ccdd51db7700a512378e312318b40047.ppt
- Количество слайдов: 52
 Targeted therapies in lung cancer - what are the limits? D. Ross Camidge, MD Ph. D Director, Thoracic Oncology Clinical Program University of Colorado Halifax, Nova Scotia, 21 st October 2011
Targeted therapies in lung cancer - what are the limits? D. Ross Camidge, MD Ph. D Director, Thoracic Oncology Clinical Program University of Colorado Halifax, Nova Scotia, 21 st October 2011
 Disclosures (DRC) • • Employment or leadership Position: None Advisory Role: Ad Hoc Advisory Boards/Consultations (most recent contact last 3 years): – 2011: Ariad, Array Biopharma, Astra. Zeneca, Aveo, Boehringer Ingelheim, Chugai, Clovis, Novartis, Synta – 2010: Millenium, Pfizer – 2009: Imclone, OSI • • • Stock Ownership: None Honoraria: Seminar/Talks to Industry (most recent contact last 3 years). – 2011: Ariad, Array Biopharma, Pfizer – 2009: Imclone, Boehringer Ingelheim, Speakers Bureau/Talks for Industry: None Research Funding: – 2010: Eli-Lilly (Translational) – 2008: Onyx/Merck (IIT) Expert Testimony: None Other Remuneration: None
Disclosures (DRC) • • Employment or leadership Position: None Advisory Role: Ad Hoc Advisory Boards/Consultations (most recent contact last 3 years): – 2011: Ariad, Array Biopharma, Astra. Zeneca, Aveo, Boehringer Ingelheim, Chugai, Clovis, Novartis, Synta – 2010: Millenium, Pfizer – 2009: Imclone, OSI • • • Stock Ownership: None Honoraria: Seminar/Talks to Industry (most recent contact last 3 years). – 2011: Ariad, Array Biopharma, Pfizer – 2009: Imclone, Boehringer Ingelheim, Speakers Bureau/Talks for Industry: None Research Funding: – 2010: Eli-Lilly (Translational) – 2008: Onyx/Merck (IIT) Expert Testimony: None Other Remuneration: None
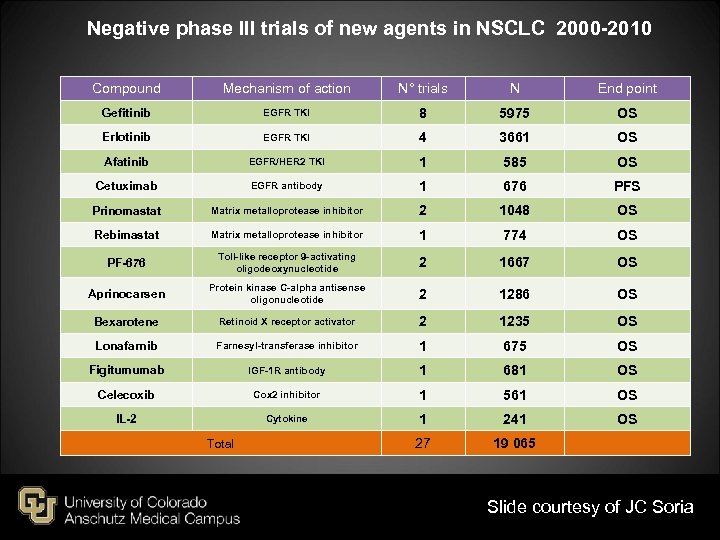 Negative phase III trials of new agents in NSCLC 2000 -2010 Compound Mechanism of action N° trials N End point Gefitinib EGFR TKI 8 5975 OS Erlotinib EGFR TKI 4 3661 OS Afatinib EGFR/HER 2 TKI 1 585 OS Cetuximab EGFR antibody 1 676 PFS Prinomastat Matrix metalloprotease inhibitor 2 1048 OS Rebimastat Matrix metalloprotease inhibitor 1 774 OS PF-676 Toll-like receptor 9 -activating oligodeoxynucleotide 2 1667 OS Aprinocarsen Protein kinase C-alpha antisense oligonucleotide 2 1286 OS Bexarotene Retinoid X receptor activator 2 1235 OS Lonafarnib Farnesyl-transferase inhibitor 1 675 OS Figitumumab IGF-1 R antibody 1 681 OS Celecoxib Cox 2 inhibitor 1 561 OS IL-2 Cytokine 1 241 OS 27 19 065 Total Slide courtesy of JC Soria
Negative phase III trials of new agents in NSCLC 2000 -2010 Compound Mechanism of action N° trials N End point Gefitinib EGFR TKI 8 5975 OS Erlotinib EGFR TKI 4 3661 OS Afatinib EGFR/HER 2 TKI 1 585 OS Cetuximab EGFR antibody 1 676 PFS Prinomastat Matrix metalloprotease inhibitor 2 1048 OS Rebimastat Matrix metalloprotease inhibitor 1 774 OS PF-676 Toll-like receptor 9 -activating oligodeoxynucleotide 2 1667 OS Aprinocarsen Protein kinase C-alpha antisense oligonucleotide 2 1286 OS Bexarotene Retinoid X receptor activator 2 1235 OS Lonafarnib Farnesyl-transferase inhibitor 1 675 OS Figitumumab IGF-1 R antibody 1 681 OS Celecoxib Cox 2 inhibitor 1 561 OS IL-2 Cytokine 1 241 OS 27 19 065 Total Slide courtesy of JC Soria
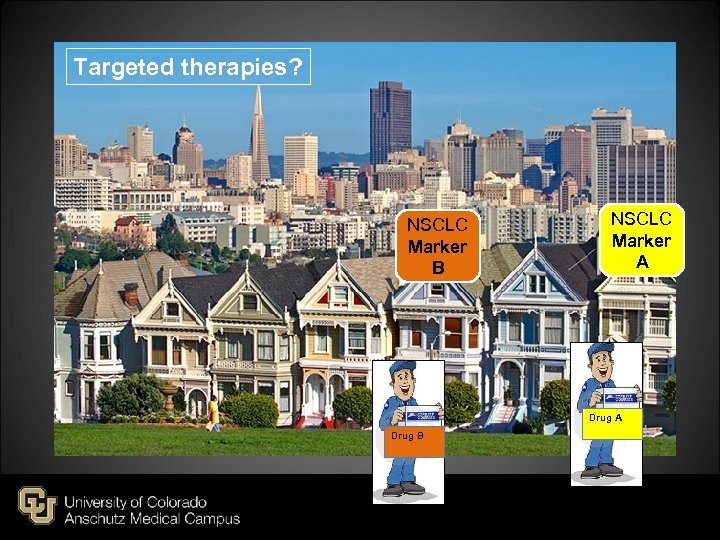 Targeted therapies? NSCLC Marker B NSCLC Marker A Drug B
Targeted therapies? NSCLC Marker B NSCLC Marker A Drug B
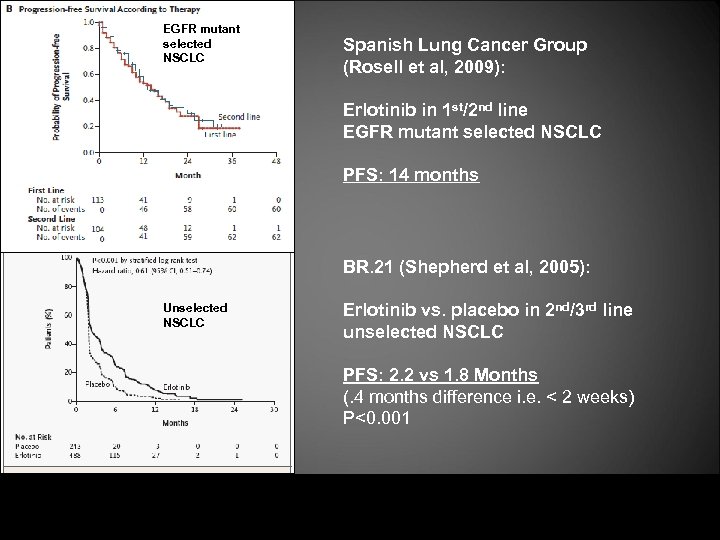 EGFR mutant selected NSCLC Spanish Lung Cancer Group (Rosell et al, 2009): Erlotinib in 1 st/2 nd line EGFR mutant selected NSCLC PFS: 14 months BR. 21 (Shepherd et al, 2005): Unselected NSCLC Erlotinib vs. placebo in 2 nd/3 rd line unselected NSCLC PFS: 2. 2 vs 1. 8 Months (. 4 months difference i. e. < 2 weeks) P<0. 001
EGFR mutant selected NSCLC Spanish Lung Cancer Group (Rosell et al, 2009): Erlotinib in 1 st/2 nd line EGFR mutant selected NSCLC PFS: 14 months BR. 21 (Shepherd et al, 2005): Unselected NSCLC Erlotinib vs. placebo in 2 nd/3 rd line unselected NSCLC PFS: 2. 2 vs 1. 8 Months (. 4 months difference i. e. < 2 weeks) P<0. 001
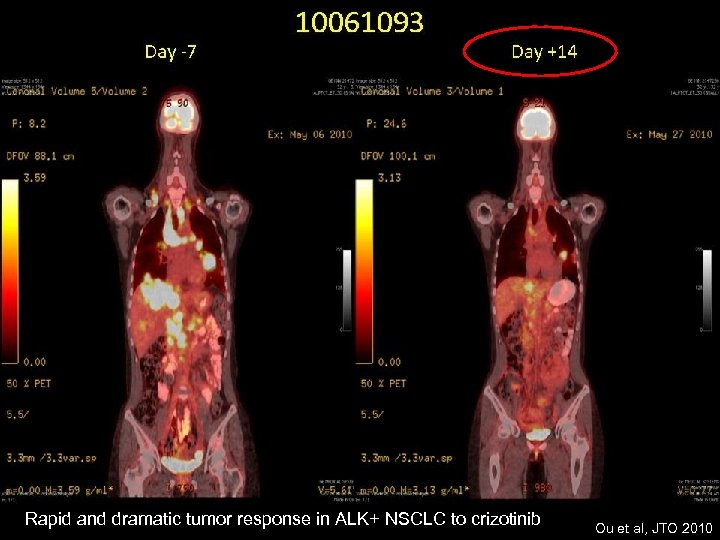 Rapid and dramatic tumor response in ALK+ NSCLC to crizotinib Ou et al, JTO 2010
