ee87d73b13d4b05af207a08d5f3b1118.ppt
- Количество слайдов: 26
 Sue Gregory Audit of IT Systems SARQA / DKG Scandinavian Conference, October 2002, Copenhagen Sue Gregory, Genmab A/S, October 2002
Sue Gregory Audit of IT Systems SARQA / DKG Scandinavian Conference, October 2002, Copenhagen Sue Gregory, Genmab A/S, October 2002
 Purpose of IT System Audit • To assure that established standards are met for all phases of the validation, operation and maintenance of computerised systems. • To monitor the Gx. P compliance of computerised systems. Sue Gregory, Genmab A/S, October 2002 2
Purpose of IT System Audit • To assure that established standards are met for all phases of the validation, operation and maintenance of computerised systems. • To monitor the Gx. P compliance of computerised systems. Sue Gregory, Genmab A/S, October 2002 2
 Types of IT System Audit • "Spot Check" – not an audit in its own right, but • • conducted as part of a facilities-type audit Vertical – (specific) looks at defined elements in great depth Horizontal – (general) looks at the entire system but in less depth Or maybe combination – review of the entire system in general and then specific elements in depth Sue Gregory, Genmab A/S, October 2002 3
Types of IT System Audit • "Spot Check" – not an audit in its own right, but • • conducted as part of a facilities-type audit Vertical – (specific) looks at defined elements in great depth Horizontal – (general) looks at the entire system but in less depth Or maybe combination – review of the entire system in general and then specific elements in depth Sue Gregory, Genmab A/S, October 2002 3
 IT System Audit - Auditor Requirements • Auditing skills • Knowledge of applicable regulations and • • • regulatory expectations Knowledge of computer system validation process Knowledge of software development life cycle (SDLC) Technical IT skills / knowledge Sue Gregory, Genmab A/S, October 2002 4
IT System Audit - Auditor Requirements • Auditing skills • Knowledge of applicable regulations and • • • regulatory expectations Knowledge of computer system validation process Knowledge of software development life cycle (SDLC) Technical IT skills / knowledge Sue Gregory, Genmab A/S, October 2002 4
 Some applicable regulations and references • GLP Consensus document, The application of the principles of GLP to computerised systems, environment monograph 116, OECD 1995 • Rules governing medicinal products in the European Community, Volume 4 Annex 11, computerised systems, Eudralex. • 21 CFR part 11 Electronic Records; Electronic Signatures, Final Rule, FDA 1997 • Guidance for Industry, Computerized Systems used in Clinical Trials, FDA 1999. Sue Gregory, Genmab A/S, October 2002 5
Some applicable regulations and references • GLP Consensus document, The application of the principles of GLP to computerised systems, environment monograph 116, OECD 1995 • Rules governing medicinal products in the European Community, Volume 4 Annex 11, computerised systems, Eudralex. • 21 CFR part 11 Electronic Records; Electronic Signatures, Final Rule, FDA 1997 • Guidance for Industry, Computerized Systems used in Clinical Trials, FDA 1999. Sue Gregory, Genmab A/S, October 2002 5
 Some applicable regulations and references • PDA Journal of Pharmaceutical Science and Technology, Technical Report No 31 – Validation and Qualification of Computerized Laboratory Data Acquisition Systems, 1999 supplement, Volume 53, Number 4 • GAMP guide for validation of automated systems in Pharmaceutical Manufacture, version 4, GAMP forum, 2001 • International Standard, ISO/IEC 12207 – Information Technology – Software life cycle processes, 1995 and amendment 1, 2002 • Guidance for industry, General principles of software validation; final guidance for Industry and FDA staff, FDA, 2002 Sue Gregory, Genmab A/S, October 2002 6
