Scleroderma integr 2006.ppt
- Количество слайдов: 67

Systemic Sclerosis (SSc) Alexandra Balbir-Gurman

Systemic Sclerosis (SSc) n n n Chronic disease Progressive course Skin thickening – scleroderma Multi-organ involvement Early and late SSc are different Subsets of SSc are different
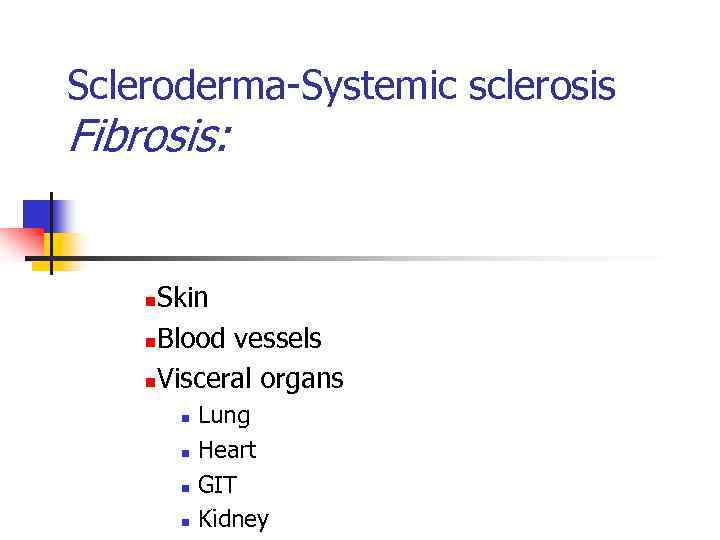
Scleroderma-Systemic sclerosis Fibrosis: Skin n. Blood vessels n. Visceral organs n n n Lung Heart GIT Kidney