Rapid and dramatic tumor response in ALK+ NSCLC to crizotinib Ou et al, JTO 2010
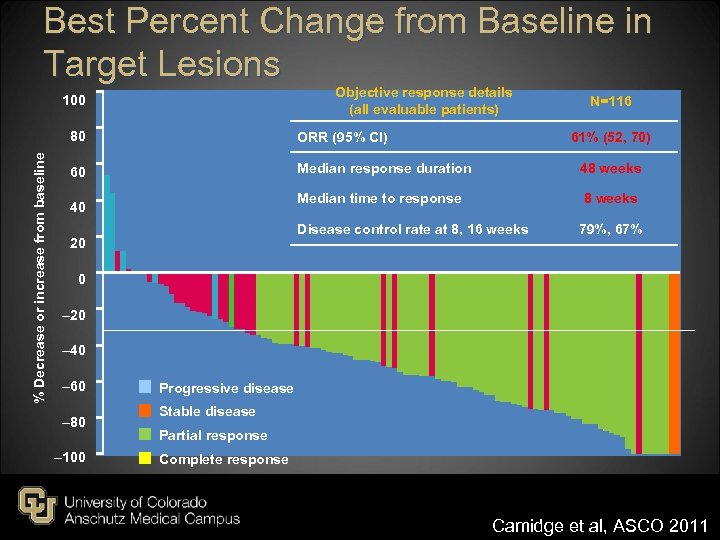 Best Percent Change from Baseline in Target Lesions Objective response details (all evaluable patients) 100 N=116 % Decrease or increase from baseline 80 ORR (95% CI) 60 Median response duration 48 weeks Median time to response 8 weeks 40 61% (52, 70) Disease control rate at 8, 16 weeks 20 79%, 67% 0 – 20 – 40 – 60 – 80 – 100 Progressive disease Stable disease Partial response Complete response Camidge et al, ASCO 2011
Best Percent Change from Baseline in Target Lesions Objective response details (all evaluable patients) 100 N=116 % Decrease or increase from baseline 80 ORR (95% CI) 60 Median response duration 48 weeks Median time to response 8 weeks 40 61% (52, 70) Disease control rate at 8, 16 weeks 20 79%, 67% 0 – 20 – 40 – 60 – 80 – 100 Progressive disease Stable disease Partial response Complete response Camidge et al, ASCO 2011
 Potential predictive biomarkers (both categorical and continuous variables) • Tumor – – – Mutations Gene rearrangements Gene copy number Protein expression/expression level Transcript(s) levels • Host – – – Immune system Vasculature Endocrine environment
Potential predictive biomarkers (both categorical and continuous variables) • Tumor – – – Mutations Gene rearrangements Gene copy number Protein expression/expression level Transcript(s) levels • Host – – – Immune system Vasculature Endocrine environment
 Personalized medicine in oncology Unselected large population, modest overall benefit Selected small population, large benefit
Personalized medicine in oncology Unselected large population, modest overall benefit Selected small population, large benefit
 From a drug-perspective, the limits will only be partly set by the targets
From a drug-perspective, the limits will only be partly set by the targets
 Kinases Protein Interactions Immune Effectors Nucleic Acid Beyond chemo repressors (e. g. YM 155) Transcriptional Si. RNA Mi. RNA Gene therapy • Challenges set by the methods of drug delivery • But required - given the challenge of most oncogenic changes being loss of function …
Kinases Protein Interactions Immune Effectors Nucleic Acid Beyond chemo repressors (e. g. YM 155) Transcriptional Si. RNA Mi. RNA Gene therapy • Challenges set by the methods of drug delivery • But required - given the challenge of most oncogenic changes being loss of function …
 Most of the limits are far more immediate and more ‘obvious’ • There are no common cancers – The practical implications of heterogeneity – The financial consequences of heterogeneity • Today’s miracles aren’t curing anyone – The brain is a special place – Addressing molecular mechanisms of resistance – Darwinian oncology The elephant(s) in the room
Most of the limits are far more immediate and more ‘obvious’ • There are no common cancers – The practical implications of heterogeneity – The financial consequences of heterogeneity • Today’s miracles aren’t curing anyone – The brain is a special place – Addressing molecular mechanisms of resistance – Darwinian oncology The elephant(s) in the room
 1. There are no common cancers
1. There are no common cancers
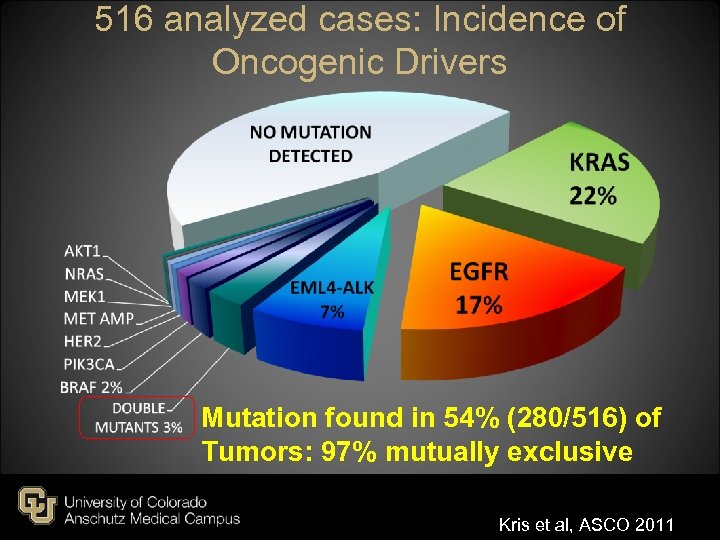 516 analyzed cases: Incidence of Oncogenic Drivers Mutation found in 54% (280/516) of Tumors: 97% mutually exclusive Kris et al, ASCO 2011
516 analyzed cases: Incidence of Oncogenic Drivers Mutation found in 54% (280/516) of Tumors: 97% mutually exclusive Kris et al, ASCO 2011
 1. 1 The practical implications of heterogeneity in clinical research
1. 1 The practical implications of heterogeneity in clinical research
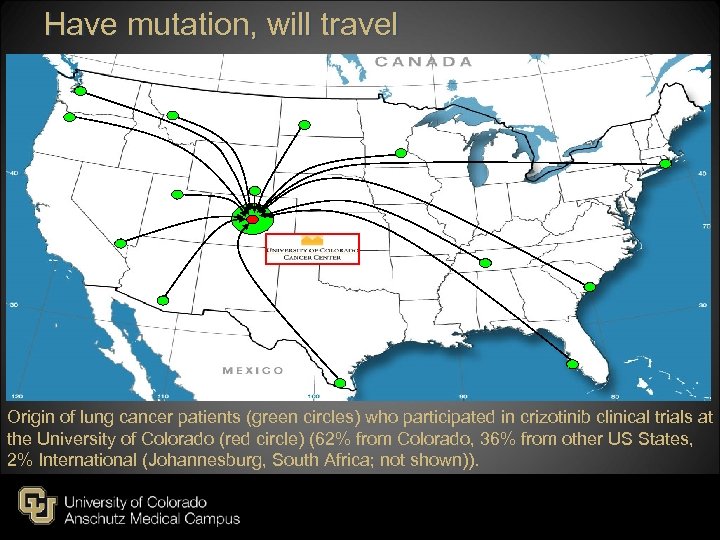 Have mutation, will travel Origin of lung cancer patients (green circles) who participated in crizotinib clinical trials at the University of Colorado (red circle) (62% from Colorado, 36% from other US States, 2% International (Johannesburg, South Africa; not shown)).
Have mutation, will travel Origin of lung cancer patients (green circles) who participated in crizotinib clinical trials at the University of Colorado (red circle) (62% from Colorado, 36% from other US States, 2% International (Johannesburg, South Africa; not shown)).