Some applicable regulations and references • PDA Journal of Pharmaceutical Science and Technology, Technical Report No 31 – Validation and Qualification of Computerized Laboratory Data Acquisition Systems, 1999 supplement, Volume 53, Number 4 • GAMP guide for validation of automated systems in Pharmaceutical Manufacture, version 4, GAMP forum, 2001 • International Standard, ISO/IEC 12207 – Information Technology – Software life cycle processes, 1995 and amendment 1, 2002 • Guidance for industry, General principles of software validation; final guidance for Industry and FDA staff, FDA, 2002 Sue Gregory, Genmab A/S, October 2002 6
 Some applicable regulations and references • And of course: – Any relevant internal policies, guidelines and procedures Bear in mind that the area is evolving and new interpretations are frequent. Monitor the literature and relevant websites for current developments, e. g. : – – FDA warning letters, GMP trends etc www. crsc. nist. gov/publications/nistpubs/index. html www. pda. org/techdocs/index. html www. groups. yahoo. com/group/21 cfrpart 11/messages Sue Gregory, Genmab A/S, October 2002 7
Some applicable regulations and references • And of course: – Any relevant internal policies, guidelines and procedures Bear in mind that the area is evolving and new interpretations are frequent. Monitor the literature and relevant websites for current developments, e. g. : – – FDA warning letters, GMP trends etc www. crsc. nist. gov/publications/nistpubs/index. html www. pda. org/techdocs/index. html www. groups. yahoo. com/group/21 cfrpart 11/messages Sue Gregory, Genmab A/S, October 2002 7
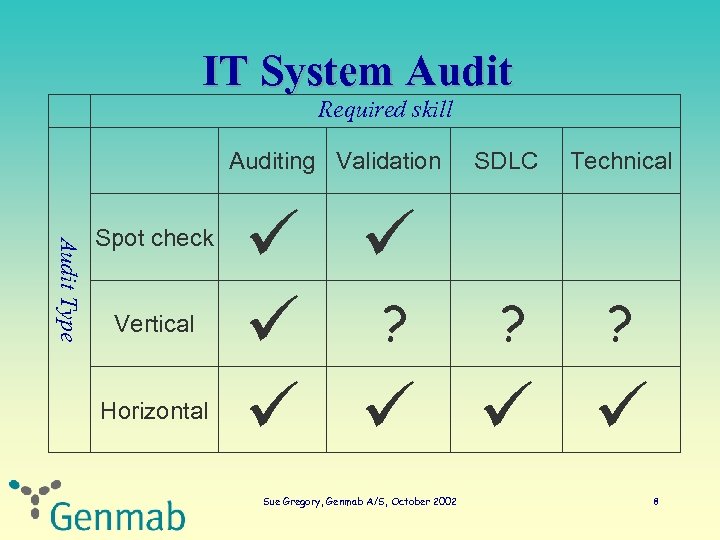 IT System Audit Required skill Auditing Validation Audit Type Spot check Vertical Horizontal SDLC Technical ? ? ? Sue Gregory, Genmab A/S, October 2002 8
IT System Audit Required skill Auditing Validation Audit Type Spot check Vertical Horizontal SDLC Technical ? ? ? Sue Gregory, Genmab A/S, October 2002 8
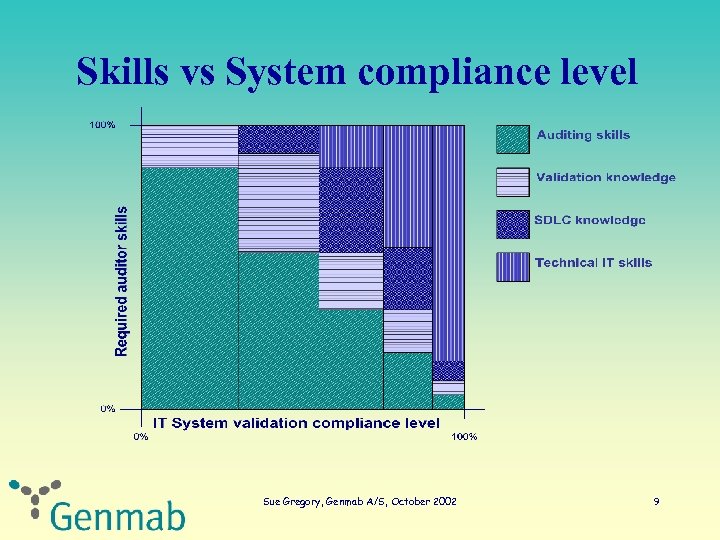 Skills vs System compliance level Sue Gregory, Genmab A/S, October 2002 9
Skills vs System compliance level Sue Gregory, Genmab A/S, October 2002 9
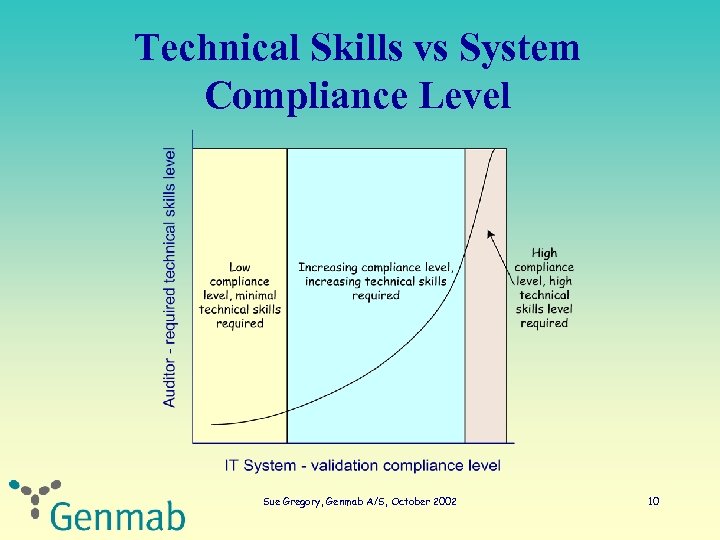 Technical Skills vs System Compliance Level Sue Gregory, Genmab A/S, October 2002 10
Technical Skills vs System Compliance Level Sue Gregory, Genmab A/S, October 2002 10
 Software Development considerations • Same standards apply to purchased software • • • and software developed in-house Documented SDLC; followed Documented specification of requirements for the system; fully traceable Documented specifications of functionality and design; fully traceable Documented standards for coding; followed Documented testing by supplier; unit, integration and system level Sue Gregory, Genmab A/S, October 2002 11
Software Development considerations • Same standards apply to purchased software • • • and software developed in-house Documented SDLC; followed Documented specification of requirements for the system; fully traceable Documented specifications of functionality and design; fully traceable Documented standards for coding; followed Documented testing by supplier; unit, integration and system level Sue Gregory, Genmab A/S, October 2002 11