Epidemiology Incidence: 1 -2 to 100000 people Age: 35 -55 y Gender: F: M = 8: 1 African Americans have more severe disease
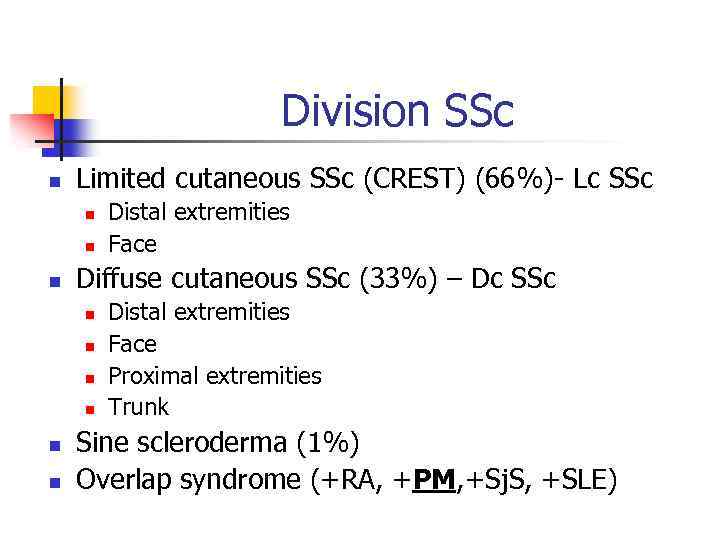
Division SSc n Limited cutaneous SSc (CREST) (66%)- Lc SSc n n n Diffuse cutaneous SSc (33%) – Dc SSc n n n Distal extremities Face Proximal extremities Trunk Sine scleroderma (1%) Overlap syndrome (+RA, +PM, +Sj. S, +SLE)
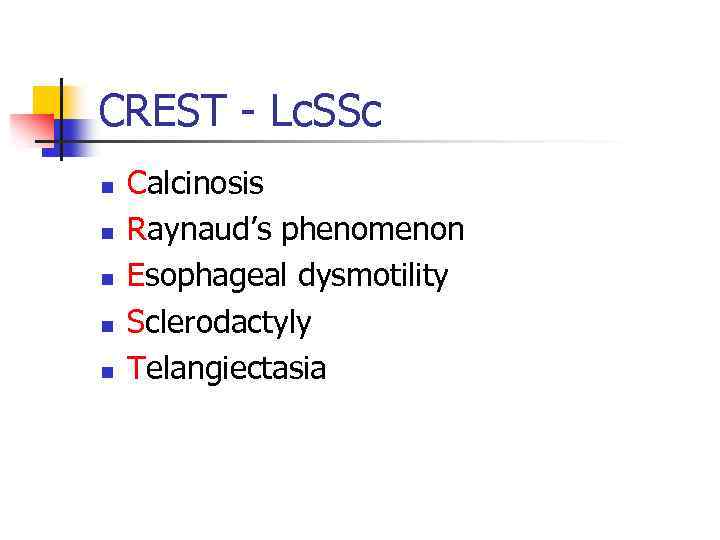
CREST - Lc. SSc n n n Calcinosis Raynaud’s phenomenon Esophageal dysmotility Sclerodactyly Telangiectasia
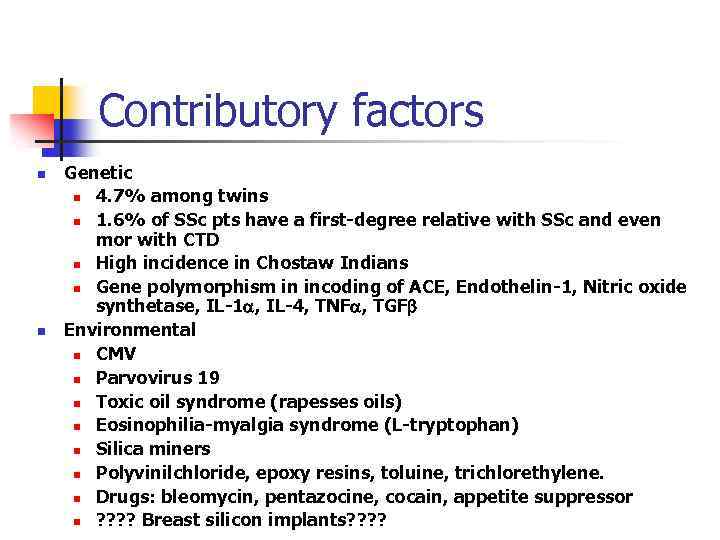
Contributory factors n n Genetic n 4. 7% among twins n 1. 6% of SSc pts have a first-degree relative with SSc and even mor with CTD n High incidence in Chostaw Indians n Gene polymorphism in incoding of ACE, Endothelin-1, Nitric oxide synthetase, IL-1 , IL-4, TNF , TGF Environmental n CMV n Parvovirus 19 n Toxic oil syndrome (rapesses oils) n Eosinophilia-myalgia syndrome (L-tryptophan) n Silica miners n Polyvinilchloride, epoxy resins, toluine, trichlorethylene. n Drugs: bleomycin, pentazocine, cocain, appetite suppressor n ? ? Breast silicon implants? ?