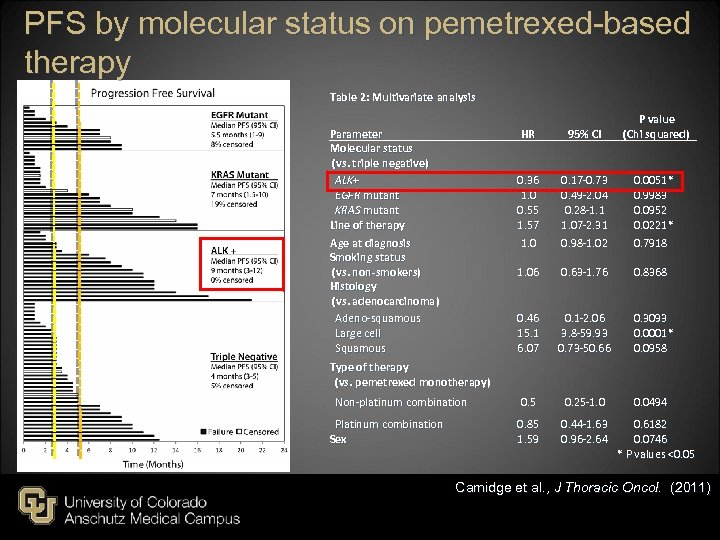 PFS by molecular status on pemetrexed-based therapy Table 2: Multivariate analysis Parameter Molecular status (vs. triple negative) ALK+ EGFR mutant KRAS mutant Line of therapy Age at diagnosis Smoking status (vs. non-smokers) Histology (vs. adenocarcinoma) Adeno-squamous Large cell Squamous P value (Chi squared) HR 95% CI 0. 36 1. 0 0. 55 1. 57 1. 0 0. 17 -0. 73 0. 49 -2. 04 0. 28 -1. 1 1. 07 -2. 31 0. 98 -1. 02 1. 06 0. 63 -1. 76 0. 8368 0. 46 15. 1 6. 07 0. 1 -2. 06 3. 8 -59. 93 0. 73 -50. 66 Non-platinum combination 0. 5 0. 25 -1. 0 Platinum combination Sex 0. 85 1. 59 0. 44 -1. 63 0. 6182 0. 96 -2. 64 0. 0746 * P values <0. 05 Type of therapy (vs. pemetrexed monotherapy) 0. 0051 * 0. 9983 0. 0952 0. 0221 * 0. 7918 0. 3093 0. 0001 * 0. 0958 0. 0494 Camidge et al. , J Thoracic Oncol. (2011)
PFS by molecular status on pemetrexed-based therapy Table 2: Multivariate analysis Parameter Molecular status (vs. triple negative) ALK+ EGFR mutant KRAS mutant Line of therapy Age at diagnosis Smoking status (vs. non-smokers) Histology (vs. adenocarcinoma) Adeno-squamous Large cell Squamous P value (Chi squared) HR 95% CI 0. 36 1. 0 0. 55 1. 57 1. 0 0. 17 -0. 73 0. 49 -2. 04 0. 28 -1. 1 1. 07 -2. 31 0. 98 -1. 02 1. 06 0. 63 -1. 76 0. 8368 0. 46 15. 1 6. 07 0. 1 -2. 06 3. 8 -59. 93 0. 73 -50. 66 Non-platinum combination 0. 5 0. 25 -1. 0 Platinum combination Sex 0. 85 1. 59 0. 44 -1. 63 0. 6182 0. 96 -2. 64 0. 0746 * P values <0. 05 Type of therapy (vs. pemetrexed monotherapy) 0. 0051 * 0. 9983 0. 0952 0. 0221 * 0. 7918 0. 3093 0. 0001 * 0. 0958 0. 0494 Camidge et al. , J Thoracic Oncol. (2011)
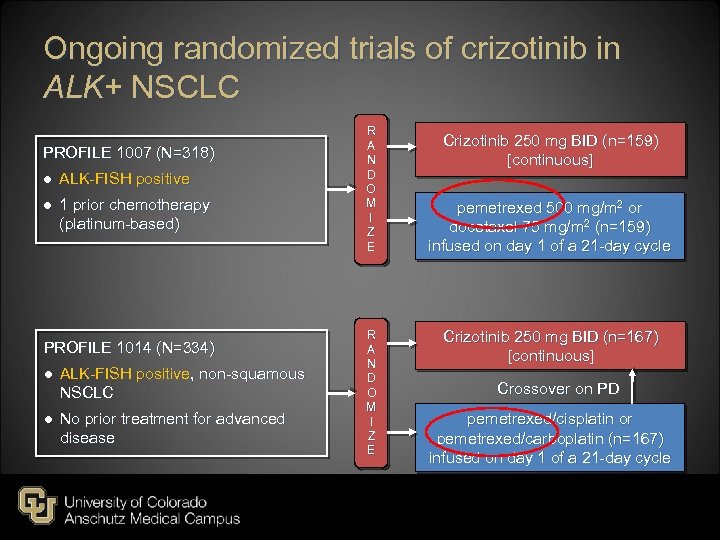 Ongoing randomized trials of crizotinib in ALK+ NSCLC PROFILE 1007 (N=318) ● ALK-FISH positive ● 1 prior chemotherapy (platinum-based) PROFILE 1014 (N=334) ● ALK-FISH positive, non-squamous NSCLC ● No prior treatment for advanced disease R A N D O M I Z E Crizotinib 250 mg BID (n=159) [continuous] pemetrexed 500 mg/m 2 or docetaxel 75 mg/m 2 (n=159) infused on day 1 of a 21 -day cycle Crizotinib 250 mg BID (n=167) [continuous] Crossover on PD pemetrexed/cisplatin or pemetrexed/carboplatin (n=167) infused on day 1 of a 21 -day cycle
Ongoing randomized trials of crizotinib in ALK+ NSCLC PROFILE 1007 (N=318) ● ALK-FISH positive ● 1 prior chemotherapy (platinum-based) PROFILE 1014 (N=334) ● ALK-FISH positive, non-squamous NSCLC ● No prior treatment for advanced disease R A N D O M I Z E Crizotinib 250 mg BID (n=159) [continuous] pemetrexed 500 mg/m 2 or docetaxel 75 mg/m 2 (n=159) infused on day 1 of a 21 -day cycle Crizotinib 250 mg BID (n=167) [continuous] Crossover on PD pemetrexed/cisplatin or pemetrexed/carboplatin (n=167) infused on day 1 of a 21 -day cycle
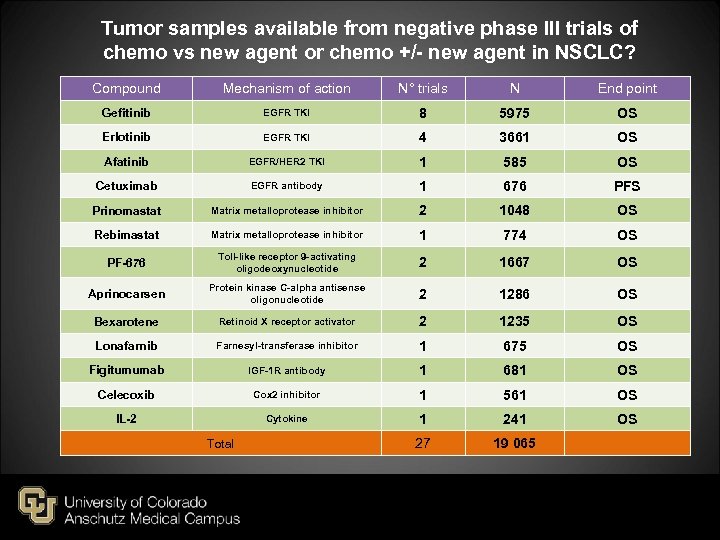 Tumor samples available from negative phase III trials of chemo vs new agent or chemo +/- new agent in NSCLC? Compound Mechanism of action N° trials N End point Gefitinib EGFR TKI 8 5975 OS Erlotinib EGFR TKI 4 3661 OS Afatinib EGFR/HER 2 TKI 1 585 OS Cetuximab EGFR antibody 1 676 PFS Prinomastat Matrix metalloprotease inhibitor 2 1048 OS Rebimastat Matrix metalloprotease inhibitor 1 774 OS PF-676 Toll-like receptor 9 -activating oligodeoxynucleotide 2 1667 OS Aprinocarsen Protein kinase C-alpha antisense oligonucleotide 2 1286 OS Bexarotene Retinoid X receptor activator 2 1235 OS Lonafarnib Farnesyl-transferase inhibitor 1 675 OS Figitumumab IGF-1 R antibody 1 681 OS Celecoxib Cox 2 inhibitor 1 561 OS IL-2 Cytokine 1 241 OS 27 19 065 Total
