74ae6ac11db55b559ce47c1b0219a1bc.ppt
- Количество слайдов: 44
 SURVEILLANCE AND PREVENTION AND CONTROLOF CENTRAL INTRAVENOUS CATHETERASSOCIATED INFECTIONS C. Glen Mayhall, M. D. Healthcare Epidemiologist Department of Healthcare Epidemiology Professor, Department of Internal Medicine Division of Infectious Diseases
SURVEILLANCE AND PREVENTION AND CONTROLOF CENTRAL INTRAVENOUS CATHETERASSOCIATED INFECTIONS C. Glen Mayhall, M. D. Healthcare Epidemiologist Department of Healthcare Epidemiology Professor, Department of Internal Medicine Division of Infectious Diseases
 CIVC – ASSOCIATED INFECTIONS OUTLINE • Types of Central IV Catheters • Pathogenesis of CIVC-associated infections • Etiologies of CIVC-associated infections • Definitions of CIVC-associated infections • Clinical signs of infection • Epidemiology • Prevention and Control
CIVC – ASSOCIATED INFECTIONS OUTLINE • Types of Central IV Catheters • Pathogenesis of CIVC-associated infections • Etiologies of CIVC-associated infections • Definitions of CIVC-associated infections • Clinical signs of infection • Epidemiology • Prevention and Control
 CIVC – ASSOCIATED INFECTIONS • The Problem Noncuffed, percutaneously inserted central venous catheters for short-term use – 7 million sold each year in the U. S. – 80, 000 catheter-related bloodstream infections per year – 28, 000 deaths per year – $45, 000 in healthcare cost per infection – $2. 3 billion annually Safdar N, Fine JP, Maki DG. Ann Intern Med 2005; 142: 451 -466 Pronovost, P, Needham D, Berenholtz S, et al. N Engl J Med 2006; 355: 27252732
CIVC – ASSOCIATED INFECTIONS • The Problem Noncuffed, percutaneously inserted central venous catheters for short-term use – 7 million sold each year in the U. S. – 80, 000 catheter-related bloodstream infections per year – 28, 000 deaths per year – $45, 000 in healthcare cost per infection – $2. 3 billion annually Safdar N, Fine JP, Maki DG. Ann Intern Med 2005; 142: 451 -466 Pronovost, P, Needham D, Berenholtz S, et al. N Engl J Med 2006; 355: 27252732
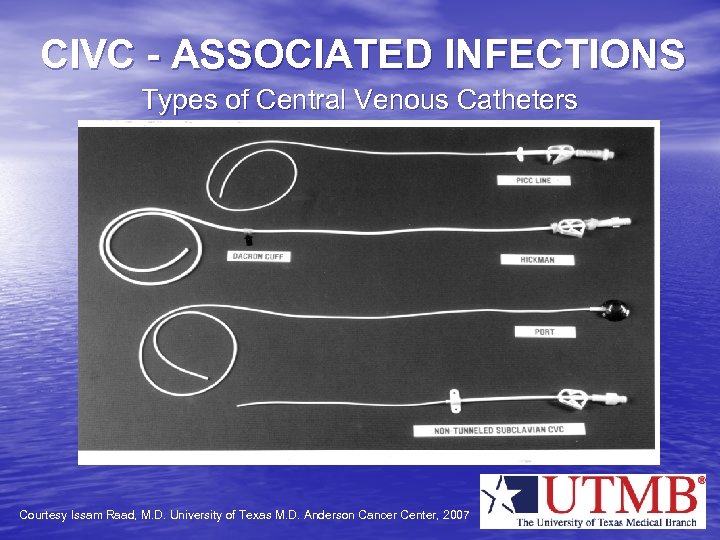 CIVC - ASSOCIATED INFECTIONS Types of Central Venous Catheters Courtesy Issam Raad, M. D. University of Texas M. D. Anderson Cancer Center, 2007
CIVC - ASSOCIATED INFECTIONS Types of Central Venous Catheters Courtesy Issam Raad, M. D. University of Texas M. D. Anderson Cancer Center, 2007
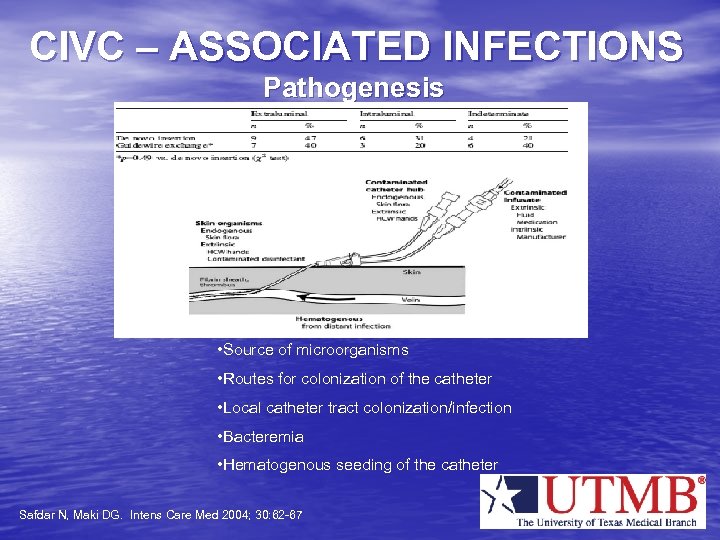 CIVC – ASSOCIATED INFECTIONS Pathogenesis • Source of microorganisms • Routes for colonization of the catheter • Local catheter tract colonization/infection • Bacteremia • Hematogenous seeding of the catheter Safdar N, Maki DG. Intens Care Med 2004; 30: 62 -67
CIVC – ASSOCIATED INFECTIONS Pathogenesis • Source of microorganisms • Routes for colonization of the catheter • Local catheter tract colonization/infection • Bacteremia • Hematogenous seeding of the catheter Safdar N, Maki DG. Intens Care Med 2004; 30: 62 -67
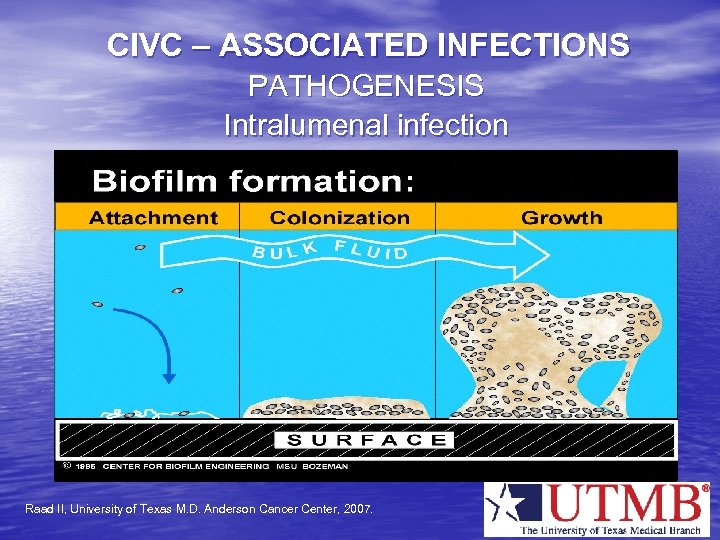 CIVC – ASSOCIATED INFECTIONS PATHOGENESIS Intralumenal infection Raad II, University of Texas M. D. Anderson Cancer Center, 2007.