Pathogenesis of SSc n n n Vasculopathy Cellular and humoral immunity Progressive visceral and vascular fibrosis n Animal models n n Tigh skin mouse Bleomycin induced in mice Chicken GVHD
Pathogenesis: Cellular and humoral autoimmunity n T-cell, monocytes, macrophages cell activation n T cells- CD 4+ have elevated level of receptors to cytokines and adhesion molecules n T cells, monocytes, macropheges show Th-2 response n Cytokine production n Excess of Th-2 cytokines IL-2, IL-4, IL-6, IL-13, Transforming Growth factor (TGF- ), CTGF n Deficit of Th-1 cytokines INF- n B-cells activation n Ab production n T-cells and fibroblasts activation Fibroblast activation n Collagen type I and III n Collagen type VII (abnormal) n
Pathogenesis: humoral immune abnormalities n n n ANA (~100%) – correlate with SSc activity and severity n Anti-topoisomerase (SCL-70) n Anti-centromere n Anti RNA-polymerase Ab against surface cell-surface Ag, secreted proteins: to fibroblasts, to EC, to PDGF receptors, to Fibrillin-1, to MMPe Other non specific Ab: n RF+ n SS-A, SS-B n Anti-mitochondrial Ab (Primary Billiary Cirrhosis- PBC)
