f5bdc865f6f2847ad3cf57198c7e3582.ppt
- Количество слайдов: 43

Systematic Analysis of Observational Data to Augment Current Pharmacovigilance Practices Stephanie Reisinger, SVP Pro. Sanos Corp. Harrisburg, PA Pro. Sanos Corporation Confidential and Proprietary 1

Today’s Agenda u Primary Sources of Safety Data Today – What are the Gaps? u Systematic Analysis of Observational Data – – What is it? Why now? What Are the Challenges? How can Systematic Analysis Augment and Inform Current Practices? Objective: Framework for thinking about the use of observational data in the context of of current pharmacovigilance practices 2 Pro. Sanos Corporation Confidential and Proprietary

Case Study and Disclosure u In 2005, Glaxo. Smith. Kline (GSK) initiated a largescale R&D project (Safety. Works) to research and develop methodologies to enable the systematic use of observational data u GSK and Pro. Sanos® worked in partnership to implement the methodologies in web based software for access by GSK Safety Scientists, which was implemented in 2008 u Pro. Sanos markets a commercial version of this software 3 Pro. Sanos Corporation Confidential and Proprietary
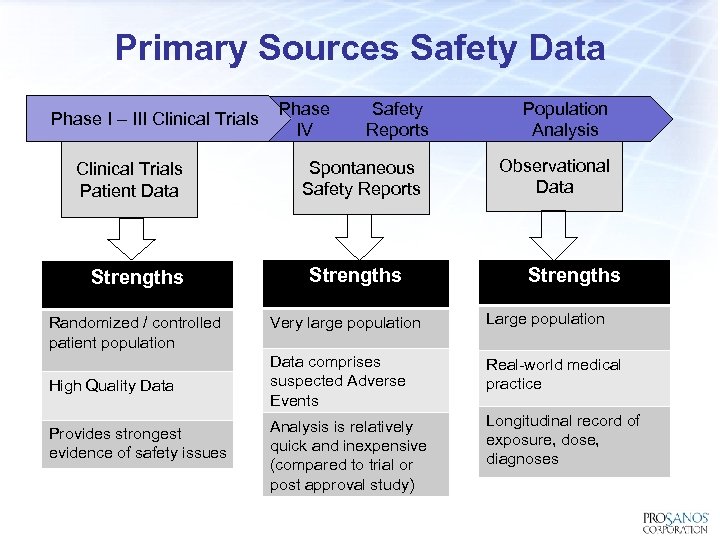
Primary Sources Safety Data Phase I – III Clinical Trials Patient Data Strengths Randomized / controlled patient population High Quality Data Provides strongest evidence of safety issues Phase IV Safety Reports Spontaneous Safety Reports Strengths Population Analysis Observational Data Strengths Very large population Large population Data comprises suspected Adverse Events Real-world medical practice Analysis is relatively quick and inexpensive (compared to trial or post approval study) Longitudinal record of exposure, dose, diagnoses
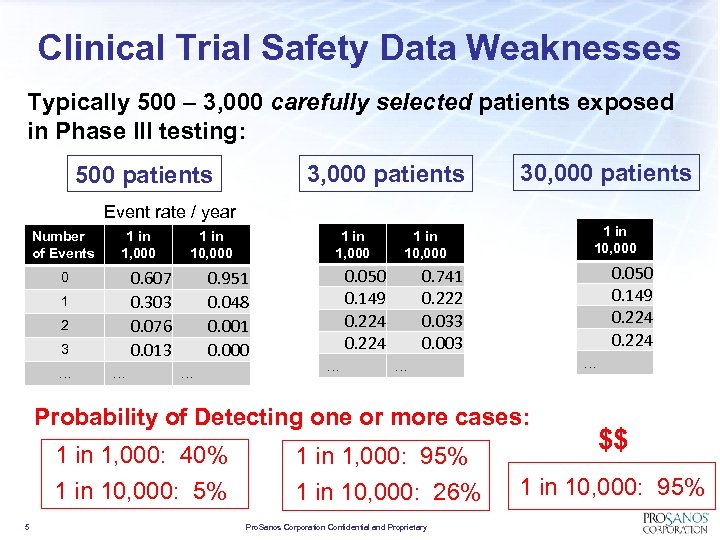
Clinical Trial Safety Data Weaknesses Typically 500 – 3, 000 carefully selected patients exposed in Phase III testing: 3, 000 patients 500 patients 30, 000 patients Event rate / year Number of Events 1 in 1, 000 0. 607 0. 303 0. 076 0. 013 0 1 2 3 … … 1 in 1, 000 1 in 10, 000 0. 951 0. 048 0. 001 0. 000 … 0. 050 0. 149 0. 224 … 1 in 10, 000 0. 050 0. 149 0. 224 0. 741 0. 222 0. 033 0. 003 … … Probability of Detecting one or more cases: 1 in 1, 000: 40% 1 in 10, 000: 5% 5 1 in 1, 000: 95% 1 in 10, 000: 26% Pro. Sanos Corporation Confidential and Proprietary $$ 1 in 10, 000: 95%
