771bf832ae9f1e8a2d28d0096b27a541.ppt
- Количество слайдов: 88
 Surgical Treatment of Malignant Melanoma Yağmur AYDIN, M. D. , Asc. Prof. University of Istanbul, Cerrahpaşa Medical School Department of Plastic, Reconstructive and Aesthetic Surgery
Surgical Treatment of Malignant Melanoma Yağmur AYDIN, M. D. , Asc. Prof. University of Istanbul, Cerrahpaşa Medical School Department of Plastic, Reconstructive and Aesthetic Surgery
 Malignant Melanoma n n n Arise from melanocytes in basal layer in the epidermis The worst prognosis and morbidity in all skin cancers Incidence is increasing by every year (% 6 per year) accounts for only 4% of all skin cancers, but responsible for more than 77% of skin cancer deaths Early detection and treatment important, because it reduces the mortality and increases survival Prognosis ( 5 years) Local disease > % 90 Regional disease % 60 Distant metastasis % 5
Malignant Melanoma n n n Arise from melanocytes in basal layer in the epidermis The worst prognosis and morbidity in all skin cancers Incidence is increasing by every year (% 6 per year) accounts for only 4% of all skin cancers, but responsible for more than 77% of skin cancer deaths Early detection and treatment important, because it reduces the mortality and increases survival Prognosis ( 5 years) Local disease > % 90 Regional disease % 60 Distant metastasis % 5
 n n n Mostly arise from skin ( 95 % ) But also found in the eyes, ears, GI tract, leptomeninges, and mucous membranes Unknown primary or metastasis (3 %) May develop in precursor melanocytic nevi(common, congenital, and atypical/dysplastic types (25 %) Commonly arise from de-novo (not from a preexisting pigmented lesion)
n n n Mostly arise from skin ( 95 % ) But also found in the eyes, ears, GI tract, leptomeninges, and mucous membranes Unknown primary or metastasis (3 %) May develop in precursor melanocytic nevi(common, congenital, and atypical/dysplastic types (25 %) Commonly arise from de-novo (not from a preexisting pigmented lesion)
 Causes n n Melanoma tends to occur at sites of intermittent, intense sun exposure an increased worldwide incidence in fair -complexioned individuals living in sunny climates and nearer the equator, suggesting a causative role for ultraviolet radiation.
Causes n n Melanoma tends to occur at sites of intermittent, intense sun exposure an increased worldwide incidence in fair -complexioned individuals living in sunny climates and nearer the equator, suggesting a causative role for ultraviolet radiation.
 Precursor Lesions n n Atypical or Displastic nevi Congenital nevi Lentigo maligna Acquired nevi
Precursor Lesions n n Atypical or Displastic nevi Congenital nevi Lentigo maligna Acquired nevi
 Risk Factors - I The development of melanoma is multifactorial and appears to be related to multiple risk factors n n n Very fair skinned, particularly those with fair or red hair Tendency to sun burn Excessive childhood sun exposure and blistering childhood burns Age : the incidence steadily rises with age. The highest incidence is in those over 80 The more moles you have on your body, the higher your risk of melanoma (more than 100 Atypical or dysplastic nevi)
Risk Factors - I The development of melanoma is multifactorial and appears to be related to multiple risk factors n n n Very fair skinned, particularly those with fair or red hair Tendency to sun burn Excessive childhood sun exposure and blistering childhood burns Age : the incidence steadily rises with age. The highest incidence is in those over 80 The more moles you have on your body, the higher your risk of melanoma (more than 100 Atypical or dysplastic nevi)