Pathogenesis: vascular damage n n n Endothelial cell (EC) injury n Endothelin 1(ET-1) excess n Nitric oxide (NO) impaired production n Prostacycline (Pg. I) impaired production n Von Willebrand factor (v. WF) activation n Adhesion molecules activation (ICAM-1) n PLT activation (thromboxane, PDGF) n VEGF Dysfunction of autonomic and peripheral nervous system Reduced number of endothelial progenitor cells Obliterative vasculopathy
Pathogenesis n n Fibroblast activation Myofibroblasts transformation Overexpression of genes related to fibrosis: collagen, fibronectin, fibrillins Overproduction and accumulation of collagen n Small vessels damage n Raynaud’s phenomenon n GIT damage n Heart n Renal ischemia n Lung – pulmonary hypertension n Tissue collagen accumulation
Pathology - SSc n n Obliterative vasculopathy Early phase – perivascular inflammatory cells accumulation n CD 4+(skin) CD 8+ (lungs) Fibrosis
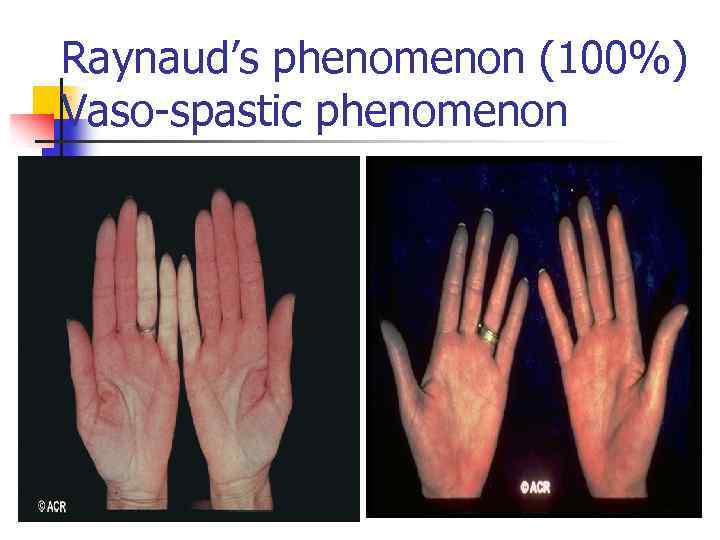
Raynaud’s phenomenon (100%) Vaso-spastic phenomenon
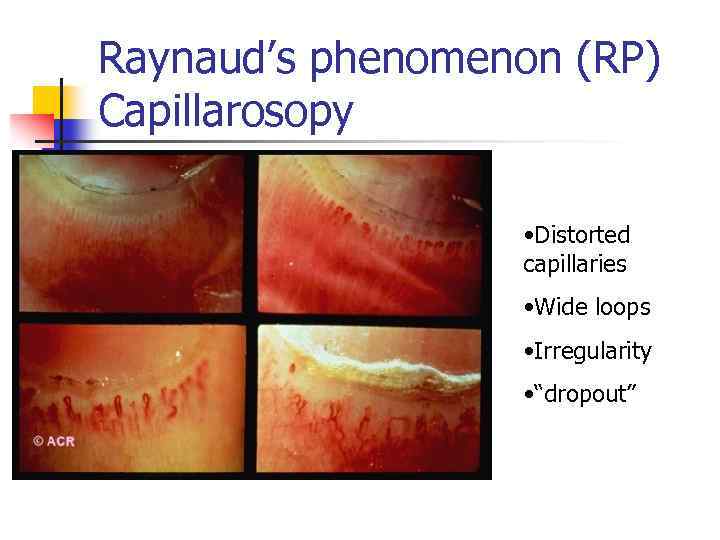
Raynaud’s phenomenon (RP) Capillarosopy • Distorted capillaries • Wide loops • Irregularity • “dropout”
