98c49dae6631d0b221d92b06020aa659.ppt
- Количество слайдов: 15
 Taq. Screen West Nile Virus Program - Roche Update James L. Gallarda, Ph. D. Roche Molecular Diagnostics June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics r
Taq. Screen West Nile Virus Program - Roche Update James L. Gallarda, Ph. D. Roche Molecular Diagnostics June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics r
 r Diagnostics Critical Milestones (Update from March BPAC) • IND filed - April 21, 2003 – Approved to allow sites to begin as test of record • Phase I – Training, Installation (5 sites - US and Canada) – Initiated studies (completed May, 2003) • Unlinked studies focused on workflow, training • Phase II – Training, Installation (completed June 14) – Safety and effectiveness in detecting WNV June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening
r Diagnostics Critical Milestones (Update from March BPAC) • IND filed - April 21, 2003 – Approved to allow sites to begin as test of record • Phase I – Training, Installation (5 sites - US and Canada) – Initiated studies (completed May, 2003) • Unlinked studies focused on workflow, training • Phase II – Training, Installation (completed June 14) – Safety and effectiveness in detecting WNV June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening
 Hamilton Pipettor r COBAS Taq. Man COBAS Ampli. Prep June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics First Fully Automated System in North America Taq. Screen™ WNV
Hamilton Pipettor r COBAS Taq. Man COBAS Ampli. Prep June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics First Fully Automated System in North America Taq. Screen™ WNV
 Non-Clinical Performance Studies & Capacity Analysis June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics r
Non-Clinical Performance Studies & Capacity Analysis June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics r
 Diagnostics r Non-Clinical Performance Studies • Clinical specificity - 400 random volunteer samples from WNV low- and high- prevalence areas • Analytical specificity studies with non-WNV microorganisms • Limit of detection studies with CDC WNV lysate • Analytical sensitivity studies with IMPATH/BCP WNV Lineage 1 isolate • Analytical sensitivity studies with BBI WNV Lineage 2 (QWN 701) Isolate • Reactivity to Japanese encephalitis serocomplex members June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening
Diagnostics r Non-Clinical Performance Studies • Clinical specificity - 400 random volunteer samples from WNV low- and high- prevalence areas • Analytical specificity studies with non-WNV microorganisms • Limit of detection studies with CDC WNV lysate • Analytical sensitivity studies with IMPATH/BCP WNV Lineage 1 isolate • Analytical sensitivity studies with BBI WNV Lineage 2 (QWN 701) Isolate • Reactivity to Japanese encephalitis serocomplex members June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening
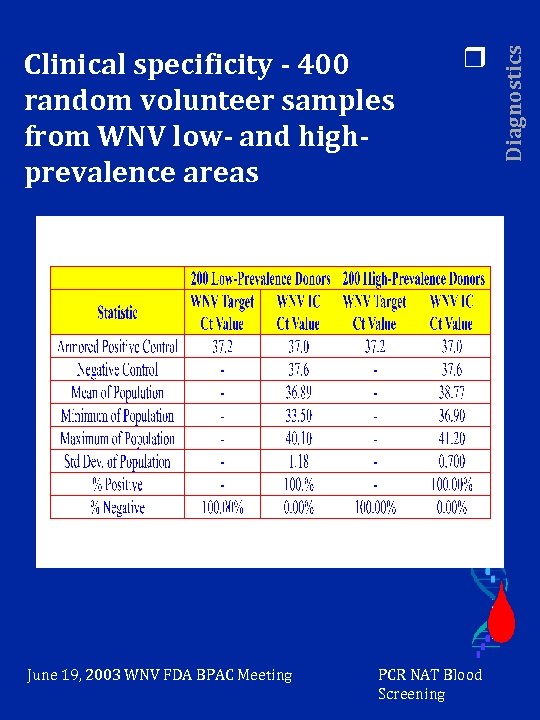 June 19, 2003 WNV FDA BPAC Meeting r PCR NAT Blood Screening Diagnostics Clinical specificity - 400 random volunteer samples from WNV low- and highprevalence areas
June 19, 2003 WNV FDA BPAC Meeting r PCR NAT Blood Screening Diagnostics Clinical specificity - 400 random volunteer samples from WNV low- and highprevalence areas
 r • Human T-cell leukemia virus (HTLV I/II) • Human papilloma virus (HPV) • Hepatitis B virus (HBV) • Varicella virus • Human immunodeficiency virus Type I (HIV-1) • Chlamydia trachomatis • Hepatitis C virus (HCV) • Staphylococcus aureus • Cytomegalovirus (CMV) • Staphylococcus epidermidis Diagnostics Analytical specificity studies with non-WNV microorganisms • Hepatitis A virus (HAV) • Adenovirus • Herpes simplex virus (HSV) • Proprionibacterium acnes • Neisseria gonorrhoeae • Candida albicans June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening
r • Human T-cell leukemia virus (HTLV I/II) • Human papilloma virus (HPV) • Hepatitis B virus (HBV) • Varicella virus • Human immunodeficiency virus Type I (HIV-1) • Chlamydia trachomatis • Hepatitis C virus (HCV) • Staphylococcus aureus • Cytomegalovirus (CMV) • Staphylococcus epidermidis Diagnostics Analytical specificity studies with non-WNV microorganisms • Hepatitis A virus (HAV) • Adenovirus • Herpes simplex virus (HSV) • Proprionibacterium acnes • Neisseria gonorrhoeae • Candida albicans June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening
 r Diagnostics Analytical specificity studies with non-WNV microorganisms • Each of the 125 samples positive for these microorganisms tested negative in the Taq. Screen WNV assay - no false-positive test results were observed. All associated Armored Internal Controls were positive for each sample tested. June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening
r Diagnostics Analytical specificity studies with non-WNV microorganisms • Each of the 125 samples positive for these microorganisms tested negative in the Taq. Screen WNV assay - no false-positive test results were observed. All associated Armored Internal Controls were positive for each sample tested. June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening
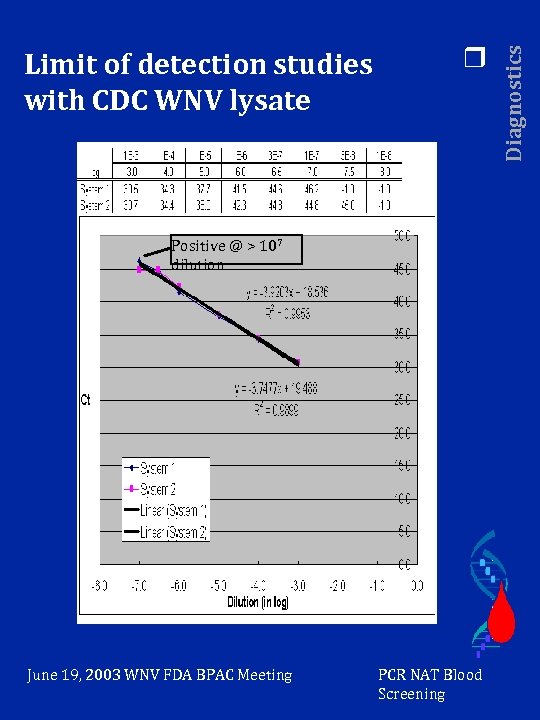 r Positive @ > 107 dilution June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics Limit of detection studies with CDC WNV lysate
r Positive @ > 107 dilution June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics Limit of detection studies with CDC WNV lysate
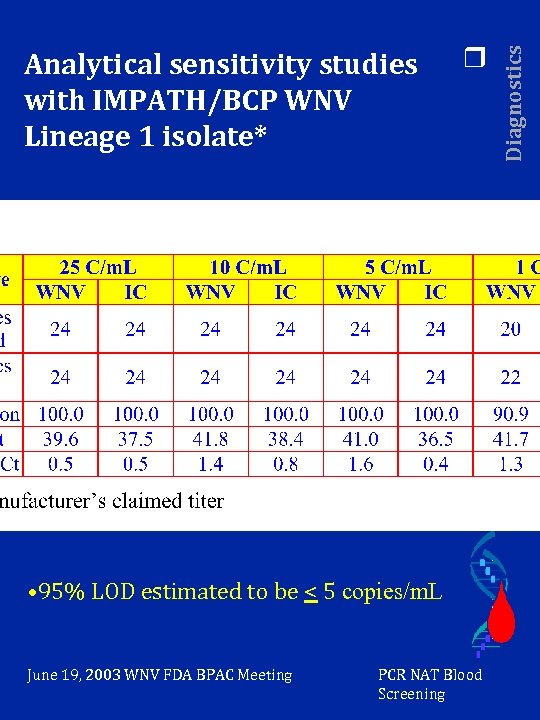 r • 95% LOD estimated to be < 5 copies/m. L June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics Analytical sensitivity studies with IMPATH/BCP WNV Lineage 1 isolate*
r • 95% LOD estimated to be < 5 copies/m. L June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics Analytical sensitivity studies with IMPATH/BCP WNV Lineage 1 isolate*
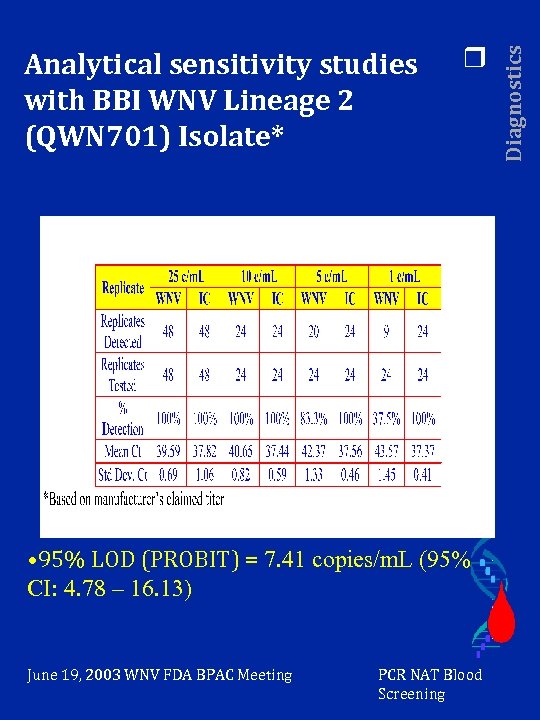 r • 95% LOD (PROBIT) = 7. 41 copies/m. L (95% CI: 4. 78 – 16. 13) June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics Analytical sensitivity studies with BBI WNV Lineage 2 (QWN 701) Isolate*
r • 95% LOD (PROBIT) = 7. 41 copies/m. L (95% CI: 4. 78 – 16. 13) June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics Analytical sensitivity studies with BBI WNV Lineage 2 (QWN 701) Isolate*
 • Additional WNV isolate (ATCC VR-1267) • St Louis encephalitis virus (ATCC VR-1265) • Murray Valley encephalitis virus (ATCC VR 77) All isolates detected June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics r Reactivity to Japanese Encephalitis serocomplex members
• Additional WNV isolate (ATCC VR-1267) • St Louis encephalitis virus (ATCC VR-1265) • Murray Valley encephalitis virus (ATCC VR 77) All isolates detected June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics r Reactivity to Japanese Encephalitis serocomplex members
 r Diagnostics Phase I Capacity Analysis (Gulf Coast Regional Blood Center) • Start pooling 0630 hrs (average pooling time 19. 5 minutes) • First extractions on COBAS Ampli. Prep begin 0649 hrs • First amplifications begin 0900 hrs • Total throughput with 2 FTE operating 2 systems = 1728 samples in 11 ½ hours • Conservative extrapolation (one shift of 8 hours with “reporting” in the last 3 ½ hours) estimates ~500, 000 samples per 2 FTE operating 2 system per year. June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening
r Diagnostics Phase I Capacity Analysis (Gulf Coast Regional Blood Center) • Start pooling 0630 hrs (average pooling time 19. 5 minutes) • First extractions on COBAS Ampli. Prep begin 0649 hrs • First amplifications begin 0900 hrs • Total throughput with 2 FTE operating 2 systems = 1728 samples in 11 ½ hours • Conservative extrapolation (one shift of 8 hours with “reporting” in the last 3 ½ hours) estimates ~500, 000 samples per 2 FTE operating 2 system per year. June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening
 • All 11 US sites and 3 Canadian sites are installed and prepared for linked testing on or before July 1 • Potential expansion to other sites • Assisting public health lab diagnostic needs • Dissemination of prevalence data through REDS • USDA permit requirements to ship WNV panels • Standardization of WNV panel • Capital, training, and support costs are substantial to execute multi-site IND June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics r Current Status & Issues
• All 11 US sites and 3 Canadian sites are installed and prepared for linked testing on or before July 1 • Potential expansion to other sites • Assisting public health lab diagnostic needs • Dissemination of prevalence data through REDS • USDA permit requirements to ship WNV panels • Standardization of WNV panel • Capital, training, and support costs are substantial to execute multi-site IND June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics r Current Status & Issues
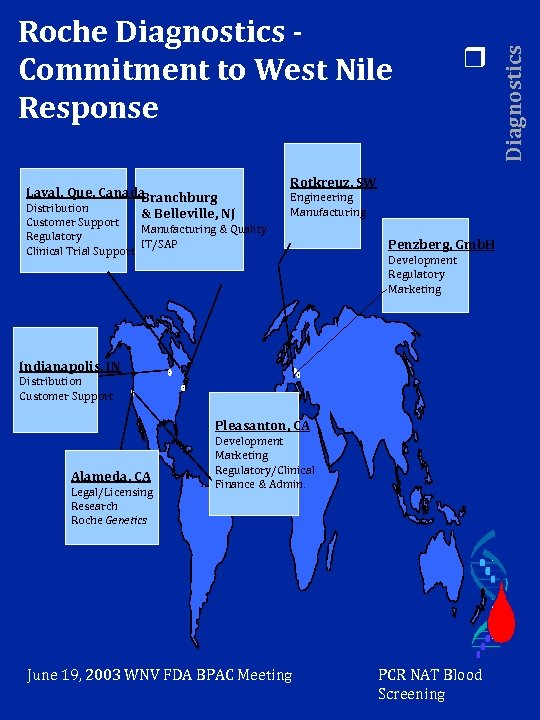 Laval, Que, Canada Branchburg Distribution & Belleville, NJ Customer Support Manufacturing & Quality Regulatory IT/SAP Clinical Trial Support r Rotkreuz, SW Engineering Manufacturing Penzberg, Gmb. H Development Regulatory Marketing Indianapolis, IN Distribution Customer Support Pleasanton, CA Alameda, CA Legal/Licensing Research Roche Genetics Development Marketing Regulatory/Clinical Finance & Admin. June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics Roche Diagnostics Commitment to West Nile Response
Laval, Que, Canada Branchburg Distribution & Belleville, NJ Customer Support Manufacturing & Quality Regulatory IT/SAP Clinical Trial Support r Rotkreuz, SW Engineering Manufacturing Penzberg, Gmb. H Development Regulatory Marketing Indianapolis, IN Distribution Customer Support Pleasanton, CA Alameda, CA Legal/Licensing Research Roche Genetics Development Marketing Regulatory/Clinical Finance & Admin. June 19, 2003 WNV FDA BPAC Meeting PCR NAT Blood Screening Diagnostics Roche Diagnostics Commitment to West Nile Response