 Approach to IT system "Spot Check" • Determine implementation date • Ascertain whethere is a validation report, • • check date, authorisation and conclusion Ascertain whethere is a log of changes since the implementation date Obtain a list of SOPs related to the system, ascertain that these are authorised and cover use, maintenance, ……… etc. Sue Gregory, Genmab A/S, October 2002 12
Approach to IT system "Spot Check" • Determine implementation date • Ascertain whethere is a validation report, • • check date, authorisation and conclusion Ascertain whethere is a log of changes since the implementation date Obtain a list of SOPs related to the system, ascertain that these are authorised and cover use, maintenance, ……… etc. Sue Gregory, Genmab A/S, October 2002 12
 Horizontal IT audit - basics • User / System Requirements Specification “It is not possible to validate software without predetermined and documented software requirements” FDA, principles of software validation, 2002 – Authorised (internally) and chronologically correct – Precise requirements covering all functions the system will perform – Uniquely identified – Verifiable Sue Gregory, Genmab A/S, October 2002 13
Horizontal IT audit - basics • User / System Requirements Specification “It is not possible to validate software without predetermined and documented software requirements” FDA, principles of software validation, 2002 – Authorised (internally) and chronologically correct – Precise requirements covering all functions the system will perform – Uniquely identified – Verifiable Sue Gregory, Genmab A/S, October 2002 13
 Horizontal IT audit - basics • Traceability – Check that each requirement is traceable through the subsequent specifications and tests – Is there evidence that each requirement has been addressed? Sue Gregory, Genmab A/S, October 2002 14
Horizontal IT audit - basics • Traceability – Check that each requirement is traceable through the subsequent specifications and tests – Is there evidence that each requirement has been addressed? Sue Gregory, Genmab A/S, October 2002 14
 Horizontal IT audit - basics • Validation Plan “The validation must be conducted in accordance with a documented protocol”FDA, principles of software validation, 2002 – Authorised and chronologically correct – Describes who does what and when – Describes or references how Sue Gregory, Genmab A/S, October 2002 15
Horizontal IT audit - basics • Validation Plan “The validation must be conducted in accordance with a documented protocol”FDA, principles of software validation, 2002 – Authorised and chronologically correct – Describes who does what and when – Describes or references how Sue Gregory, Genmab A/S, October 2002 15
 Horizontal IT audit - basics • User Testing – Test Plan – Test acceptance criteria – Test records – Final test report • Ensure the system can properly perform its • intended functions Ensure the users can understand use the system Sue Gregory, Genmab A/S, October 2002 16
Horizontal IT audit - basics • User Testing – Test Plan – Test acceptance criteria – Test records – Final test report • Ensure the system can properly perform its • intended functions Ensure the users can understand use the system Sue Gregory, Genmab A/S, October 2002 16
 Horizontal IT audit - basics • Validation Report – Authorised and chronologically correct – Summarises the validation exercise – Describes deviations and errors encountered – Includes clear statement of success or otherwise of validation Sue Gregory, Genmab A/S, October 2002 17
Horizontal IT audit - basics • Validation Report – Authorised and chronologically correct – Summarises the validation exercise – Describes deviations and errors encountered – Includes clear statement of success or otherwise of validation Sue Gregory, Genmab A/S, October 2002 17