 Risk Factors -II n n n Born in a hot country, such as Australia or Israel Malignant melanoma in the first degree relative (3 -10 %) Giant congenital nevi (5 -40 ) Second malignant melanoma (3 -6 %) Sunbeds (Solarium) People who work outdoors and so are in the sun (sailors, farmers. . )
Risk Factors -II n n n Born in a hot country, such as Australia or Israel Malignant melanoma in the first degree relative (3 -10 %) Giant congenital nevi (5 -40 ) Second malignant melanoma (3 -6 %) Sunbeds (Solarium) People who work outdoors and so are in the sun (sailors, farmers. . )
 People who have risk factors should be follow and have preventive efforts
People who have risk factors should be follow and have preventive efforts
 Early Diagnosis A changing mole is the most common warning sign for melanoma
Early Diagnosis A changing mole is the most common warning sign for melanoma
 Early Signs of Melanoma n n The most common early sign of melanoma is pruritis A, B, C, D, E warnin signs n n n Asymmetry: One half of the lesion does not match the other half. Border irregularity: The edges are ragged, notched, or blurred. Color variegation: Pigmentation is not uniform and may display shades of tan, brown, or black; white, reddish, or blue discoloration is of particular concern. Diameter: A diameter greater than 6 mm is characteristic, although some melanomas may have smaller diameters; any growth in a nevus warrants an evaluation. Evolving: Changes in the lesion over time Bleeding and ulceration are late signs showing advanced disease
Early Signs of Melanoma n n The most common early sign of melanoma is pruritis A, B, C, D, E warnin signs n n n Asymmetry: One half of the lesion does not match the other half. Border irregularity: The edges are ragged, notched, or blurred. Color variegation: Pigmentation is not uniform and may display shades of tan, brown, or black; white, reddish, or blue discoloration is of particular concern. Diameter: A diameter greater than 6 mm is characteristic, although some melanomas may have smaller diameters; any growth in a nevus warrants an evaluation. Evolving: Changes in the lesion over time Bleeding and ulceration are late signs showing advanced disease
 All suspected lesions are removed for histopathologic examination
All suspected lesions are removed for histopathologic examination
 Biopsy n n Excision (Golden standard) *İncision biopsy *Punch biopsy Partial thickness or shaving biopsies are contraindicated *All dermis layers should be removed
Biopsy n n Excision (Golden standard) *İncision biopsy *Punch biopsy Partial thickness or shaving biopsies are contraindicated *All dermis layers should be removed
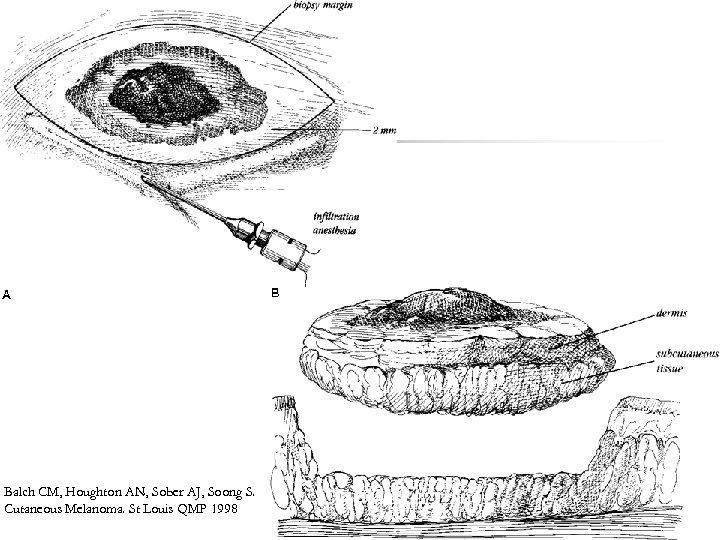 Balch CM, Houghton AN, Sober AJ, Soong S. Cutaneous Melanoma. St Louis QMP 1998
Balch CM, Houghton AN, Sober AJ, Soong S. Cutaneous Melanoma. St Louis QMP 1998
 Melanoma is divided into the four major subtypes 1. 2. 3. 4. Superficial spreading melanoma Nodular melanoma Lentigo Maligna Melanoma Acral Lentiginous Melanoma
Melanoma is divided into the four major subtypes 1. 2. 3. 4. Superficial spreading melanoma Nodular melanoma Lentigo Maligna Melanoma Acral Lentiginous Melanoma
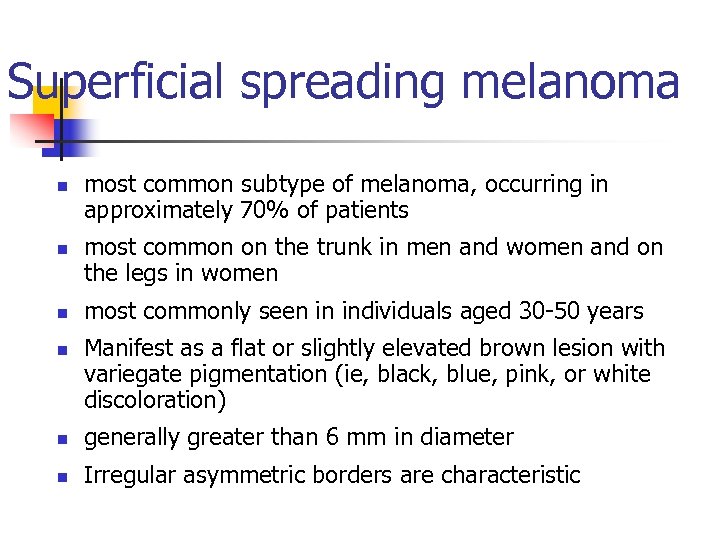 Superficial spreading melanoma n n most common subtype of melanoma, occurring in approximately 70% of patients most common on the trunk in men and women and on the legs in women most commonly seen in individuals aged 30 -50 years Manifest as a flat or slightly elevated brown lesion with variegate pigmentation (ie, black, blue, pink, or white discoloration) n generally greater than 6 mm in diameter n Irregular asymmetric borders are characteristic
Superficial spreading melanoma n n most common subtype of melanoma, occurring in approximately 70% of patients most common on the trunk in men and women and on the legs in women most commonly seen in individuals aged 30 -50 years Manifest as a flat or slightly elevated brown lesion with variegate pigmentation (ie, black, blue, pink, or white discoloration) n generally greater than 6 mm in diameter n Irregular asymmetric borders are characteristic