CIVC – ASSOCIATED INFECTIONS PATHOGENESIS Intralumenal infection Raad II, University of Texas M. D. Anderson Cancer Center, 2007.
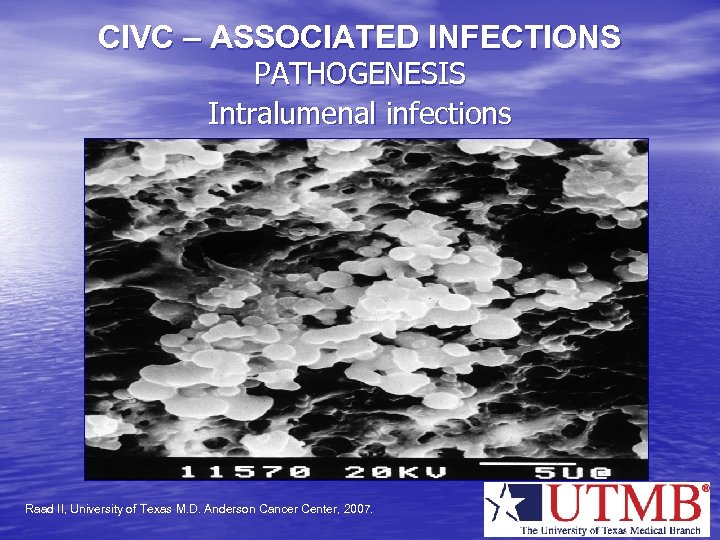 CIVC – ASSOCIATED INFECTIONS PATHOGENESIS Intralumenal infections Raad II, University of Texas M. D. Anderson Cancer Center, 2007.
CIVC – ASSOCIATED INFECTIONS PATHOGENESIS Intralumenal infections Raad II, University of Texas M. D. Anderson Cancer Center, 2007.
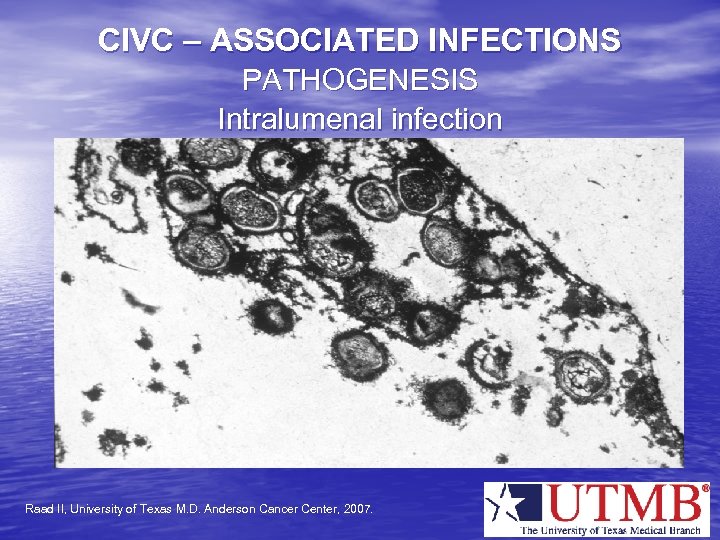 CIVC – ASSOCIATED INFECTIONS PATHOGENESIS Intralumenal infection Raad II, University of Texas M. D. Anderson Cancer Center, 2007.
CIVC – ASSOCIATED INFECTIONS PATHOGENESIS Intralumenal infection Raad II, University of Texas M. D. Anderson Cancer Center, 2007.
 CIVC – ASSOCIATED INFECTIONS • ETIOLOGIES OF INFECTIONS 1992 – 1999 – Coagulase – negative staphylococci – 37% – Staphylococcus aureus – 13% – Enterococcus species – 13% – Escherichia coli – 2% – Enterobacter species – 5% – Pseudomonas aeruginosa – 4% – Klebsiella pneumoniae – 3% – Candida species – 8% Centers for Disease Control and Prevention, 2002
CIVC – ASSOCIATED INFECTIONS • ETIOLOGIES OF INFECTIONS 1992 – 1999 – Coagulase – negative staphylococci – 37% – Staphylococcus aureus – 13% – Enterococcus species – 13% – Escherichia coli – 2% – Enterobacter species – 5% – Pseudomonas aeruginosa – 4% – Klebsiella pneumoniae – 3% – Candida species – 8% Centers for Disease Control and Prevention, 2002
 CIVC – ASSOCIATED INFECTIONS DEFINITIONS OF INTRAVENOUS CATHETER-ASSOCIATED BLOODSTREAM INFECTIONS • CENTERS FOR DISEASE CONTROL NAD PREVENTION (CDC) – Laboratory-confirmed bloodstream infection (LCBI) • Recognized pathogen cultured from one or more blood cultures • Organism cultured from blood not related to infection at another site
CIVC – ASSOCIATED INFECTIONS DEFINITIONS OF INTRAVENOUS CATHETER-ASSOCIATED BLOODSTREAM INFECTIONS • CENTERS FOR DISEASE CONTROL NAD PREVENTION (CDC) – Laboratory-confirmed bloodstream infection (LCBI) • Recognized pathogen cultured from one or more blood cultures • Organism cultured from blood not related to infection at another site
 CIVC – ASSOCIATED INFECTIONS • DEFINITIONS (cont. ) CDC (cont. ) – LCBI (cont. ) • Fever (>38 C), chills, hypotension ( > 1) – Skin contaminant cultured from two or more blood cultures – Skin contaminant cultured from one blood culture and physician initiates treatment – Positive antigen test for microorganism in blood
CIVC – ASSOCIATED INFECTIONS • DEFINITIONS (cont. ) CDC (cont. ) – LCBI (cont. ) • Fever (>38 C), chills, hypotension ( > 1) – Skin contaminant cultured from two or more blood cultures – Skin contaminant cultured from one blood culture and physician initiates treatment – Positive antigen test for microorganism in blood
