7499d8be3c00f4a9cfe38d50bbd6ed54.ppt
- Количество слайдов: 22
 Study program to Evaluate the Prevention of Ischemia with direct Anti-Xa inhibition: SEPIA-ACS 1 – TIMI 42 A randomized, double-blind, triple-dummy, dose-ranging study to evaluate the clinical efficacy and safety of OTAMIXABAN in patients with NSTEACS and planned early invasive strategy Marc S. Sabatine, MD, MPH on behalf of the SEPIA-ACS 1 TIMI 42 Investigators M Sabatine has received honoraria and consulting fees from sanofi-aventis and honoraria from Bristol-Myers Squibb. SEPIA-ACS 1 TIMI 42 was supported by a research grant from sanofi-aventis 1
Study program to Evaluate the Prevention of Ischemia with direct Anti-Xa inhibition: SEPIA-ACS 1 – TIMI 42 A randomized, double-blind, triple-dummy, dose-ranging study to evaluate the clinical efficacy and safety of OTAMIXABAN in patients with NSTEACS and planned early invasive strategy Marc S. Sabatine, MD, MPH on behalf of the SEPIA-ACS 1 TIMI 42 Investigators M Sabatine has received honoraria and consulting fees from sanofi-aventis and honoraria from Bristol-Myers Squibb. SEPIA-ACS 1 TIMI 42 was supported by a research grant from sanofi-aventis 1
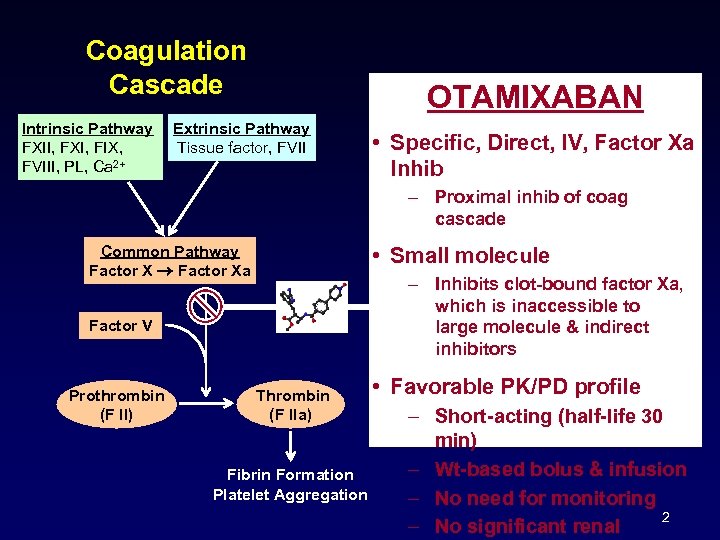 Coagulation Cascade Intrinsic Pathway FXII, FXI, FIX, FVIII, PL, Ca 2+ OTAMIXABAN Extrinsic Pathway Tissue factor, FVII • Specific, Direct, IV, Factor Xa Inhib – Proximal inhib of coag cascade Common Pathway Factor Xa • Small molecule – Inhibits clot-bound factor Xa, which is inaccessible to large molecule & indirect inhibitors Factor V Prothrombin (F II) Thrombin (F IIa) Fibrin Formation Platelet Aggregation • Favorable PK/PD profile – Short-acting (half-life 30 min) – Wt-based bolus & infusion – No need for monitoring 2 – No significant renal