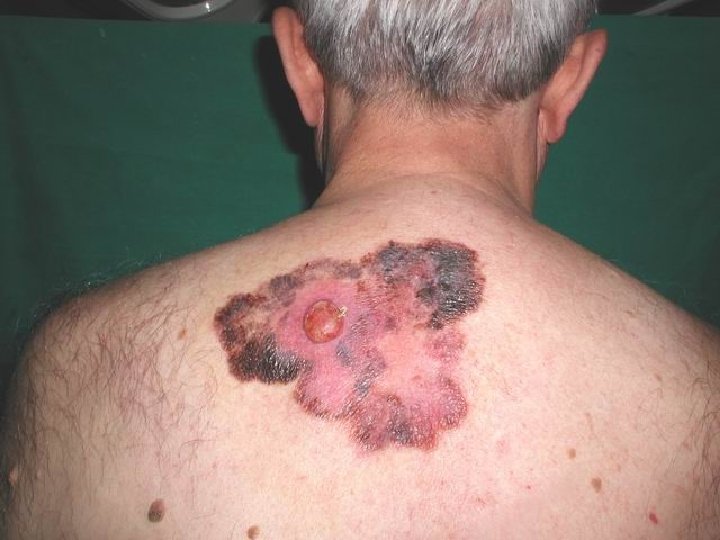
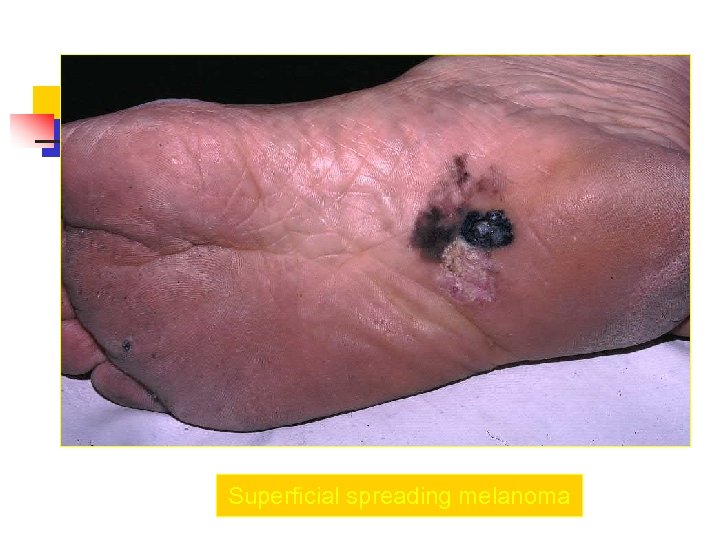 Superficial spreading melanoma
Superficial spreading melanoma
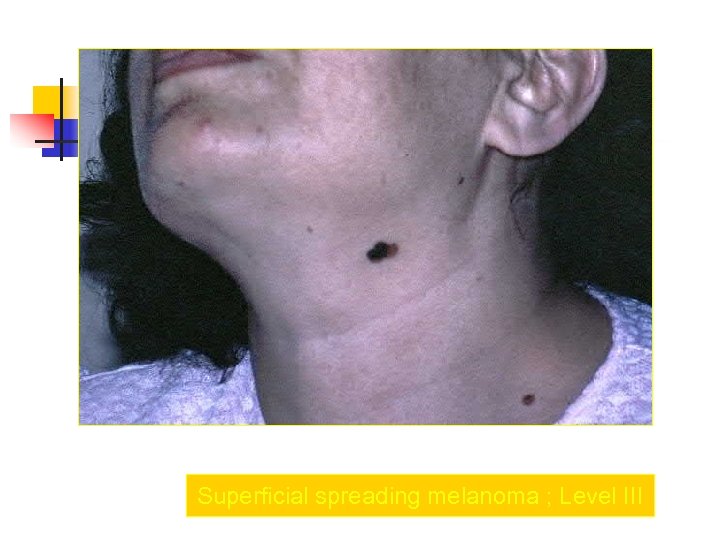 Superficial spreading melanoma ; Level III
Superficial spreading melanoma ; Level III
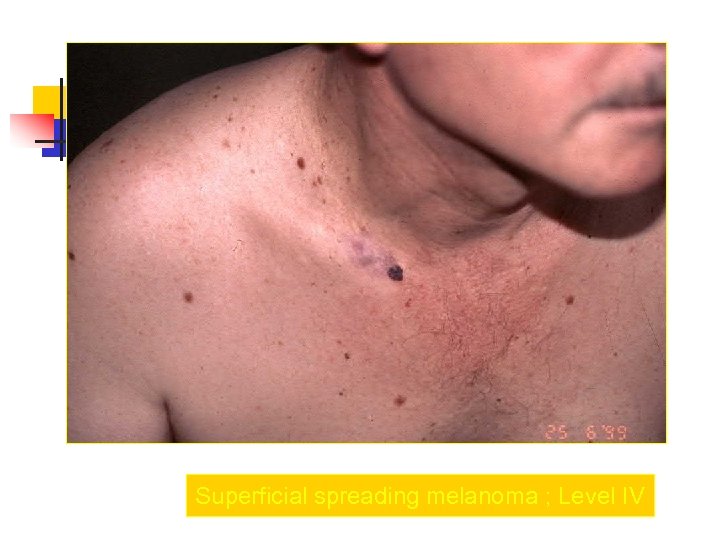 Superficial spreading melanoma ; Level IV
Superficial spreading melanoma ; Level IV
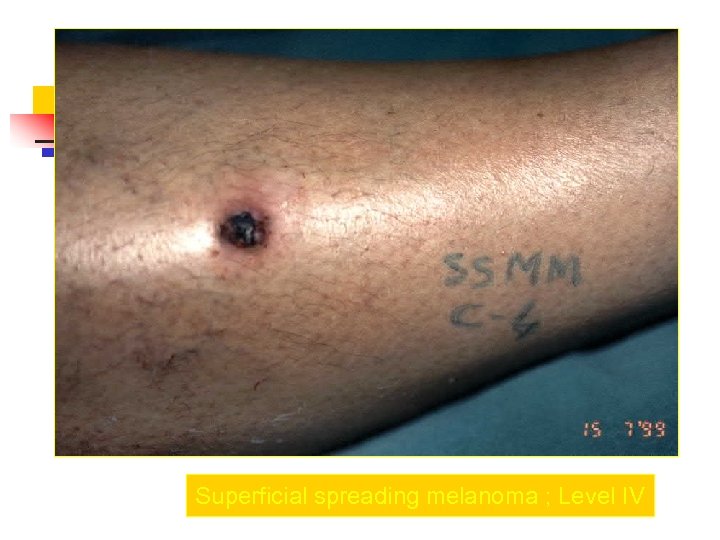 Superficial spreading melanoma ; Level IV
Superficial spreading melanoma ; Level IV
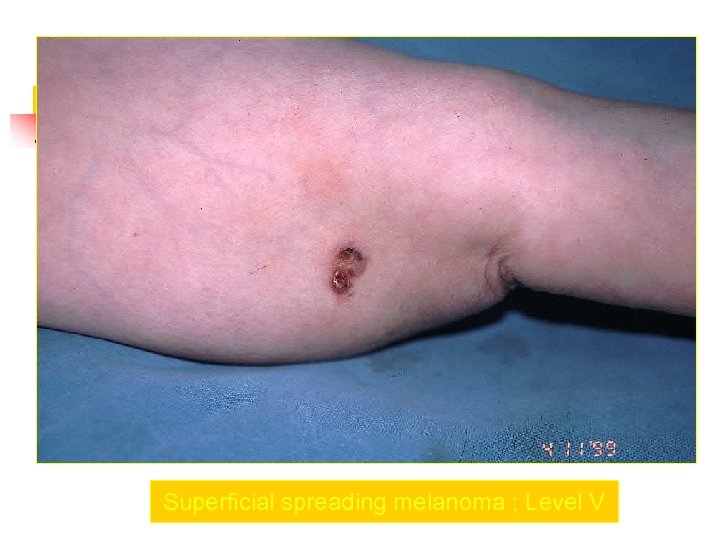 Superficial spreading melanoma ; Level V
Superficial spreading melanoma ; Level V
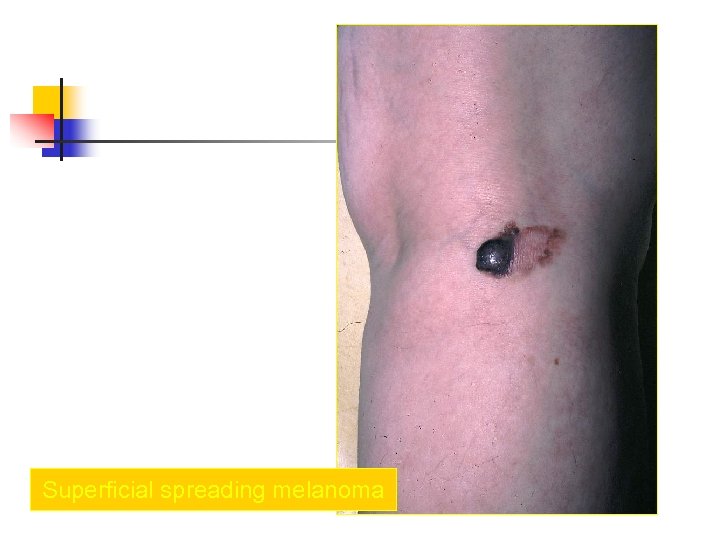 Superficial spreading melanoma
Superficial spreading melanoma
 Superficial spreading melanoma n The most common melanoma mimickers are seborrheic keratoses Qenign keratinocytic proliferations) and traumatized nevi, which often present as a "bleeding mole. " A mole showing severely atypical features may be clinically indistinguishable from a melanoma
Superficial spreading melanoma n The most common melanoma mimickers are seborrheic keratoses Qenign keratinocytic proliferations) and traumatized nevi, which often present as a "bleeding mole. " A mole showing severely atypical features may be clinically indistinguishable from a melanoma
 Nodular Melanoma n n n n Nodular melanoma is the second most common subtype of melanoma, occurs in 15 -30% of patients Seen most commonly on the legs and trunk Rapid growth occurs over weeks to months Lack of an identifiable in situ (or radial growth) phase Responsible for most thick melanomas Manifests as a dark brown-to-black papule or domeshaped nodule, which may ulcerate and bleed with minor trauma Tends to lack the typical ABCDE melanoma warning signs
Nodular Melanoma n n n n Nodular melanoma is the second most common subtype of melanoma, occurs in 15 -30% of patients Seen most commonly on the legs and trunk Rapid growth occurs over weeks to months Lack of an identifiable in situ (or radial growth) phase Responsible for most thick melanomas Manifests as a dark brown-to-black papule or domeshaped nodule, which may ulcerate and bleed with minor trauma Tends to lack the typical ABCDE melanoma warning signs
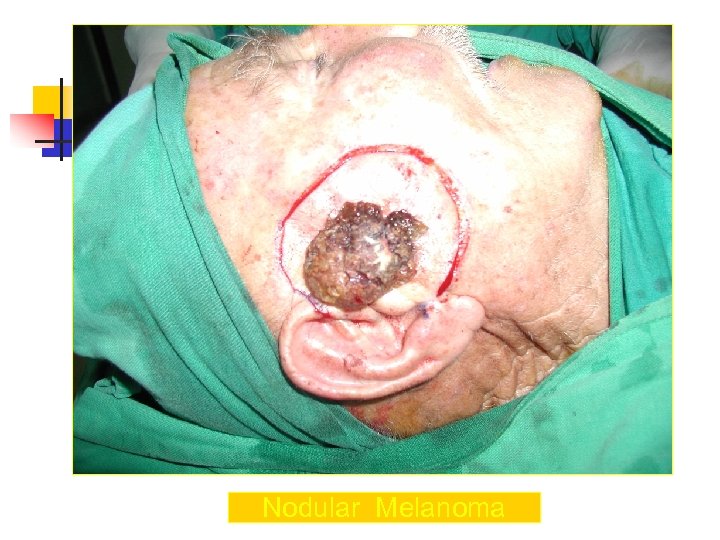 Nodular Melanoma
Nodular Melanoma
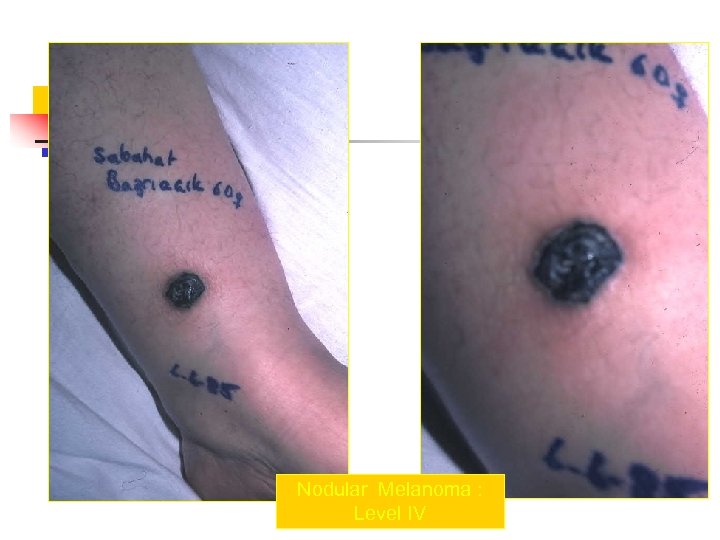 Nodular Melanoma : Level IV
Nodular Melanoma : Level IV
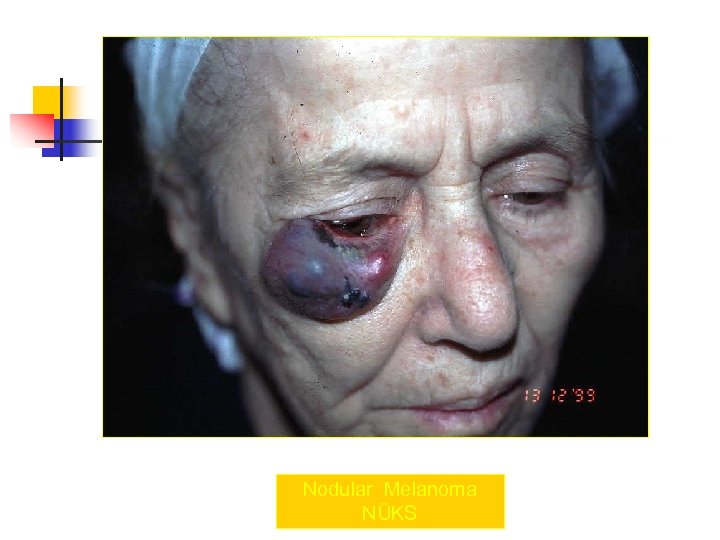 Nodular Melanoma NÜKS
Nodular Melanoma NÜKS
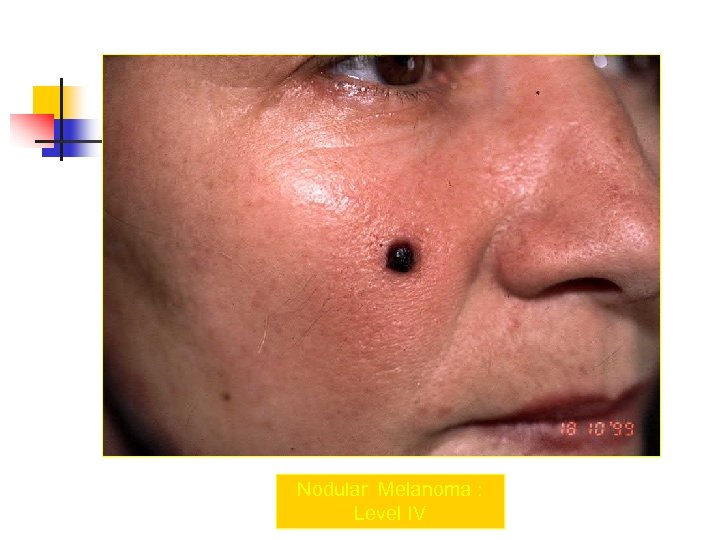 Nodular Melanoma : Level IV
Nodular Melanoma : Level IV
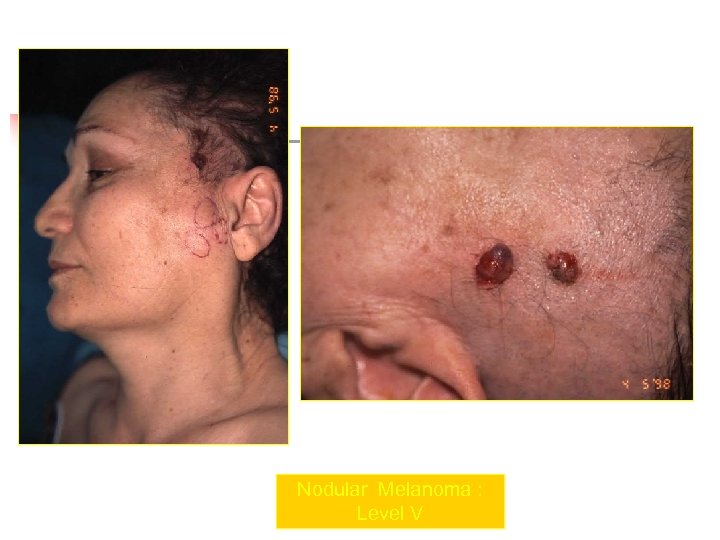 Nodular Melanoma : Level V
Nodular Melanoma : Level V
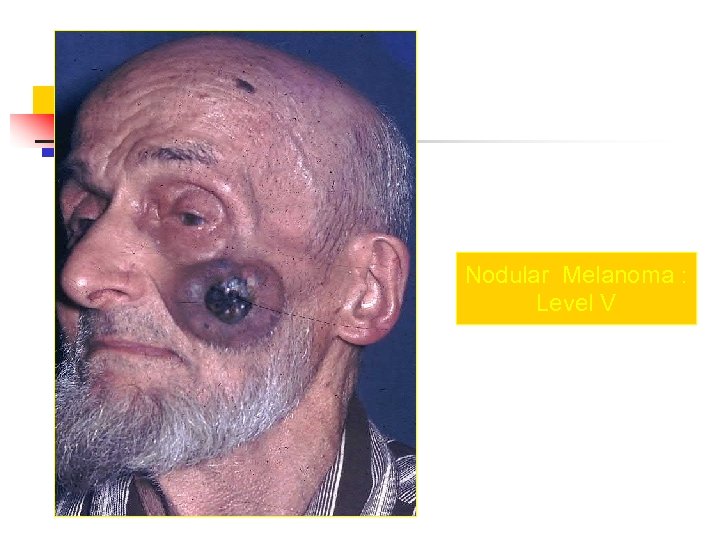 Nodular Melanoma : Level V
Nodular Melanoma : Level V
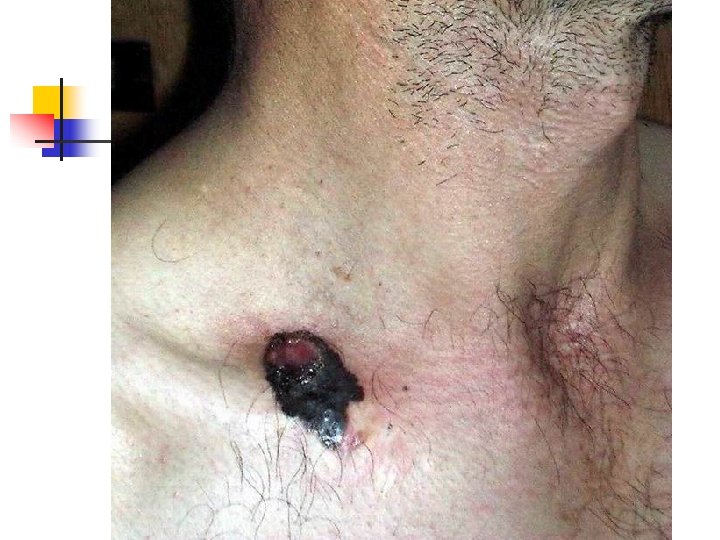
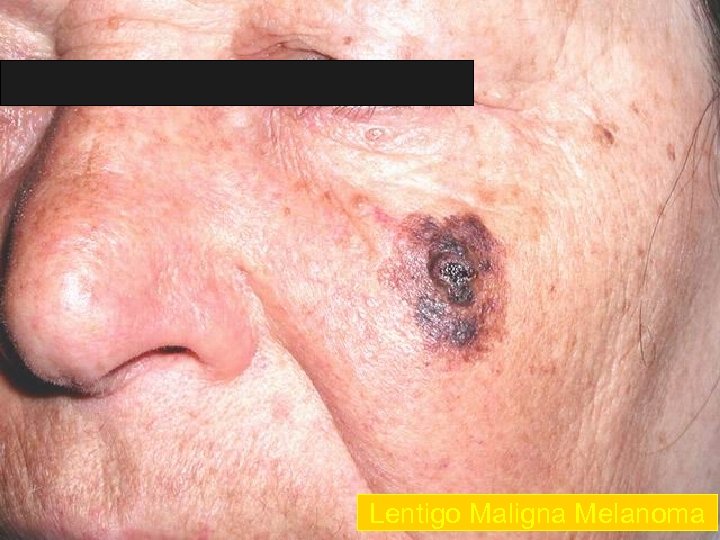
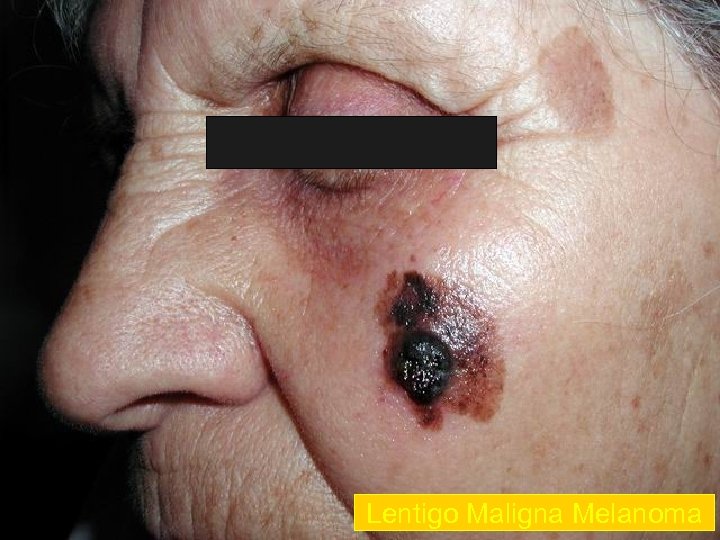
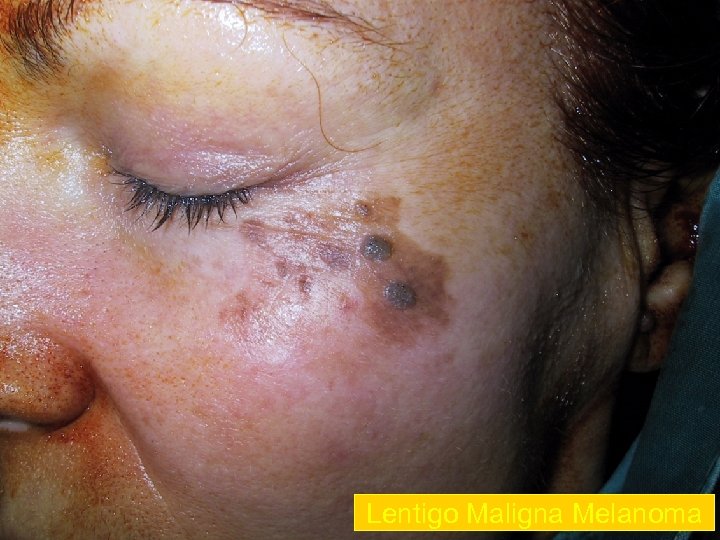
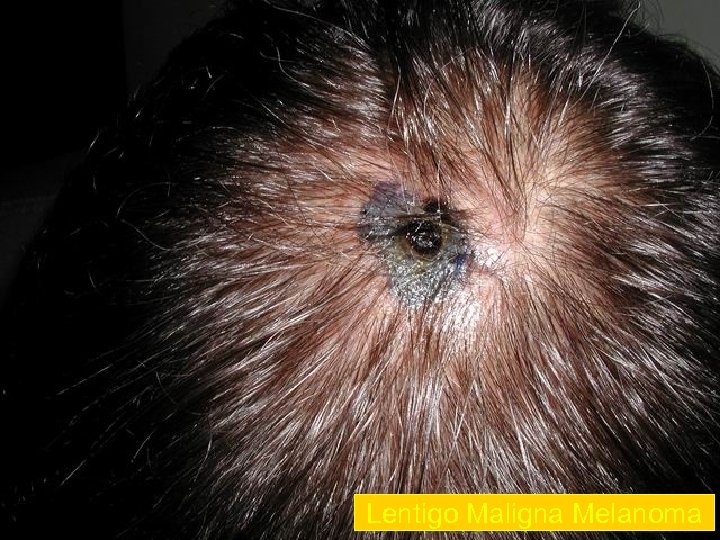
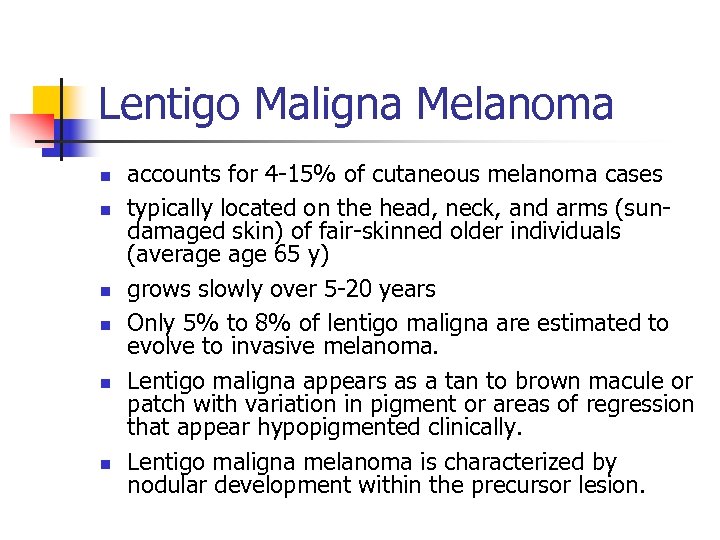 Lentigo Maligna Melanoma n n n accounts for 4 -15% of cutaneous melanoma cases typically located on the head, neck, and arms (sundamaged skin) of fair-skinned older individuals (average 65 y) grows slowly over 5 -20 years Only 5% to 8% of lentigo maligna are estimated to evolve to invasive melanoma. Lentigo maligna appears as a tan to brown macule or patch with variation in pigment or areas of regression that appear hypopigmented clinically. Lentigo maligna melanoma is characterized by nodular development within the precursor lesion.