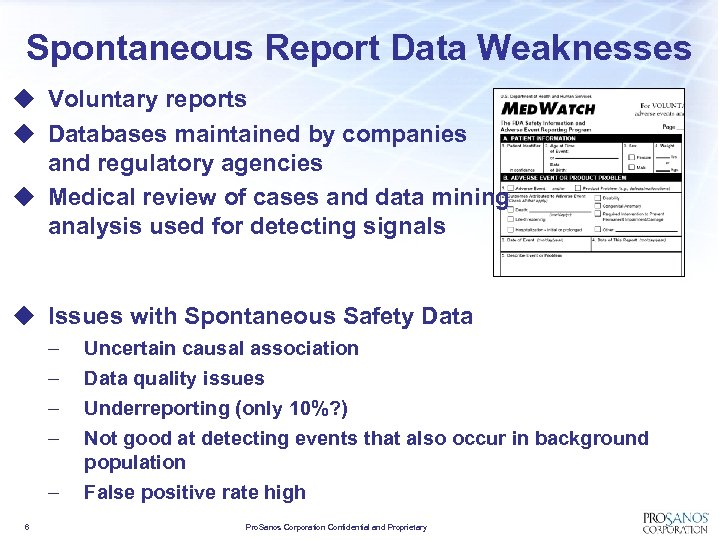
Spontaneous Report Data Weaknesses u Voluntary reports u Databases maintained by companies and regulatory agencies u Medical review of cases and data mining analysis used for detecting signals u Issues with Spontaneous Safety Data – – – 6 Uncertain causal association Data quality issues Underreporting (only 10%? ) Not good at detecting events that also occur in background population False positive rate high Pro. Sanos Corporation Confidential and Proprietary
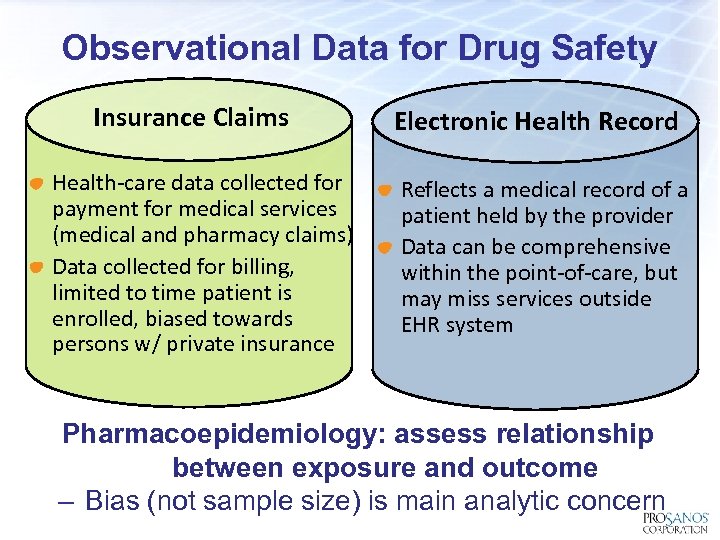
Observational Data for Drug Safety Insurance Claims Health-care data collected for payment for medical services (medical and pharmacy claims) Data collected for billing, limited to time patient is enrolled, biased towards persons w/ private insurance Electronic Health Record Reflects a medical record of a patient held by the provider Data can be comprehensive within the point-of-care, but may miss services outside EHR system Pharmacoepidemiology: assess relationship between exposure and outcome – Bias (not sample size) is main analytic concern

Weaknesses of Current Observational Data Paradigm u “One –off” studies analyses not reusable on disparate databases due data format differences u Expensive cost of individual pharmacoepidemiology studies*: – $30 K-50 K for a feasibility/protocol assessments – $80 K-150 K (<$200 K) for a descriptive study – $200 K-400 K for a single case-control/analytical studies (no validation) u Time-consuming 6 weeks to over 6 months depending on study *Internal estimate based on studies at GSK 8 Pro. Sanos Corporation Confidential and Proprietary

Opportunities for Observational Data u Cost-effective approaches to address current drug safety gaps: – Detection of rare adverse events, especially those occurring in background population (MI, stroke, etc. ) – Rapid evaluation of potential safety signals – Information to inform and focus expensive pharmaco-epidemiology studies 9 Pro. Sanos Corporation Confidential and Proprietary

Today’s Agenda u Primary Sources of Safety Data Today – What are the Gaps? u Systematic Analysis of Observational Data – – What is it? Why now? What Are the Challenges? How can Systematic Analysis Augment and Inform Current Practices? Objective: Framework for thinking about the use of observational data in the context of of current pharmacovigilance practices 10 Pro. Sanos Corporation Confidential and Proprietary