 Horizontal IT audit - basics • Authorised operating procedures covering: – Maintenance and repair – Disaster recovery – Security – Back-up and restore – Administration – Periodic review – Data collection and handling – Change and configuration management • Evidence of their implementation Sue Gregory, Genmab A/S, October 2002 18
Horizontal IT audit - basics • Authorised operating procedures covering: – Maintenance and repair – Disaster recovery – Security – Back-up and restore – Administration – Periodic review – Data collection and handling – Change and configuration management • Evidence of their implementation Sue Gregory, Genmab A/S, October 2002 18
 Horizontal IT audit - basics • Training – Staff involved in the validation – Staff involved in routine use of the system – Staff involved in development and maintenance of the system Sue Gregory, Genmab A/S, October 2002 19
Horizontal IT audit - basics • Training – Staff involved in the validation – Staff involved in routine use of the system – Staff involved in development and maintenance of the system Sue Gregory, Genmab A/S, October 2002 19
 Additional considerations • Vendor Audit • Installation • Development Processes • Internal IT department Sue Gregory, Genmab A/S, October 2002 20
Additional considerations • Vendor Audit • Installation • Development Processes • Internal IT department Sue Gregory, Genmab A/S, October 2002 20
 Additional considerations • Vendor Audit (software development) – ISO Quality Systems – SDLC Sue Gregory, Genmab A/S, October 2002 21
Additional considerations • Vendor Audit (software development) – ISO Quality Systems – SDLC Sue Gregory, Genmab A/S, October 2002 21
 Additional considerations • Development Processes – Coding – written standards, followed – Code review – pre-planned, documented – Unit tests – owned by developers, documented – Configuration management – Testing: • Test Strategy • Test Plan, scripts, cases – Error reporting – Release procedure – User documentation (help files, user manual etc) Sue Gregory, Genmab A/S, October 2002 22
Additional considerations • Development Processes – Coding – written standards, followed – Code review – pre-planned, documented – Unit tests – owned by developers, documented – Configuration management – Testing: • Test Strategy • Test Plan, scripts, cases – Error reporting – Release procedure – User documentation (help files, user manual etc) Sue Gregory, Genmab A/S, October 2002 22
 Additional considerations • Installation – IT department SOP – Protocol, pre-approved and followed – Records – Report Sue Gregory, Genmab A/S, October 2002 23
Additional considerations • Installation – IT department SOP – Protocol, pre-approved and followed – Records – Report Sue Gregory, Genmab A/S, October 2002 23
 Additional considerations • Internal IT Department processes – Installation – Change Control – Security – Training – Document control etc. Sue Gregory, Genmab A/S, October 2002 24
Additional considerations • Internal IT Department processes – Installation – Change Control – Security – Training – Document control etc. Sue Gregory, Genmab A/S, October 2002 24
 Practice makes perfect…. . • Start small • Define audit’s scope • Allow plenty of time • Start with the general requirements • Focus on the words audit and system Sue Gregory, Genmab A/S, October 2002 25
Practice makes perfect…. . • Start small • Define audit’s scope • Allow plenty of time • Start with the general requirements • Focus on the words audit and system Sue Gregory, Genmab A/S, October 2002 25
 …. start practising! Sue Gregory, Genmab A/S, October 2002 26
…. start practising! Sue Gregory, Genmab A/S, October 2002 26