Lentigo Maligna Melanoma n n n accounts for 4 -15% of cutaneous melanoma cases typically located on the head, neck, and arms (sundamaged skin) of fair-skinned older individuals (average 65 y) grows slowly over 5 -20 years Only 5% to 8% of lentigo maligna are estimated to evolve to invasive melanoma. Lentigo maligna appears as a tan to brown macule or patch with variation in pigment or areas of regression that appear hypopigmented clinically. Lentigo maligna melanoma is characterized by nodular development within the precursor lesion.
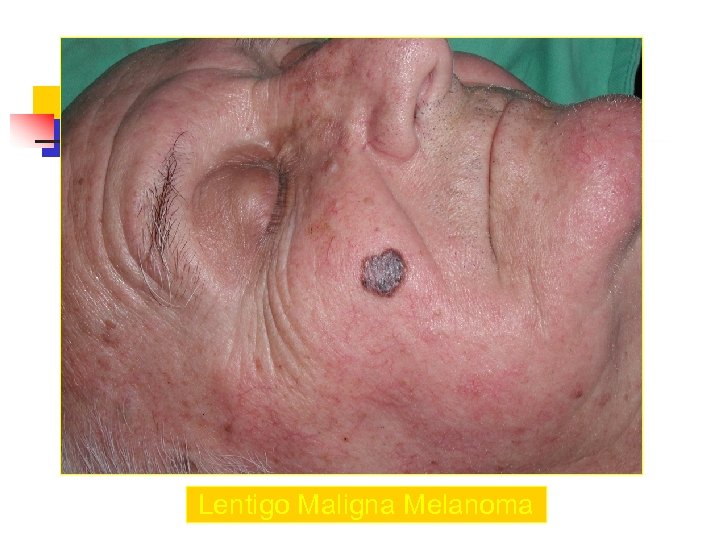 Lentigo Maligna Melanoma
Lentigo Maligna Melanoma
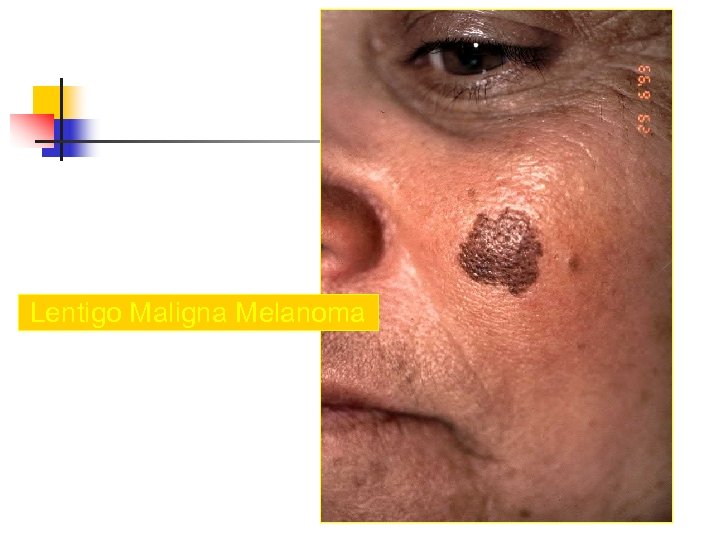 Lentigo Maligna Melanoma
Lentigo Maligna Melanoma
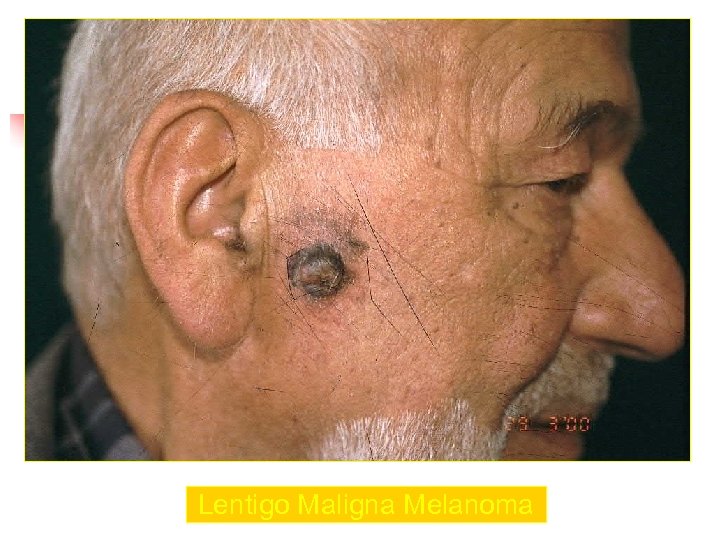 Lentigo Maligna Melanoma
Lentigo Maligna Melanoma
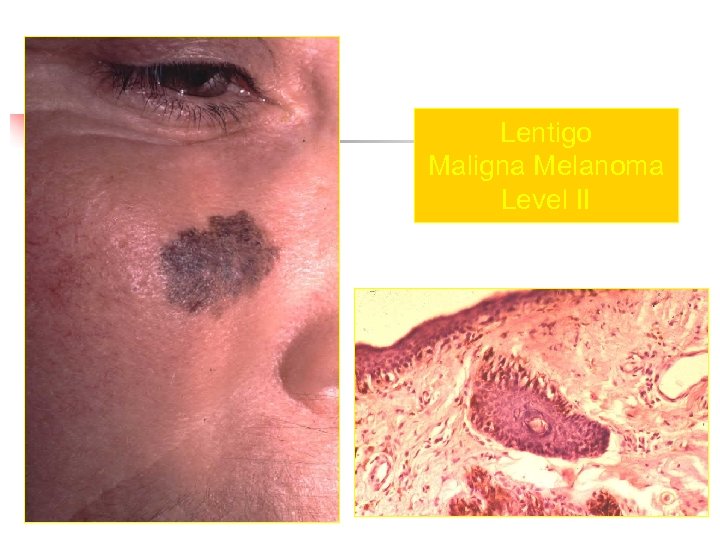 Lentigo Maligna Melanoma Level II
Lentigo Maligna Melanoma Level II
 Lentigo Maligna Melanoma
Lentigo Maligna Melanoma
 Lentigo Maligna Melanoma
Lentigo Maligna Melanoma
 Lentigo Maligna Melanoma
Lentigo Maligna Melanoma
 Lentigo Maligna Melanoma
Lentigo Maligna Melanoma
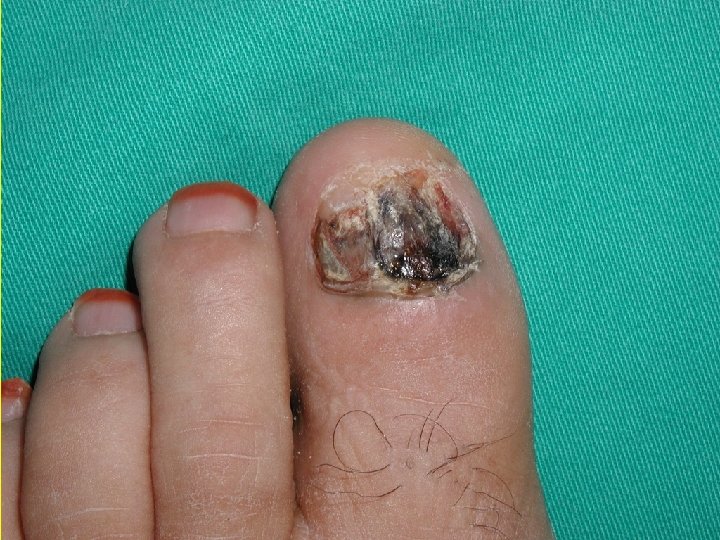
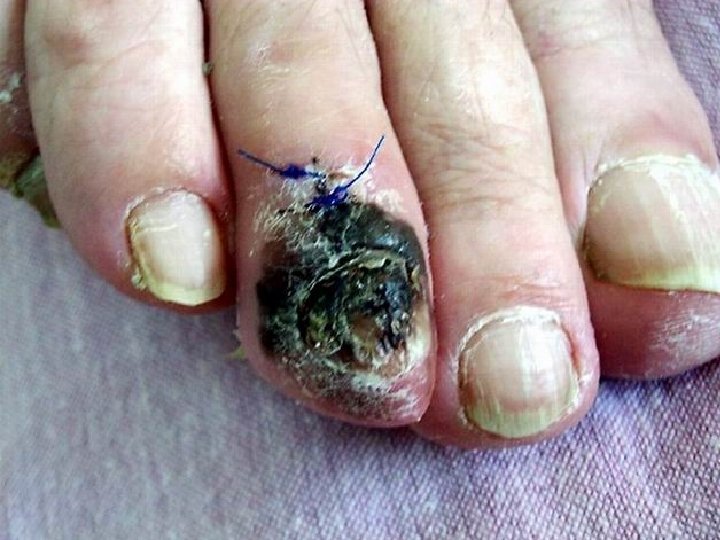
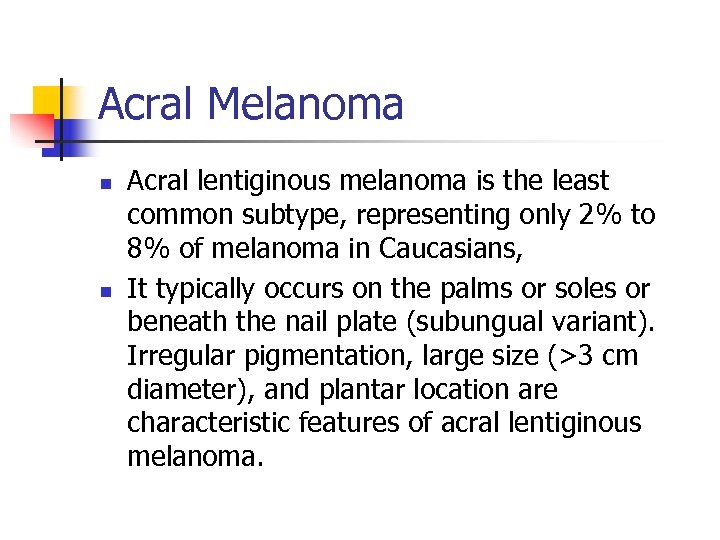 Acral Melanoma n n Acral lentiginous melanoma is the least common subtype, representing only 2% to 8% of melanoma in Caucasians, It typically occurs on the palms or soles or beneath the nail plate (subungual variant). Irregular pigmentation, large size (>3 cm diameter), and plantar location are characteristic features of acral lentiginous melanoma.