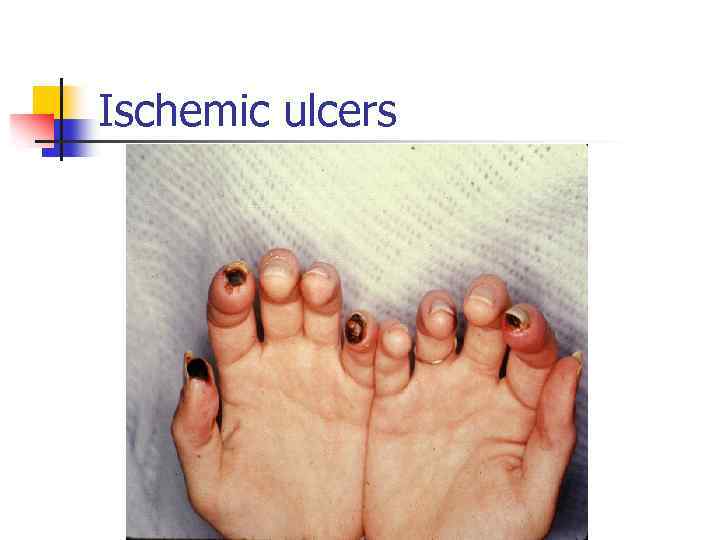
Ischemic ulcers
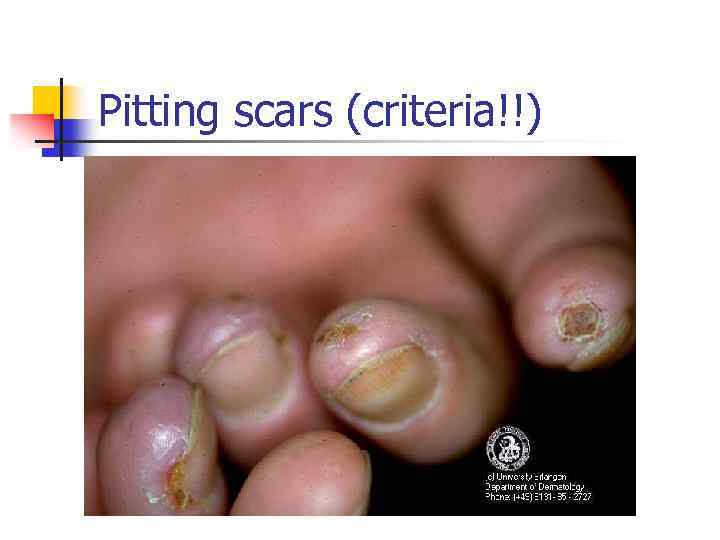
Pitting scars (criteria!!)
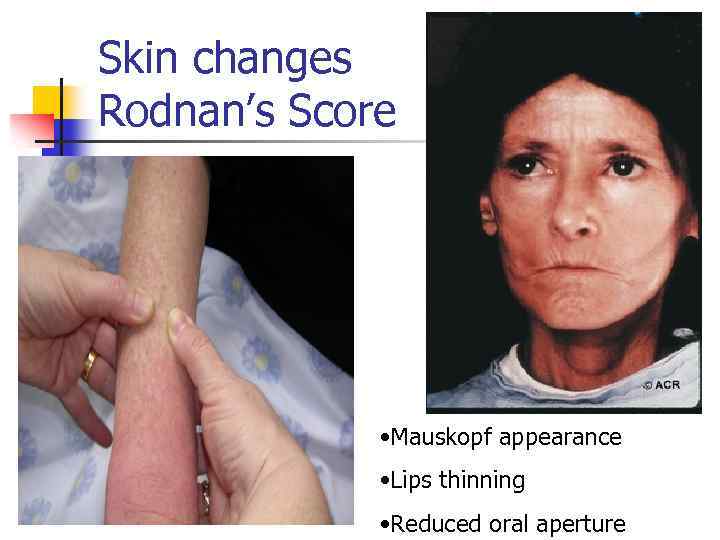
Skin changes Rodnan’s Score • Mauskopf appearance • Lips thinning • Reduced oral aperture
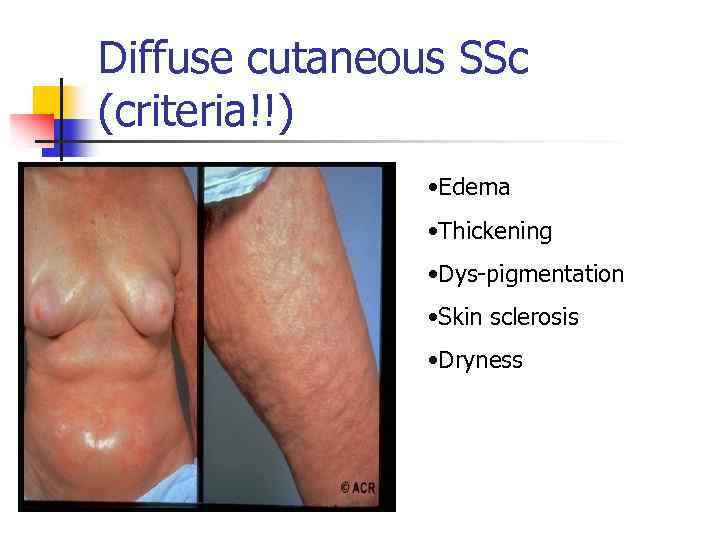
Diffuse cutaneous SSc (criteria!!) • Edema • Thickening • Dys-pigmentation • Skin sclerosis • Dryness
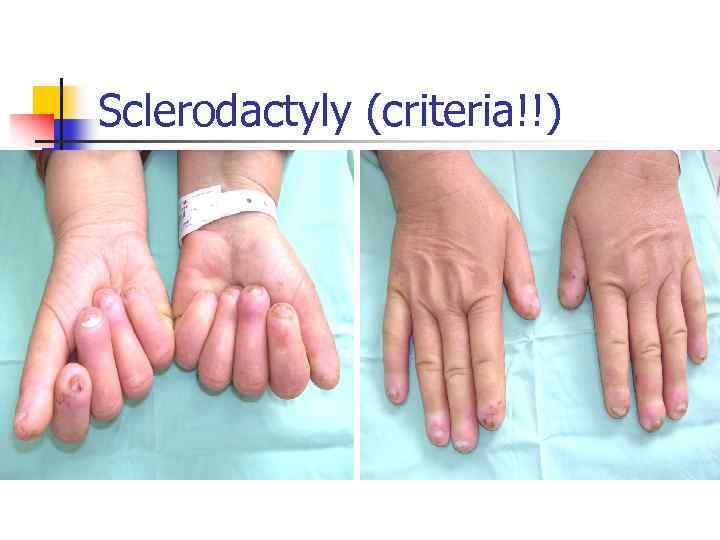
Sclerodactyly (criteria!!)
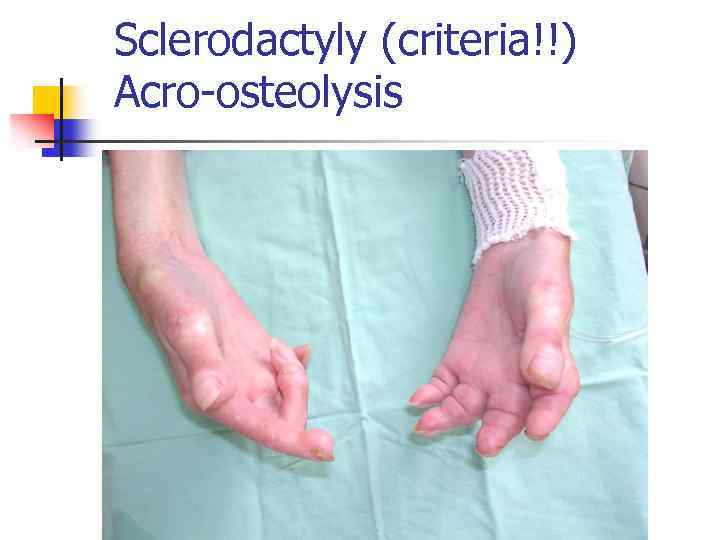
Sclerodactyly (criteria!!) Acro-osteolysis
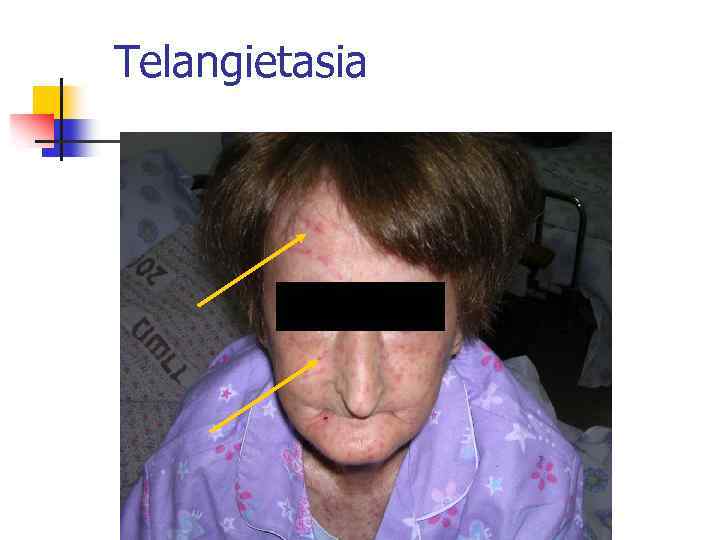
Telangietasia
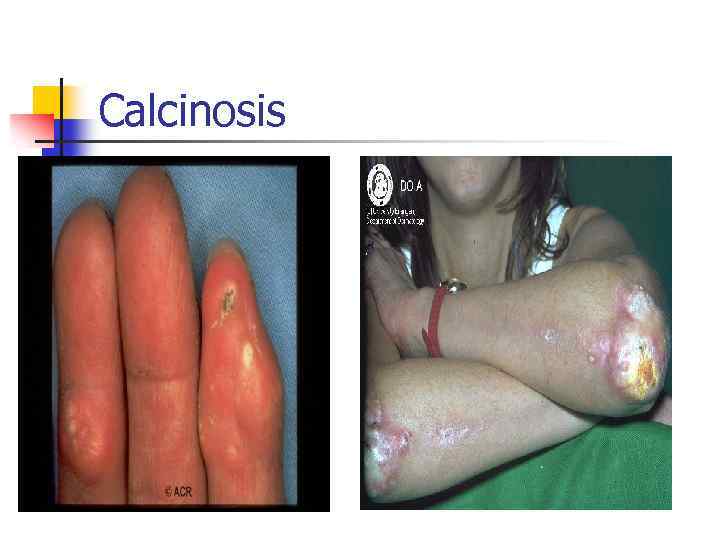
Calcinosis
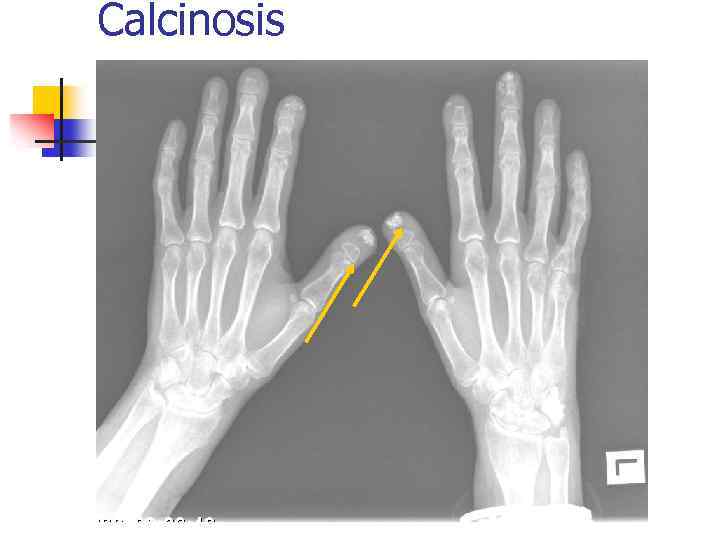
Calcinosis
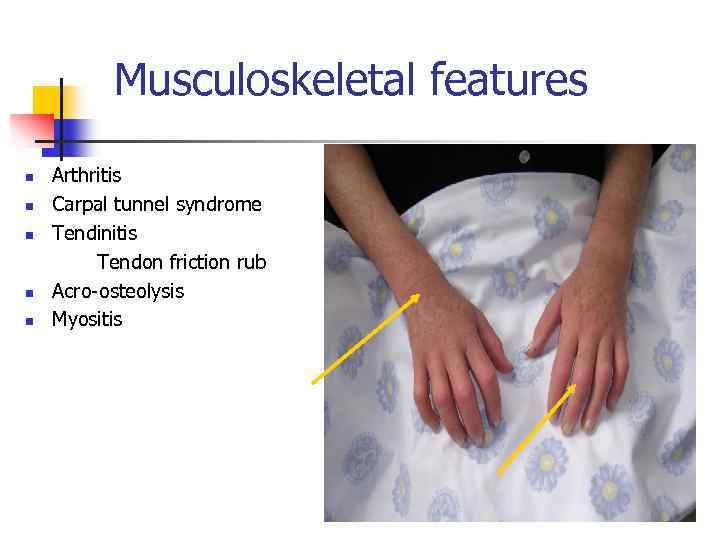
Musculoskeletal features n n n Arthritis Carpal tunnel syndrome Tendinitis Tendon friction rub Acro-osteolysis Myositis
GIT involvement (50 -90%) Xerostomia, periodontitis n Esophageal dysmotility and reflux – GERD n n n n n Heartburn Regurgitation Dysphagia Esophagitis Stricture Barret’s esophagus Hoarsness Aspiration pneumonitis