Coagulation Cascade Intrinsic Pathway FXII, FXI, FIX, FVIII, PL, Ca 2+ OTAMIXABAN Extrinsic Pathway Tissue factor, FVII • Specific, Direct, IV, Factor Xa Inhib – Proximal inhib of coag cascade Common Pathway Factor Xa • Small molecule – Inhibits clot-bound factor Xa, which is inaccessible to large molecule & indirect inhibitors Factor V Prothrombin (F II) Thrombin (F IIa) Fibrin Formation Platelet Aggregation • Favorable PK/PD profile – Short-acting (half-life 30 min) – Wt-based bolus & infusion – No need for monitoring 2 – No significant renal
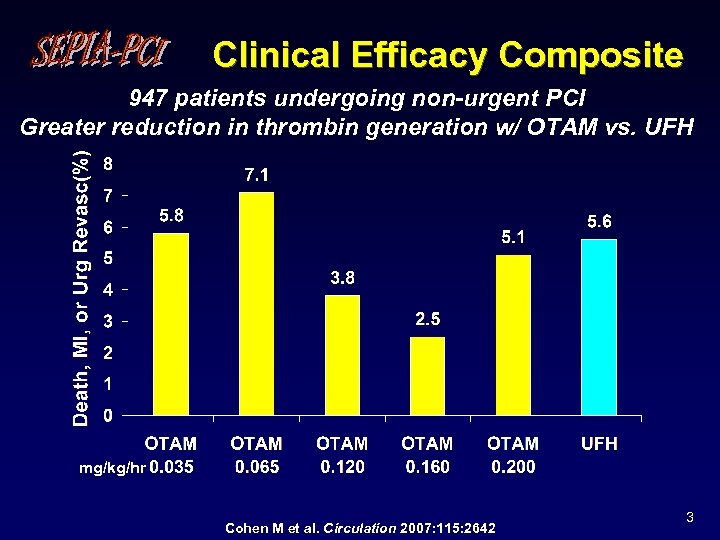 Clinical Efficacy Composite 947 patients undergoing non-urgent PCI Greater reduction in thrombin generation w/ OTAM vs. UFH mg/kg/hr Cohen M et al. Circulation 2007: 115: 2642 3
Clinical Efficacy Composite 947 patients undergoing non-urgent PCI Greater reduction in thrombin generation w/ OTAM vs. UFH mg/kg/hr Cohen M et al. Circulation 2007: 115: 2642 3
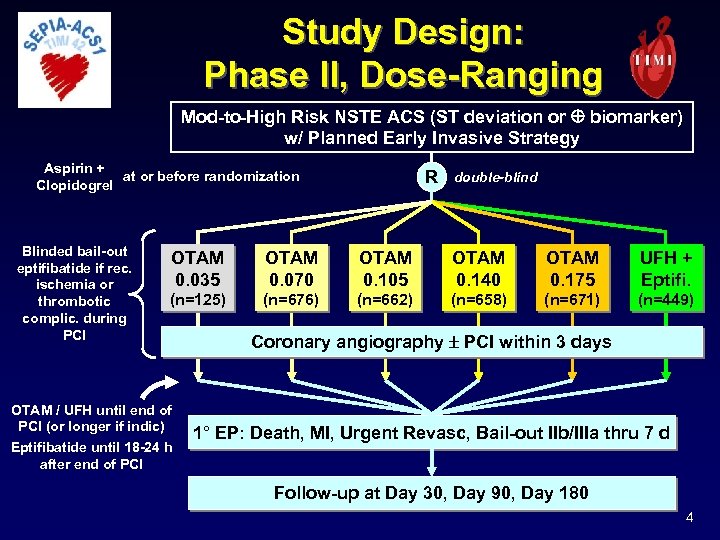 Study Design: Phase II, Dose-Ranging Mod-to-High Risk NSTE ACS (ST deviation or biomarker) w/ Planned Early Invasive Strategy Aspirin + at or before randomization Clopidogrel Blinded bail-out eptifibatide if rec. ischemia or thrombotic complic. during PCI R double-blind OTAM 0. 035 OTAM 0. 070 OTAM 0. 105 OTAM 0. 140 OTAM 0. 175 UFH + Eptifi. (n=125) (n=676) (n=662) (n=658) (n=671) (n=449) OTAM / UFH until end of PCI (or longer if indic) Eptifibatide until 18 -24 h after end of PCI Coronary angiography PCI within 3 days 1° EP: Death, MI, Urgent Revasc, Bail-out IIb/IIIa thru 7 d Follow-up at Day 30, Day 90, Day 180 4