Acral Melanoma n n Acral lentiginous melanoma is the least common subtype, representing only 2% to 8% of melanoma in Caucasians, It typically occurs on the palms or soles or beneath the nail plate (subungual variant). Irregular pigmentation, large size (>3 cm diameter), and plantar location are characteristic features of acral lentiginous melanoma.
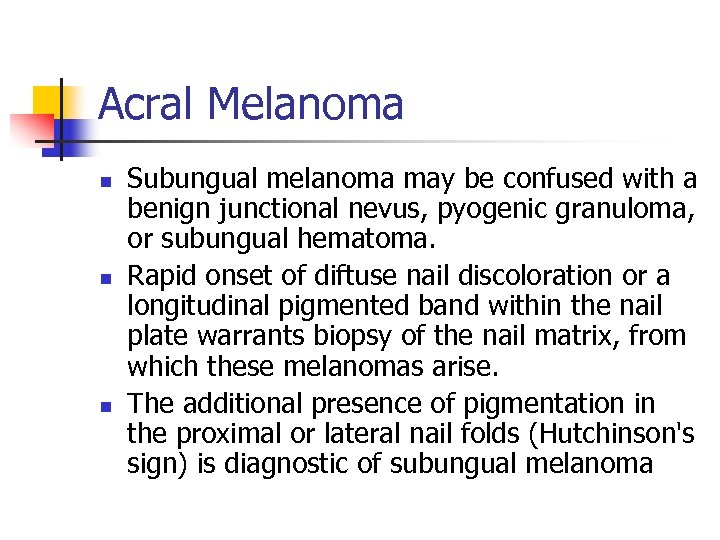 Acral Melanoma n n n Subungual melanoma may be confused with a benign junctional nevus, pyogenic granuloma, or subungual hematoma. Rapid onset of diftuse nail discoloration or a longitudinal pigmented band within the nail plate warrants biopsy of the nail matrix, from which these melanomas arise. The additional presence of pigmentation in the proximal or lateral nail folds (Hutchinson's sign) is diagnostic of subungual melanoma
Acral Melanoma n n n Subungual melanoma may be confused with a benign junctional nevus, pyogenic granuloma, or subungual hematoma. Rapid onset of diftuse nail discoloration or a longitudinal pigmented band within the nail plate warrants biopsy of the nail matrix, from which these melanomas arise. The additional presence of pigmentation in the proximal or lateral nail folds (Hutchinson's sign) is diagnostic of subungual melanoma
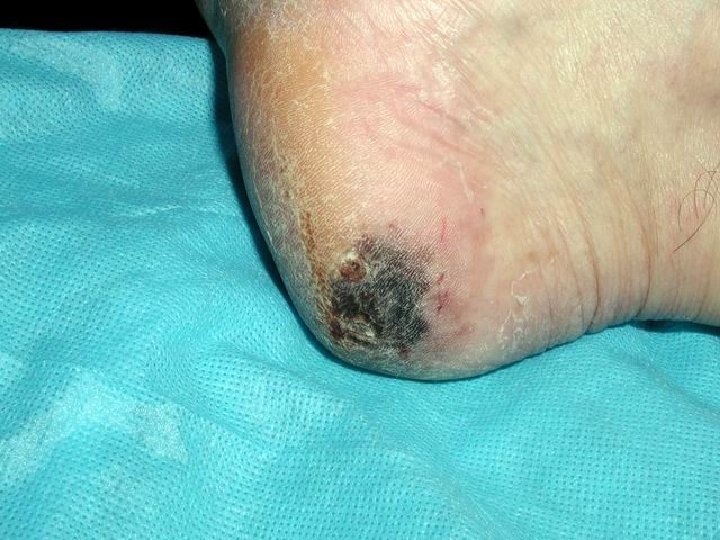
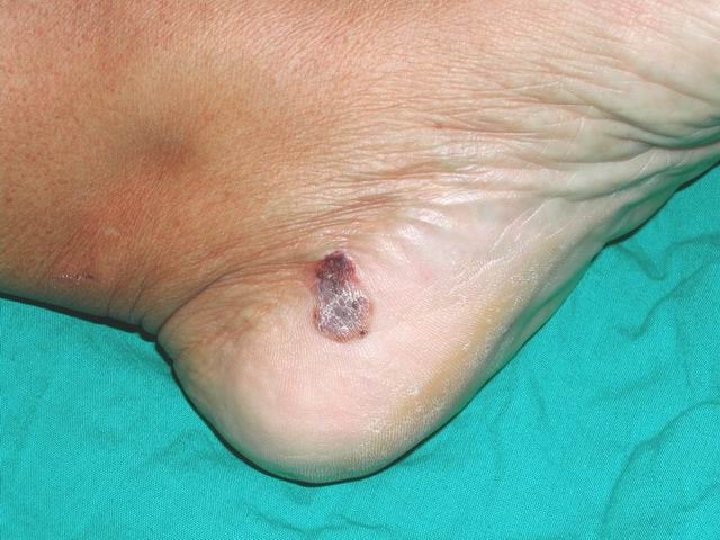
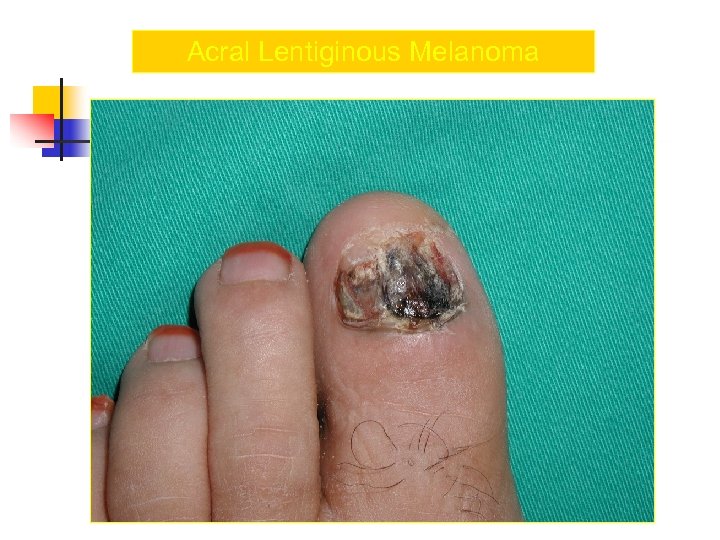 Acral Lentiginous Melanoma
Acral Lentiginous Melanoma
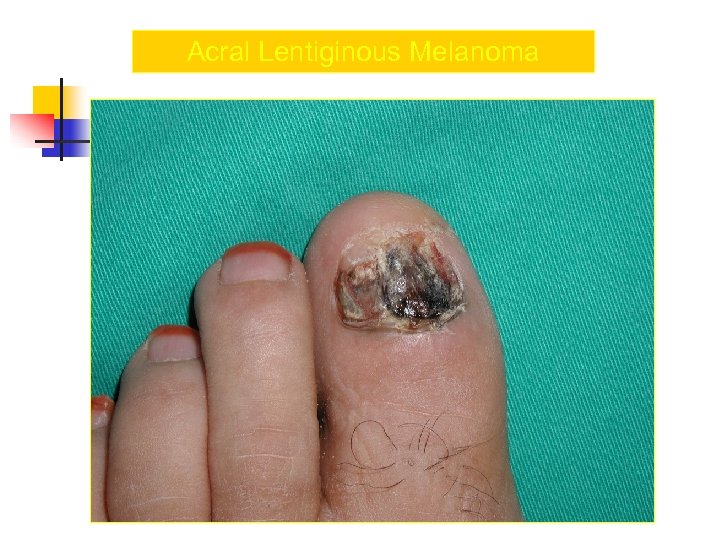 Acral Lentiginous Melanoma
Acral Lentiginous Melanoma
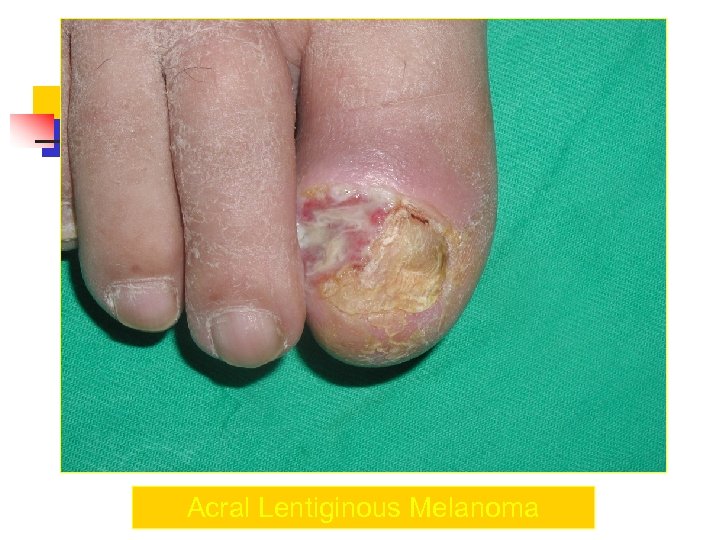 Acral Lentiginous Melanoma
Acral Lentiginous Melanoma
 Histopathologic Examination
Histopathologic Examination
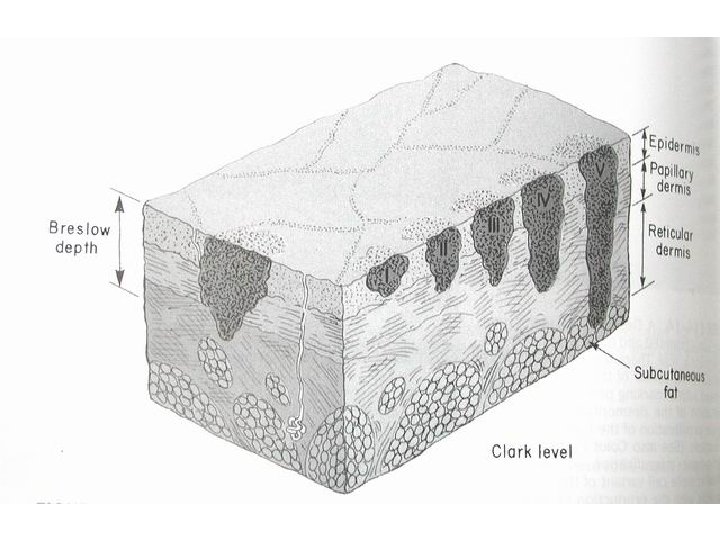
 The histopathologic classification of the Melanoma by Clark Spread of melanoma with the depth of invasion from its origin in the epidermis. There are five levels of invasion
The histopathologic classification of the Melanoma by Clark Spread of melanoma with the depth of invasion from its origin in the epidermis. There are five levels of invasion
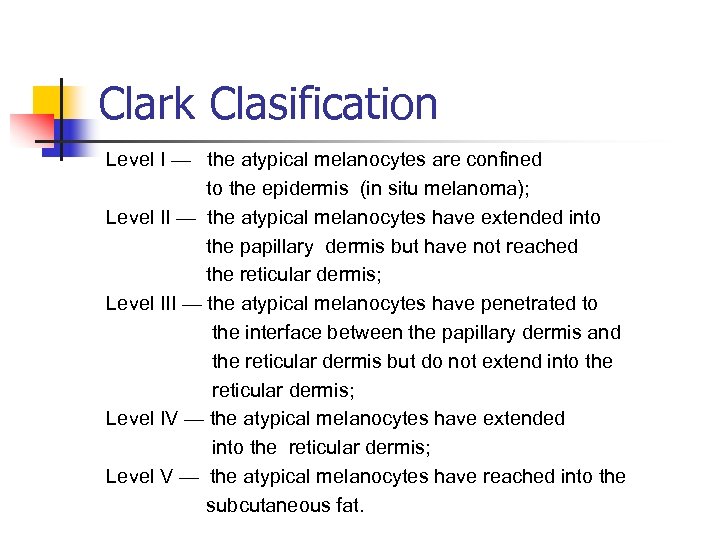 Clark Clasification Level I — the atypical melanocytes are confined to the epidermis (in situ melanoma); Level II — the atypical melanocytes have extended into the papillary dermis but have not reached the reticular dermis; Level III — the atypical melanocytes have penetrated to the interface between the papillary dermis and the reticular dermis but do not extend into the reticular dermis; Level IV — the atypical melanocytes have extended into the reticular dermis; Level V — the atypical melanocytes have reached into the subcutaneous fat.
