44010aa30d3674ad4b79985c216d48b2.ppt
- Количество слайдов: 38

 Supporting multiple e. DC systems – the CRO dilemma Rob Nichols, Quintiles GDRU Director of Data Operations and Business Solutions CCRA @ UKTI 01 NOV 05
Supporting multiple e. DC systems – the CRO dilemma Rob Nichols, Quintiles GDRU Director of Data Operations and Business Solutions CCRA @ UKTI 01 NOV 05
 Outline 4 e. DC - desirable or reality 4 Outline of the potential problems 4 How can these issues be managed 1. Adapt to multiple systems 2. Drive forward in-house solution 4 Conclusion
Outline 4 e. DC - desirable or reality 4 Outline of the potential problems 4 How can these issues be managed 1. Adapt to multiple systems 2. Drive forward in-house solution 4 Conclusion
 e. DC – Definition 4 An electronic system (e. CRF) to directly capture data historically captured via traditional paper CRFs (note however that remote data entry is still used in 9/10 ‘e. DC’ studies) 4 An extension takes this to capturing data direct from devices (e. g. BP & ECG), merging in external data (e. g. laboratory data), using barcodes to capture subject’s id, nurses id, sample times and dates (the 3 -way swipe)
e. DC – Definition 4 An electronic system (e. CRF) to directly capture data historically captured via traditional paper CRFs (note however that remote data entry is still used in 9/10 ‘e. DC’ studies) 4 An extension takes this to capturing data direct from devices (e. g. BP & ECG), merging in external data (e. g. laboratory data), using barcodes to capture subject’s id, nurses id, sample times and dates (the 3 -way swipe)
 e. DC Trial Uptake - desirable The benefits of 4 Full engagement for all in clinical process with data availability in real time 4 Quicker lock / report 4 Lower direct costs (over 30 subject trial) 4 Lower indirect costs (quicker to market / quicker to kill) are leading to the push towards e. DC replacing paper solutions
e. DC Trial Uptake - desirable The benefits of 4 Full engagement for all in clinical process with data availability in real time 4 Quicker lock / report 4 Lower direct costs (over 30 subject trial) 4 Lower indirect costs (quicker to market / quicker to kill) are leading to the push towards e. DC replacing paper solutions
 e. DC Trial Uptake – the dream Hypothetical indirect savings for big pharma with integrated protocol (4 parts) 1 week delay for significant new product = £ 19. 2 M* Saving per part from LPO to Lock = up to 3 weeks Total saving = 12 weeks =£ 229 M =$447 M Smaller companies benefit from a quicker sell / investment = improved cash flow * Source UKTI and CCRA survey on UK CRO industry
e. DC Trial Uptake – the dream Hypothetical indirect savings for big pharma with integrated protocol (4 parts) 1 week delay for significant new product = £ 19. 2 M* Saving per part from LPO to Lock = up to 3 weeks Total saving = 12 weeks =£ 229 M =$447 M Smaller companies benefit from a quicker sell / investment = improved cash flow * Source UKTI and CCRA survey on UK CRO industry
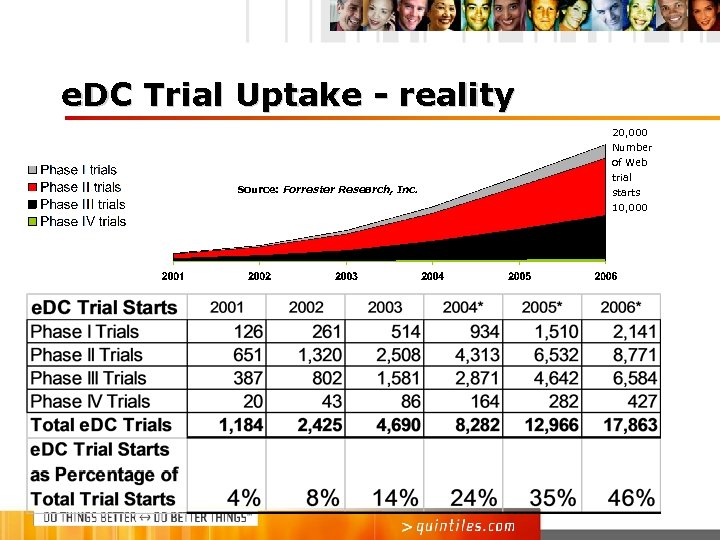 e. DC Trial Uptake - reality 20, 000 Number Source: Forrester Research, Inc. of Web trial starts 10, 000
e. DC Trial Uptake - reality 20, 000 Number Source: Forrester Research, Inc. of Web trial starts 10, 000
 e. DC Trial Uptake - reality Centre watch (03) estimated around 40% in 2004 would use e. DC CDISC (04 survey) – 30% of responders trials were using e. DC
e. DC Trial Uptake - reality Centre watch (03) estimated around 40% in 2004 would use e. DC CDISC (04 survey) – 30% of responders trials were using e. DC
 Outline 4 e. DC - desirable or reality 4 Outline of the potential problems 4 How can these issues be managed 1. Adapt to multiple systems 2. Drive forward in-house solution 4 Conclusion
Outline 4 e. DC - desirable or reality 4 Outline of the potential problems 4 How can these issues be managed 1. Adapt to multiple systems 2. Drive forward in-house solution 4 Conclusion
 Phase I - Definition Will vary but here built around: 4 A dedicated Phase I/IIa clinical unit 4 Majority of work is first in man 4 75 beds in 4 wards 4 60 protocols per year 4 CRO Content of presentation should though be widely applicable
Phase I - Definition Will vary but here built around: 4 A dedicated Phase I/IIa clinical unit 4 Majority of work is first in man 4 75 beds in 4 wards 4 60 protocols per year 4 CRO Content of presentation should though be widely applicable
 Clinical e. DC experience 4 Quintiles GDRU has been running sponsor-driven Phase I e. DC studies for around 6 years (30+ to date) 4 Partnered a pharmaceutical industry leader as they have developed and delivered 3 e. DC systems 4 Have developed our own e. DC solution (e. Phase I Lite) with around 15 studies won over the last 9 months