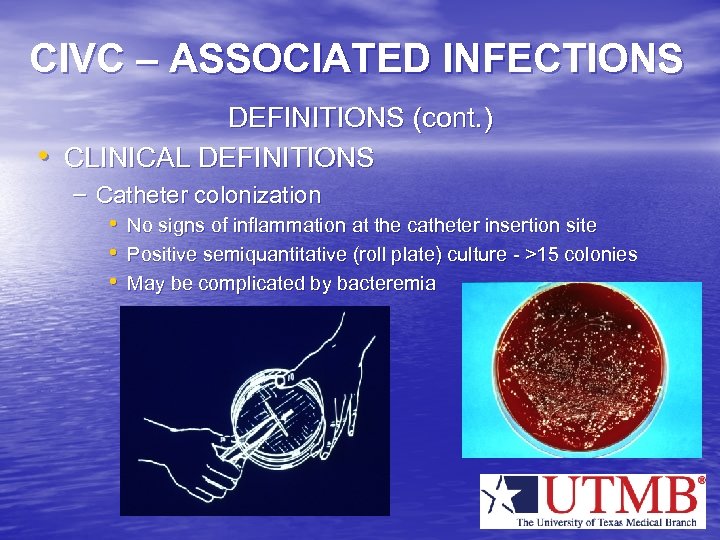 CIVC – ASSOCIATED INFECTIONS • DEFINITIONS (cont. ) CLINICAL DEFINITIONS – Catheter colonization • No signs of inflammation at the catheter insertion site • Positive semiquantitative (roll plate) culture - >15 colonies • May be complicated by bacteremia
CIVC – ASSOCIATED INFECTIONS • DEFINITIONS (cont. ) CLINICAL DEFINITIONS – Catheter colonization • No signs of inflammation at the catheter insertion site • Positive semiquantitative (roll plate) culture - >15 colonies • May be complicated by bacteremia
 CIVC – ASSOCIATED INFECTIONS DEFINITIONS (cont. ) • CLINICAL DEFINITIONS (cont. ) – Catheter – associated infection • Signs of inflammation at catheter insertion site – – – Erythema Warmth Swelling Tenderness Purulent drainage • Positive semiquantitative catheter culture
CIVC – ASSOCIATED INFECTIONS DEFINITIONS (cont. ) • CLINICAL DEFINITIONS (cont. ) – Catheter – associated infection • Signs of inflammation at catheter insertion site – – – Erythema Warmth Swelling Tenderness Purulent drainage • Positive semiquantitative catheter culture
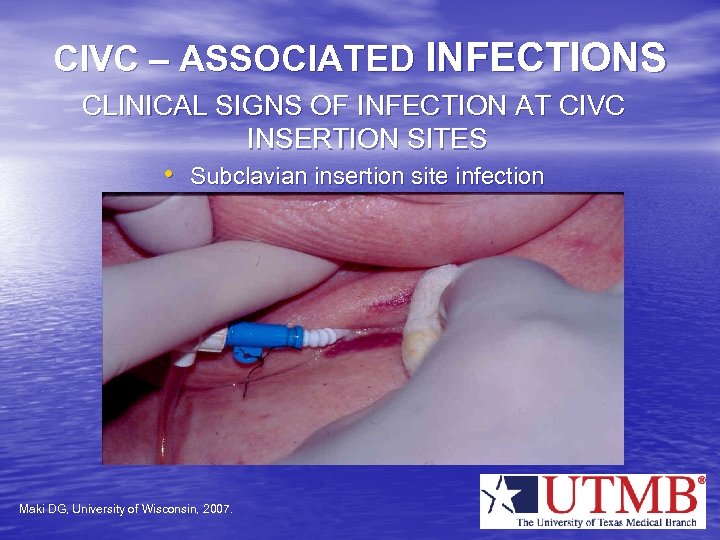 CIVC – ASSOCIATED INFECTIONS CLINICAL SIGNS OF INFECTION AT CIVC INSERTION SITES • Subclavian insertion site infection Maki DG, University of Wisconsin, 2007.
CIVC – ASSOCIATED INFECTIONS CLINICAL SIGNS OF INFECTION AT CIVC INSERTION SITES • Subclavian insertion site infection Maki DG, University of Wisconsin, 2007.
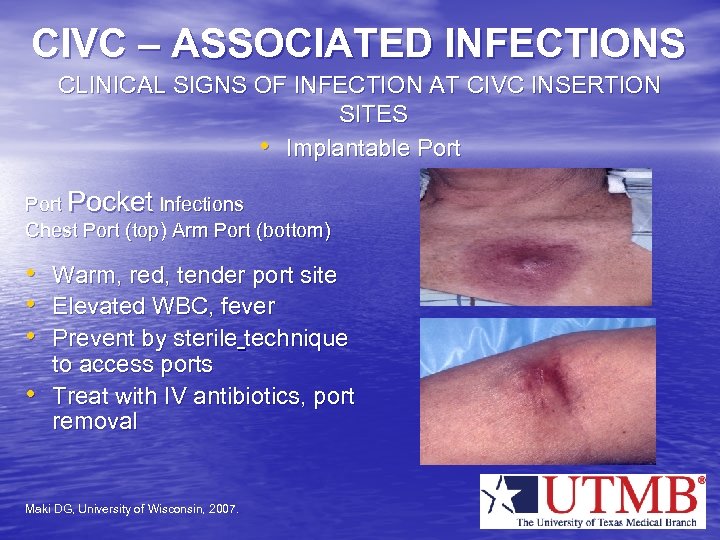 CIVC – ASSOCIATED INFECTIONS CLINICAL SIGNS OF INFECTION AT CIVC INSERTION SITES • Implantable Port Pocket Infections Chest Port (top) Arm Port (bottom) • Warm, red, tender port site • Elevated WBC, fever • Prevent by sterile technique • to access ports Treat with IV antibiotics, port removal Maki DG, University of Wisconsin, 2007.
CIVC – ASSOCIATED INFECTIONS CLINICAL SIGNS OF INFECTION AT CIVC INSERTION SITES • Implantable Port Pocket Infections Chest Port (top) Arm Port (bottom) • Warm, red, tender port site • Elevated WBC, fever • Prevent by sterile technique • to access ports Treat with IV antibiotics, port removal Maki DG, University of Wisconsin, 2007.
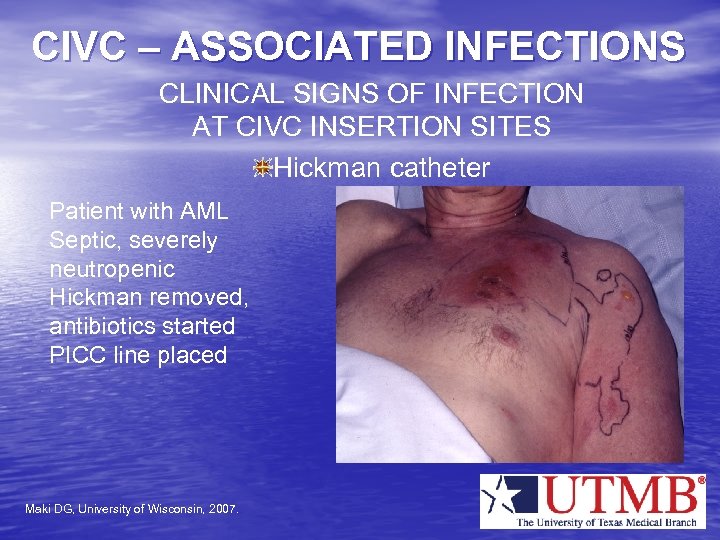 CIVC – ASSOCIATED INFECTIONS CLINICAL SIGNS OF INFECTION AT CIVC INSERTION SITES Hickman catheter Patient with AML Septic, severely neutropenic Hickman removed, antibiotics started PICC line placed Maki DG, University of Wisconsin, 2007.