What is Systematic Observational Analysis? Library of analytic routines that can be applied to any observational database without requiring custom programming for each data source Interface between medical knowledge, statistics/epidemiology, and computer science 11 Pro. Sanos Corporation Confidential and Proprietary
Why Now? Increased Data Availability Technology Advances Industry & Regulatory Focus A Perfect Storm 12 Pro. Sanos Corporation Confidential and Proprietary
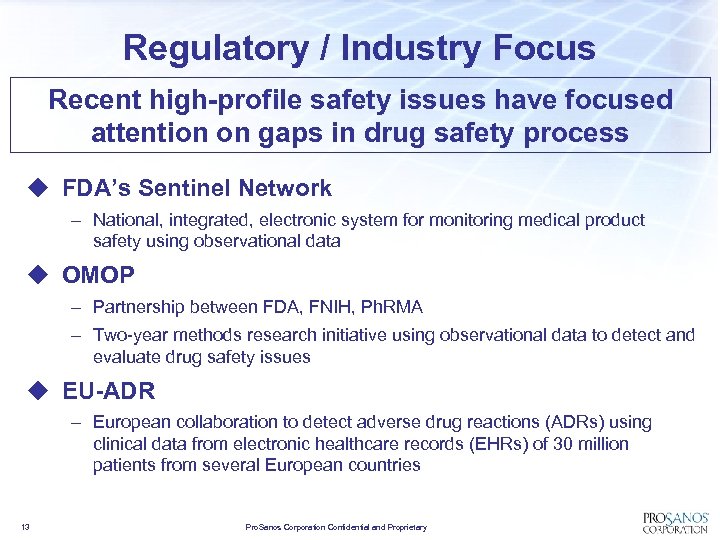
Regulatory / Industry Focus Recent high-profile safety issues have focused attention on gaps in drug safety process u FDA’s Sentinel Network – National, integrated, electronic system for monitoring medical product safety using observational data u OMOP – Partnership between FDA, FNIH, Ph. RMA – Two-year methods research initiative using observational data to detect and evaluate drug safety issues u EU-ADR – European collaboration to detect adverse drug reactions (ADRs) using clinical data from electronic healthcare records (EHRs) of 30 million patients from several European countries 13 Pro. Sanos Corporation Confidential and Proprietary
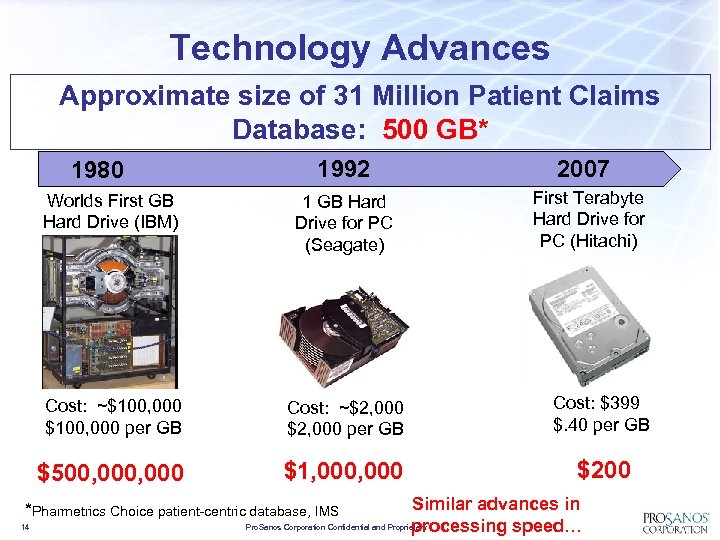
Technology Advances Approximate size of 31 Million Patient Claims Database: 500 GB* 1992 2007 Worlds First GB Hard Drive (IBM) 1 GB Hard Drive for PC (Seagate) First Terabyte Hard Drive for PC (Hitachi) Cost: ~$100, 000 per GB Cost: ~$2, 000 per GB Cost: $399 $. 40 per GB $500, 000 $1, 000 $200 1980 Similar advances in Pro. Sanos Corporation Confidential and Proprietary processing speed… *Pharmetrics Choice patient-centric database, IMS 14

What are the Major Technical Challenges? Goal: Develop library of analytic routines that can be applied by a drug safety scientist to any observational database without requiring custom programming for each data source u Challenge “normalize” disparate vocabulary, data format and structures – Create standardized vocabulary for drugs and conditions – Develop a common data format 15 Pro. Sanos Corporation Confidential and Proprietary