Tumor samples available from negative phase III trials of chemo vs new agent or chemo +/- new agent in NSCLC? Compound Mechanism of action N° trials N End point Gefitinib EGFR TKI 8 5975 OS Erlotinib EGFR TKI 4 3661 OS Afatinib EGFR/HER 2 TKI 1 585 OS Cetuximab EGFR antibody 1 676 PFS Prinomastat Matrix metalloprotease inhibitor 2 1048 OS Rebimastat Matrix metalloprotease inhibitor 1 774 OS PF-676 Toll-like receptor 9 -activating oligodeoxynucleotide 2 1667 OS Aprinocarsen Protein kinase C-alpha antisense oligonucleotide 2 1286 OS Bexarotene Retinoid X receptor activator 2 1235 OS Lonafarnib Farnesyl-transferase inhibitor 1 675 OS Figitumumab IGF-1 R antibody 1 681 OS Celecoxib Cox 2 inhibitor 1 561 OS IL-2 Cytokine 1 241 OS 27 19 065 Total
 1. 2 The financial consequences of heterogeneity
1. 2 The financial consequences of heterogeneity
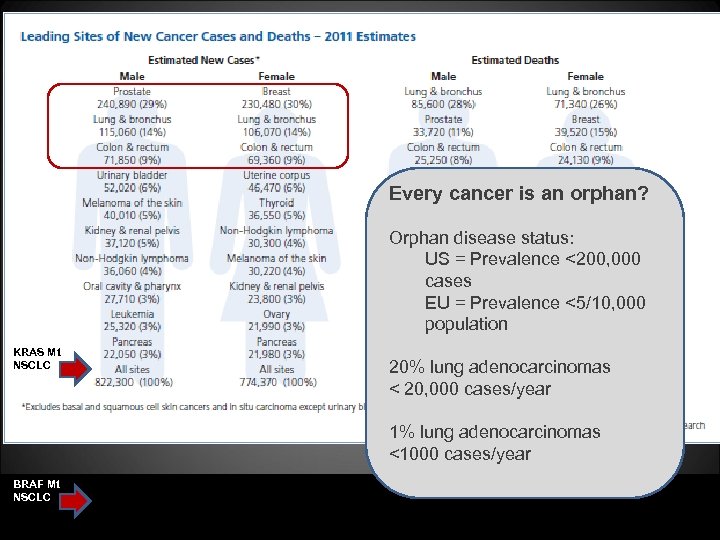 Every cancer is an orphan? Orphan disease status: US = Prevalence <200, 000 cases EU = Prevalence <5/10, 000 population KRAS Mt NSCLC 20% lung adenocarcinomas < 20, 000 cases/year 1% lung adenocarcinomas <1000 cases/year BRAF Mt NSCLC
Every cancer is an orphan? Orphan disease status: US = Prevalence <200, 000 cases EU = Prevalence <5/10, 000 population KRAS Mt NSCLC 20% lung adenocarcinomas < 20, 000 cases/year 1% lung adenocarcinomas <1000 cases/year BRAF Mt NSCLC
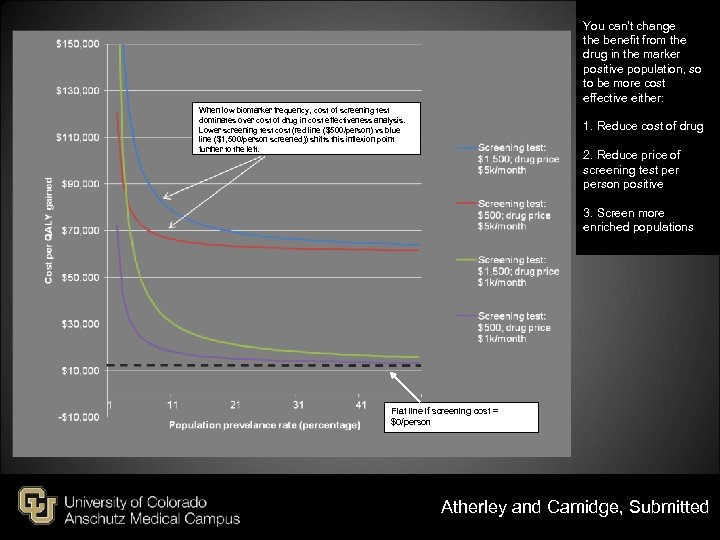 You can’t change the benefit from the drug in the marker positive population, so to be more cost effective either: When low biomarker frequency, cost of screening test dominates over cost of drug in cost effectiveness analysis. Lower screening test cost (red line ($500/person) vs blue line ($1, 500/person screened)) shifts this inflexion point further to the left. 1. Reduce cost of drug 2. Reduce price of screening test person positive 3. Screen more enriched populations Flat line if screening cost = $0/person Atherley and Camidge, Submitted
You can’t change the benefit from the drug in the marker positive population, so to be more cost effective either: When low biomarker frequency, cost of screening test dominates over cost of drug in cost effectiveness analysis. Lower screening test cost (red line ($500/person) vs blue line ($1, 500/person screened)) shifts this inflexion point further to the left. 1. Reduce cost of drug 2. Reduce price of screening test person positive 3. Screen more enriched populations Flat line if screening cost = $0/person Atherley and Camidge, Submitted
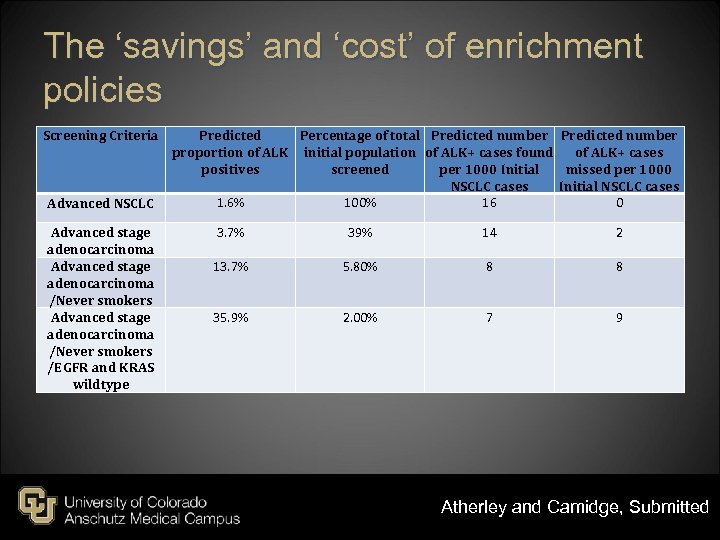 The ‘savings’ and ‘cost’ of enrichment policies Screening Criteria Advanced NSCLC Advanced stage adenocarcinoma /Never smokers /EGFR and KRAS wildtype Predicted Percentage of total Predicted number proportion of ALK initial population of ALK+ cases found of ALK+ cases positives screened per 1000 Initial missed per 1000 NSCLC cases Initial NSCLC cases 1. 6% 100% 16 0 3. 7% 39% 14 2 13. 7% 5. 80% 8 8 35. 9% 2. 00% 7 9 Atherley and Camidge, Submitted
The ‘savings’ and ‘cost’ of enrichment policies Screening Criteria Advanced NSCLC Advanced stage adenocarcinoma /Never smokers /EGFR and KRAS wildtype Predicted Percentage of total Predicted number proportion of ALK initial population of ALK+ cases found of ALK+ cases positives screened per 1000 Initial missed per 1000 NSCLC cases Initial NSCLC cases 1. 6% 100% 16 0 3. 7% 39% 14 2 13. 7% 5. 80% 8 8 35. 9% 2. 00% 7 9 Atherley and Camidge, Submitted
 2. Today’s miracles aren’t curing anyone
2. Today’s miracles aren’t curing anyone
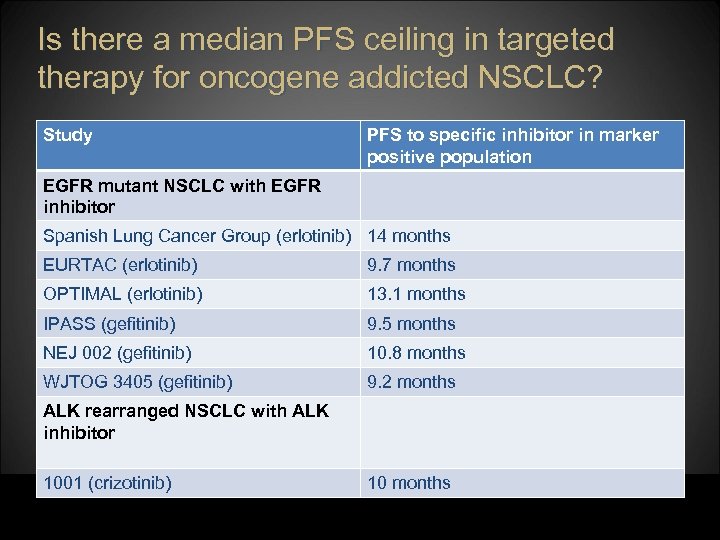 Is there a median PFS ceiling in targeted therapy for oncogene addicted NSCLC? Study PFS to specific inhibitor in marker positive population EGFR mutant NSCLC with EGFR inhibitor Spanish Lung Cancer Group (erlotinib) 14 months EURTAC (erlotinib) 9. 7 months OPTIMAL (erlotinib) 13. 1 months IPASS (gefitinib) 9. 5 months NEJ 002 (gefitinib) 10. 8 months WJTOG 3405 (gefitinib) 9. 2 months ALK rearranged NSCLC with ALK inhibitor 1001 (crizotinib) 10 months