GIT involvement n n n Gastroparesis Watermelon stomach - GAVE n (GIT bleeding) Bacterial overgrowth Malabsorption Diarrhea Malnutrition Weight loss Pseudoileus - obstruction Fecal incontinence Rectal prolapse Pneumatosis cystoides intestinalis Primary billiary cirrhosis (PBC)

Pulmonary involvement n n n Pleuritis Interstitial lung disease Pulmonary hypertension Both Leading cause of death
Pulmonary fibrosis (PF) (criteria!!) n n n n Cough Effort dyspnea Rest dyspnea Hypoxemia Secondary PAH Right CHF Right ventricular arrhythmias (VF) Death
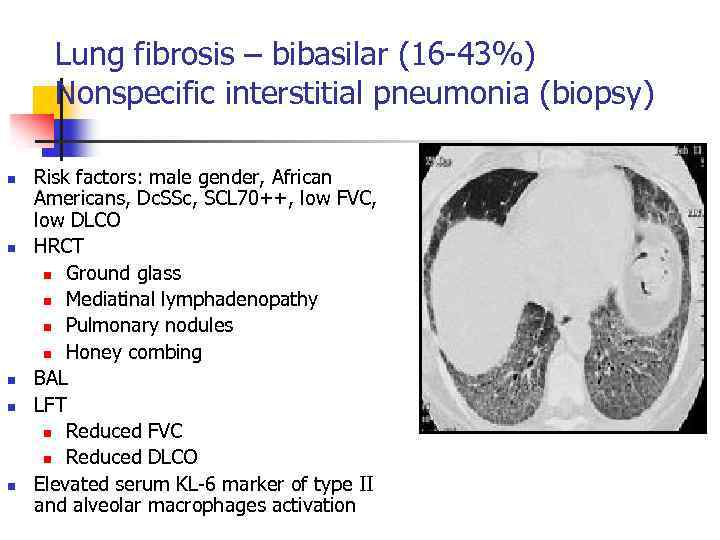
Lung fibrosis – bibasilar (16 -43%) Nonspecific interstitial pneumonia (biopsy) n n n Risk factors: male gender, African Americans, Dc. SSc, SCL 70++, low FVC, low DLCO HRCT n Ground glass n Mediatinal lymphadenopathy n Pulmonary nodules n Honey combing BAL LFT n Reduced FVC n Reduced DLCO Elevated serum KL-6 marker of type II and alveolar macrophages activation
Pulmonary hypertension (PAH-primary) n n n n Effort dyspnea Fatigue Syncope Rest dyspnea Hypoxemia Right CHF Right ventricular arrhythmias (VF) Death
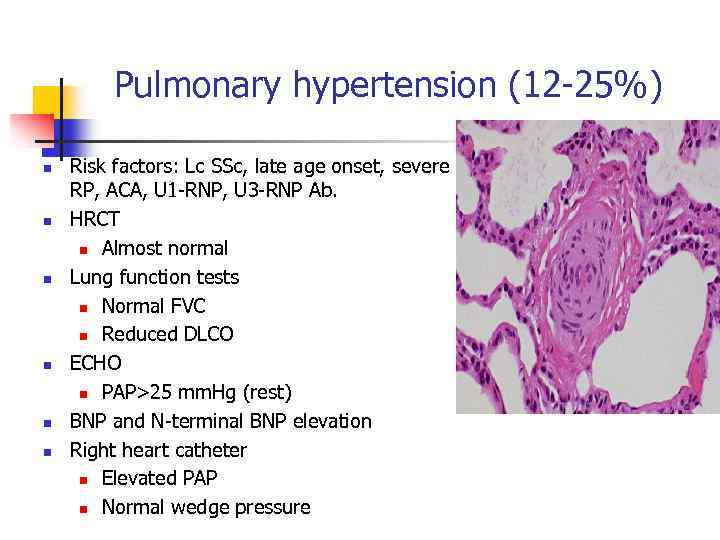
Pulmonary hypertension (12 -25%) n n n Risk factors: Lc SSc, late age onset, severe RP, ACA, U 1 -RNP, U 3 -RNP Ab. HRCT n Almost normal Lung function tests n Normal FVC n Reduced DLCO ECHO n PAP>25 mm. Hg (rest) BNP and N-terminal BNP elevation Right heart catheter n Elevated PAP n Normal wedge pressure
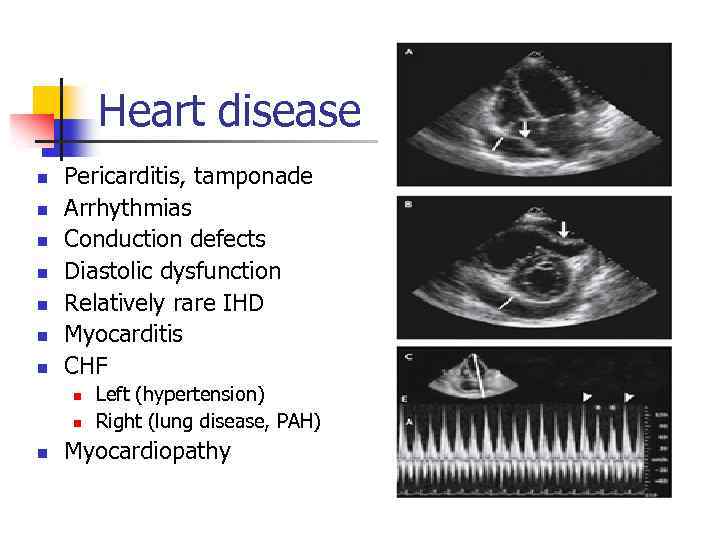
Heart disease n n n n Pericarditis, tamponade Arrhythmias Conduction defects Diastolic dysfunction Relatively rare IHD Myocarditis CHF n n n Left (hypertension) Right (lung disease, PAH) Myocardiopathy