Considerations for a Selection of a Common Vocabulary u Correct and uniform classification for drugs and conditions u Comprehensive with respect to source data u Hierarchical structure to adequately represent relationships – Drugs, ingredients, classes – Conditions and related condition groups u Easy to view and navigate graphically
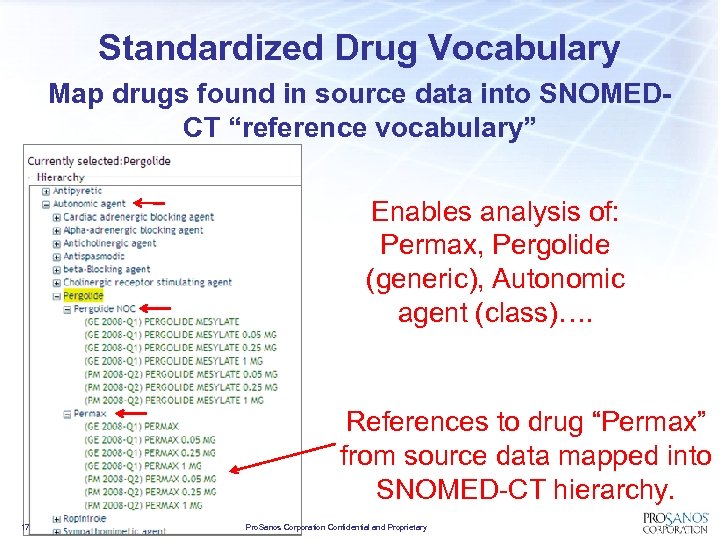
Standardized Drug Vocabulary Map drugs found in source data into SNOMEDCT “reference vocabulary” Enables analysis of: Permax, Pergolide (generic), Autonomic agent (class)…. References to drug “Permax” from source data mapped into SNOMED-CT hierarchy. 17 Pro. Sanos Corporation Confidential and Proprietary
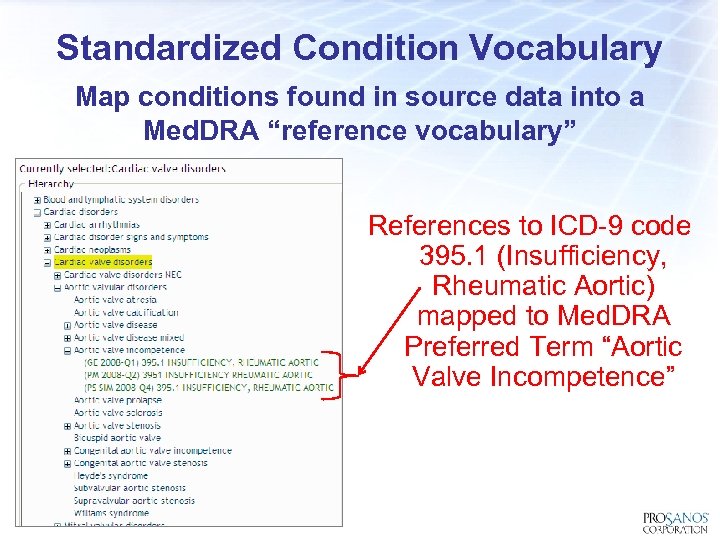
Standardized Condition Vocabulary Map conditions found in source data into a Med. DRA “reference vocabulary” References to ICD-9 code 395. 1 (Insufficiency, Rheumatic Aortic) mapped to Med. DRA Preferred Term “Aortic Valve Incompetence”
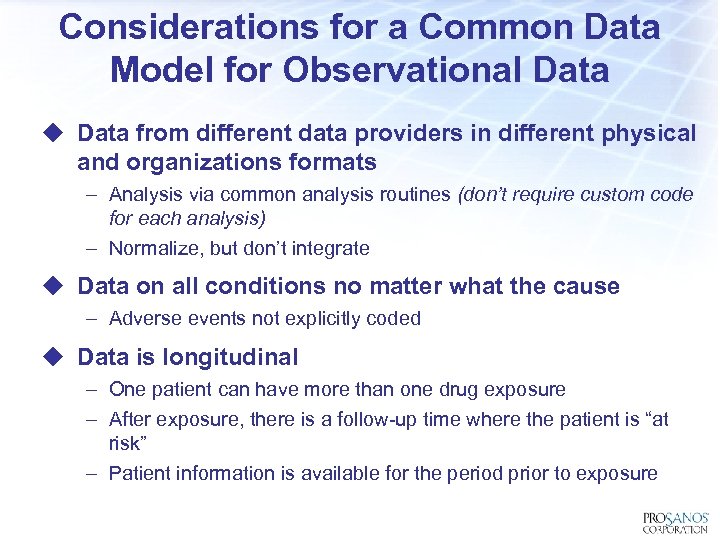
Considerations for a Common Data Model for Observational Data u Data from different data providers in different physical and organizations formats – Analysis via common analysis routines (don’t require custom code for each analysis) – Normalize, but don’t integrate u Data on all conditions no matter what the cause – Adverse events not explicitly coded u Data is longitudinal – One patient can have more than one drug exposure – After exposure, there is a follow-up time where the patient is “at risk” – Patient information is available for the period prior to exposure
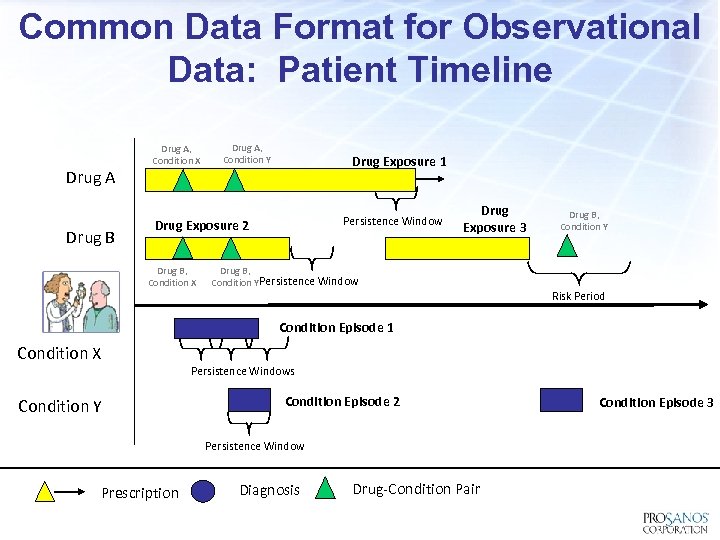
Common Data Format for Observational Data: Patient Timeline Drug A Drug B Drug A, Condition X Drug A, Condition Y Drug Exposure 1 Persistence Window Drug Exposure 2 Drug B, Condition X Drug Exposure 3 Drug B, Condition YPersistence Window Drug B, Condition Y Risk Period Condition Episode 1 Condition X Persistence Windows Condition Y Condition Episode 2 Persistence Window Prescription Diagnosis Drug-Condition Pair Condition Episode 3
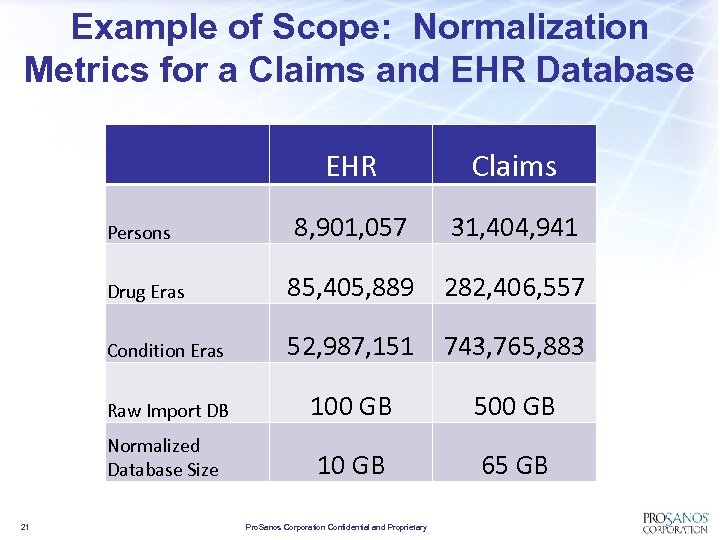
Example of Scope: Normalization Metrics for a Claims and EHR Database EHR Claims 8, 901, 057 31, 404, 941 Drug Eras 85, 405, 889 282, 406, 557 Condition Eras 52, 987, 151 743, 765, 883 Raw Import DB 100 GB 500 GB Normalized Database Size 10 GB 65 GB Persons 21 Pro. Sanos Corporation Confidential and Proprietary