Is there a median PFS ceiling in targeted therapy for oncogene addicted NSCLC? Study PFS to specific inhibitor in marker positive population EGFR mutant NSCLC with EGFR inhibitor Spanish Lung Cancer Group (erlotinib) 14 months EURTAC (erlotinib) 9. 7 months OPTIMAL (erlotinib) 13. 1 months IPASS (gefitinib) 9. 5 months NEJ 002 (gefitinib) 10. 8 months WJTOG 3405 (gefitinib) 9. 2 months ALK rearranged NSCLC with ALK inhibitor 1001 (crizotinib) 10 months
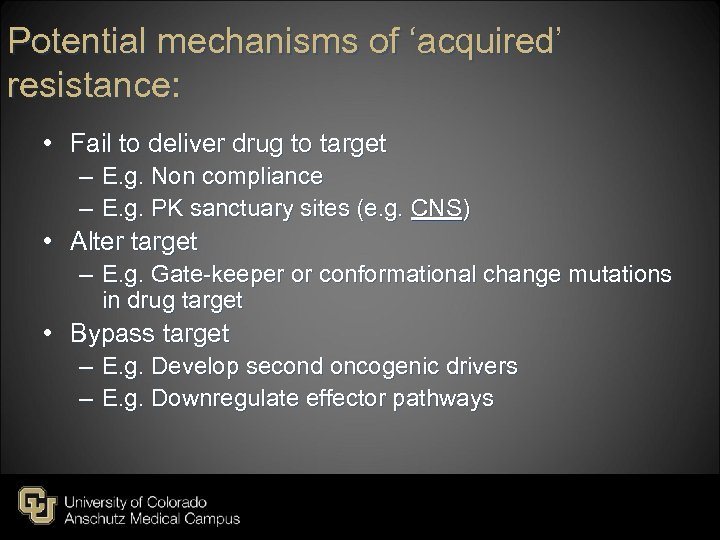 Potential mechanisms of ‘acquired’ resistance: • Fail to deliver drug to target – E. g. Non compliance – E. g. PK sanctuary sites (e. g. CNS) • Alter target – E. g. Gate-keeper or conformational change mutations in drug target • Bypass target – E. g. Develop second oncogenic drivers – E. g. Downregulate effector pathways
Potential mechanisms of ‘acquired’ resistance: • Fail to deliver drug to target – E. g. Non compliance – E. g. PK sanctuary sites (e. g. CNS) • Alter target – E. g. Gate-keeper or conformational change mutations in drug target • Bypass target – E. g. Develop second oncogenic drivers – E. g. Downregulate effector pathways
 2. 1 The brain is a special place
2. 1 The brain is a special place
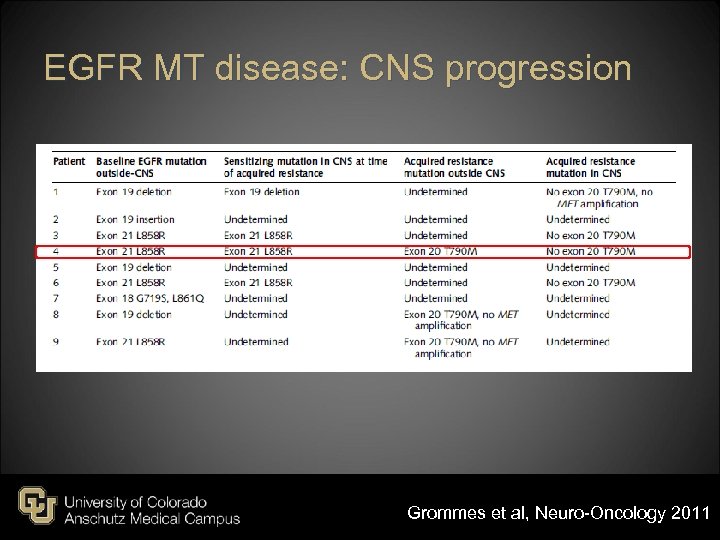 EGFR MT disease: CNS progression Grommes et al, Neuro-Oncology 2011
EGFR MT disease: CNS progression Grommes et al, Neuro-Oncology 2011
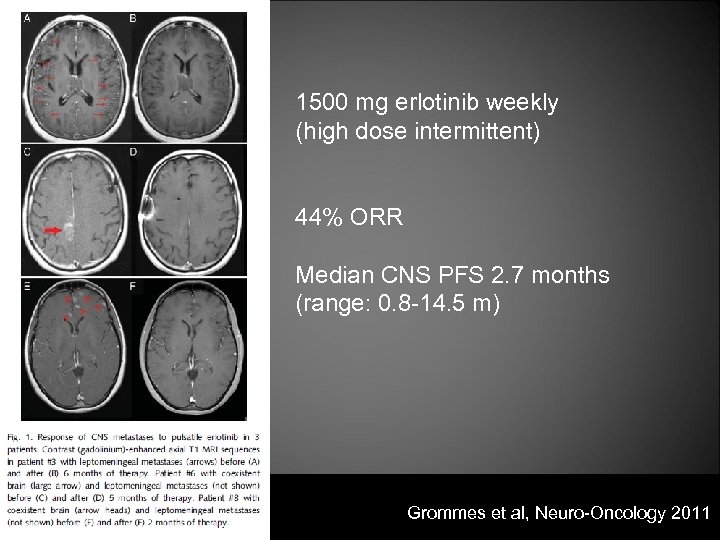 1500 mg erlotinib weekly (high dose intermittent) 44% ORR Median CNS PFS 2. 7 months (range: 0. 8 -14. 5 m) Grommes et al, Neuro-Oncology 2011
1500 mg erlotinib weekly (high dose intermittent) 44% ORR Median CNS PFS 2. 7 months (range: 0. 8 -14. 5 m) Grommes et al, Neuro-Oncology 2011
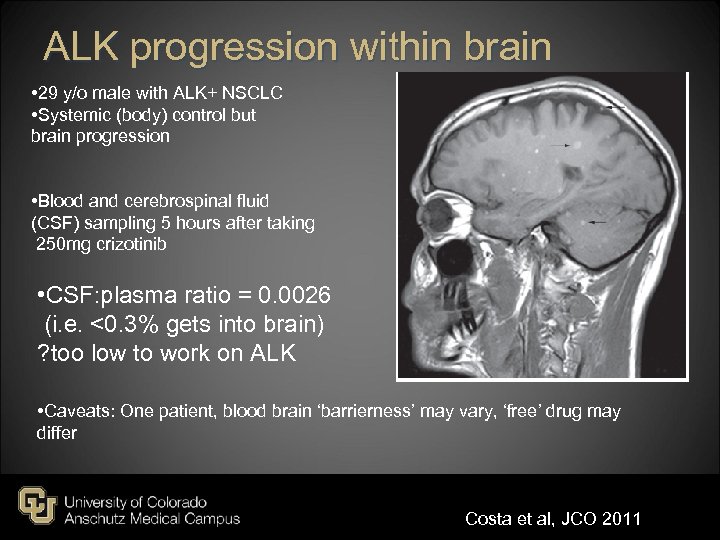 ALK progression within brain • 29 y/o male with ALK+ NSCLC • Systemic (body) control but brain progression • Blood and cerebrospinal fluid (CSF) sampling 5 hours after taking 250 mg crizotinib • CSF: plasma ratio = 0. 0026 (i. e. <0. 3% gets into brain) ? too low to work on ALK • Caveats: One patient, blood brain ‘barrierness’ may vary, ‘free’ drug may differ Costa et al, JCO 2011
ALK progression within brain • 29 y/o male with ALK+ NSCLC • Systemic (body) control but brain progression • Blood and cerebrospinal fluid (CSF) sampling 5 hours after taking 250 mg crizotinib • CSF: plasma ratio = 0. 0026 (i. e. <0. 3% gets into brain) ? too low to work on ALK • Caveats: One patient, blood brain ‘barrierness’ may vary, ‘free’ drug may differ Costa et al, JCO 2011
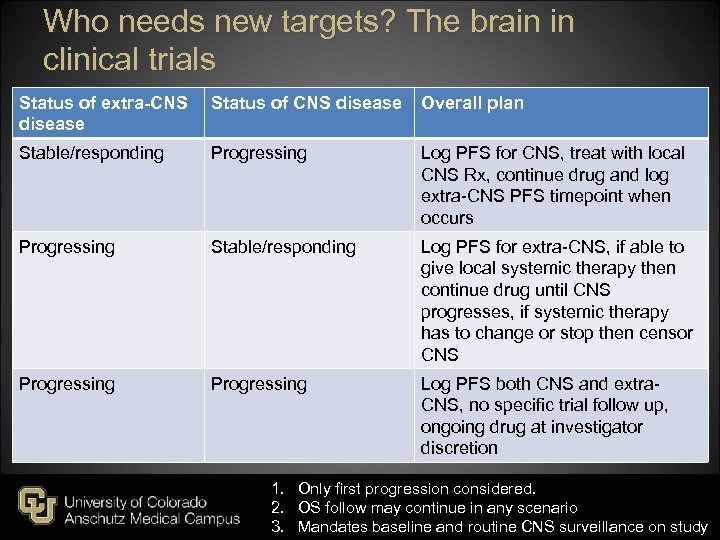 Who needs new targets? The brain in clinical trials Status of extra-CNS disease Status of CNS disease Overall plan Stable/responding Progressing Log PFS for CNS, treat with local CNS Rx, continue drug and log extra-CNS PFS timepoint when occurs Progressing Stable/responding Log PFS for extra-CNS, if able to give local systemic therapy then continue drug until CNS progresses, if systemic therapy has to change or stop then censor CNS Progressing Log PFS both CNS and extra. CNS, no specific trial follow up, ongoing drug at investigator discretion 1. Only first progression considered. 2. OS follow may continue in any scenario 3. Mandates baseline and routine CNS surveillance on study
Who needs new targets? The brain in clinical trials Status of extra-CNS disease Status of CNS disease Overall plan Stable/responding Progressing Log PFS for CNS, treat with local CNS Rx, continue drug and log extra-CNS PFS timepoint when occurs Progressing Stable/responding Log PFS for extra-CNS, if able to give local systemic therapy then continue drug until CNS progresses, if systemic therapy has to change or stop then censor CNS Progressing Log PFS both CNS and extra. CNS, no specific trial follow up, ongoing drug at investigator discretion 1. Only first progression considered. 2. OS follow may continue in any scenario 3. Mandates baseline and routine CNS surveillance on study
 2. 2 Addressing molecular mechanisms of resistance