CIVC – ASSOCIATED INFECTIONS CLINICAL SIGNS OF INFECTION AT CIVC INSERTION SITES Hickman catheter Patient with AML Septic, severely neutropenic Hickman removed, antibiotics started PICC line placed Maki DG, University of Wisconsin, 2007.
 CIVC – ASSOCIATED INFECTIONS CLINICAL SIGNS OF INFECTION AT CIVC INSERTION SITES • PICC line • Another example of the • • • importance of patient education Patient would take showers with dressing off and then do site care after Patient thought cleaning site in shower was a good idea Exposure to tap water has been shown to cause CRBSIs with GNR water organisms Maki DG, University of Wisconsin, 2007.
CIVC – ASSOCIATED INFECTIONS CLINICAL SIGNS OF INFECTION AT CIVC INSERTION SITES • PICC line • Another example of the • • • importance of patient education Patient would take showers with dressing off and then do site care after Patient thought cleaning site in shower was a good idea Exposure to tap water has been shown to cause CRBSIs with GNR water organisms Maki DG, University of Wisconsin, 2007.
 CIVC – ASSOCIATED INFECTIONS CLINICAL SIGNS OF INFECTION AT CIVC INSERTION SITES • PICC line • Bleeding into arm after • • • PICC placed Patient with thrombocytopenia, did not receive platelets before procedure Bleeding into tissue resulted in infection PICC removed Maki DG, University of Wisconsin, 2007.
CIVC – ASSOCIATED INFECTIONS CLINICAL SIGNS OF INFECTION AT CIVC INSERTION SITES • PICC line • Bleeding into arm after • • • PICC placed Patient with thrombocytopenia, did not receive platelets before procedure Bleeding into tissue resulted in infection PICC removed Maki DG, University of Wisconsin, 2007.
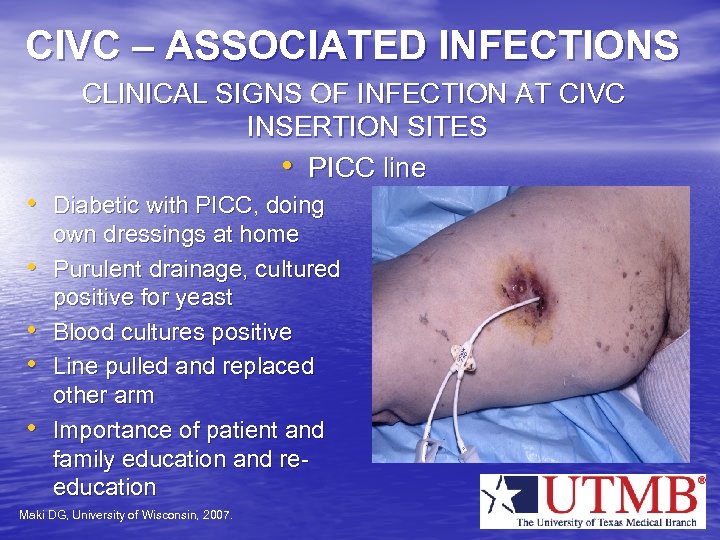 CIVC – ASSOCIATED INFECTIONS CLINICAL SIGNS OF INFECTION AT CIVC INSERTION SITES • PICC line • Diabetic with PICC, doing • • own dressings at home Purulent drainage, cultured positive for yeast Blood cultures positive Line pulled and replaced other arm Importance of patient and family education and reeducation Maki DG, University of Wisconsin, 2007.
CIVC – ASSOCIATED INFECTIONS CLINICAL SIGNS OF INFECTION AT CIVC INSERTION SITES • PICC line • Diabetic with PICC, doing • • own dressings at home Purulent drainage, cultured positive for yeast Blood cultures positive Line pulled and replaced other arm Importance of patient and family education and reeducation Maki DG, University of Wisconsin, 2007.
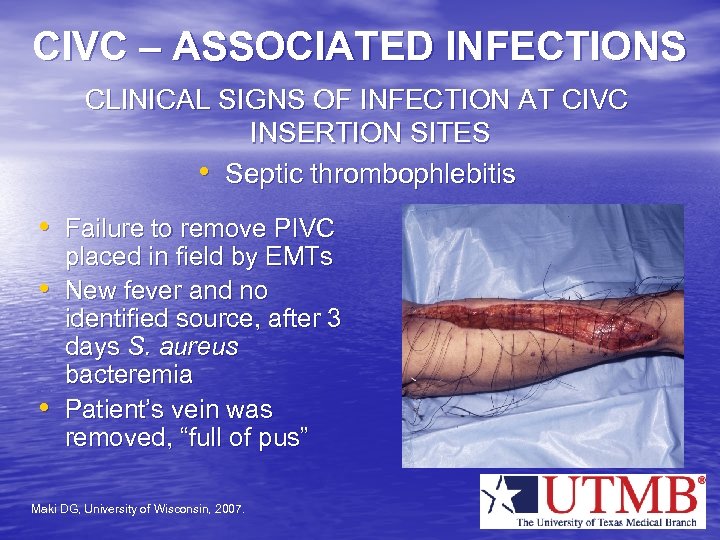 CIVC – ASSOCIATED INFECTIONS CLINICAL SIGNS OF INFECTION AT CIVC INSERTION SITES • Septic thrombophlebitis • Failure to remove PIVC • • placed in field by EMTs New fever and no identified source, after 3 days S. aureus bacteremia Patient’s vein was removed, “full of pus” Maki DG, University of Wisconsin, 2007.
CIVC – ASSOCIATED INFECTIONS CLINICAL SIGNS OF INFECTION AT CIVC INSERTION SITES • Septic thrombophlebitis • Failure to remove PIVC • • placed in field by EMTs New fever and no identified source, after 3 days S. aureus bacteremia Patient’s vein was removed, “full of pus” Maki DG, University of Wisconsin, 2007.