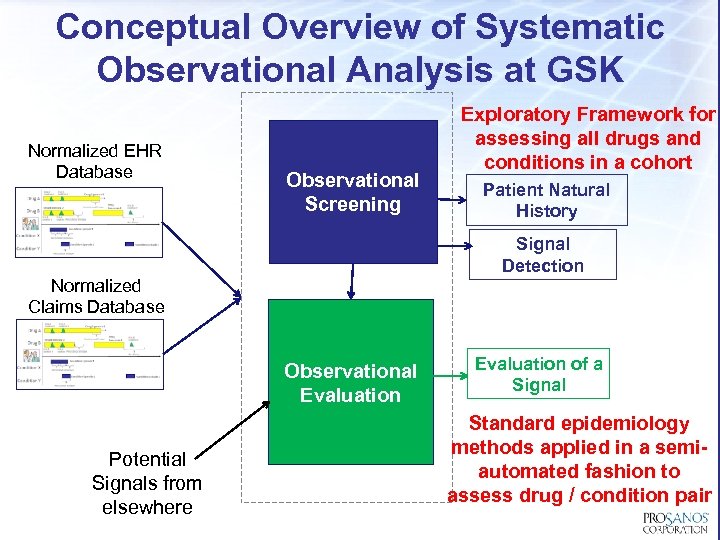
Conceptual Overview of Systematic Observational Analysis at GSK Normalized EHR Database Observational Screening Patient Natural History Signal Detection Normalized Claims Database Observational Evaluation Potential Signals from elsewhere Exploratory Framework for assessing all drugs and conditions in a cohort Evaluation of a Signal Standard epidemiology methods applied in a semiautomated fashion to assess drug / condition pair
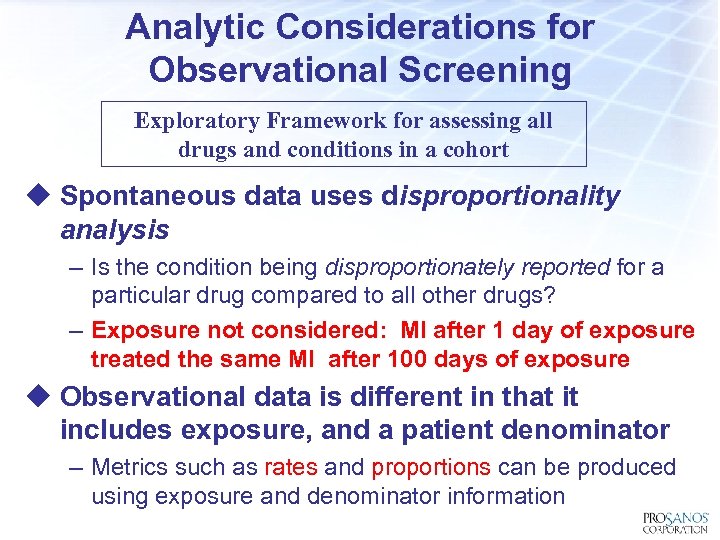
Analytic Considerations for Observational Screening Exploratory Framework for assessing all drugs and conditions in a cohort u Spontaneous data uses disproportionality analysis – Is the condition being disproportionately reported for a particular drug compared to all other drugs? – Exposure not considered: MI after 1 day of exposure treated the same MI after 100 days of exposure u Observational data is different in that it includes exposure, and a patient denominator – Metrics such as rates and proportions can be produced using exposure and denominator information

Calculation of a “Screening Proportion” • Screening Proportion: The proportion of exposed persons who have had at least one occurrence of a condition in a given timeframe Screening Proportion = # persons with >= 1 occurrence in time (t) prior to exposure / total number of persons exposed
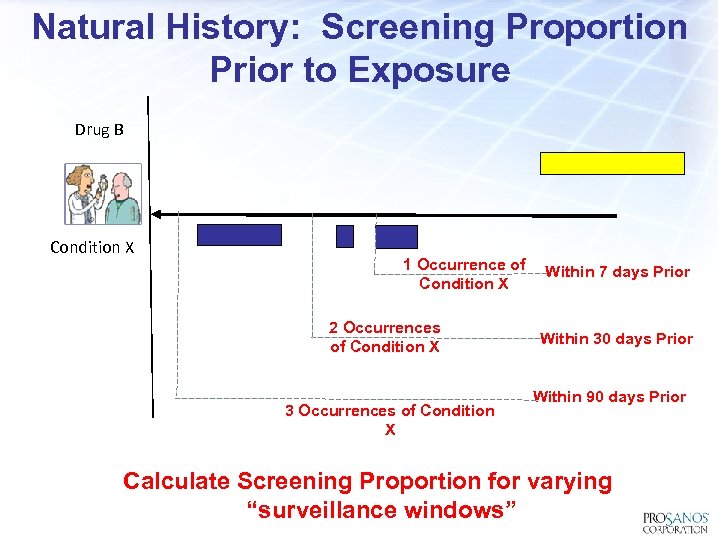
Natural History: Screening Proportion Prior to Exposure Drug B Condition X 1 Occurrence of Condition X 2 Occurrences of Condition X 3 Occurrences of Condition X Within 7 days Prior Within 30 days Prior Within 90 days Prior Calculate Screening Proportion for varying “surveillance windows”
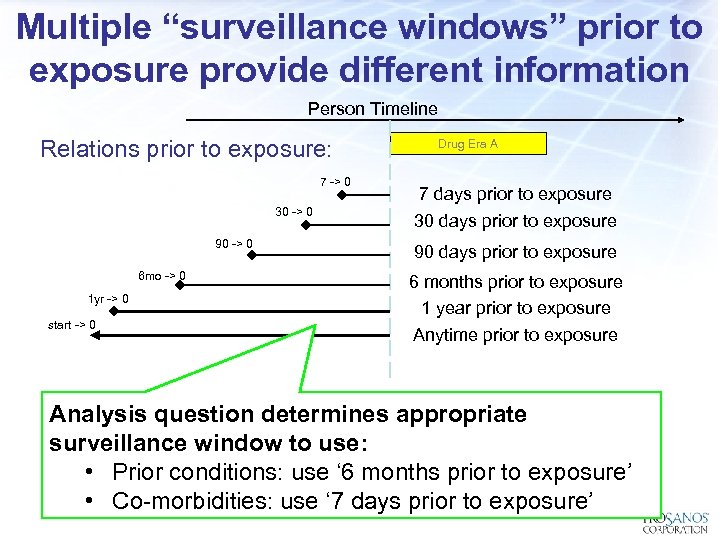
Multiple “surveillance windows” prior to exposure provide different information Person Timeline Relations prior to exposure: 7 -> 0 30 -> 0 90 -> 0 6 mo -> 0 1 yr -> 0 start -> 0 Drug Era A 7 days prior to exposure 30 days prior to exposure 90 days prior to exposure 6 months prior to exposure 1 year prior to exposure Anytime prior to exposure Analysis question determines appropriate surveillance window to use: • Prior conditions: use ‘ 6 months prior to exposure’ • Co-morbidities: use ‘ 7 days prior to exposure’
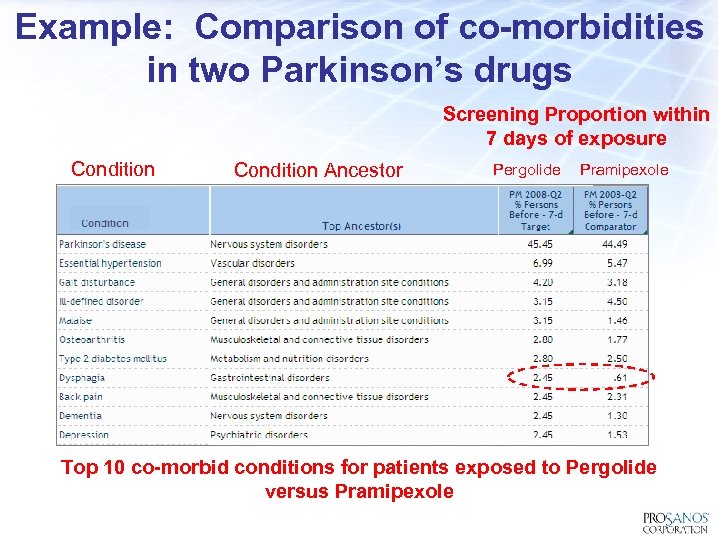