2. 2 Addressing molecular mechanisms of resistance
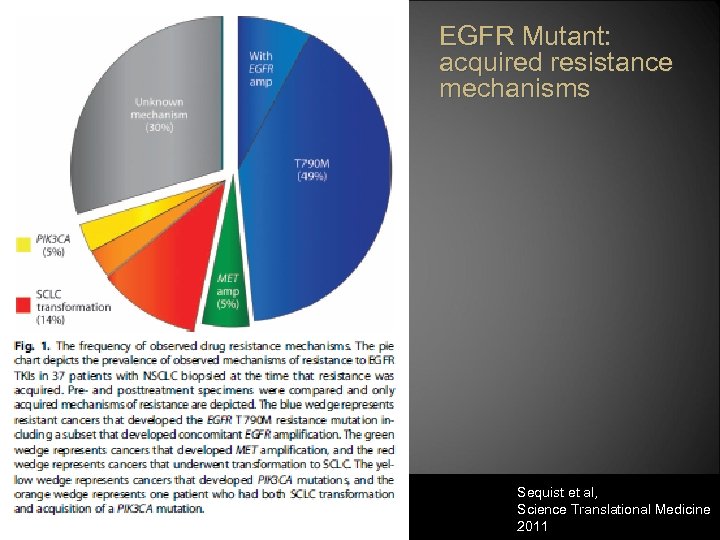 EGFR Mutant: acquired resistance mechanisms Sequist et al, Science Translational Medicine 2011
EGFR Mutant: acquired resistance mechanisms Sequist et al, Science Translational Medicine 2011
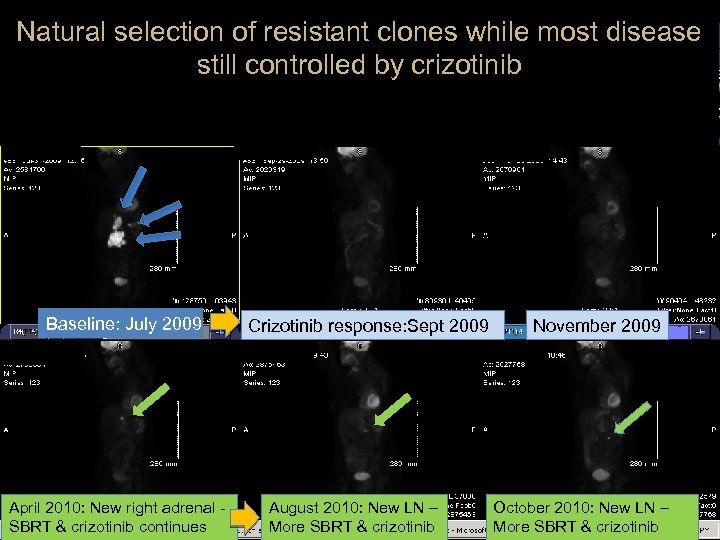 Natural selection of resistant clones while most disease still controlled by crizotinib Baseline: July 2009 April 2010: New right adrenal SBRT & crizotinib continues Crizotinib response: Sept 2009 August 2010: New LN – More SBRT & crizotinib November 2009 October 2010: New LN – More SBRT & crizotinib
Natural selection of resistant clones while most disease still controlled by crizotinib Baseline: July 2009 April 2010: New right adrenal SBRT & crizotinib continues Crizotinib response: Sept 2009 August 2010: New LN – More SBRT & crizotinib November 2009 October 2010: New LN – More SBRT & crizotinib
 Drugs for AR: Rapid Deployment Forces vs. Armies of Occupation? • Adding in/replacing with each new drug at time of AR? • Or combine up front? • Tolerability of new drug(s)?
Drugs for AR: Rapid Deployment Forces vs. Armies of Occupation? • Adding in/replacing with each new drug at time of AR? • Or combine up front? • Tolerability of new drug(s)?
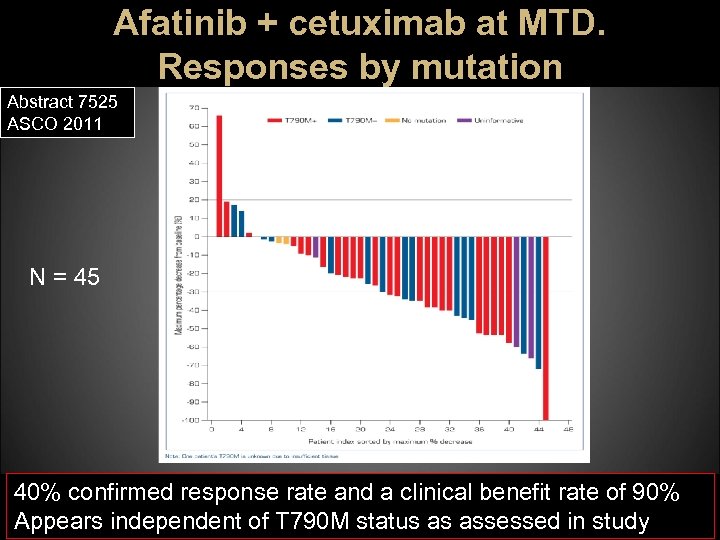 Afatinib + cetuximab at MTD. Responses by mutation Abstract 7525 ASCO 2011 N = 45 40% confirmed response rate and a clinical benefit rate of 90% Appears independent of T 790 M status as assessed in study
Afatinib + cetuximab at MTD. Responses by mutation Abstract 7525 ASCO 2011 N = 45 40% confirmed response rate and a clinical benefit rate of 90% Appears independent of T 790 M status as assessed in study
 2. 3 Darwinian Oncology
2. 3 Darwinian Oncology
![Pt 9 T 790 M present [low levels] in CTCs – still ‘responds’ to Pt 9 T 790 M present [low levels] in CTCs – still ‘responds’ to](https://present5.com/presentation/ccdd51db7700a512378e312318b40047/image-38.jpg) Pt 9 T 790 M present [low levels] in CTCs – still ‘responds’ to reversible TKIs as T 790 M is not an all/none event Turke et al, Cancer Cell 2010 Maheswaran et al, NEJM 2008 Diversity pre-exists (? hardwired) In patients who manifested MET gene amp as mechanism of Number of CTCs (micropost) and frequency of different alleles (L 858 R/Del/T 790 M) acquired resistance, rare amplified radiographic relative to control, alters with treatment and parallels cells were seen pre-EGFR response (Top panels – [X] = low frequency allele) (Bottom panels – TKI treatment cycles for detection – left to right panel at number of amplification different points in treatment)
Pt 9 T 790 M present [low levels] in CTCs – still ‘responds’ to reversible TKIs as T 790 M is not an all/none event Turke et al, Cancer Cell 2010 Maheswaran et al, NEJM 2008 Diversity pre-exists (? hardwired) In patients who manifested MET gene amp as mechanism of Number of CTCs (micropost) and frequency of different alleles (L 858 R/Del/T 790 M) acquired resistance, rare amplified radiographic relative to control, alters with treatment and parallels cells were seen pre-EGFR response (Top panels – [X] = low frequency allele) (Bottom panels – TKI treatment cycles for detection – left to right panel at number of amplification different points in treatment)
 Why is the TKI naïve molecular status as it is? • Why don’t T 790 M and MET amplified EGFR mutants dominate prior to EGFR TKI? – Easier to develop the basic model? – T 790 M and/or MET plus EGFR MT have some disadvantage in the absence of a specific selection pressure?
Why is the TKI naïve molecular status as it is? • Why don’t T 790 M and MET amplified EGFR mutants dominate prior to EGFR TKI? – Easier to develop the basic model? – T 790 M and/or MET plus EGFR MT have some disadvantage in the absence of a specific selection pressure?