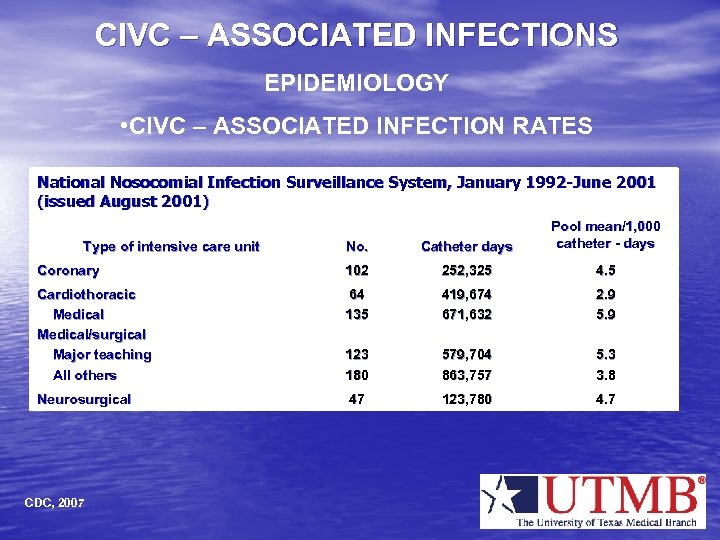 CIVC – ASSOCIATED INFECTIONS EPIDEMIOLOGY • CIVC – ASSOCIATED INFECTION RATES National Nosocomial Infection Surveillance System, January 1992 -June 2001 (issued August 2001) No. Catheter days Pool mean/1, 000 catheter - days Coronary 102 252, 325 4. 5 Cardiothoracic Medical/surgical Major teaching All others 64 135 419, 674 671, 632 2. 9 5. 9 123 180 579, 704 863, 757 5. 3 3. 8 Neurosurgical 47 123, 780 4. 7 Type of intensive care unit CDC, 2007
CIVC – ASSOCIATED INFECTIONS EPIDEMIOLOGY • CIVC – ASSOCIATED INFECTION RATES National Nosocomial Infection Surveillance System, January 1992 -June 2001 (issued August 2001) No. Catheter days Pool mean/1, 000 catheter - days Coronary 102 252, 325 4. 5 Cardiothoracic Medical/surgical Major teaching All others 64 135 419, 674 671, 632 2. 9 5. 9 123 180 579, 704 863, 757 5. 3 3. 8 Neurosurgical 47 123, 780 4. 7 Type of intensive care unit CDC, 2007
 CIVC – ASSOCIATED INFECTIONS EPIDMIOLOGY • RISK FACTORS FOR INFECTION – Site of insertion • Subclavian vein – Lowest infection rate – Highest risk for mechanical complications • Internal jugular vein – Intermediate risk of infection – Lower risk for mechanical complications • Femoral vein – Highest risk for infection – Lower risk for mechanical complications Goetz AM, Wagener MM, Miller JM, Muder RR. Infect Cont Hosp Epidemiol 1998; 19: 842 -845 Lorente L, Henry C, Martin MM, et al. Crit Care 2005; 9: R 631 -R 635
CIVC – ASSOCIATED INFECTIONS EPIDMIOLOGY • RISK FACTORS FOR INFECTION – Site of insertion • Subclavian vein – Lowest infection rate – Highest risk for mechanical complications • Internal jugular vein – Intermediate risk of infection – Lower risk for mechanical complications • Femoral vein – Highest risk for infection – Lower risk for mechanical complications Goetz AM, Wagener MM, Miller JM, Muder RR. Infect Cont Hosp Epidemiol 1998; 19: 842 -845 Lorente L, Henry C, Martin MM, et al. Crit Care 2005; 9: R 631 -R 635
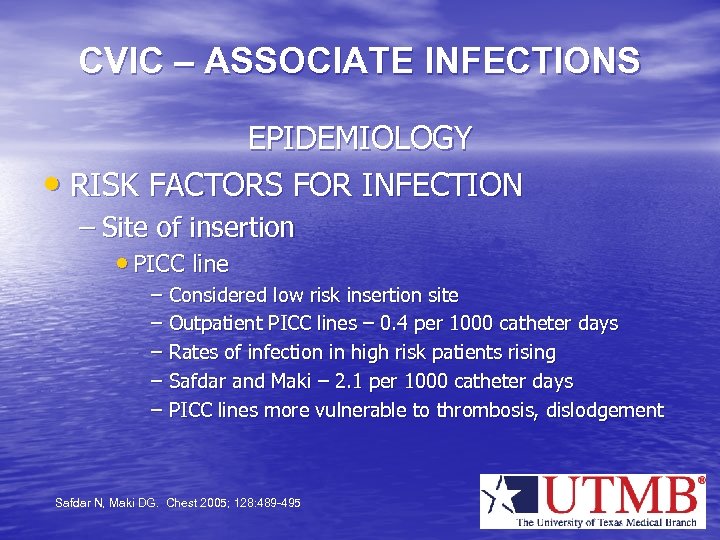 CVIC – ASSOCIATE INFECTIONS EPIDEMIOLOGY • RISK FACTORS FOR INFECTION – Site of insertion • PICC line – Considered low risk insertion site – Outpatient PICC lines – 0. 4 per 1000 catheter days – Rates of infection in high risk patients rising – Safdar and Maki – 2. 1 per 1000 catheter days – PICC lines more vulnerable to thrombosis, dislodgement Safdar N, Maki DG. Chest 2005; 128: 489 -495
CVIC – ASSOCIATE INFECTIONS EPIDEMIOLOGY • RISK FACTORS FOR INFECTION – Site of insertion • PICC line – Considered low risk insertion site – Outpatient PICC lines – 0. 4 per 1000 catheter days – Rates of infection in high risk patients rising – Safdar and Maki – 2. 1 per 1000 catheter days – PICC lines more vulnerable to thrombosis, dislodgement Safdar N, Maki DG. Chest 2005; 128: 489 -495
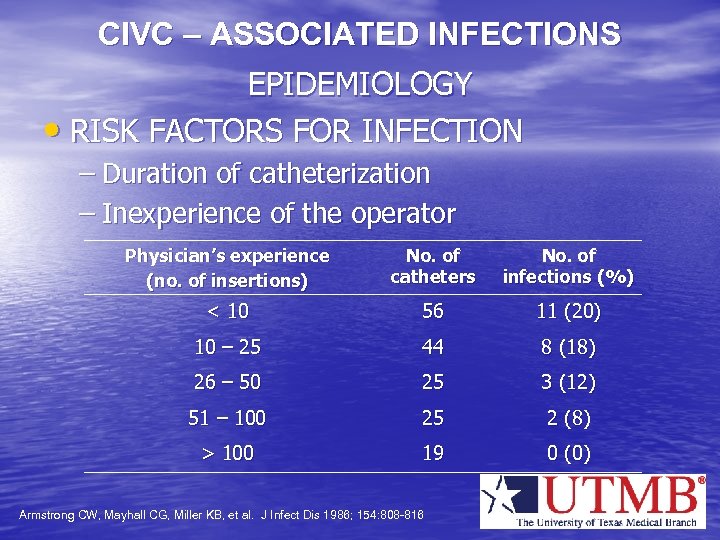 CIVC – ASSOCIATED INFECTIONS EPIDEMIOLOGY • RISK FACTORS FOR INFECTION – Duration of catheterization – Inexperience of the operator Physician’s experience (no. of insertions) No. of catheters No. of infections (%) < 10 56 11 (20) 10 – 25 44 8 (18) 26 – 50 25 3 (12) 51 – 100 25 2 (8) > 100 19 0 (0) Armstrong CW, Mayhall CG, Miller KB, et al. J Infect Dis 1986; 154: 808 -816
CIVC – ASSOCIATED INFECTIONS EPIDEMIOLOGY • RISK FACTORS FOR INFECTION – Duration of catheterization – Inexperience of the operator Physician’s experience (no. of insertions) No. of catheters No. of infections (%) < 10 56 11 (20) 10 – 25 44 8 (18) 26 – 50 25 3 (12) 51 – 100 25 2 (8) > 100 19 0 (0) Armstrong CW, Mayhall CG, Miller KB, et al. J Infect Dis 1986; 154: 808 -816
 CIVC – ASSOCIATED INFECTIONS EPIDEMIOLOGY • RISK FACTORS FOR INFECTION – Ineffective insertion site antisepsis • Chlorhexidine the agent of choice – 2% aqueous or 2% tincture – More effect than povidone-iodine or alcohol – 0. 5% tincture of chlorhexidine is no more effective than alcohol Maki DG, Ringer M, Alvarado CJ. Lancet 1991; 338: 339 -343. Humar A, Ostromecki A, Direnfeld J, et al. Clin Infect Dis 2000; 31: 1001 -1007.