Clark Clasification Level I — the atypical melanocytes are confined to the epidermis (in situ melanoma); Level II — the atypical melanocytes have extended into the papillary dermis but have not reached the reticular dermis; Level III — the atypical melanocytes have penetrated to the interface between the papillary dermis and the reticular dermis but do not extend into the reticular dermis; Level IV — the atypical melanocytes have extended into the reticular dermis; Level V — the atypical melanocytes have reached into the subcutaneous fat.
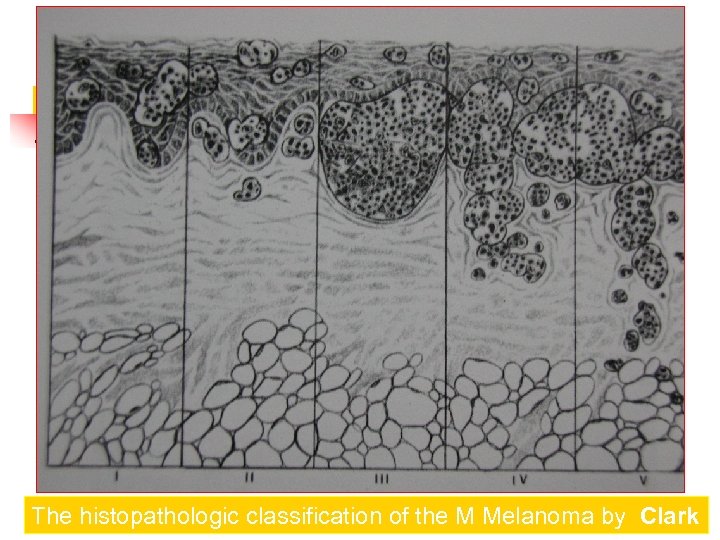 The histopathologic classification of the M Melanoma by Clark
The histopathologic classification of the M Melanoma by Clark
 The histopathologic classification by Breslow According to the thickness of the lesion as measured by an ocular micrometer from the top of the granular zone of the epidermis to the base of the neoplasm
The histopathologic classification by Breslow According to the thickness of the lesion as measured by an ocular micrometer from the top of the granular zone of the epidermis to the base of the neoplasm
 Breslow Classification n Level 1: less than 0. 76 mm thick n Level 2: between 0. 76– 1. 50 mm n Level 3: between 1. 50 - 4. 00 mm n Level 3: exceed 4. 00 mm in thickness
Breslow Classification n Level 1: less than 0. 76 mm thick n Level 2: between 0. 76– 1. 50 mm n Level 3: between 1. 50 - 4. 00 mm n Level 3: exceed 4. 00 mm in thickness
 Prognostic Factors n n n Breslow thickness (most important) Clark invasion level Ulceration Age, sex, location Size and surgical margins Others (Mitotic index, growth phase, regression. . . )
Prognostic Factors n n n Breslow thickness (most important) Clark invasion level Ulceration Age, sex, location Size and surgical margins Others (Mitotic index, growth phase, regression. . . )
 Staging
Staging
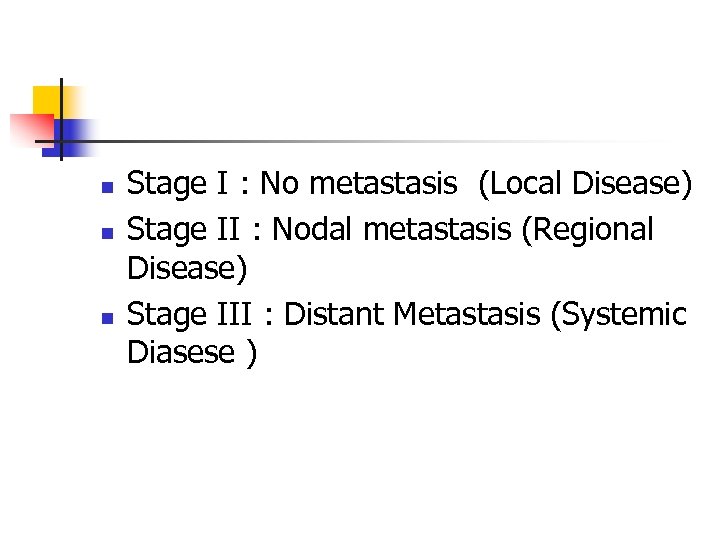 n n n Stage I : No metastasis (Local Disease) Stage II : Nodal metastasis (Regional Disease) Stage III : Distant Metastasis (Systemic Diasese )
n n n Stage I : No metastasis (Local Disease) Stage II : Nodal metastasis (Regional Disease) Stage III : Distant Metastasis (Systemic Diasese )
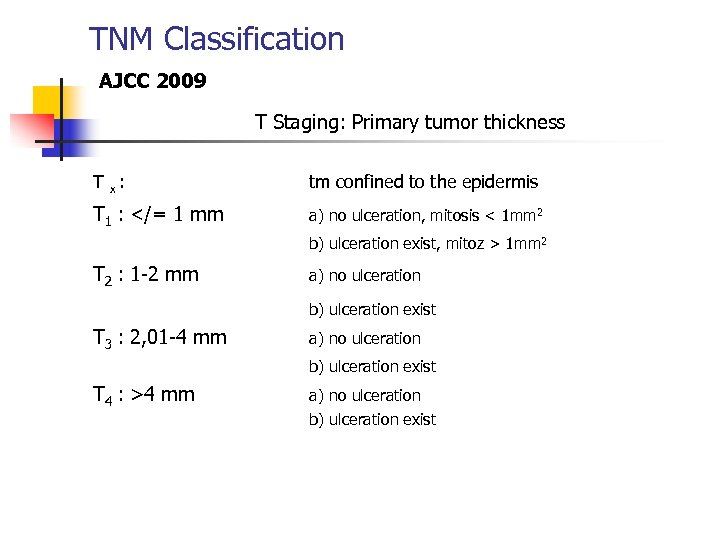 TNM Classification AJCC 2009 T Staging: Primary tumor thickness T x : tm confined to the epidermis T 1 : 1 mm 2 T 2 : 1 -2 mm a) no ulceration b) ulceration exist T 3 : 2, 01 -4 mm a) no ulceration b) ulceration exist T 4 : >4 mm a) no ulceration b) ulceration exist
TNM Classification AJCC 2009 T Staging: Primary tumor thickness T x : tm confined to the epidermis T 1 : 1 mm 2 T 2 : 1 -2 mm a) no ulceration b) ulceration exist T 3 : 2, 01 -4 mm a) no ulceration b) ulceration exist T 4 : >4 mm a) no ulceration b) ulceration exist
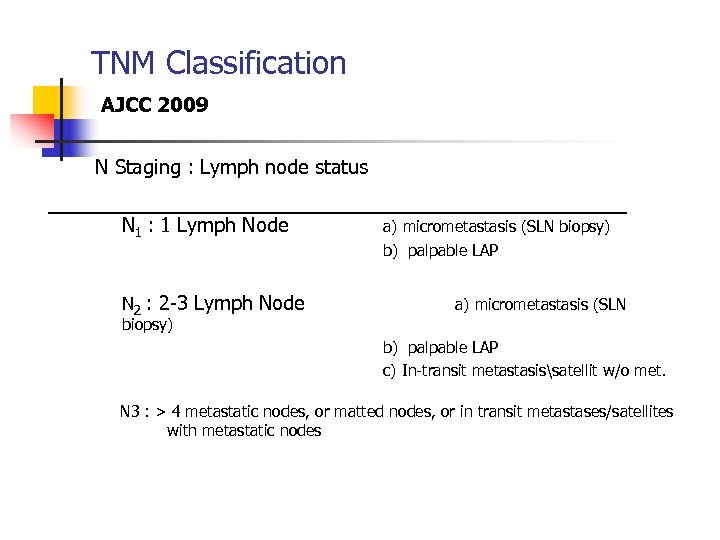 TNM Classification AJCC 2009 N Staging : Lymph node status N 1 : 1 Lymph Node a) micrometastasis (SLN biopsy) b) palpable LAP N 2 : 2 -3 Lymph Node a) micrometastasis (SLN biopsy) b) palpable LAP c) In-transit metastasissatellit w/o met. N 3 : > 4 metastatic nodes, or matted nodes, or in transit metastases/satellites with metastatic nodes
TNM Classification AJCC 2009 N Staging : Lymph node status N 1 : 1 Lymph Node a) micrometastasis (SLN biopsy) b) palpable LAP N 2 : 2 -3 Lymph Node a) micrometastasis (SLN biopsy) b) palpable LAP c) In-transit metastasissatellit w/o met. N 3 : > 4 metastatic nodes, or matted nodes, or in transit metastases/satellites with metastatic nodes
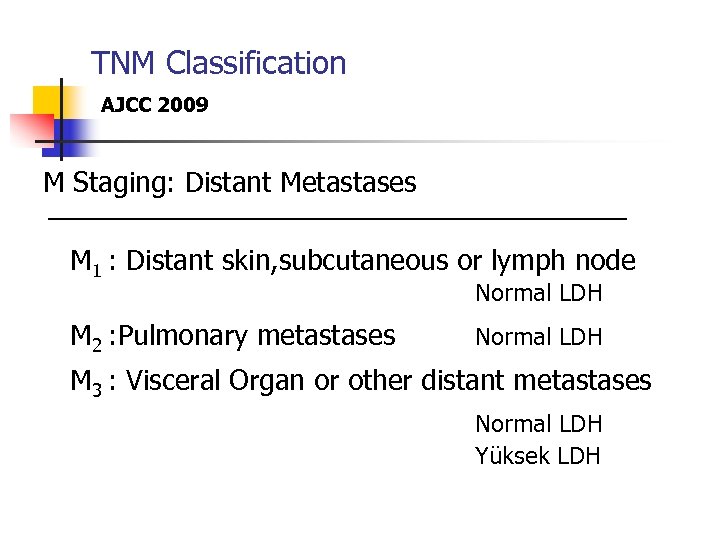 TNM Classification AJCC 2009 M Staging: Distant Metastases M 1 : Distant skin, subcutaneous or lymph node M 2 : Pulmonary metastases Normal LDH M 3 : Visceral Organ or other distant metastases Normal LDH Yüksek LDH
TNM Classification AJCC 2009 M Staging: Distant Metastases M 1 : Distant skin, subcutaneous or lymph node M 2 : Pulmonary metastases Normal LDH M 3 : Visceral Organ or other distant metastases Normal LDH Yüksek LDH
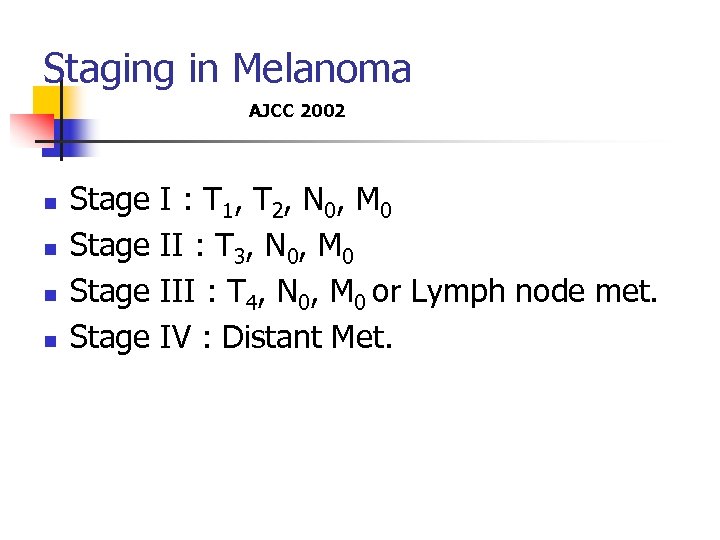 Staging in Melanoma AJCC 2002 n n Stage I : T 1, T 2, N 0, M 0 Stage II : T 3, N 0, M 0 Stage III : T 4, N 0, M 0 or Lymph node met. Stage IV : Distant Met.