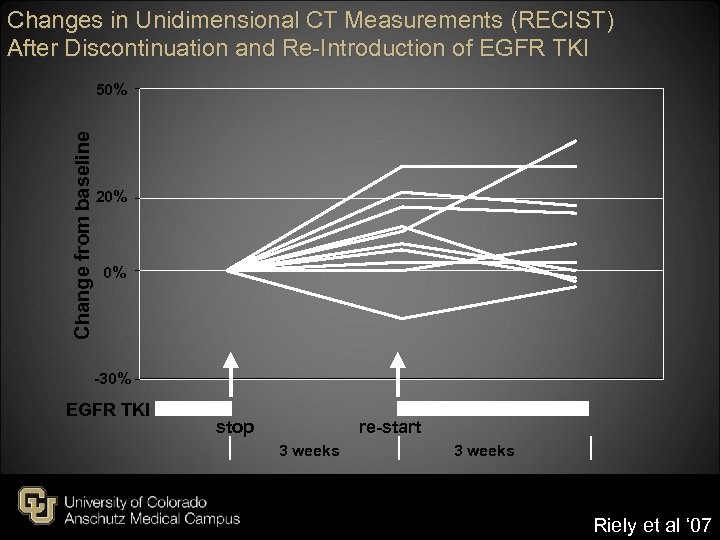 Changes in Unidimensional CT Measurements (RECIST) After Discontinuation and Re-Introduction of EGFR TKI Change from baseline 50% 20% 0% -30% EGFR TKI re-start stop 3 weeks Riely et al ‘ 07
Changes in Unidimensional CT Measurements (RECIST) After Discontinuation and Re-Introduction of EGFR TKI Change from baseline 50% 20% 0% -30% EGFR TKI re-start stop 3 weeks Riely et al ‘ 07
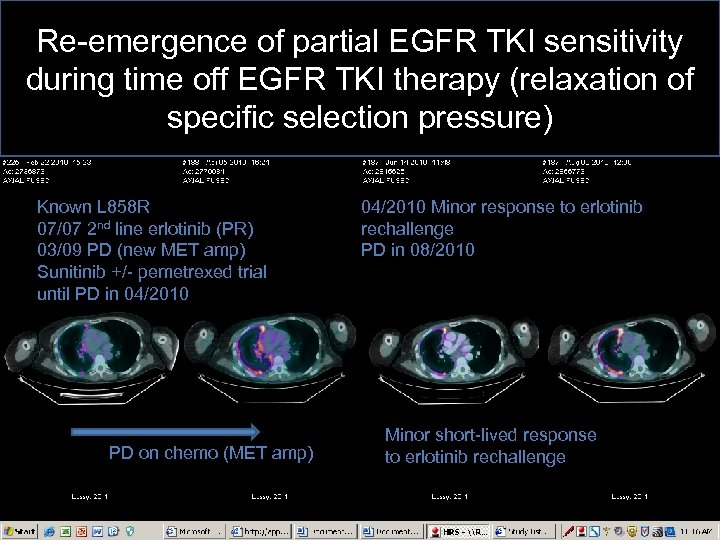 Re-emergence of partial EGFR TKI sensitivity during time off EGFR TKI therapy (relaxation of specific selection pressure) Known L 858 R 07/07 2 nd line erlotinib (PR) 03/09 PD (new MET amp) Sunitinib +/- pemetrexed trial until PD in 04/2010 PD on chemo (MET amp) 04/2010 Minor response to erlotinib rechallenge PD in 08/2010 Minor short-lived response to erlotinib rechallenge
Re-emergence of partial EGFR TKI sensitivity during time off EGFR TKI therapy (relaxation of specific selection pressure) Known L 858 R 07/07 2 nd line erlotinib (PR) 03/09 PD (new MET amp) Sunitinib +/- pemetrexed trial until PD in 04/2010 PD on chemo (MET amp) 04/2010 Minor response to erlotinib rechallenge PD in 08/2010 Minor short-lived response to erlotinib rechallenge
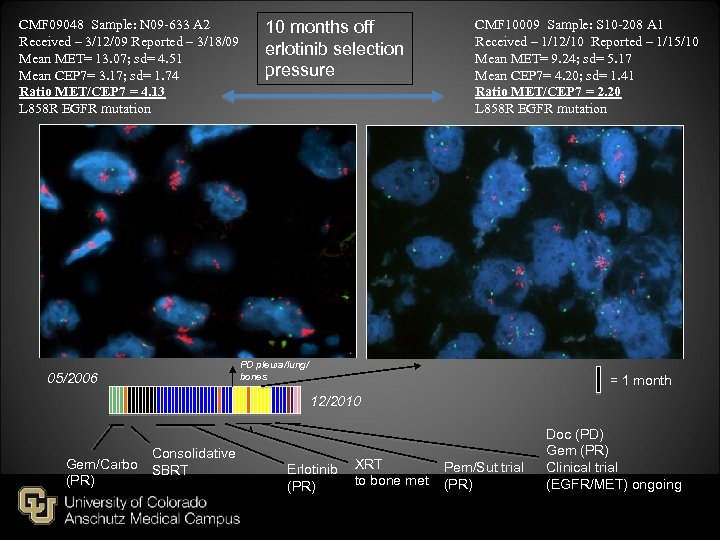 CMF 09048 Sample: N 09 -633 A 2 Received – 3/12/09 Reported – 3/18/09 Mean MET= 13. 07; sd= 4. 51 Mean CEP 7= 3. 17; sd= 1. 74 Ratio MET/CEP 7 = 4. 13 L 858 R EGFR mutation 05/2006 10 months off erlotinib selection pressure CMF 10009 Sample: S 10 -208 A 1 Received – 1/12/10 Reported – 1/15/10 Mean MET= 9. 24; sd= 5. 17 Mean CEP 7= 4. 20; sd= 1. 41 Ratio MET/CEP 7 = 2. 20 L 858 R EGFR mutation PD pleura/lung/ bones = 1 month 12/2010 Consolidative Gem/Carbo SBRT (PR) Erlotinib (PR) XRT to bone met Pem/Sut trial (PR) Doc (PD) Gem (PR) Clinical trial (EGFR/MET) ongoing
CMF 09048 Sample: N 09 -633 A 2 Received – 3/12/09 Reported – 3/18/09 Mean MET= 13. 07; sd= 4. 51 Mean CEP 7= 3. 17; sd= 1. 74 Ratio MET/CEP 7 = 4. 13 L 858 R EGFR mutation 05/2006 10 months off erlotinib selection pressure CMF 10009 Sample: S 10 -208 A 1 Received – 1/12/10 Reported – 1/15/10 Mean MET= 9. 24; sd= 5. 17 Mean CEP 7= 4. 20; sd= 1. 41 Ratio MET/CEP 7 = 2. 20 L 858 R EGFR mutation PD pleura/lung/ bones = 1 month 12/2010 Consolidative Gem/Carbo SBRT (PR) Erlotinib (PR) XRT to bone met Pem/Sut trial (PR) Doc (PD) Gem (PR) Clinical trial (EGFR/MET) ongoing
 Biston betularia (morpha typica) 1848 – carbonaria morph first described By 1895 – 98% carbonaria Natural selection, secondary to industrial soot blackening the environment, would not have been obvious if all local insects, rather than just the peppered moth, had been studied together as a single group.
Biston betularia (morpha typica) 1848 – carbonaria morph first described By 1895 – 98% carbonaria Natural selection, secondary to industrial soot blackening the environment, would not have been obvious if all local insects, rather than just the peppered moth, had been studied together as a single group.
 Changes in Unidimensional CT Measurements (RECIST) After Discontinuation and Re-Introduction of EGFR TKI 50% Change from baseline Drug sensitive cells survive drug 20% 0% -30% EGFR TKI re-start stop 3 weeks Riely et al ‘ 07
Changes in Unidimensional CT Measurements (RECIST) After Discontinuation and Re-Introduction of EGFR TKI 50% Change from baseline Drug sensitive cells survive drug 20% 0% -30% EGFR TKI re-start stop 3 weeks Riely et al ‘ 07
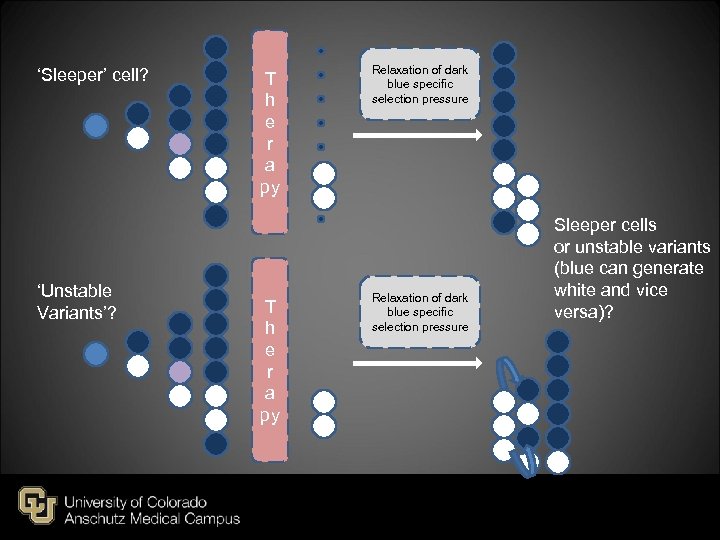 ‘Sleeper’ cell? ‘Unstable Variants’? T h e r a py Relaxation of dark blue specific selection pressure Sleeper cells or unstable variants (blue can generate white and vice versa)?