Clinical e. DC experience 4 Quintiles GDRU has been running sponsor-driven Phase I e. DC studies for around 6 years (30+ to date) 4 Partnered a pharmaceutical industry leader as they have developed and delivered 3 e. DC systems 4 Have developed our own e. DC solution (e. Phase I Lite) with around 15 studies won over the last 9 months
 Current clinical position 4 Staff enjoy using e. DC and clinically it works well 4 How to escalate and meet future demand
Current clinical position 4 Staff enjoy using e. DC and clinically it works well 4 How to escalate and meet future demand
 Differing Systems – the CRO Dilemma 4 Pharma want to commit to one approach 4 Pharma companies work with CRO 4 CRO have multiple systems dilemma… 4 An extreme example is a CRO phase I clinical unit (also an issue for clinical units working on multiple phase III studies)
Differing Systems – the CRO Dilemma 4 Pharma want to commit to one approach 4 Pharma companies work with CRO 4 CRO have multiple systems dilemma… 4 An extreme example is a CRO phase I clinical unit (also an issue for clinical units working on multiple phase III studies)
 Differing Systems – the CRO Dilemma 4 There are currently an estimated 200 different suppliers of ‘e. DC’ solutions 4 This has chaotic implications for a CRO working with 40 different sponsors a year if each sponsor arrives with a different e. DC solution
Differing Systems – the CRO Dilemma 4 There are currently an estimated 200 different suppliers of ‘e. DC’ solutions 4 This has chaotic implications for a CRO working with 40 different sponsors a year if each sponsor arrives with a different e. DC solution
 Differing Systems – the CRO Dilemma Training 4 Staff require in-depth familiarity with multiple types of software (all [~70] clinical staff are trained in all systems) 4 ½ - 5 days per system for generic training and also study specific training
Differing Systems – the CRO Dilemma Training 4 Staff require in-depth familiarity with multiple types of software (all [~70] clinical staff are trained in all systems) 4 ½ - 5 days per system for generic training and also study specific training
 Differing Systems – the CRO Dilemma Logistics 4 Ever increasing number of passwords to be remembered (and ~$200 a reset!) User Support 4 Need to be familiar with the contact details and working processes for several external helpdesks
Differing Systems – the CRO Dilemma Logistics 4 Ever increasing number of passwords to be remembered (and ~$200 a reset!) User Support 4 Need to be familiar with the contact details and working processes for several external helpdesks
 Differing Systems – the CRO Dilemma Resource 4 Where remote data entry is used data entry support required (clinical, secretarial/admin, temporary staff? ) 4 Role of project manager can cross over with traditional data manager roles 4 Depending on technology local involvement of IT
Differing Systems – the CRO Dilemma Resource 4 Where remote data entry is used data entry support required (clinical, secretarial/admin, temporary staff? ) 4 Role of project manager can cross over with traditional data manager roles 4 Depending on technology local involvement of IT
 Differing Systems – the CRO Dilemma IT 4 Regulatory compliance of systems 4 Security may be an issue if sponsors place their infrastructure (e. g. server, network, PCs) into the clinical unit 4 Interaction with current systems (cardiac monitoring) 4 Regular access for sponsor staff may be required 4 Space may be an issue with new servers, networks, and 15+ collection devices per project
Differing Systems – the CRO Dilemma IT 4 Regulatory compliance of systems 4 Security may be an issue if sponsors place their infrastructure (e. g. server, network, PCs) into the clinical unit 4 Interaction with current systems (cardiac monitoring) 4 Regular access for sponsor staff may be required 4 Space may be an issue with new servers, networks, and 15+ collection devices per project
 Outline 4 e. DC - desirable or reality 4 Outline of the potential problems 4 How can these issues be managed 1. Adapt to multiple systems 2. Drive forward in-house solution 4 Conclusion
Outline 4 e. DC - desirable or reality 4 Outline of the potential problems 4 How can these issues be managed 1. Adapt to multiple systems 2. Drive forward in-house solution 4 Conclusion
 Differing Systems – the CRO solution Current strategy Two pronged approach 1 – support ‘numerous’ sponsor driven solutions 2 – develop an in-house solution
Differing Systems – the CRO solution Current strategy Two pronged approach 1 – support ‘numerous’ sponsor driven solutions 2 – develop an in-house solution