CIVC – ASSOCIATED INFECTIONS EPIDEMIOLOGY • RISK FACTORS FOR INFECTION – Ineffective insertion site antisepsis • Chlorhexidine the agent of choice – 2% aqueous or 2% tincture – More effect than povidone-iodine or alcohol – 0. 5% tincture of chlorhexidine is no more effective than alcohol Maki DG, Ringer M, Alvarado CJ. Lancet 1991; 338: 339 -343. Humar A, Ostromecki A, Direnfeld J, et al. Clin Infect Dis 2000; 31: 1001 -1007.
 CIVC – ASSOCIATED INFECTIONS EPIDEMIOLOGY • RISK FACTORS FOR INFECTION – Failure to use appropriate insertion technique – Appropriate technique is maximal sterile barrier precautions – Use of a topical antibiotic ointment or cream may result in fungemia Raad II, Hohn DC, Gilbreath BJ, et al. Infect Control Hosp Epidemiol 1994; 15: 231 -238.
CIVC – ASSOCIATED INFECTIONS EPIDEMIOLOGY • RISK FACTORS FOR INFECTION – Failure to use appropriate insertion technique – Appropriate technique is maximal sterile barrier precautions – Use of a topical antibiotic ointment or cream may result in fungemia Raad II, Hohn DC, Gilbreath BJ, et al. Infect Control Hosp Epidemiol 1994; 15: 231 -238.
 CIVC – ASSOCIATED INFECTIONS • PREVENTION AND CONTROL Surveillance – Clinical – check insertion sites for signs of inflammation, ask patients about pain • Encourage patients to report pain, discomfort at insertion site to healthcare providers • Record operator, date and time of catheter insertion and dressing changes on a standard form • Do not routinely culture catheter tips CDC. MMWR 2002; 51 (No. RR-10): 1 -36.
CIVC – ASSOCIATED INFECTIONS • PREVENTION AND CONTROL Surveillance – Clinical – check insertion sites for signs of inflammation, ask patients about pain • Encourage patients to report pain, discomfort at insertion site to healthcare providers • Record operator, date and time of catheter insertion and dressing changes on a standard form • Do not routinely culture catheter tips CDC. MMWR 2002; 51 (No. RR-10): 1 -36.
 CIVC – ASSOCIATED INFECTIONS • PREVENTION AND CONTROL Surveillance – Epidemiologic • Count catheter days • Calculate cases of primary bacteremia per 1000 catheter days • This is how rates for public reporting of bacteremia will be reported
CIVC – ASSOCIATED INFECTIONS • PREVENTION AND CONTROL Surveillance – Epidemiologic • Count catheter days • Calculate cases of primary bacteremia per 1000 catheter days • This is how rates for public reporting of bacteremia will be reported
 CIVC – ASSOCIATED INFECTIONS • • PREVENTION AND CONTROL Hand hygiene is necessary before sterile gloves are donned and after they are doffed Choose CIVC with least ports needed for care of the patient CDC. MMWR 2002; 51 (No. RR-10): 1 -36. Mc. Gee DC, Gould MK. N Engl J Med 2003; 348: 1123 -1133.
CIVC – ASSOCIATED INFECTIONS • • PREVENTION AND CONTROL Hand hygiene is necessary before sterile gloves are donned and after they are doffed Choose CIVC with least ports needed for care of the patient CDC. MMWR 2002; 51 (No. RR-10): 1 -36. Mc. Gee DC, Gould MK. N Engl J Med 2003; 348: 1123 -1133.
 CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Insertion site selection – Subclavian – Internal jugular – Femoral – Site selection based on the relative risks for infectious vs mechanical complications Lorente L, Henry C, Martin MM, et al. Crit Care 2005; 9: R 631 -R 635
CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Insertion site selection – Subclavian – Internal jugular – Femoral – Site selection based on the relative risks for infectious vs mechanical complications Lorente L, Henry C, Martin MM, et al. Crit Care 2005; 9: R 631 -R 635
 CIVC – ASSOCIATED INFECTIONS • • PREVENTION AND CONTROL Type of catheter – Coated with antimicrobial agents or not Types of coated catheters – Chlorhexidine – silver – sulfadiazine – 4 studies • All showed decreased rate of catheter colonization • Only one showed a significant decrease in catheter –associated bacteremia Maki DG, Stolz SM, Wheeler S, Mermel LA. Ann Intern Med 1997; 127: 257 -266. Rupp ME, Lisco SJ, Lipsett PA, et al. Ann Intern Med 2005; 143: 570 -580.
CIVC – ASSOCIATED INFECTIONS • • PREVENTION AND CONTROL Type of catheter – Coated with antimicrobial agents or not Types of coated catheters – Chlorhexidine – silver – sulfadiazine – 4 studies • All showed decreased rate of catheter colonization • Only one showed a significant decrease in catheter –associated bacteremia Maki DG, Stolz SM, Wheeler S, Mermel LA. Ann Intern Med 1997; 127: 257 -266. Rupp ME, Lisco SJ, Lipsett PA, et al. Ann Intern Med 2005; 143: 570 -580.
 CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Types of coated catheters – Minocycline – rifampin coated catheters • Significant reduction in catheter colonization and bacteremia compared to uncoated catheters • Significantly greater reduction in catheter colonization and bacteremia when compared with a chlorhexidine – silver sulfadiazine coated catheter Raad I, Darouiche R, Dupuis J, et al. Ann Intern Med 1997; 127: 267 -274. Darouiche RO, Raad II, Heard SO, et al. N Engl J Med 1999; 340: 1 -8.
CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Types of coated catheters – Minocycline – rifampin coated catheters • Significant reduction in catheter colonization and bacteremia compared to uncoated catheters • Significantly greater reduction in catheter colonization and bacteremia when compared with a chlorhexidine – silver sulfadiazine coated catheter Raad I, Darouiche R, Dupuis J, et al. Ann Intern Med 1997; 127: 267 -274. Darouiche RO, Raad II, Heard SO, et al. N Engl J Med 1999; 340: 1 -8.
 CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Should catheters coated with antimicrobials be used? – More expensive than uncoated catheters – May select for antimicrobial resistance – May be used to cover up poor aseptic technique – May have limited effect on reducing bacteremias
CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Should catheters coated with antimicrobials be used? – More expensive than uncoated catheters – May select for antimicrobial resistance – May be used to cover up poor aseptic technique – May have limited effect on reducing bacteremias
 CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Catheter insertion – Maximal sterile barrier precautions • Cap • Mask • Sterile Gown • Sterile gloves • Large sterile drape – Skin asepsis – 2% chlorhexidine – Sterile sleeve to protect pulmonary artery catheters Raad II, Hohn DC, Gilbreath J, Infect Control Hosp Epidemiol 1994; 15: 231 -238 CDC. MMWR 2002; 51(No. RR-10): 1 -36
CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Catheter insertion – Maximal sterile barrier precautions • Cap • Mask • Sterile Gown • Sterile gloves • Large sterile drape – Skin asepsis – 2% chlorhexidine – Sterile sleeve to protect pulmonary artery catheters Raad II, Hohn DC, Gilbreath J, Infect Control Hosp Epidemiol 1994; 15: 231 -238 CDC. MMWR 2002; 51(No. RR-10): 1 -36
 CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Maximal sterile barrier precautions – PICC lines – Guidewire exchanges • Trainees must be supervised by personnel who have been trained and exhibit competency in the insertion of CIVCs • REMOVE CIVCs AS SOON AS POSSIBLE
CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Maximal sterile barrier precautions – PICC lines – Guidewire exchanges • Trainees must be supervised by personnel who have been trained and exhibit competency in the insertion of CIVCs • REMOVE CIVCs AS SOON AS POSSIBLE
 CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Catheter insertion site care – Replace catheter site dressings • Damp • Loosened • Soiled • When dressing removed for site inspection – Dressing changes • Gauze and tape – every 2 days • Transparent – every 7 days CDC. MMWR 2002; 51(No. RR-10): 1 -36
CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Catheter insertion site care – Replace catheter site dressings • Damp • Loosened • Soiled • When dressing removed for site inspection – Dressing changes • Gauze and tape – every 2 days • Transparent – every 7 days CDC. MMWR 2002; 51(No. RR-10): 1 -36
 CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Should CIVCs be changed to a new site or be changed over a guidewire at routine intervals to prevent CIVC – associated infections?
CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Should CIVCs be changed to a new site or be changed over a guidewire at routine intervals to prevent CIVC – associated infections?
 CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL – No • Four randomized clinical trials indicated that routine rotation of CIVCs does not significantly reduce infection rates • Includes guidewire exchanges • Also applies to hemodialysis catheters Uldall PR, Merchant N, Woods F, et al. Lancet 1981; 1: 1373 Powell C, Kudsk KA, Kulich PA, et al. J Parenter Enteral Nurt 1988; 12: 4621 -464 Eyer S, Brummitt C, Crossley K, et al. Crit Care Med 1990; 18: 1073 -1079 Cobb DK, High KP, Sawyer RG, et al. N Engl J Med 1992; 327: 1062 -1068
CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL – No • Four randomized clinical trials indicated that routine rotation of CIVCs does not significantly reduce infection rates • Includes guidewire exchanges • Also applies to hemodialysis catheters Uldall PR, Merchant N, Woods F, et al. Lancet 1981; 1: 1373 Powell C, Kudsk KA, Kulich PA, et al. J Parenter Enteral Nurt 1988; 12: 4621 -464 Eyer S, Brummitt C, Crossley K, et al. Crit Care Med 1990; 18: 1073 -1079 Cobb DK, High KP, Sawyer RG, et al. N Engl J Med 1992; 327: 1062 -1068
 CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Guidewire exchanges – May be used to replace a malfunctioning catheter when no evidence of infection – Must never be used to replace an infected catheter – Use a new set of sterile gloves before handling the new catheter
CIVC – ASSOCIATED INFECTIONS PREVENTION AND CONTROL • Guidewire exchanges – May be used to replace a malfunctioning catheter when no evidence of infection – Must never be used to replace an infected catheter – Use a new set of sterile gloves before handling the new catheter
 CIVC - ASSOCIATED INFECTIONS • PREVENTION AND CONTROL Infection control bundle for the prevention of catheter-associated bloodstream infection – Prospective observational study – Multicenter study - 103 ICUs – 1981 ICU months – 375, 757 catheter-days Pronovost P, Needham D, Berenholtz S, et al. N Engl J Med 2006; 355: 2725 -2732
CIVC - ASSOCIATED INFECTIONS • PREVENTION AND CONTROL Infection control bundle for the prevention of catheter-associated bloodstream infection – Prospective observational study – Multicenter study - 103 ICUs – 1981 ICU months – 375, 757 catheter-days Pronovost P, Needham D, Berenholtz S, et al. N Engl J Med 2006; 355: 2725 -2732