Example: Comparison of co-morbidities in two Parkinson’s drugs Screening Proportion within 7 days of exposure Condition Ancestor Pergolide Pramipexole Top 10 co-morbid conditions for patients exposed to Pergolide versus Pramipexole
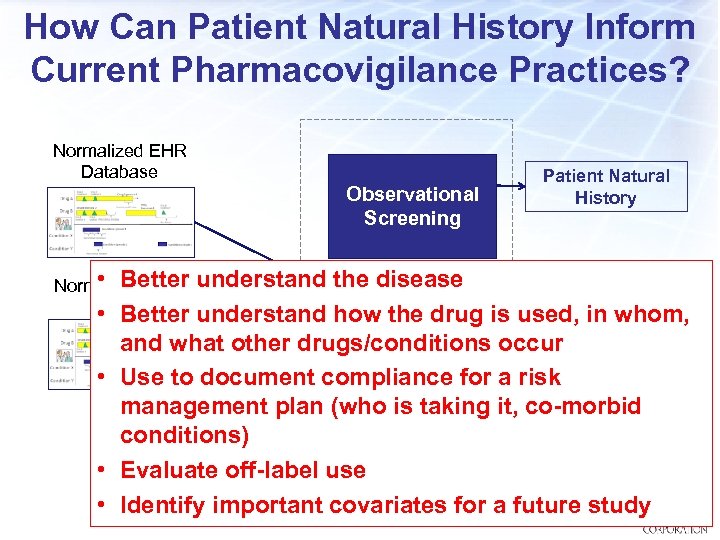
How Can Patient Natural History Inform Current Pharmacovigilance Practices? Normalized EHR Database Observational Screening Patient Natural History • Better understand the disease Normalized Claims Database • Better understand how the drug is used, in whom, and what other drugs/conditions occur • Use to document compliance for a risk management plan (who is taking it, co-morbid conditions) • Evaluate off-label use • Identify important covariates for a future study
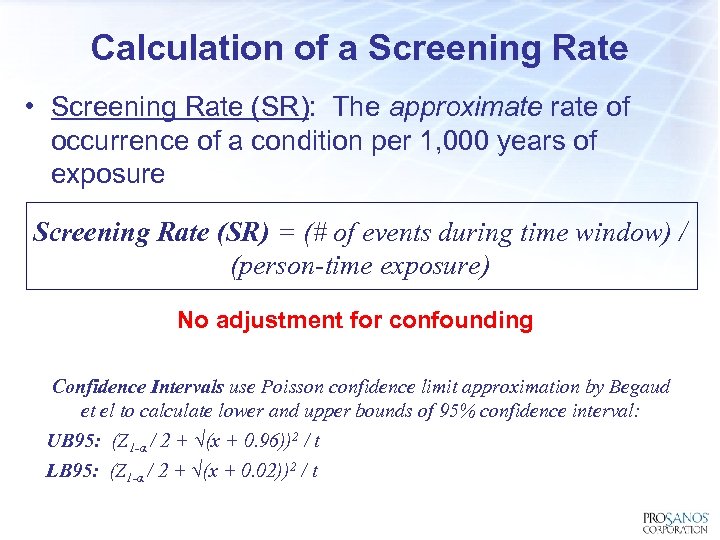
Calculation of a Screening Rate • Screening Rate (SR): The approximate rate of occurrence of a condition per 1, 000 years of exposure Screening Rate (SR) = (# of events during time window) / (person-time exposure) No adjustment for confounding Confidence Intervals use Poisson confidence limit approximation by Begaud et el to calculate lower and upper bounds of 95% confidence interval: UB 95: (Z 1 -α / 2 + √(x + 0. 96))2 / t LB 95: (Z 1 -α / 2 + √(x + 0. 02))2 / t
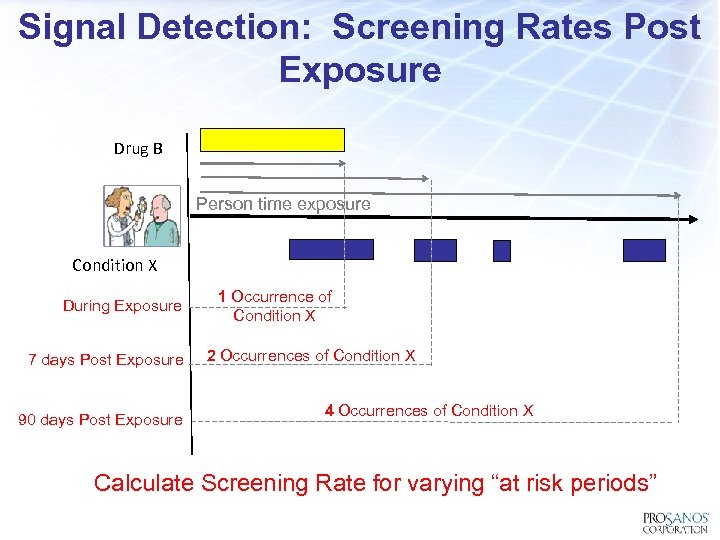
Signal Detection: Screening Rates Post Exposure Drug B Person time exposure Condition X During Exposure 7 days Post Exposure 90 days Post Exposure 1 Occurrence of Condition X 2 Occurrences of Condition X 4 Occurrences of Condition X Calculate Screening Rate for varying “at risk periods”
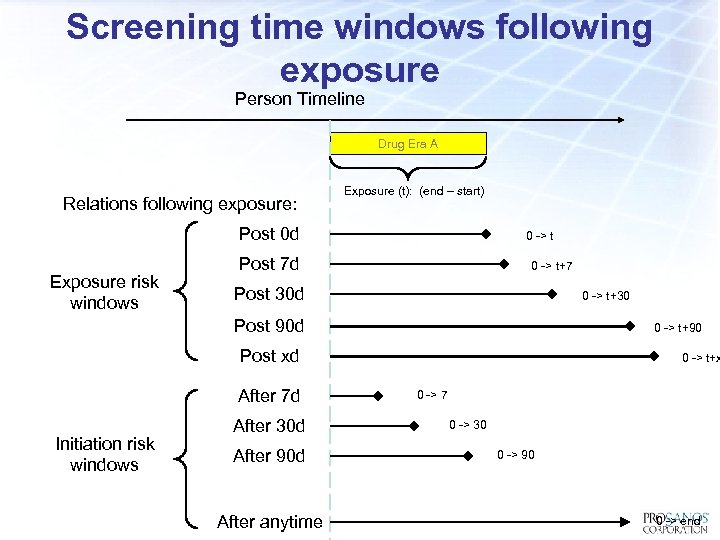
Screening time windows following exposure Person Timeline Drug Era A Relations following exposure: Exposure (t): (end – start) Post 0 d Exposure risk windows 0 -> t Post 7 d 0 -> t+7 Post 30 d 0 -> t+30 Post 90 d 0 -> t+90 Post xd After 7 d Initiation risk windows After 30 d After 90 d After anytime 0 -> t+x 0 -> 7 0 -> 30 0 -> 90 0 -> end
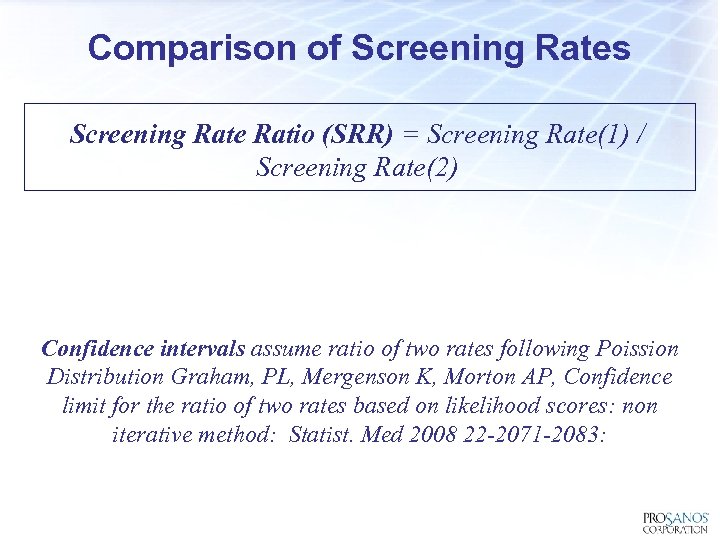
Comparison of Screening Rates Screening Rate Ratio (SRR) = Screening Rate(1) / Screening Rate(2) Confidence intervals assume ratio of two rates following Poission Distribution Graham, PL, Mergenson K, Morton AP, Confidence limit for the ratio of two rates based on likelihood scores: non iterative method: Statist. Med 2008 22 -2071 -2083:
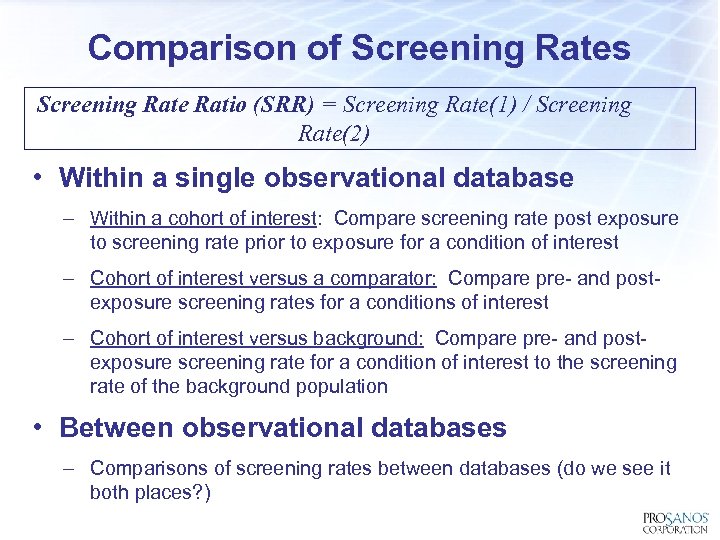
Comparison of Screening Rates Screening Rate Ratio (SRR) = Screening Rate(1) / Screening Rate(2) • Within a single observational database – Within a cohort of interest: Compare screening rate post exposure to screening rate prior to exposure for a condition of interest – Cohort of interest versus a comparator: Compare pre- and postexposure screening rates for a conditions of interest – Cohort of interest versus background: Compare pre- and postexposure screening rate for a condition of interest to the screening rate of the background population • Between observational databases – Comparisons of screening rates between databases (do we see it both places? )
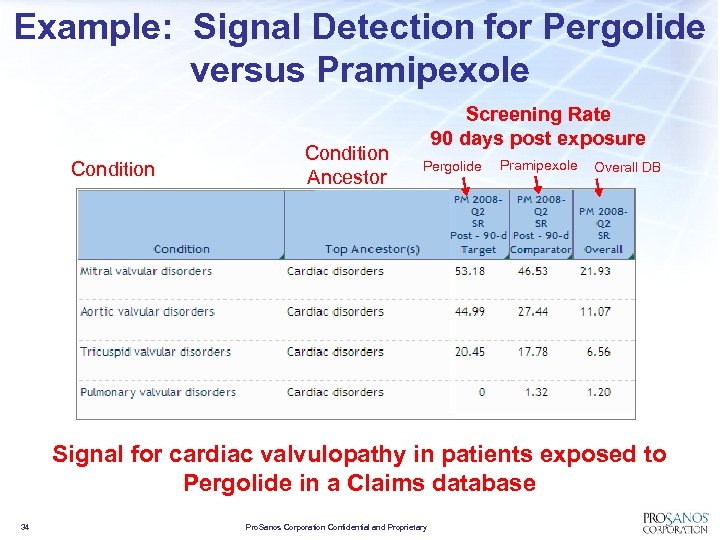
Example: Signal Detection for Pergolide versus Pramipexole Condition Ancestor Screening Rate 90 days post exposure Pergolide Pramipexole Overall DB Signal for cardiac valvulopathy in patients exposed to Pergolide in a Claims database 34 Pro. Sanos Corporation Confidential and Proprietary
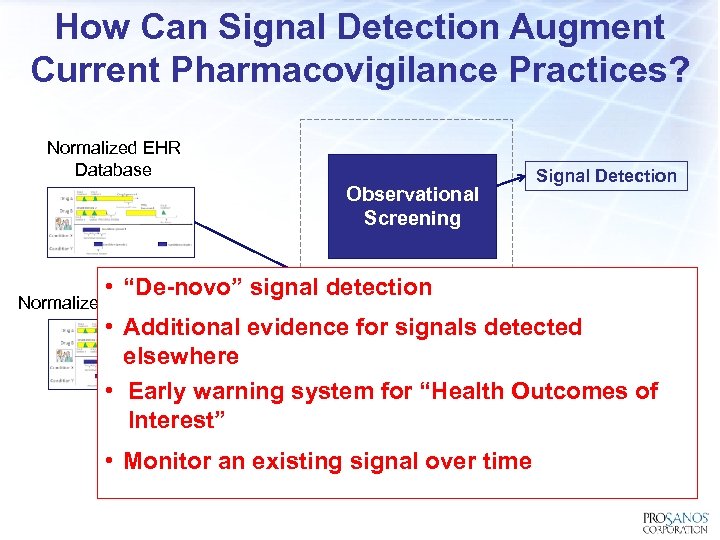
How Can Signal Detection Augment Current Pharmacovigilance Practices? Normalized EHR Database Observational Screening Signal Detection • “De-novo” signal detection Normalized Claims Database • Additional evidence for signals detected elsewhere • Early warning system for “Health Outcomes of Interest” • Monitor an existing signal over time
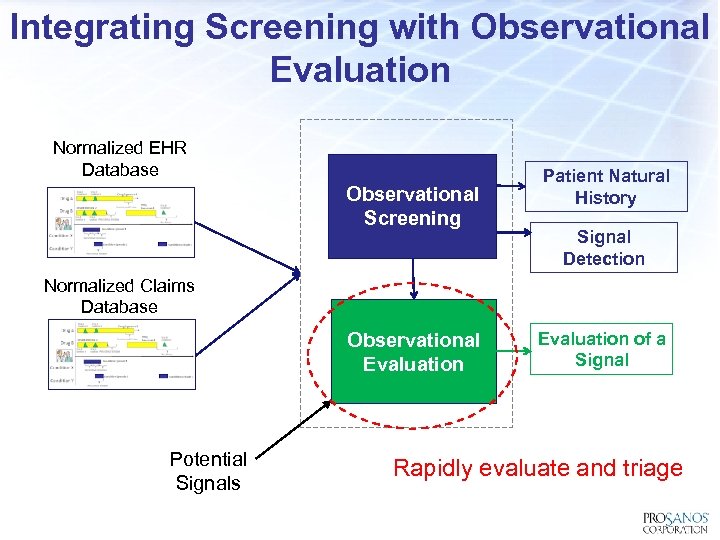
Integrating Screening with Observational Evaluation Normalized EHR Database Observational Screening Patient Natural History Signal Detection Normalized Claims Database Observational Evaluation Potential Signals Evaluation of a Signal Rapidly evaluate and triage
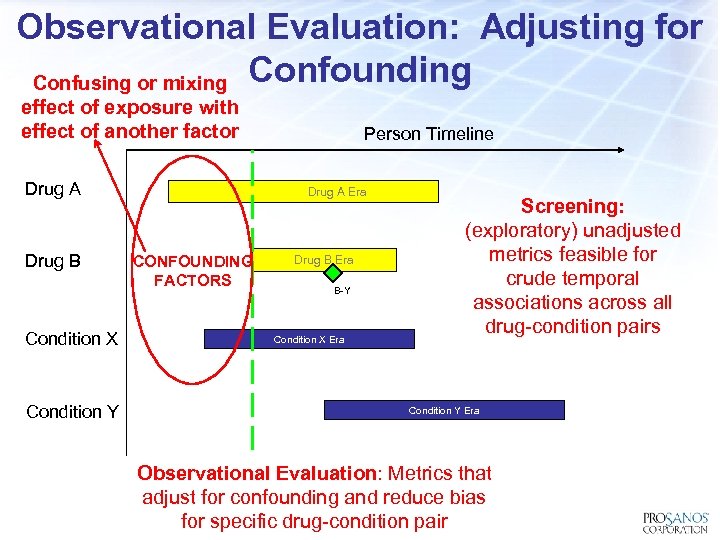
Observational Evaluation: Adjusting for Confusing or mixing Confounding effect of exposure with effect of another factor Drug A Drug B Condition X Condition Y Person Timeline Drug A Era CONFOUNDING FACTORS Drug B Era B-Y Condition X Era Screening: (exploratory) unadjusted metrics feasible for crude temporal associations across all drug-condition pairs Condition Y Era Observational Evaluation: Metrics that adjust for confounding and reduce bias for specific drug-condition pair
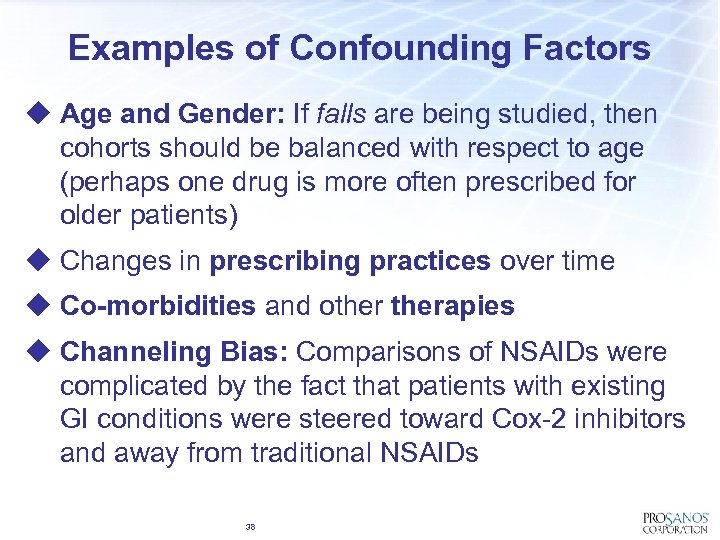