Study Design: Phase II, Dose-Ranging Mod-to-High Risk NSTE ACS (ST deviation or biomarker) w/ Planned Early Invasive Strategy Aspirin + at or before randomization Clopidogrel Blinded bail-out eptifibatide if rec. ischemia or thrombotic complic. during PCI R double-blind OTAM 0. 035 OTAM 0. 070 OTAM 0. 105 OTAM 0. 140 OTAM 0. 175 UFH + Eptifi. (n=125) (n=676) (n=662) (n=658) (n=671) (n=449) OTAM / UFH until end of PCI (or longer if indic) Eptifibatide until 18 -24 h after end of PCI Coronary angiography PCI within 3 days 1° EP: Death, MI, Urgent Revasc, Bail-out IIb/IIIa thru 7 d Follow-up at Day 30, Day 90, Day 180 4
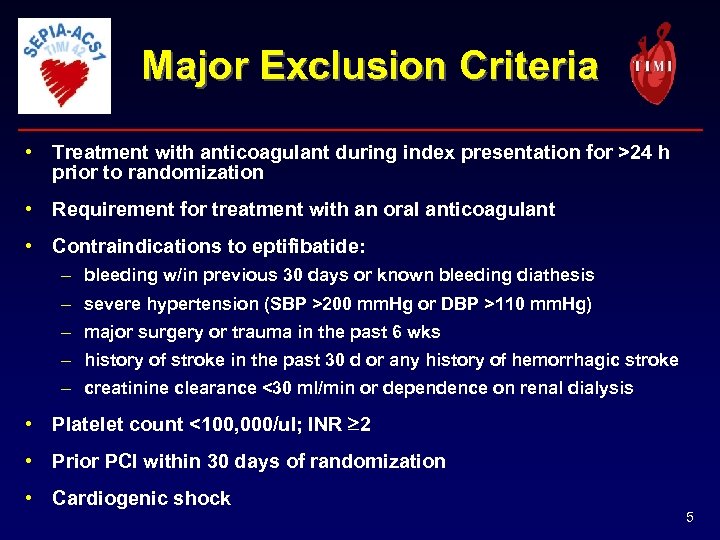 Major Exclusion Criteria • Treatment with anticoagulant during index presentation for >24 h prior to randomization • Requirement for treatment with an oral anticoagulant • Contraindications to eptifibatide: – bleeding w/in previous 30 days or known bleeding diathesis – severe hypertension (SBP >200 mm. Hg or DBP >110 mm. Hg) – major surgery or trauma in the past 6 wks – history of stroke in the past 30 d or any history of hemorrhagic stroke – creatinine clearance <30 ml/min or dependence on renal dialysis • Platelet count <100, 000/ul; INR 2 • Prior PCI within 30 days of randomization • Cardiogenic shock 5
Major Exclusion Criteria • Treatment with anticoagulant during index presentation for >24 h prior to randomization • Requirement for treatment with an oral anticoagulant • Contraindications to eptifibatide: – bleeding w/in previous 30 days or known bleeding diathesis – severe hypertension (SBP >200 mm. Hg or DBP >110 mm. Hg) – major surgery or trauma in the past 6 wks – history of stroke in the past 30 d or any history of hemorrhagic stroke – creatinine clearance <30 ml/min or dependence on renal dialysis • Platelet count <100, 000/ul; INR 2 • Prior PCI within 30 days of randomization • Cardiogenic shock 5