 CIVC – ASSOCIATED INFECTIONS • PREVENTION AND CONTROL Infection control bundle – First step • Implement use of daily goals sheet to improve clinician-to-clinician communication* • Instituted program to control ventilator-associated pneumonia† • Comprehensive unit-based safety program‡§ *Pronovost P, Berenholtz S, Dorman T, et al. J Crit Care Med 2003; 18: 71 -75 †Berenholtz SM, Milanovich S, Faircloth A, et al. Jt Comm J Qual Saf 2004; 30: 195 -204 ‡Pronovost P, Weast B, Rosenstein B, et al. J Patient Saf 2005; 1: 33 -40 §Pronovost PJ, Weast B, Bishop K, et al. Jt Comm J Qual Saf 2004; 30: 59 -68
CIVC – ASSOCIATED INFECTIONS • PREVENTION AND CONTROL Infection control bundle – First step • Implement use of daily goals sheet to improve clinician-to-clinician communication* • Instituted program to control ventilator-associated pneumonia† • Comprehensive unit-based safety program‡§ *Pronovost P, Berenholtz S, Dorman T, et al. J Crit Care Med 2003; 18: 71 -75 †Berenholtz SM, Milanovich S, Faircloth A, et al. Jt Comm J Qual Saf 2004; 30: 195 -204 ‡Pronovost P, Weast B, Rosenstein B, et al. J Patient Saf 2005; 1: 33 -40 §Pronovost PJ, Weast B, Bishop K, et al. Jt Comm J Qual Saf 2004; 30: 59 -68
 CIVC – ASSOCIATED INFECTIONS • PREVENTION AND CONTROL Interventions – evidence based – Hand washing – Maximal sterile barrier precautions – Chlorhexidine preparation of the skin – Avoiding femoral site, if possible – Removing unnecessary catheters
CIVC – ASSOCIATED INFECTIONS • PREVENTION AND CONTROL Interventions – evidence based – Hand washing – Maximal sterile barrier precautions – Chlorhexidine preparation of the skin – Avoiding femoral site, if possible – Removing unnecessary catheters
 CIVC – ASSOCIATED INFECTIONS • PREVENTION AND CONTROL Promoters of the interventions – – Clinician education Central line cart Infection control procedures checklist Providers stopped if not following infection control procedures – Feedback of rates to teams – Letters to CEOs of hospitals to stock chlorhexidine
CIVC – ASSOCIATED INFECTIONS • PREVENTION AND CONTROL Promoters of the interventions – – Clinician education Central line cart Infection control procedures checklist Providers stopped if not following infection control procedures – Feedback of rates to teams – Letters to CEOs of hospitals to stock chlorhexidine
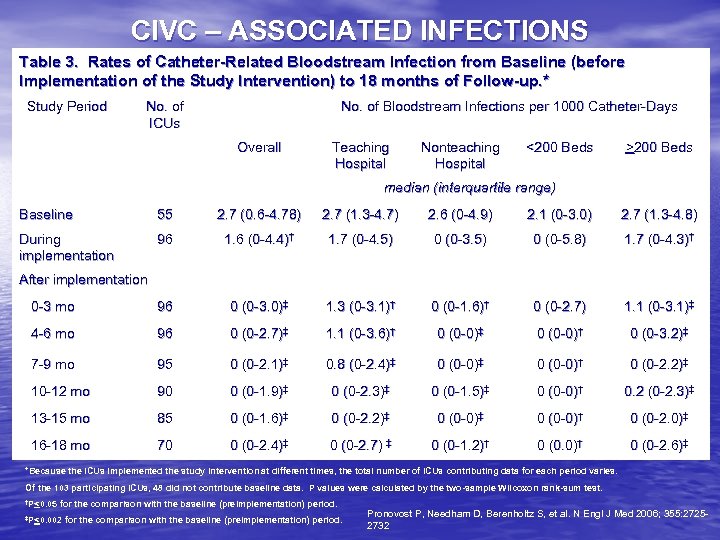 CIVC – ASSOCIATED INFECTIONS Table 3. Rates of Catheter-Related Bloodstream Infection from Baseline (before Implementation of the Study Intervention) to 18 months of Follow-up. * Study Period No. of ICUs No. of Bloodstream Infections per 1000 Catheter-Days Overall Teaching Hospital Nonteaching Hospital <200 Beds >200 Beds median (interquartile range) Baseline 55 2. 7 (0. 6 -4. 78) 2. 7 (1. 3 -4. 7) 2. 6 (0 -4. 9) 2. 1 (0 -3. 0) 2. 7 (1. 3 -4. 8) During implementation 96 1. 6 (0 -4. 4)† 1. 7 (0 -4. 5) 0 (0 -3. 5) 0 (0 -5. 8) 1. 7 (0 -4. 3)† 0 -3 mo 96 0 (0 -3. 0)‡ 1. 3 (0 -3. 1)† 0 (0 -1. 6)† 0 (0 -2. 7) 1. 1 (0 -3. 1)‡ 4 -6 mo 96 0 (0 -2. 7)‡ 1. 1 (0 -3. 6)† 0 (0 -0)‡ 0 (0 -0)† 0 (0 -3. 2)‡ 7 -9 mo 95 0 (0 -2. 1)‡ 0. 8 (0 -2. 4)‡ 0 (0 -0)† 0 (0 -2. 2)‡ 10 -12 mo 90 0 (0 -1. 9)‡ 0 (0 -2. 3)‡ 0 (0 -1. 5)‡ 0 (0 -0)† 0. 2 (0 -2. 3)‡ 13 -15 mo 85 0 (0 -1. 6)‡ 0 (0 -2. 2)‡ 0 (0 -0)† 0 (0 -2. 0)‡ 16 -18 mo 70 0 (0 -2. 4)‡ 0 (0 -2. 7) ‡ 0 (0 -1. 2)† 0 (0. 0)† 0 (0 -2. 6)‡ After implementation *Because the ICUs implemented the study intervention at different times, the total number of ICUs contributing data for each period varies. Of the 103 participating ICUs, 48 did not contribute baseline data. P values were calculated by the two-sample Wilcoxon rank-sum test. †P<0. 05 for the comparison with the baseline (preimplementation) period. ‡P<0. 002 for the comparison with the baseline (preimplementation) period. Pronovost P, Needham D, Berenholtz S, et al. N Engl J Med 2006; 355: 27252732
CIVC – ASSOCIATED INFECTIONS Table 3. Rates of Catheter-Related Bloodstream Infection from Baseline (before Implementation of the Study Intervention) to 18 months of Follow-up. * Study Period No. of ICUs No. of Bloodstream Infections per 1000 Catheter-Days Overall Teaching Hospital Nonteaching Hospital <200 Beds >200 Beds median (interquartile range) Baseline 55 2. 7 (0. 6 -4. 78) 2. 7 (1. 3 -4. 7) 2. 6 (0 -4. 9) 2. 1 (0 -3. 0) 2. 7 (1. 3 -4. 8) During implementation 96 1. 6 (0 -4. 4)† 1. 7 (0 -4. 5) 0 (0 -3. 5) 0 (0 -5. 8) 1. 7 (0 -4. 3)† 0 -3 mo 96 0 (0 -3. 0)‡ 1. 3 (0 -3. 1)† 0 (0 -1. 6)† 0 (0 -2. 7) 1. 1 (0 -3. 1)‡ 4 -6 mo 96 0 (0 -2. 7)‡ 1. 1 (0 -3. 6)† 0 (0 -0)‡ 0 (0 -0)† 0 (0 -3. 2)‡ 7 -9 mo 95 0 (0 -2. 1)‡ 0. 8 (0 -2. 4)‡ 0 (0 -0)† 0 (0 -2. 2)‡ 10 -12 mo 90 0 (0 -1. 9)‡ 0 (0 -2. 3)‡ 0 (0 -1. 5)‡ 0 (0 -0)† 0. 2 (0 -2. 3)‡ 13 -15 mo 85 0 (0 -1. 6)‡ 0 (0 -2. 2)‡ 0 (0 -0)† 0 (0 -2. 0)‡ 16 -18 mo 70 0 (0 -2. 4)‡ 0 (0 -2. 7) ‡ 0 (0 -1. 2)† 0 (0. 0)† 0 (0 -2. 6)‡ After implementation *Because the ICUs implemented the study intervention at different times, the total number of ICUs contributing data for each period varies. Of the 103 participating ICUs, 48 did not contribute baseline data. P values were calculated by the two-sample Wilcoxon rank-sum test. †P<0. 05 for the comparison with the baseline (preimplementation) period. ‡P<0. 002 for the comparison with the baseline (preimplementation) period. Pronovost P, Needham D, Berenholtz S, et al. N Engl J Med 2006; 355: 27252732