Staging in Melanoma AJCC 2002 n n Stage I : T 1, T 2, N 0, M 0 Stage II : T 3, N 0, M 0 Stage III : T 4, N 0, M 0 or Lymph node met. Stage IV : Distant Met.
 Surgical Treatment
Surgical Treatment
 Surgical Treatment n n n Biopsy Wide Local Exsicion Staging with Sentinel Lymph Node biopsy Therapeutic Lymph Node Dissection Treatment of Distant Metastasis
Surgical Treatment n n n Biopsy Wide Local Exsicion Staging with Sentinel Lymph Node biopsy Therapeutic Lymph Node Dissection Treatment of Distant Metastasis
 Main treatment in melanoma is wide surgical excision of the tumor
Main treatment in melanoma is wide surgical excision of the tumor
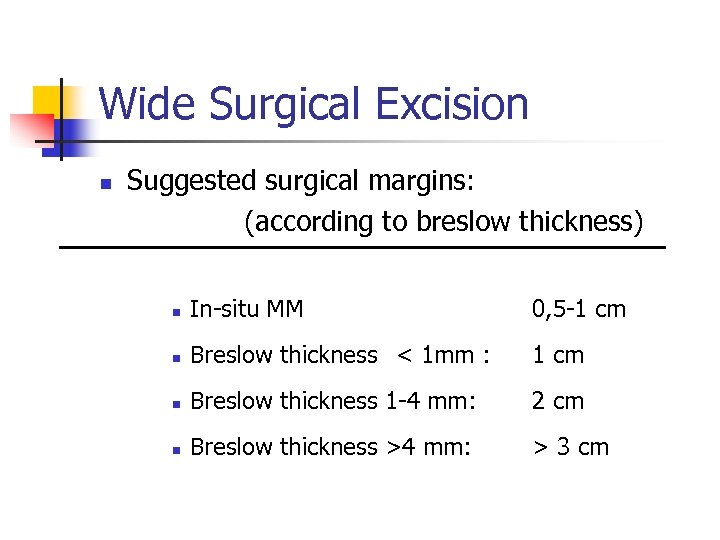 Wide Surgical Excision n Suggested surgical margins: (according to breslow thickness) n In-situ MM 0, 5 -1 cm n Breslow thickness < 1 mm : 1 cm n Breslow thickness 1 -4 mm: 2 cm n Breslow thickness >4 mm: > 3 cm
Wide Surgical Excision n Suggested surgical margins: (according to breslow thickness) n In-situ MM 0, 5 -1 cm n Breslow thickness < 1 mm : 1 cm n Breslow thickness 1 -4 mm: 2 cm n Breslow thickness >4 mm: > 3 cm
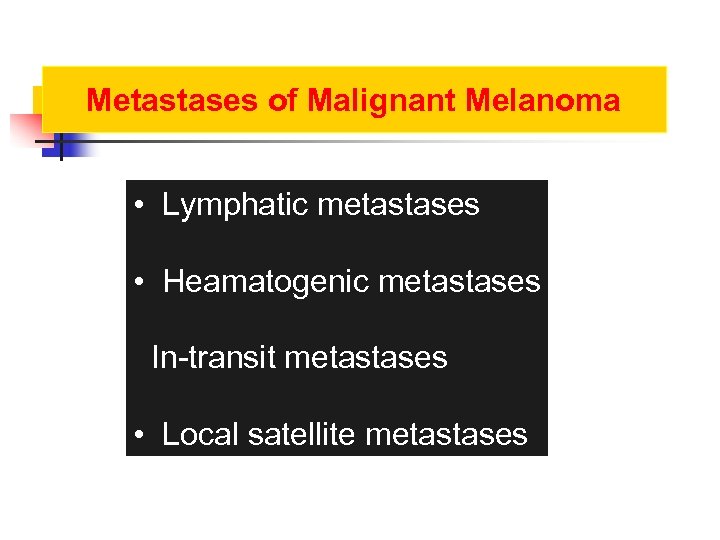 Metastases of Malignant Melanoma • Lymphatic metastases • Heamatogenic metastases In-transit metastases • Local satellite metastases
Metastases of Malignant Melanoma • Lymphatic metastases • Heamatogenic metastases In-transit metastases • Local satellite metastases
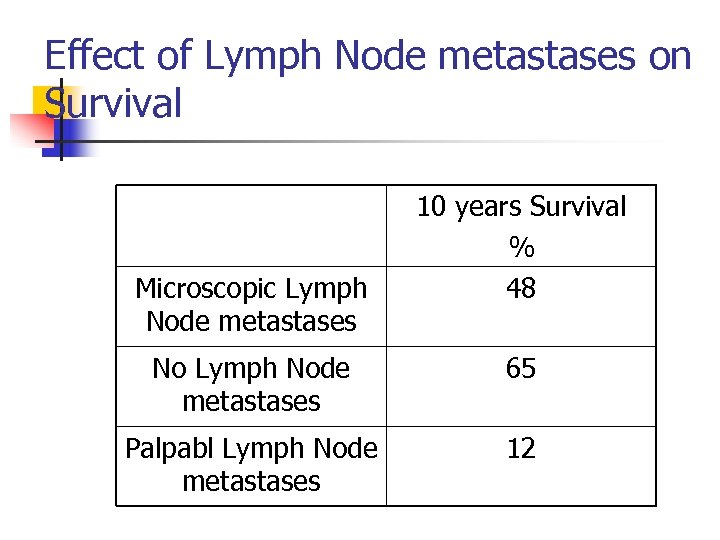 Effect of Lymph Node metastases on Survival Microscopic Lymph Node metastases 10 years Survival % 48 No Lymph Node metastases 65 Palpabl Lymph Node metastases 12
Effect of Lymph Node metastases on Survival Microscopic Lymph Node metastases 10 years Survival % 48 No Lymph Node metastases 65 Palpabl Lymph Node metastases 12
 Treatment of Lymph Nodes n n n Wait and see (no treatment) Elective Lymph Node Dissection Sentinel Lymphadenectomy
Treatment of Lymph Nodes n n n Wait and see (no treatment) Elective Lymph Node Dissection Sentinel Lymphadenectomy
 Elective Lymph Node Dissection n n Removing the regional lymph nodes for prevention in no clinical palpable lymph node There is no consensus on survival advantage of Elective Lymph Node Dissection (ELND) n Survival advantage has been shown on retrospective studies but not in progressive studies
Elective Lymph Node Dissection n n Removing the regional lymph nodes for prevention in no clinical palpable lymph node There is no consensus on survival advantage of Elective Lymph Node Dissection (ELND) n Survival advantage has been shown on retrospective studies but not in progressive studies
 Complications for ELND n Early: Wound healing problems, necrosis, and wound seperation n Infection n n Late: n Lymphedema
Complications for ELND n Early: Wound healing problems, necrosis, and wound seperation n Infection n n Late: n Lymphedema
 Sentinel Lymphadenectomy
Sentinel Lymphadenectomy
 Sentinel Lymphadenectomy n n n Morton introduced for Stage I Melanoma patients in 1992 Vital blue dye intradermal injection was used for lymphatic mapping Lymphatic drainage of primary tumor goes specific lymph node in regional lymph basin. This is the first node in the basin. This is called as sentinel lymph node
Sentinel Lymphadenectomy n n n Morton introduced for Stage I Melanoma patients in 1992 Vital blue dye intradermal injection was used for lymphatic mapping Lymphatic drainage of primary tumor goes specific lymph node in regional lymph basin. This is the first node in the basin. This is called as sentinel lymph node
 Sentinel Lymphadenectomy n n n Sentinel lymph node shows the regional node status If sentinel lymph node negative, others lymph nodes in the basin are also negative If sentinel lymph node contains tumor cells, It means disease spread to the regional nodal basin
Sentinel Lymphadenectomy n n n Sentinel lymph node shows the regional node status If sentinel lymph node negative, others lymph nodes in the basin are also negative If sentinel lymph node contains tumor cells, It means disease spread to the regional nodal basin
 Sentinel Lymphadenectomy n Sentinel node negative n n no additional treatment, follow the patient Sentinel lymph node positive n Therapeutic lymph node dissection