 Trial Organization TIMI Study Group Brigham and Women’s Hospital Harvard Medical School E Braunwald, MD (Chair) MS Sabatine, MD, MPH (PI) EM Antman, MD (Co-PI) CH Mc. Cabe (Director) S Crugnale (Project Manager) CF Contant (Director of Biostats) Clinical Events Cmte SD Wiviott, MD (Chair) Data Monitoring Cmte P Theroux, MD (Chair) JP Bassand S Kelsey Sponsor A Moryusef, M Brynildsen, S Hitier, T Colineau, S Ribadeau-Dumas, P Taistra, C Mahdi Sanofi-Aventis 6
Trial Organization TIMI Study Group Brigham and Women’s Hospital Harvard Medical School E Braunwald, MD (Chair) MS Sabatine, MD, MPH (PI) EM Antman, MD (Co-PI) CH Mc. Cabe (Director) S Crugnale (Project Manager) CF Contant (Director of Biostats) Clinical Events Cmte SD Wiviott, MD (Chair) Data Monitoring Cmte P Theroux, MD (Chair) JP Bassand S Kelsey Sponsor A Moryusef, M Brynildsen, S Hitier, T Colineau, S Ribadeau-Dumas, P Taistra, C Mahdi Sanofi-Aventis 6
 Steering Committee Argentina JL Navaro Estrada Israel H Hod Brazil JC Nicolau Italy G De. Ferrari Canada S Goodman Mexico F Petersen Chile R Corbalan Netherlands F Verheugt Croatia M Bergovec Poland W Ruzyllo Czech Republic P Widimsky Portugal R Ferreira Denmark P Clemmensen Russia M Ruda Estonia J Voitk South Africa IO Ebrahim Finland I Tierala South Korea M Jeong France G Montalescot Southeast Asia R Zambahari Germany C Bode Spain J Ruiz-Nodar Greece D Alexopoulos Turkey Z Ongen Hungary RG Kiss United States MS Sabatine India BS Raju 7
Steering Committee Argentina JL Navaro Estrada Israel H Hod Brazil JC Nicolau Italy G De. Ferrari Canada S Goodman Mexico F Petersen Chile R Corbalan Netherlands F Verheugt Croatia M Bergovec Poland W Ruzyllo Czech Republic P Widimsky Portugal R Ferreira Denmark P Clemmensen Russia M Ruda Estonia J Voitk South Africa IO Ebrahim Finland I Tierala South Korea M Jeong France G Montalescot Southeast Asia R Zambahari Germany C Bode Spain J Ruiz-Nodar Greece D Alexopoulos Turkey Z Ongen Hungary RG Kiss United States MS Sabatine India BS Raju 7
