve • Multilumen catheter 3 position and fill lumen 1 guide wire lumen • Flexible nose cone – Non-metallic delivery system and valve Ventricular Ring • Flexible and trackable – Precise positioning Guidewire Lumen – Retrievable with deflation and withdrawl into retrieval basket Grube ISET Miami 2012 41
Direct Flow Medical Position & Fill Lumens Aortic Ring • Design Features – Delivery System • Polymer nylon sleeve • Multilumen catheter 3 position and fill lumen 1 guide wire lumen • Flexible nose cone – Non-metallic delivery system and valve Ventricular Ring • Flexible and trackable – Precise positioning Guidewire Lumen – Retrievable with deflation and withdrawl into retrieval basket Grube ISET Miami 2012 41
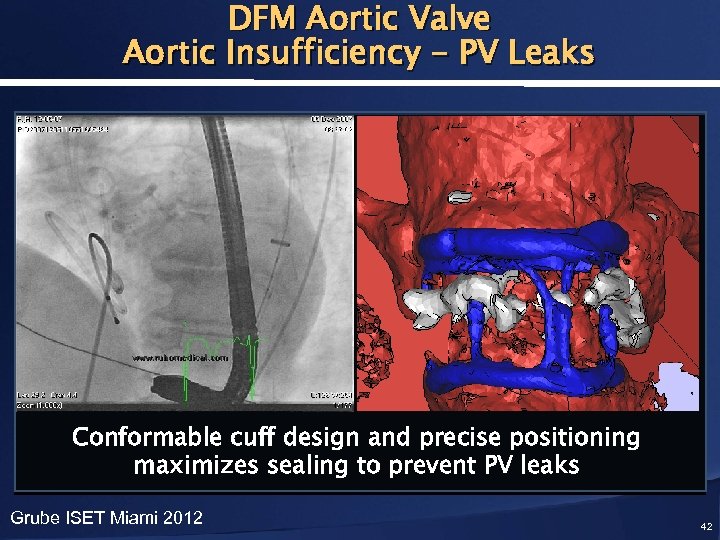 DFM Aortic Valve Aortic Insufficiency - PV Leaks Conformable cuff design and precise positioning maximizes sealing to prevent PV leaks Grube ISET Miami 2012 42
DFM Aortic Valve Aortic Insufficiency - PV Leaks Conformable cuff design and precise positioning maximizes sealing to prevent PV leaks Grube ISET Miami 2012 42
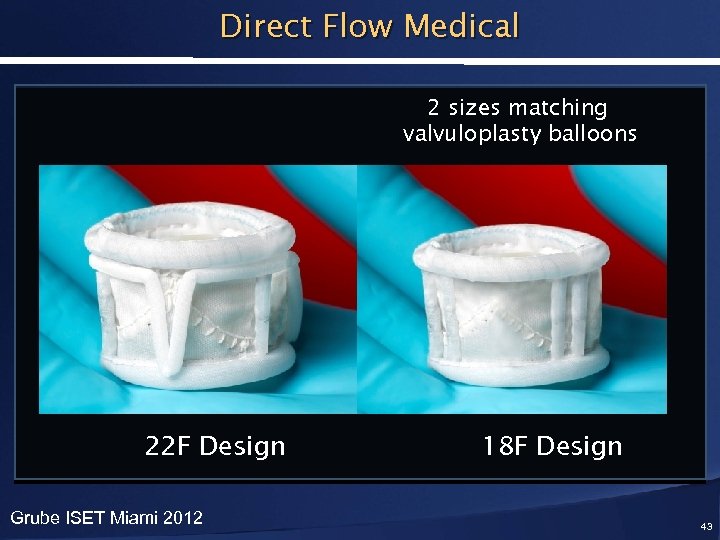 Direct Flow Medical 2 sizes matching valvuloplasty balloons 22 F Design Grube ISET Miami 2012 18 F Design 43
Direct Flow Medical 2 sizes matching valvuloplasty balloons 22 F Design Grube ISET Miami 2012 18 F Design 43
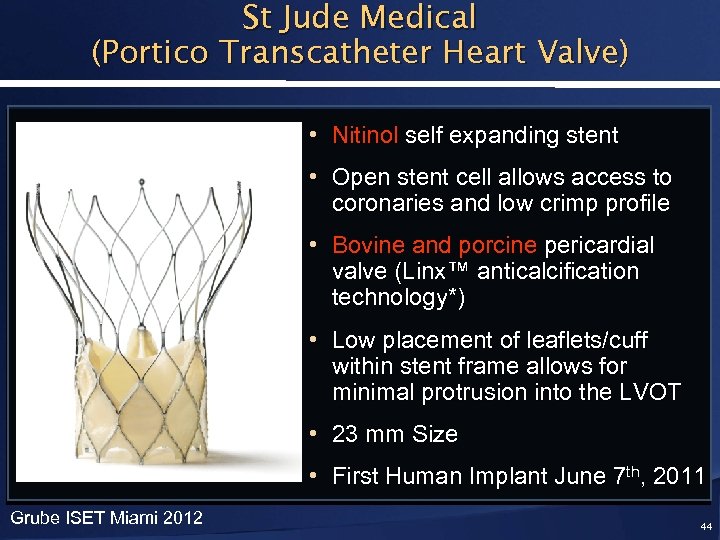 St Jude Medical (Portico Transcatheter Heart Valve) • Nitinol self expanding stent • Open stent cell allows access to coronaries and low crimp profile • Bovine and porcine pericardial valve (Linx™ anticalcification technology*) • Low placement of leaflets/cuff within stent frame allows for minimal protrusion into the LVOT • 23 mm Size • First Human Implant June 7 th, 2011 Grube ISET Miami 2012 44