Renal disease (crisis) Early disease n n n Precipitating factors n Dehydration, hypotension n Pregnancy n Dc. SSc, male gender, anti RNA polymerase III Ab n High doses of Cs Renal crisis n Hypertension n Headache n Blurred vision n Chest pain n Dyspnea n Renal failure n Microangiopathic hemolytic anemia, thrombocytopenia n Left CHF n Encephalopthy Mild proteinuria

Others +SSc n n n n n PBC in Limited SSc Sicca syndrome Impotence Hypothyroidism Celiac disease B 12 deficiency Neuropathy Depression Pregnancy problems:

Diagnostic criteria SSc n Major n n Diffuse cutaneous SSc Minor n n n Pitting scars Sclerodactyly Pulmonary fibrosis

Laboratory tests n Anemia n n n n Iron deficiency B 12, FA deficiency Microangiopathic hemolytic anemia ESR elevation not prominent Hupoalbuminemia CK elevation ANA, SCL 70, ACA, anti RNAIII polymerase, KL -6, AMA, Gliadin Ab etc

DD in SSc n n n Scleredema Scleromyxedema Nephrogenic systemic fibrosis GVHD Eosinophilic fasceitis

Treatment SSc: medicamentous, education n RP Keep worm, wax n CCB n AIIRB n Prostacyclin (Ilomedin) n ET-1 RB n PDE n Statins n Local nitrate n Sympathectomy Ulcers/calcinosis n Antibiotics n Debridement n n

Treatment SSc n GIT n Supportive n PPI inhibitors (H 2 -blockers) n Laser coagulation for GAVE n Broad spectrum antibiotics for overgrowth n Hyperalimentation parenteral n Octreotide for severe hypomotility n Surgery occasionally

Treatment SSc n Skin n Joints n n Small doses of steroids Cytotoxic drugs (cyclophosphamide, MTX) Small doses of steroids DMARD’s (MTX, Mycophenolate Mofetil - MMF) Physiotherapy Myositis n n Steroids DMARD’s (MTX), IVIG

Treatment SSc n n Pulmonary fibrosis n Steroids? ? n Cyclophosphamide n MMF n Oxygen Pulmonary hypertension n ET-1 receptor inhibitors (Tracleer) n Sildenafil n Synthetic prostacyclins (Flolan, Ilomedin, Treprostenil) n Oxygen n Diuretics, oral anticoagulants, digoxin n Lung transplantation
Treatment SSc n Renal n n n To avoid NSAID, Cs, B-blockers ACE inhibitors AIIRB Dialysis Late transplantation Heart n n Pacemaker Symptomatic
Prognosis SSc n n Diffuse 10 -year 55% n n n SCL-70 Anti RNA polymerase Pulmonary fibrosis Renal crisis Heart damage n n Limited 10 -year 75% n n PAH Heart damage
Multidisciplinary approach Oral special ist Rheumatolog ist Nurse Social worker Obstetrics & gynecologi st Pulmonolo gist Scleroderma patient Dermatol ogist Orthopedic surgeon Primar y care physici an Nephrologi st Cardiologist Gastroenterol ogist Physiother apist
Centers Patient s Medical teams Concentration Facilities Early diagnosis Experience Early treatment Clinical trials Patients education Since Patients supporting groups

Mixed Connective Tissue Disease - MCTD Alexandra Balbir-Gurman
MCTD n Coexisting n n n SLE SSc - limited Polymyositis RA Antibodies to U 1 -RNP

MCTD- clinical features n n n RP, puffy hands, myalgia Sclerodactyly, calcinosis, telangiectasias, esophageal dysmotility Malar rash, photosensitivity Heliotropic rash, nephritis Erosive arthritis Sicca syndrome (Sjogren’s syndrome)
MCTD – life threatening conditions n n Pneumonitis and pulmonary fibrosis PAH Pericarditis Nephrotic syndrome – membranous nephritis
MCTD – Laboratory data n High inflammatory markers n n ESR CRP Hyperglobulinemia ANA, U 1 -RNP

MCTD- treatment n n Cs high doses DMARD’s CYC Prognosis – better then SSc

Eosinophilic fasciitis n Scleroderma-like skin disease n n n n Cobblestone “peau d’orange”skin Fingers are spared No internal organ involvement Transient eosinophilia High ESR, CRP No antibodies Deep skin biopsy with fascia: inflammatory infiltrate with eosinophils Treatment: Cs +/- DMARD’s
INFLAMMATORY MYOPATHY EPIDEMIOLOGY Incidence: 1 -8/1 000 Age: 10 -15 years 45 -60 years Gender: F: M = 2: 1 (IBM F: M = 1: 2) Secondary myopathy: statins rabdomyolysis

Classification n n n Polymyositis Dermatomyositis Childhood dermatomyositis Myositis associated with Collagen diseases Myositis associated with malignancy Inclusion body myositis

Polymyositis n n Progressive course Proxymal muscles weakness** Dysphagia Dyspnea n n Chest muscles weakness Lung fibrosis Cardiomyopathy Weight loss, low grade fever, fatigue