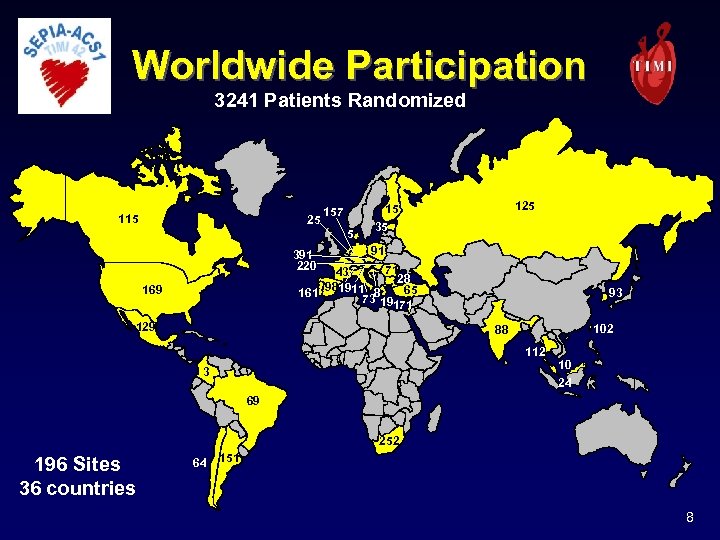 Worldwide Participation 3241 Patients Randomized 115 25 157 5 391 220 125 15 35 91 71 43 28 981911 8 65 161 73 19171 169 129 93 102 88 112 3 10 24 69 252 196 Sites 36 countries 64 151 8
Worldwide Participation 3241 Patients Randomized 115 25 157 5 391 220 125 15 35 91 71 43 28 981911 8 65 161 73 19171 169 129 93 102 88 112 3 10 24 69 252 196 Sites 36 countries 64 151 8
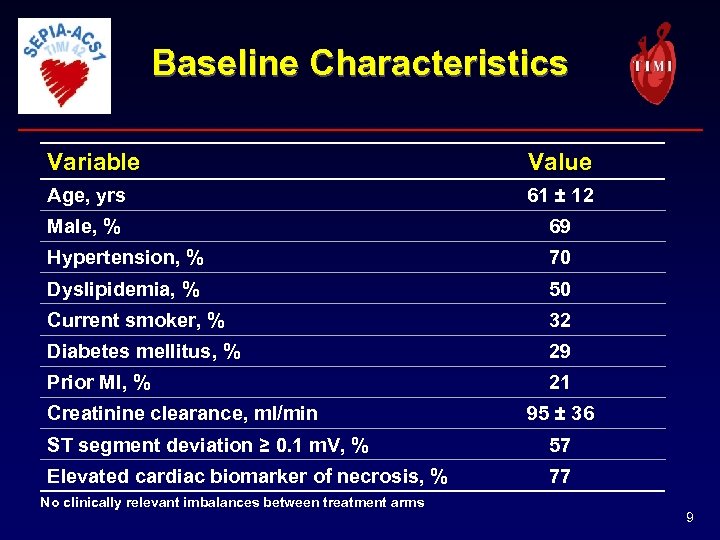 Baseline Characteristics Variable Value Age, yrs 61 ± 12 Male, % 69 Hypertension, % 70 Dyslipidemia, % 50 Current smoker, % 32 Diabetes mellitus, % 29 Prior MI, % 21 Creatinine clearance, ml/min 95 ± 36 ST segment deviation ≥ 0. 1 m. V, % 57 Elevated cardiac biomarker of necrosis, % 77 No clinically relevant imbalances between treatment arms 9
Baseline Characteristics Variable Value Age, yrs 61 ± 12 Male, % 69 Hypertension, % 70 Dyslipidemia, % 50 Current smoker, % 32 Diabetes mellitus, % 29 Prior MI, % 21 Creatinine clearance, ml/min 95 ± 36 ST segment deviation ≥ 0. 1 m. V, % 57 Elevated cardiac biomarker of necrosis, % 77 No clinically relevant imbalances between treatment arms 9
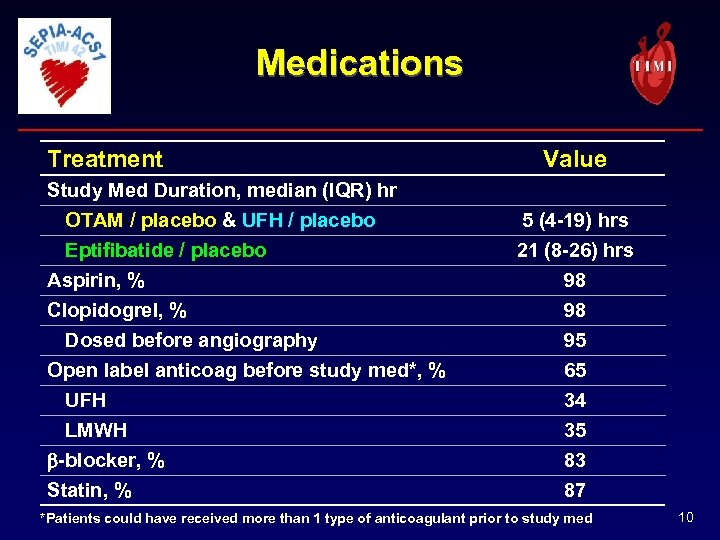 Medications Treatment Study Med Duration, median (IQR) hr OTAM / placebo & UFH / placebo Eptifibatide / placebo Aspirin, % Clopidogrel, % Dosed before angiography Open label anticoag before study med*, % UFH LMWH b-blocker, % Statin, % Value 5 (4 -19) hrs 21 (8 -26) hrs 98 98 95 65 34 35 83 87 *Patients could have received more than 1 type of anticoagulant prior to study med 10