‘Sleeper’ cell? ‘Unstable Variants’? T h e r a py Relaxation of dark blue specific selection pressure Sleeper cells or unstable variants (blue can generate white and vice versa)?
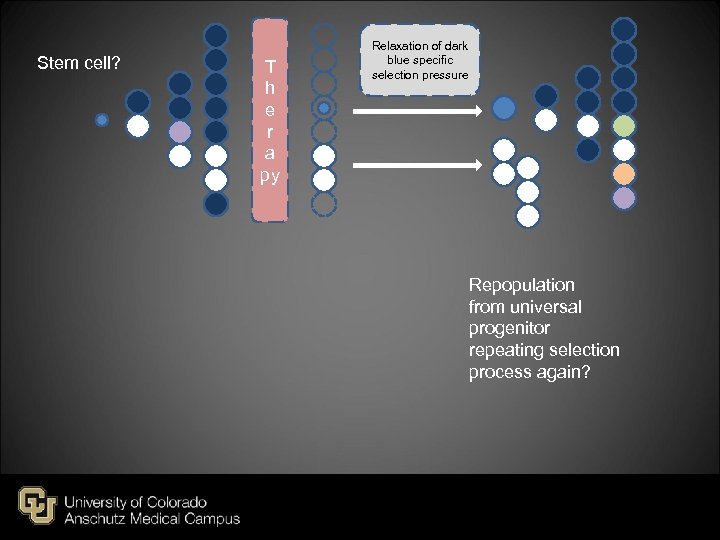 Stem cell? T h e r a py Relaxation of dark blue specific selection pressure Repopulation from universal progenitor repeating selection process again?
Stem cell? T h e r a py Relaxation of dark blue specific selection pressure Repopulation from universal progenitor repeating selection process again?
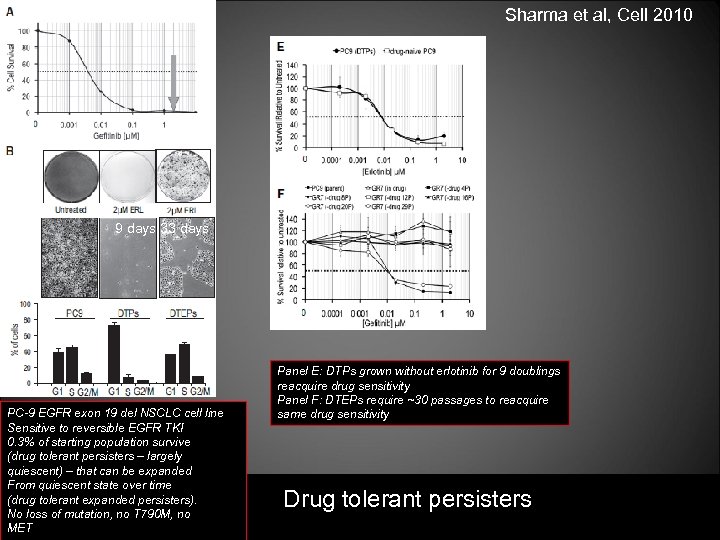 Sharma et al, Cell 2010 9 days 33 days PC-9 EGFR exon 19 del NSCLC cell line Sensitive to reversible EGFR TKI 0. 3% of starting population survive (drug tolerant persisters – largely quiescent) – that can be expanded From quiescent state over time (drug tolerant expanded persisters). No loss of mutation, no T 790 M, no MET Panel E: DTPs grown without erlotinib for 9 doublings reacquire drug sensitivity Panel F: DTEPs require ~30 passages to reacquire same drug sensitivity Drug tolerant persisters
Sharma et al, Cell 2010 9 days 33 days PC-9 EGFR exon 19 del NSCLC cell line Sensitive to reversible EGFR TKI 0. 3% of starting population survive (drug tolerant persisters – largely quiescent) – that can be expanded From quiescent state over time (drug tolerant expanded persisters). No loss of mutation, no T 790 M, no MET Panel E: DTPs grown without erlotinib for 9 doublings reacquire drug sensitivity Panel F: DTEPs require ~30 passages to reacquire same drug sensitivity Drug tolerant persisters
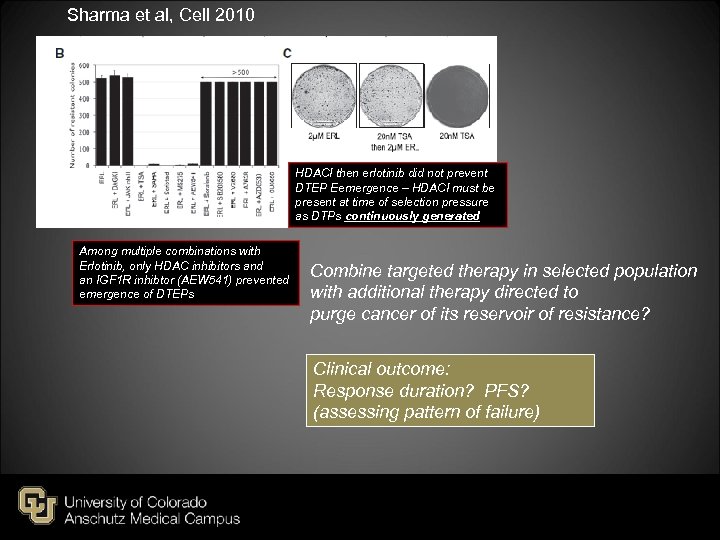 Sharma et al, Cell 2010 HDACI then erlotinib did not prevent DTEP Eemergence – HDACI must be present at time of selection pressure as DTPs continuously generated Among multiple combinations with Erlotinib, only HDAC inhibitors and an IGF 1 R inhibtor (AEW 541) prevented emergence of DTEPs Combine targeted therapy in selected population with additional therapy directed to purge cancer of its reservoir of resistance? Clinical outcome: Response duration? PFS? (assessing pattern of failure)
Sharma et al, Cell 2010 HDACI then erlotinib did not prevent DTEP Eemergence – HDACI must be present at time of selection pressure as DTPs continuously generated Among multiple combinations with Erlotinib, only HDAC inhibitors and an IGF 1 R inhibtor (AEW 541) prevented emergence of DTEPs Combine targeted therapy in selected population with additional therapy directed to purge cancer of its reservoir of resistance? Clinical outcome: Response duration? PFS? (assessing pattern of failure)
 The Limits: • Making non-chemo nucleic acid targeted therapies deliverable and addressing loss of function drivers More immediately… • Addressing heterogeneity: – In trial design and interpretation – In the financial aspects of developing new treatments • Giving the CNS credit for being different • Optimally monitoring, defining and treating acquired resistance • Addressing the existence of ‘reservoirs of resistance’
The Limits: • Making non-chemo nucleic acid targeted therapies deliverable and addressing loss of function drivers More immediately… • Addressing heterogeneity: – In trial design and interpretation – In the financial aspects of developing new treatments • Giving the CNS credit for being different • Optimally monitoring, defining and treating acquired resistance • Addressing the existence of ‘reservoirs of resistance’
 The End of the Beginning…
The End of the Beginning…
 University of Colorado Thoracic Oncology Program Positions now available for outstanding: Senior Clinical Fellows Post-Doctoral Scientists Contact: D. Ross Camidge MD, Ph. D Ross. camidge@ucdenver. edu
University of Colorado Thoracic Oncology Program Positions now available for outstanding: Senior Clinical Fellows Post-Doctoral Scientists Contact: D. Ross Camidge MD, Ph. D Ross. camidge@ucdenver. edu
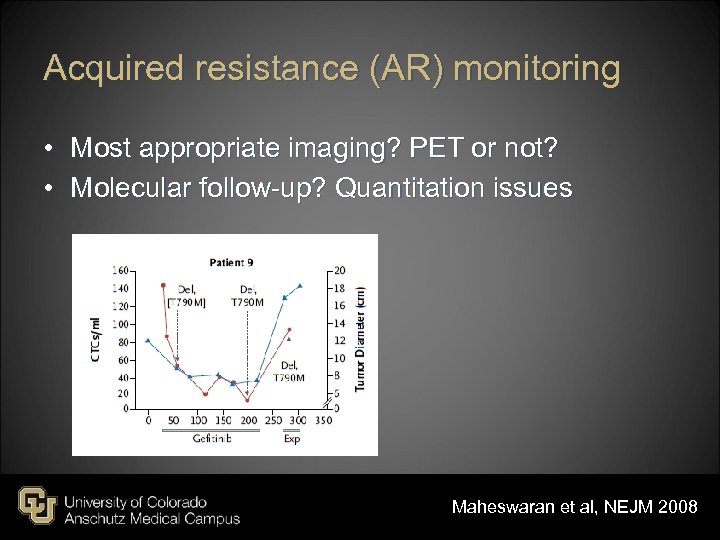 Acquired resistance (AR) monitoring • Most appropriate imaging? PET or not? • Molecular follow-up? Quantitation issues Maheswaran et al, NEJM 2008
Acquired resistance (AR) monitoring • Most appropriate imaging? PET or not? • Molecular follow-up? Quantitation issues Maheswaran et al, NEJM 2008