 Outline 4 e. DC - desirable or reality 4 Outline of the potential problems 4 How can these issues be managed 1. Adapt to multiple systems 2. Drive forward in-house solution 4 Conclusion
Outline 4 e. DC - desirable or reality 4 Outline of the potential problems 4 How can these issues be managed 1. Adapt to multiple systems 2. Drive forward in-house solution 4 Conclusion
 Support sponsor driven solutions + The industry will move towards fewer standard e. DC software solutions (as with SAS®, Clintrial®, Oracle Clinical®)
Support sponsor driven solutions + The industry will move towards fewer standard e. DC software solutions (as with SAS®, Clintrial®, Oracle Clinical®)
 Support sponsor driven solutions + Will tend to be driven by bigger companies who can bring multiple studies, allowing learning curve (training, new procedures) to be reused + Data collection part of software is standardising - Systems may now all look very similar but way sponsors are using them still tend to differ (even same software used in different ways)
Support sponsor driven solutions + Will tend to be driven by bigger companies who can bring multiple studies, allowing learning curve (training, new procedures) to be reused + Data collection part of software is standardising - Systems may now all look very similar but way sponsors are using them still tend to differ (even same software used in different ways)
 Support sponsor driven solutions - May put a lot of resource into educating the sponsor, and vendor (knowledge transfer) + Internet based solutions will help address the space required as can run all systems off same hardware + Increased acceptance of e. Source + Biometric passwords
Support sponsor driven solutions - May put a lot of resource into educating the sponsor, and vendor (knowledge transfer) + Internet based solutions will help address the space required as can run all systems off same hardware + Increased acceptance of e. Source + Biometric passwords
 Support sponsor driven solutions Portals Develop central interface through which both CRO and sponsor can access multiple products and studies 4 One password per user 4 Access restricted 4 Easier management of multiple e. DC systems
Support sponsor driven solutions Portals Develop central interface through which both CRO and sponsor can access multiple products and studies 4 One password per user 4 Access restricted 4 Easier management of multiple e. DC systems
 Support sponsor driven solutions Potential support available House server Supply/support network/hardware Database build for non supported software e. Source or paper source (remote data entry) Staff available for training Query management Query resolution ? Data management Backend services (data conversion, SAS, statistics, medical writing)
Support sponsor driven solutions Potential support available House server Supply/support network/hardware Database build for non supported software e. Source or paper source (remote data entry) Staff available for training Query management Query resolution ? Data management Backend services (data conversion, SAS, statistics, medical writing)
 Support sponsor driven solutions Strategy Manage the sponsors expectations. Put in place an internal implementation team who focus the sponsors on standardising where possible. 4 Input into e. CRF design 4 Use CRO infrastructure 4 Training – timing (inc for shift staff) and content 4 Query management
Support sponsor driven solutions Strategy Manage the sponsors expectations. Put in place an internal implementation team who focus the sponsors on standardising where possible. 4 Input into e. CRF design 4 Use CRO infrastructure 4 Training – timing (inc for shift staff) and content 4 Query management
 Outline 4 e. DC - desirable or reality 4 Outline of the potential problems 4 How can these issues be managed 1. Adapt to multiple systems 2. Drive forward in-house solution 4 Conclusion
Outline 4 e. DC - desirable or reality 4 Outline of the potential problems 4 How can these issues be managed 1. Adapt to multiple systems 2. Drive forward in-house solution 4 Conclusion
 In-house e. DC solution - remit 4 Processes to fit the rapid and flexible Phase I (not Phase III) environment 4 Direct data capture at bedside 4 High quality data collected in real time 4 Secure, on demand, online access to data for all role players 4 Set-up times that do not impact on clinical start 4 Timelines ‘dramatically’ reduced after lastpatient-out