Medications Treatment Study Med Duration, median (IQR) hr OTAM / placebo & UFH / placebo Eptifibatide / placebo Aspirin, % Clopidogrel, % Dosed before angiography Open label anticoag before study med*, % UFH LMWH b-blocker, % Statin, % Value 5 (4 -19) hrs 21 (8 -26) hrs 98 98 95 65 34 35 83 87 *Patients could have received more than 1 type of anticoagulant prior to study med 10
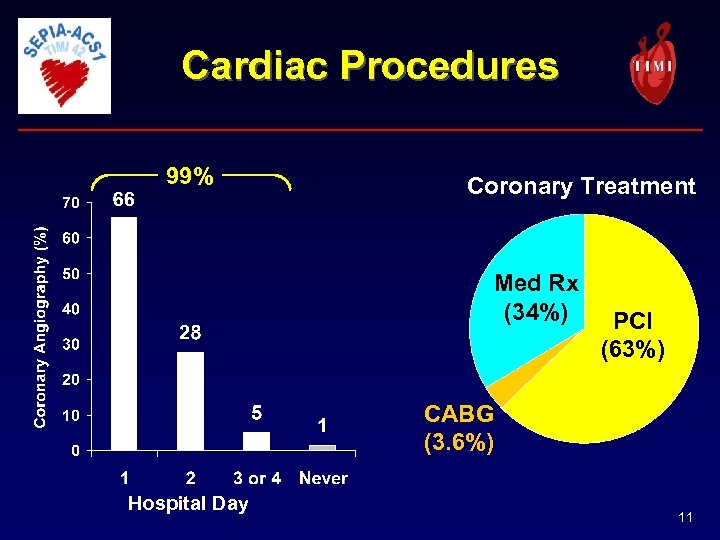 Cardiac Procedures 99% Coronary Treatment Med Rx (34%) PCI (63%) CABG (3. 6%) Hospital Day 11
Cardiac Procedures 99% Coronary Treatment Med Rx (34%) PCI (63%) CABG (3. 6%) Hospital Day 11
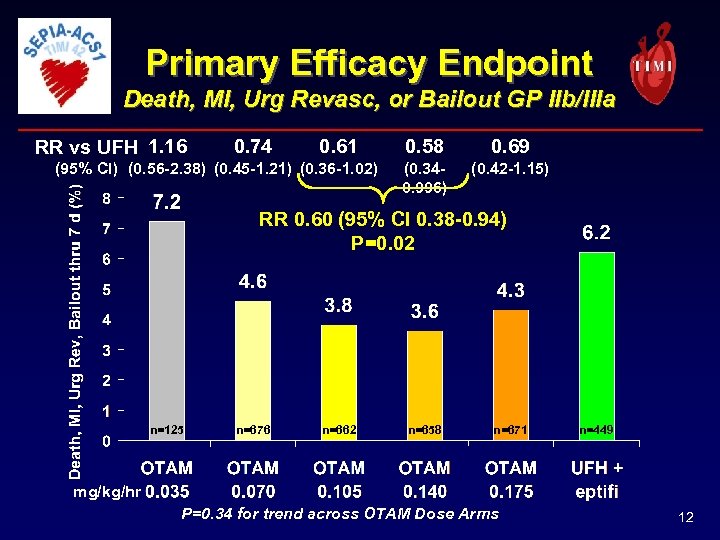 Primary Efficacy Endpoint Death, MI, Urg Revasc, or Bailout GP IIb/IIIa RR vs UFH 1. 16 0. 74 0. 61 (95% CI) (0. 56 -2. 38) (0. 45 -1. 21) (0. 36 -1. 02) 0. 58 0. 69 (0. 340. 996) (0. 42 -1. 15) RR 0. 60 (95% CI 0. 38 -0. 94) P=0. 02 n=125 n=676 n=662 n=658 n=671 n=449 mg/kg/hr P=0. 34 for trend across OTAM Dose Arms 12
Primary Efficacy Endpoint Death, MI, Urg Revasc, or Bailout GP IIb/IIIa RR vs UFH 1. 16 0. 74 0. 61 (95% CI) (0. 56 -2. 38) (0. 45 -1. 21) (0. 36 -1. 02) 0. 58 0. 69 (0. 340. 996) (0. 42 -1. 15) RR 0. 60 (95% CI 0. 38 -0. 94) P=0. 02 n=125 n=676 n=662 n=658 n=671 n=449 mg/kg/hr P=0. 34 for trend across OTAM Dose Arms 12
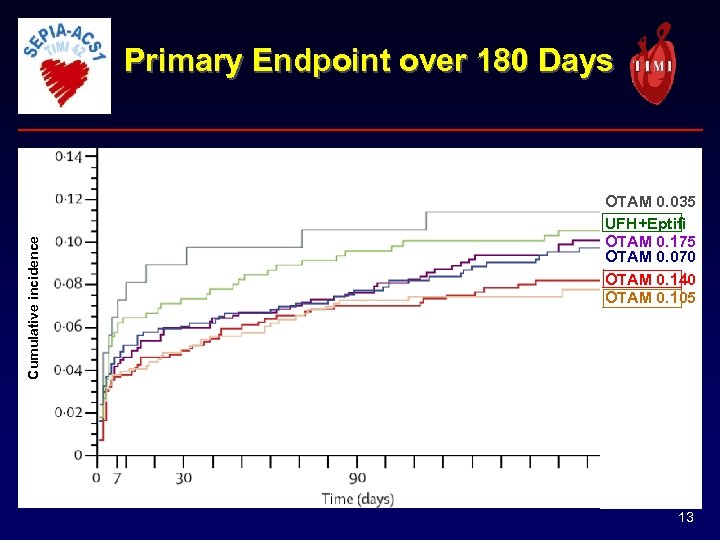 Cumulative incidence Primary Endpoint over 180 Days OTAM 0. 035 UFH+Eptifi OTAM 0. 175 OTAM 0. 070 OTAM 0. 140 OTAM 0. 105 13
Cumulative incidence Primary Endpoint over 180 Days OTAM 0. 035 UFH+Eptifi OTAM 0. 175 OTAM 0. 070 OTAM 0. 140 OTAM 0. 105 13
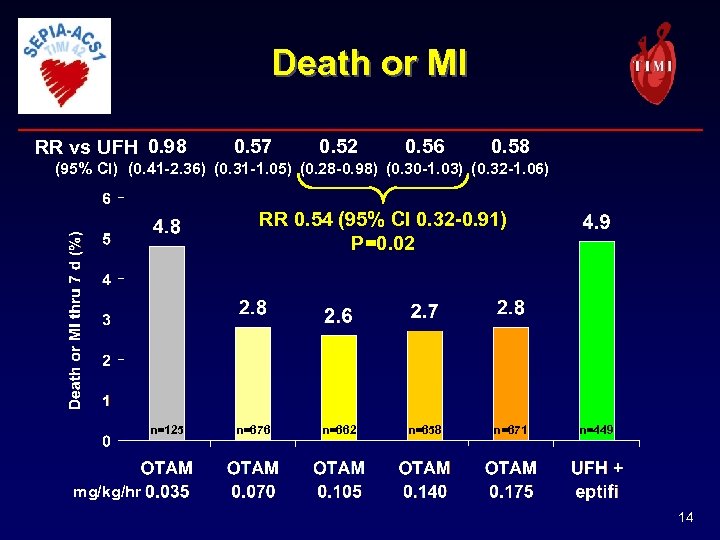 Death or MI RR vs UFH 0. 98 0. 57 0. 52 0. 56 0. 58 (95% CI) (0. 41 -2. 36) (0. 31 -1. 05) (0. 28 -0. 98) (0. 30 -1. 03) (0. 32 -1. 06) RR 0. 54 (95% CI 0. 32 -0. 91) P=0. 02 n=125 n=676 n=662 n=658 n=671 n=449 mg/kg/hr 14