Examples of Confounding Factors u Age and Gender: If falls are being studied, then cohorts should be balanced with respect to age (perhaps one drug is more often prescribed for older patients) u Changes in prescribing practices over time u Co-morbidities and otherapies u Channeling Bias: Comparisons of NSAIDs were complicated by the fact that patients with existing GI conditions were steered toward Cox-2 inhibitors and away from traditional NSAIDs 38
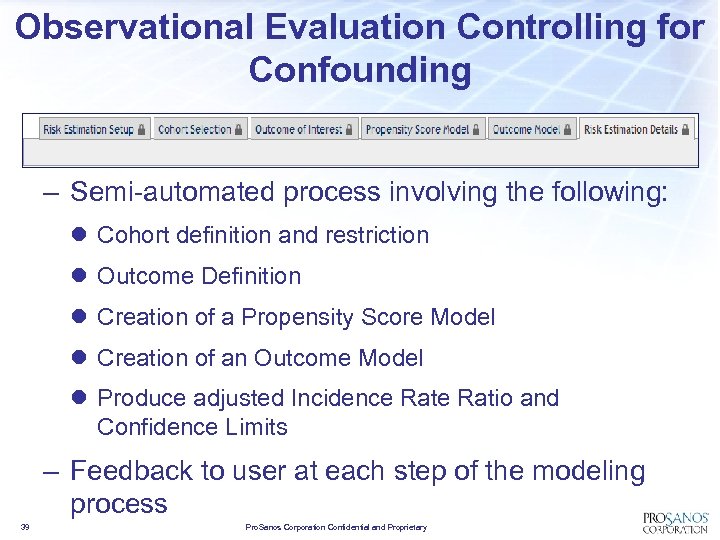
Observational Evaluation Controlling for Confounding – Semi-automated process involving the following: l Cohort definition and restriction l Outcome Definition l Creation of a Propensity Score Model l Creation of an Outcome Model l Produce adjusted Incidence Ratio and Confidence Limits – Feedback to user at each step of the modeling process 39 Pro. Sanos Corporation Confidential and Proprietary
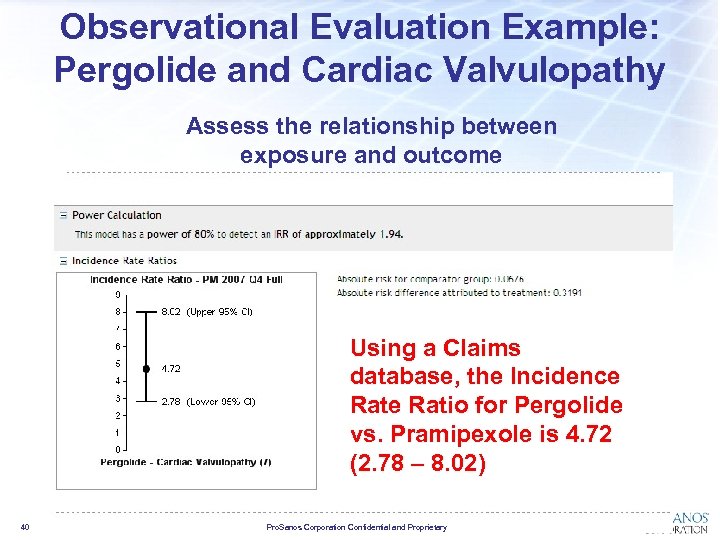
Observational Evaluation Example: Pergolide and Cardiac Valvulopathy Assess the relationship between exposure and outcome Using a Claims database, the Incidence Ratio for Pergolide vs. Pramipexole is 4. 72 (2. 78 – 8. 02) 40 Pro. Sanos Corporation Confidential and Proprietary
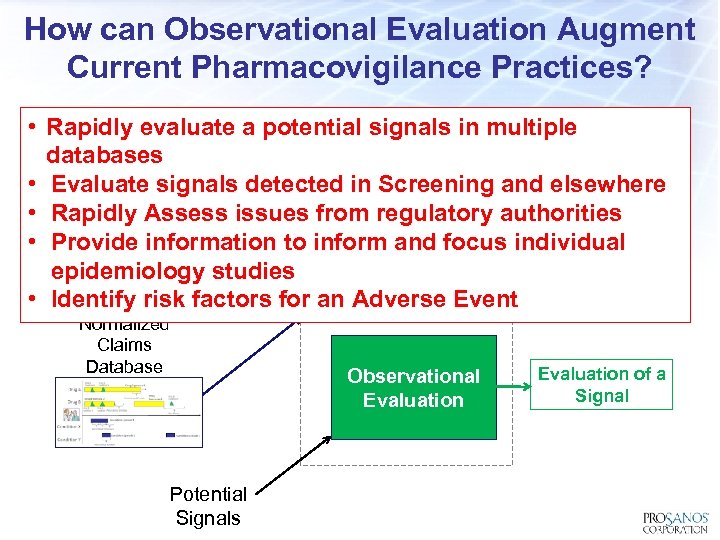
How can Observational Evaluation Augment Current Pharmacovigilance Practices? • Rapidly evaluate a potential signals in multiple databases Normalized EHR • Evaluate signals detected in Screening and elsewhere Database Patient Natural • Rapidly Assess issues from regulatory authorities Observational History • Provide information to inform and focus individual Screening epidemiology studies Signal Detection • Identify risk factors for an Adverse Event Normalized Claims Database Potential Signals Observational Evaluation of a Signal
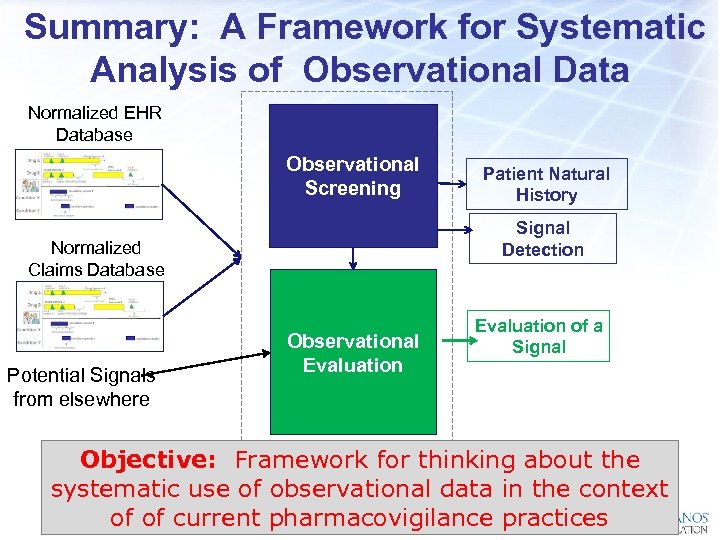
Summary: A Framework for Systematic Analysis of Observational Data Normalized EHR Database Observational Screening Signal Detection Normalized Claims Database Potential Signals from elsewhere Patient Natural History Observational Evaluation of a Signal Objective: Framework for thinking about the systematic use of observational data in the context of of current pharmacovigilance practices

steph. reisinger@prosanos. com 43 Pro. Sanos Corporation Confidential and Proprietary
f5bdc865f6f2847ad3cf57198c7e3582.ppt