In-house e. DC solution - remit 4 Processes to fit the rapid and flexible Phase I (not Phase III) environment 4 Direct data capture at bedside 4 High quality data collected in real time 4 Secure, on demand, online access to data for all role players 4 Set-up times that do not impact on clinical start 4 Timelines ‘dramatically’ reduced after lastpatient-out
 In-house e. DC solution We went for 4 Database and edit checks on a central server, use internet browser to connect to server via tablet PCs and a wireless network 4 Phase. Forward’s In. Formtm 4 An Industry Leader in e. DC technology 4 But processes are software independent
In-house e. DC solution We went for 4 Database and edit checks on a central server, use internet browser to connect to server via tablet PCs and a wireless network 4 Phase. Forward’s In. Formtm 4 An Industry Leader in e. DC technology 4 But processes are software independent

 Develop in-house solution + Will be utilized by smaller/medium sponsors who will not have own solution + Efficiencies of scale bring down price (less than paper) and setup timelines (match MHRA) + Processes will be repeated on each study ensuring high quality levels from staff very familiar with system - Can we motivate bigger companies to adopt
Develop in-house solution + Will be utilized by smaller/medium sponsors who will not have own solution + Efficiencies of scale bring down price (less than paper) and setup timelines (match MHRA) + Processes will be repeated on each study ensuring high quality levels from staff very familiar with system - Can we motivate bigger companies to adopt
 Develop in-house solution Strategy Motivate sponsors to take in-house solution. How Key requirement for most sponsors is how final delivered data look (CDISC or sponsor format), not behind the scene processes. => Get all benefits of e. DC with focus on saving of several weeks from LPO to report, data in required format and lower costs…
Develop in-house solution Strategy Motivate sponsors to take in-house solution. How Key requirement for most sponsors is how final delivered data look (CDISC or sponsor format), not behind the scene processes. => Get all benefits of e. DC with focus on saving of several weeks from LPO to report, data in required format and lower costs…
 Outline 4 e. DC - desirable or reality 4 Outline of the potential problems 4 How can these issues be managed 1. Adapt to multiple systems 2. Drive forward in-house solution 4 Conclusion
Outline 4 e. DC - desirable or reality 4 Outline of the potential problems 4 How can these issues be managed 1. Adapt to multiple systems 2. Drive forward in-house solution 4 Conclusion
 Conclusion 4 In-house solution will be utilized by the majority of sponsors. 4 A flexible approach to those sponsors who wish to implement their own solutions - while trying to develop a level of standardisation in approach across different systems/sponsors. 4 With the increasing number of preferred agreements, hybrids may well develop – i. e. sponsor specific in-house solutions (or inhouse sponsor specific solution).
Conclusion 4 In-house solution will be utilized by the majority of sponsors. 4 A flexible approach to those sponsors who wish to implement their own solutions - while trying to develop a level of standardisation in approach across different systems/sponsors. 4 With the increasing number of preferred agreements, hybrids may well develop – i. e. sponsor specific in-house solutions (or inhouse sponsor specific solution).
 © Quintiles Limited 2005 rob. nichols@quintiles. com
© Quintiles Limited 2005 rob. nichols@quintiles. com
 e. DC solution – which software Still a very large number of potential vendors with fragile existences. Assess what will work best in local environment. 4 Long term stability 4 Still riding the Dot-com vision of fast profit, short duration 4 Consolidation, acquisition, bankruptcy of e. DC vendors 4 A partner to grow with you
e. DC solution – which software Still a very large number of potential vendors with fragile existences. Assess what will work best in local environment. 4 Long term stability 4 Still riding the Dot-com vision of fast profit, short duration 4 Consolidation, acquisition, bankruptcy of e. DC vendors 4 A partner to grow with you
 e. DC solution – which software 4 Current customer base 4 Previous experience – total trials 4 Check regulatory compliance 4 Front ends are harmonising but unreliable products still exist 4 Different functionality, export/reporting tools, approaches to pricing
e. DC solution – which software 4 Current customer base 4 Previous experience – total trials 4 Check regulatory compliance 4 Front ends are harmonising but unreliable products still exist 4 Different functionality, export/reporting tools, approaches to pricing