Death or MI RR vs UFH 0. 98 0. 57 0. 52 0. 56 0. 58 (95% CI) (0. 41 -2. 36) (0. 31 -1. 05) (0. 28 -0. 98) (0. 30 -1. 03) (0. 32 -1. 06) RR 0. 54 (95% CI 0. 32 -0. 91) P=0. 02 n=125 n=676 n=662 n=658 n=671 n=449 mg/kg/hr 14
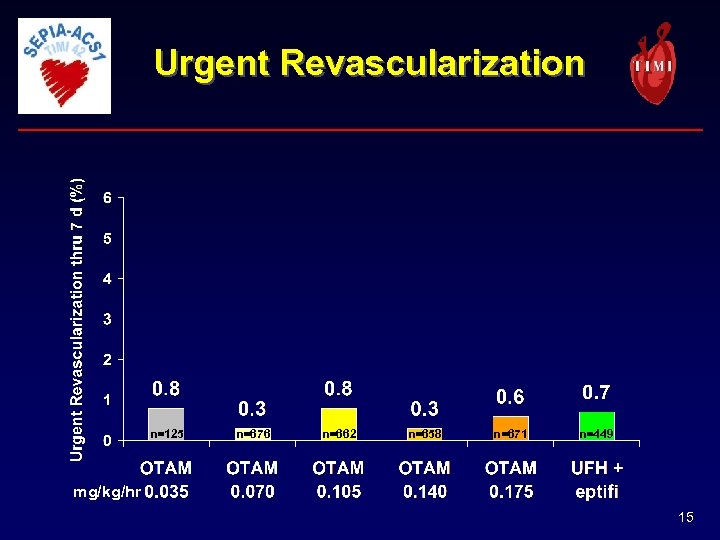 Urgent Revascularization n=125 n=676 n=662 n=658 n=671 n=449 mg/kg/hr 15
Urgent Revascularization n=125 n=676 n=662 n=658 n=671 n=449 mg/kg/hr 15
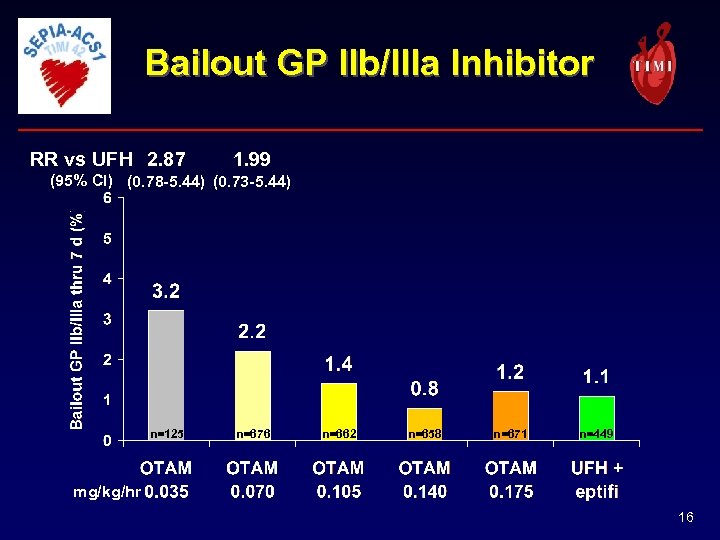 Bailout GP IIb/IIIa Inhibitor RR vs UFH 2. 87 1. 99 (95% CI) (0. 78 -5. 44) (0. 73 -5. 44) n=125 n=676 n=662 n=658 n=671 n=449 mg/kg/hr 16
Bailout GP IIb/IIIa Inhibitor RR vs UFH 2. 87 1. 99 (95% CI) (0. 78 -5. 44) (0. 73 -5. 44) n=125 n=676 n=662 n=658 n=671 n=449 mg/kg/hr 16
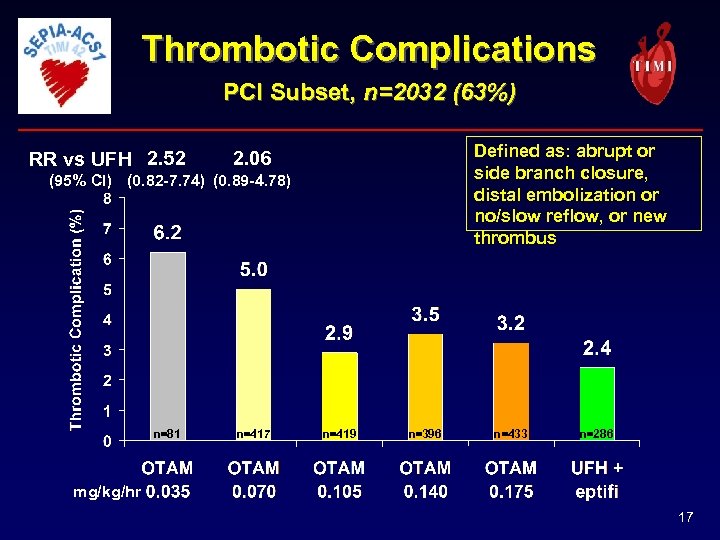 Thrombotic Complications PCI Subset, n=2032 (63%) RR vs UFH 2. 52 Defined as: abrupt or side branch closure, distal embolization or no/slow reflow, or new thrombus 2. 06 (95% CI) (0. 82 -7. 74) (0. 89 -4. 78) n=81 n=417 n=419 n=396 n=433 n=286 mg/kg/hr 17
Thrombotic Complications PCI Subset, n=2032 (63%) RR vs UFH 2. 52 Defined as: abrupt or side branch closure, distal embolization or no/slow reflow, or new thrombus 2. 06 (95% CI) (0. 82 -7. 74) (0. 89 -4. 78) n=81 n=417 n=419 n=396 n=433 n=286 mg/kg/hr 17
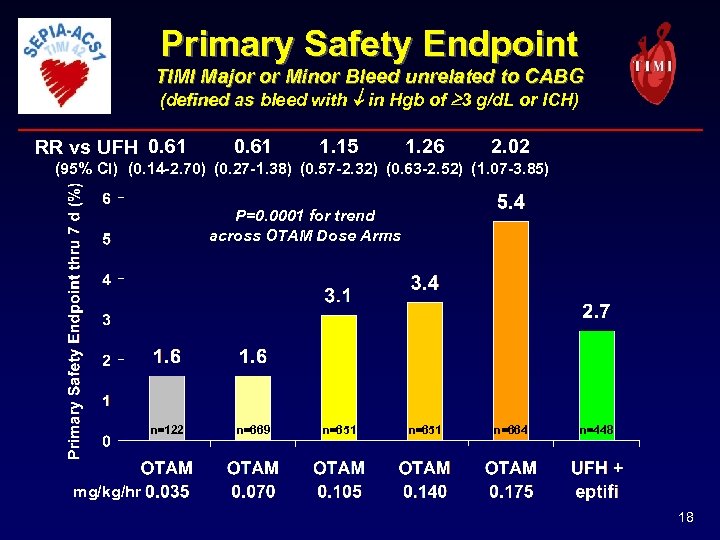 Primary Safety Endpoint TIMI Major or Minor Bleed unrelated to CABG (defined as bleed with in Hgb of 3 g/d. L or ICH) RR vs UFH 0. 61 1. 15 1. 26 2. 02 (95% CI) (0. 14 -2. 70) (0. 27 -1. 38) (0. 57 -2. 32) (0. 63 -2. 52) (1. 07 -3. 85) P=0. 0001 for trend across OTAM Dose Arms n=122 n=669 n=651 n=664 n=448 mg/kg/hr 18