Dermatomyositis n Skin changes n n Proxymal muscles weakness Dysphagia Dyspnea n n n Heliotrope rash (eyes, neck, fingers) Chest muscles weakness Lung fibrosis Cardiomyopathy Raynaud’s phenomenon Calcinosis Weight loss, fever, fatigue
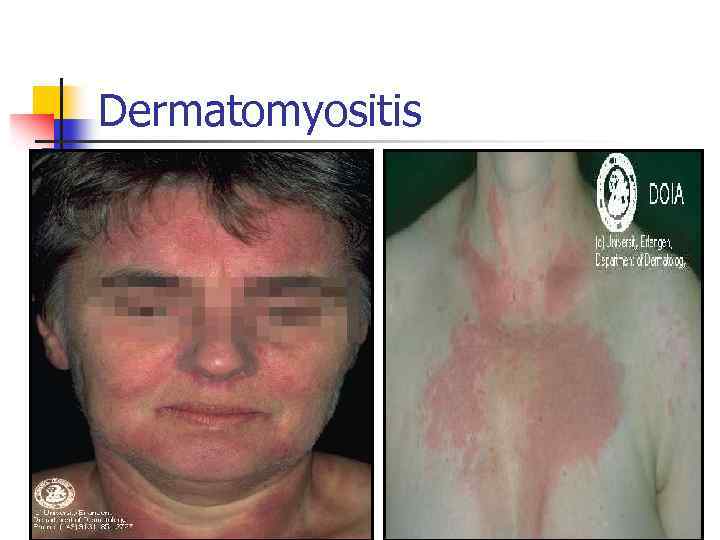
Dermatomyositis
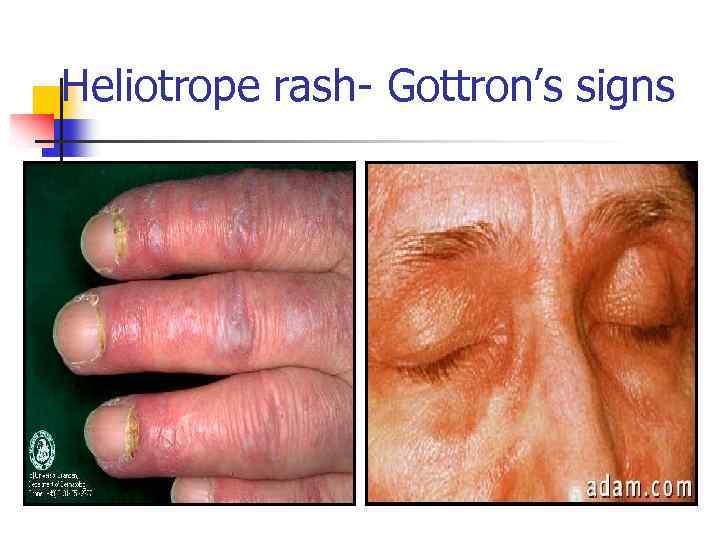
Heliotrope rash- Gottron’s signs
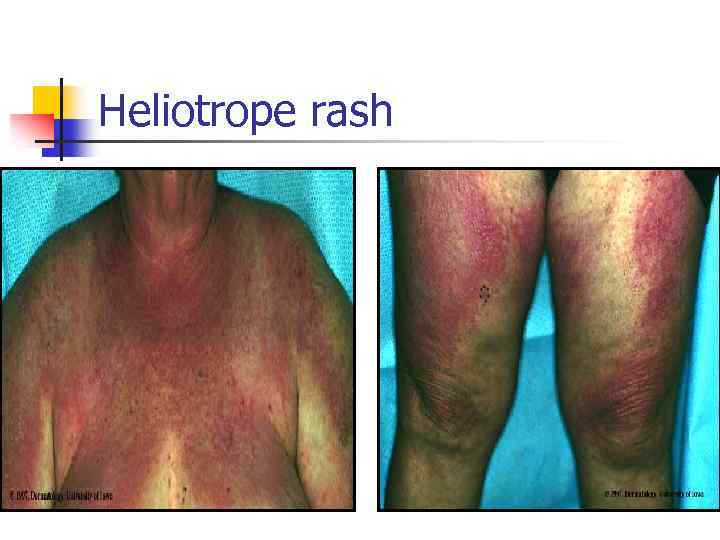
Heliotrope rash

PM/DM with malignancy n Age >60 years n n n Breast Ovary Rectal Lung Lymphomas

Myositis and CVD n Overlap n n n Systemic sclerosis SLE RA MCTD Sjogren’s syndrome Crohn’s disease

Diagnosis - criteria Symmetric muscle weakness (+/-dysphagia, +/-respiratory weakness) Elevation of muscle enzymes (AST, ALT, CK, LDH, aldolase) EMG changes (polyphasic motor units, fibrillation) Biopsy (necrosis, atrophy, cell infiltration) Rash (Dermatomyositis)

Myositis – additional laboratory tests n n Elevated ESR Elevated CRP Antinuclear antibodies 30% Anti synthetase- anti Jo-1 n n Pulmonary fibrosis Differential diagnosis n n n HIV related HCV related Drug related (statins, bezafibrate)
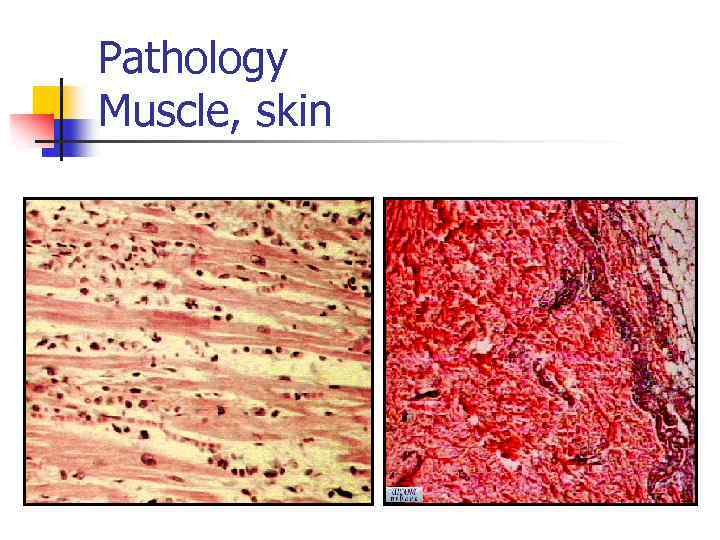
Pathology Muscle, skin

Treatment n n n Steroids DMARD’s n Methotrexate n Imurane n MMF IV gamma-globulins-high doses Pulmonary fibrosis, myocarditis n Cyclophosphamide GIT n PPI n Prokinetic drugs RP n CCB, Ilomedin

Prognosis n n n 5 -year survival – 75% Severe muscle wasting Muscle contractures Pulmonary fibrosis CHF Malignancy +/- 3 years
Scleroderma integr 2006.ppt