St Jude Medical (Portico Transcatheter Heart Valve) • Nitinol self expanding stent • Open stent cell allows access to coronaries and low crimp profile • Bovine and porcine pericardial valve (Linx™ anticalcification technology*) • Low placement of leaflets/cuff within stent frame allows for minimal protrusion into the LVOT • 23 mm Size • First Human Implant June 7 th, 2011 Grube ISET Miami 2012 44
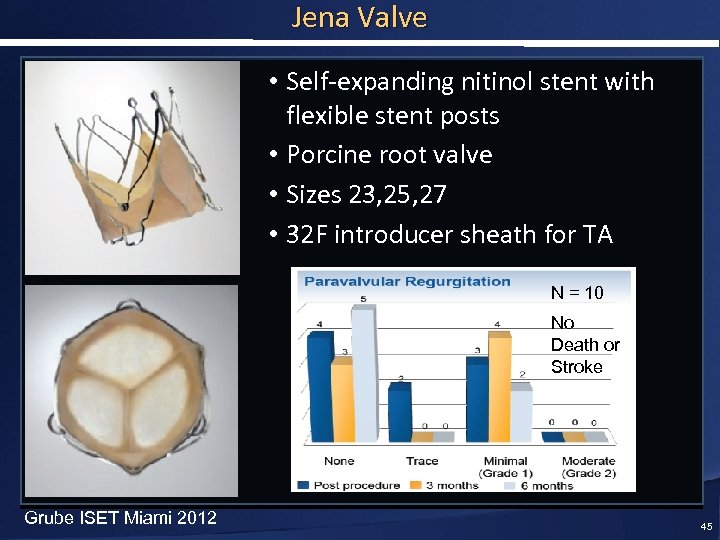 Jena Valve • Self-expanding nitinol stent with flexible stent posts • Porcine root valve • Sizes 23, 25, 27 • 32 F introducer sheath for TA N = 10 No Death or Stroke Grube ISET Miami 2012 45
Jena Valve • Self-expanding nitinol stent with flexible stent posts • Porcine root valve • Sizes 23, 25, 27 • 32 F introducer sheath for TA N = 10 No Death or Stroke Grube ISET Miami 2012 45
 TAVR – What We Have Learned • Most “High Risk” “High Gain Procedure • Present focus in clinical trials will be on stroke detection and prevention cerebroprotection devices will provide only part of the answer • Better imaging (CTA, intraprocedural echocardiography) will help reduce perivalvular regurgitation and vascular complications • Dedicated vascular closure devices in development • New TAVR designs (TF and TA) will also help reduce procedural complications 46
TAVR – What We Have Learned • Most “High Risk” “High Gain Procedure • Present focus in clinical trials will be on stroke detection and prevention cerebroprotection devices will provide only part of the answer • Better imaging (CTA, intraprocedural echocardiography) will help reduce perivalvular regurgitation and vascular complications • Dedicated vascular closure devices in development • New TAVR designs (TF and TA) will also help reduce procedural complications 46