Primary Safety Endpoint TIMI Major or Minor Bleed unrelated to CABG (defined as bleed with in Hgb of 3 g/d. L or ICH) RR vs UFH 0. 61 1. 15 1. 26 2. 02 (95% CI) (0. 14 -2. 70) (0. 27 -1. 38) (0. 57 -2. 32) (0. 63 -2. 52) (1. 07 -3. 85) P=0. 0001 for trend across OTAM Dose Arms n=122 n=669 n=651 n=664 n=448 mg/kg/hr 18
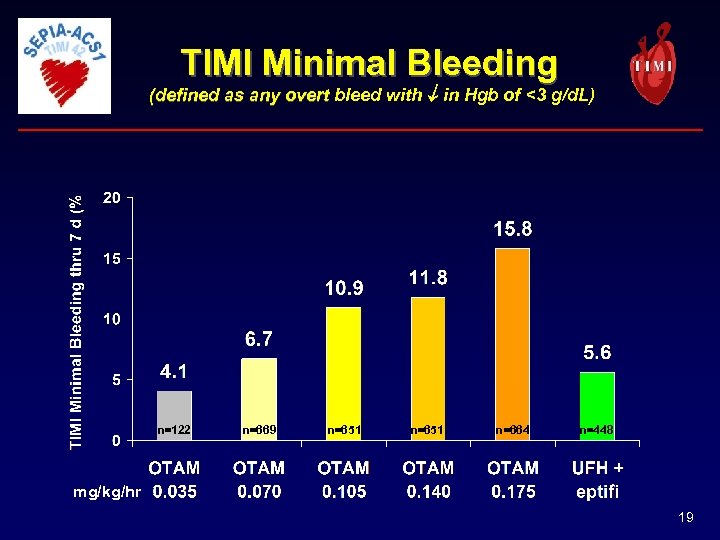 TIMI Minimal Bleeding (defined as any overt bleed with in Hgb of <3 g/d. L) n=122 n=669 n=651 n=664 n=448 mg/kg/hr 19
TIMI Minimal Bleeding (defined as any overt bleed with in Hgb of <3 g/d. L) n=122 n=669 n=651 n=664 n=448 mg/kg/hr 19
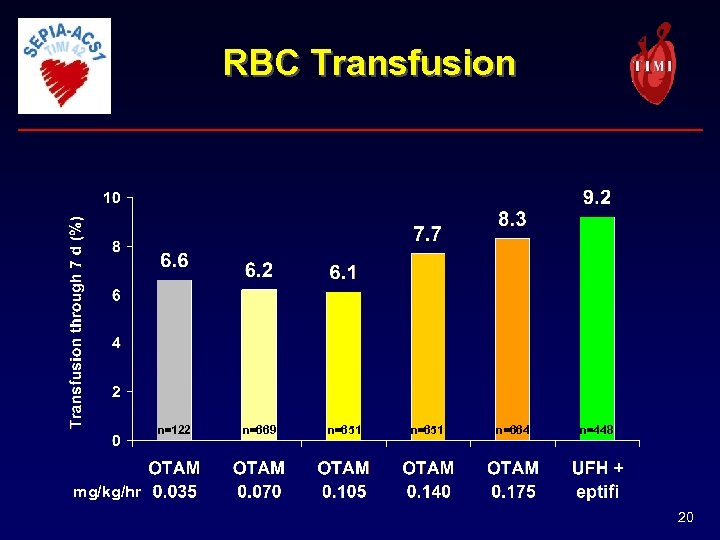 RBC Transfusion n=122 n=669 n=651 n=664 n=448 mg/kg/hr 20
RBC Transfusion n=122 n=669 n=651 n=664 n=448 mg/kg/hr 20
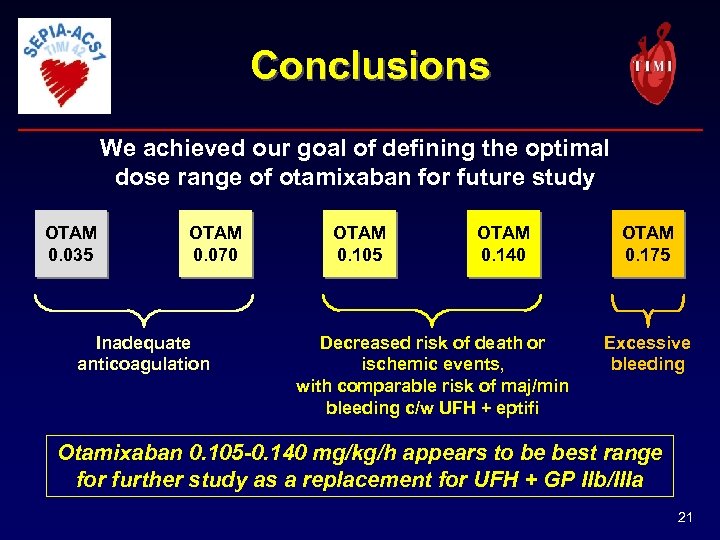 Conclusions We achieved our goal of defining the optimal dose range of otamixaban for future study OTAM 0. 035 OTAM 0. 070 Inadequate anticoagulation OTAM 0. 105 OTAM 0. 140 Decreased risk of death or ischemic events, with comparable risk of maj/min bleeding c/w UFH + eptifi OTAM 0. 175 Excessive bleeding Otamixaban 0. 105 -0. 140 mg/kg/h appears to be best range for further study as a replacement for UFH + GP IIb/IIIa 21
Conclusions We achieved our goal of defining the optimal dose range of otamixaban for future study OTAM 0. 035 OTAM 0. 070 Inadequate anticoagulation OTAM 0. 105 OTAM 0. 140 Decreased risk of death or ischemic events, with comparable risk of maj/min bleeding c/w UFH + eptifi OTAM 0. 175 Excessive bleeding Otamixaban 0. 105 -0. 140 mg/kg/h appears to be best range for further study as a replacement for UFH + GP IIb/IIIa 21
 THE LANCET Available at www. thelancet. com Presentation slides available at www. timi. org 22
THE LANCET Available at www. thelancet. com Presentation slides available at www. timi. org 22