Sentinel Lymphadenectomy n Sentinel node negative n n no additional treatment, follow the patient Sentinel lymph node positive n Therapeutic lymph node dissection
 Multidiscipliner Team n n n Surgery Nuclear Medicine Pathology
Multidiscipliner Team n n n Surgery Nuclear Medicine Pathology
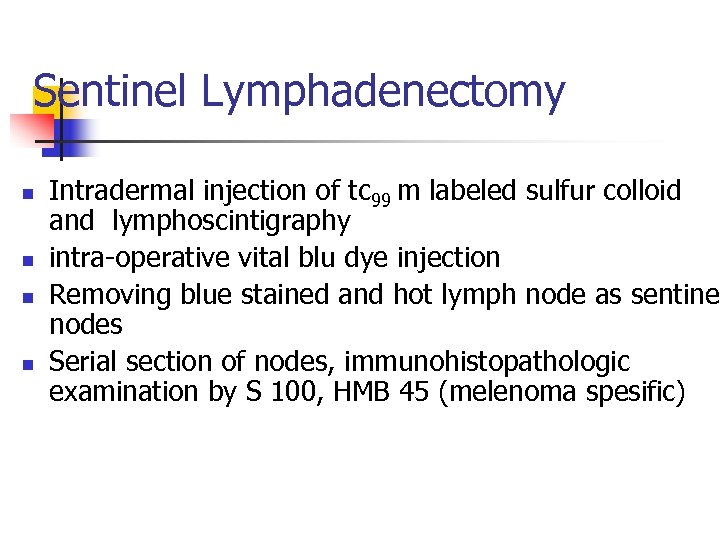 Sentinel Lymphadenectomy n n Intradermal injection of tc 99 m labeled sulfur colloid and lymphoscintigraphy intra-operative vital blu dye injection Removing blue stained and hot lymph node as sentinel nodes Serial section of nodes, immunohistopathologic examination by S 100, HMB 45 (melenoma spesific)
Sentinel Lymphadenectomy n n Intradermal injection of tc 99 m labeled sulfur colloid and lymphoscintigraphy intra-operative vital blu dye injection Removing blue stained and hot lymph node as sentinel nodes Serial section of nodes, immunohistopathologic examination by S 100, HMB 45 (melenoma spesific)
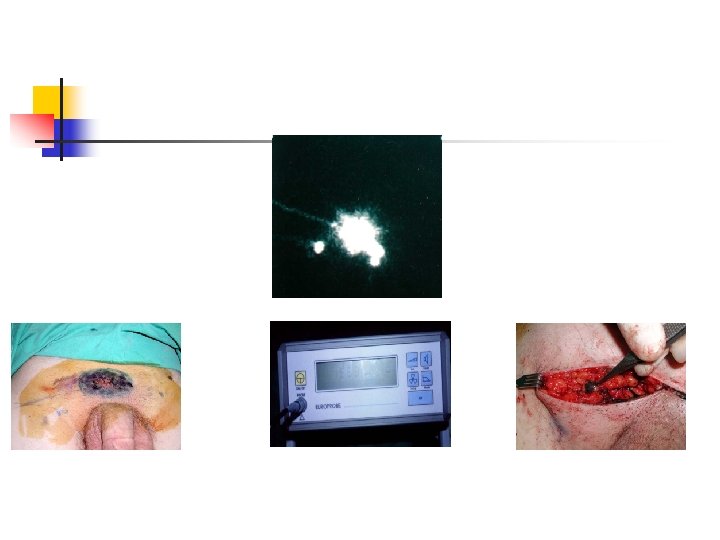
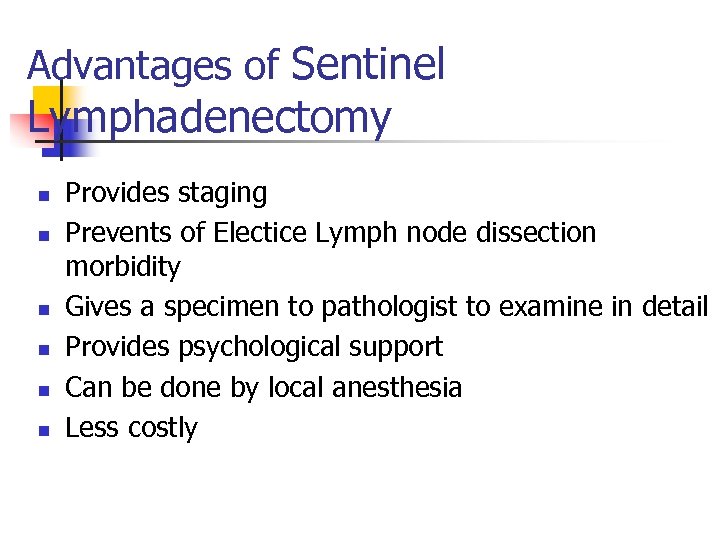 Advantages of Sentinel Lymphadenectomy n n n Provides staging Prevents of Electice Lymph node dissection morbidity Gives a specimen to pathologist to examine in detail Provides psychological support Can be done by local anesthesia Less costly
Advantages of Sentinel Lymphadenectomy n n n Provides staging Prevents of Electice Lymph node dissection morbidity Gives a specimen to pathologist to examine in detail Provides psychological support Can be done by local anesthesia Less costly
 Distant Metastases
Distant Metastases
 Sites of Distant Metastasis n n n n Skin Subcutaneous Tissue Distant Lymph Nodes Pulmonary Liver Brain Bone Intestine
Sites of Distant Metastasis n n n n Skin Subcutaneous Tissue Distant Lymph Nodes Pulmonary Liver Brain Bone Intestine
 Treatment of Distant Metastases n n Soliter skin and distant lymph nodes are removed İsoleted pulmonary metastases can be removed in selected cases Brain and GI metastases are removed for palliation Radiotherapy may be benefical for selected patients (mainly for pain treatment)
Treatment of Distant Metastases n n Soliter skin and distant lymph nodes are removed İsoleted pulmonary metastases can be removed in selected cases Brain and GI metastases are removed for palliation Radiotherapy may be benefical for selected patients (mainly for pain treatment)
 Treatment of Distant Metastases n n Avarege life expentancy is 6 -9 months There is no real treatment for Stage IV patients Most realistic goal is palliation and preservation of quality of life Experimental therapies may be considered in the first line therpeutic approach
Treatment of Distant Metastases n n Avarege life expentancy is 6 -9 months There is no real treatment for Stage IV patients Most realistic goal is palliation and preservation of quality of life Experimental therapies may be considered in the first line therpeutic approach
 Cases
Cases
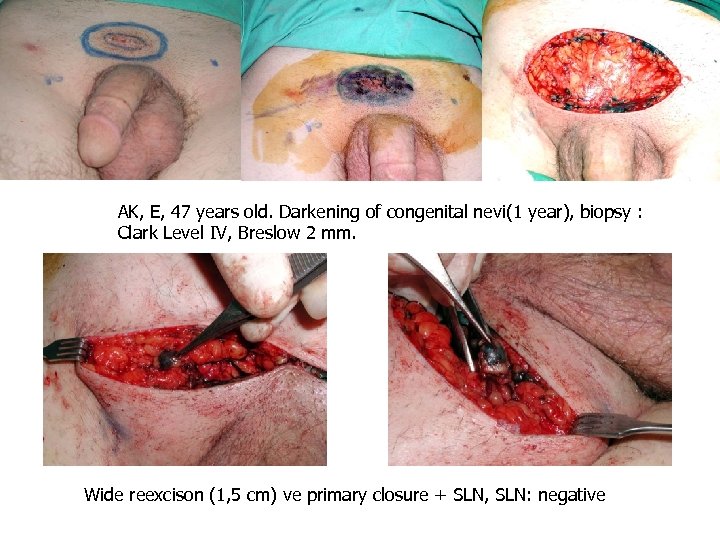 AK, E, 47 years old. Darkening of congenital nevi(1 year), biopsy : Clark Level IV, Breslow 2 mm. Wide reexcison (1, 5 cm) ve primary closure + SLN, SLN: negative
AK, E, 47 years old. Darkening of congenital nevi(1 year), biopsy : Clark Level IV, Breslow 2 mm. Wide reexcison (1, 5 cm) ve primary closure + SLN, SLN: negative
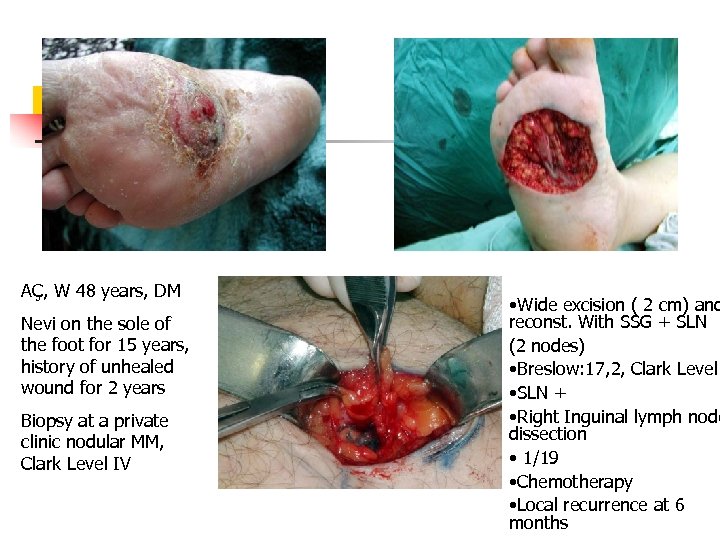 AÇ, W 48 years, DM Nevi on the sole of the foot for 15 years, history of unhealed wound for 2 years Biopsy at a private clinic nodular MM, Clark Level IV • Wide excision ( 2 cm) and reconst. With SSG + SLN (2 nodes) • Breslow: 17, 2, Clark Level • SLN + • Right Inguinal lymph node dissection • 1/19 • Chemotherapy • Local recurrence at 6 months
AÇ, W 48 years, DM Nevi on the sole of the foot for 15 years, history of unhealed wound for 2 years Biopsy at a private clinic nodular MM, Clark Level IV • Wide excision ( 2 cm) and reconst. With SSG + SLN (2 nodes) • Breslow: 17, 2, Clark Level • SLN + • Right Inguinal lymph node dissection • 1/19 • Chemotherapy • Local recurrence at 6 months
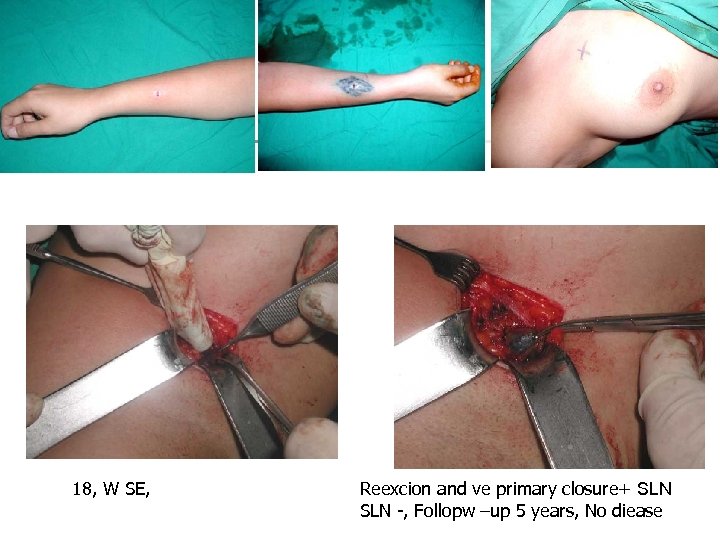 18, W SE, Reexcion and ve primary closure+ SLN -, Follopw –up 5 years, No diease
18, W SE, Reexcion and ve primary closure+ SLN -, Follopw –up 5 years, No diease


