2f77ed526b4c0967100b376d773e9827.ppt
- Количество слайдов: 23

Studio 3 a: Atomic Hotel
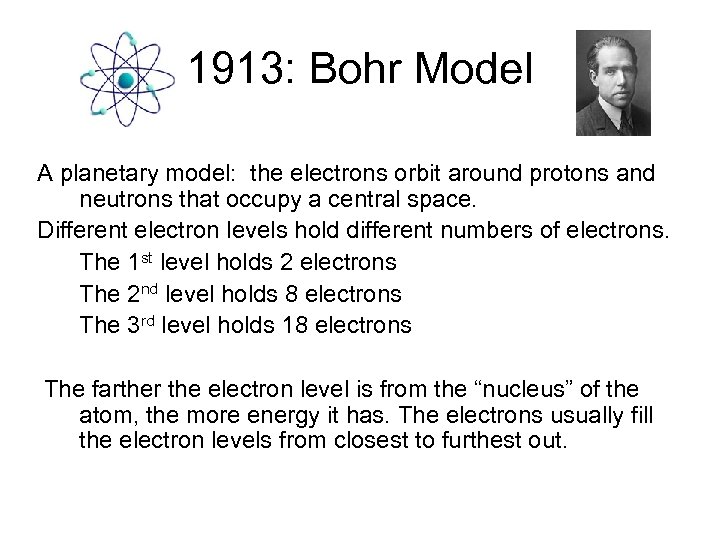
1913: Bohr Model A planetary model: the electrons orbit around protons and neutrons that occupy a central space. Different electron levels hold different numbers of electrons. The 1 st level holds 2 electrons The 2 nd level holds 8 electrons The 3 rd level holds 18 electrons The farther the electron level is from the “nucleus” of the atom, the more energy it has. The electrons usually fill the electron levels from closest to furthest out.
Bohr Model Flaws Ineffective explanation for bonding between atoms. Little ability to predict molecular shapes. Could not predict how many bonds a particular atom is likely to form
Atomic Orbital: Today’s Working Model of an Atomic orbital theory places the electrons in specific regions of space called orbitals. These orbitals can be mathematically derived through quantum mechanics.

Use the Atomic Hotel exercise (I-IV) in your coursepack to explore how electrons occupy atoms in accordance with quantum mechanics.

Analogies to Atomic Hotels • Floors = shells • Rooms = orbitals

Floors = Shells • Every atom has many shells around it. • Each shell is spherical and fully encompasses the nucleus…. More like an onion (3 D) than a solar system or hotel floor (2 D) • Shells represent the distance from the nucleus. . . they get larger as they go outward. Pictures from: www. gojskoj. se/ arkiv 03 a. asp? id=20030305 and http: //mymiraclebaby. com/Merchant 2/graphics/00000001/stack-cups. gif

Shells (n) • Shells are named using integers numbers (n). The innermost shell is 1, the next is 2, etc. • Each shell (floor) is made of up or one or more types of orbitals (rooms)
Rooms = Orbitals • Orbitals are representations of the probability that the electron/s that occupy it will be in the space they defined. • Orbitals of different shapes (different room layouts) are distinguished with letters (s, p, d, f. . . ) or numbers (0, 1, 2, 3…) (room layout l)
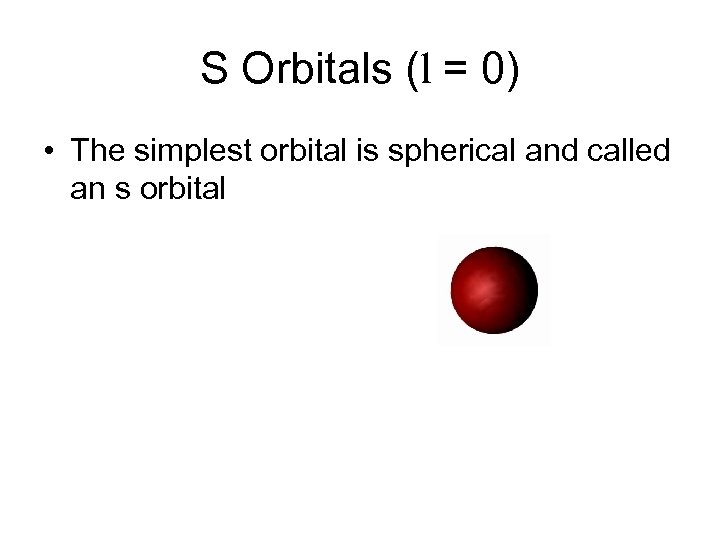
S Orbitals (l = 0) • The simplest orbital is spherical and called an s orbital
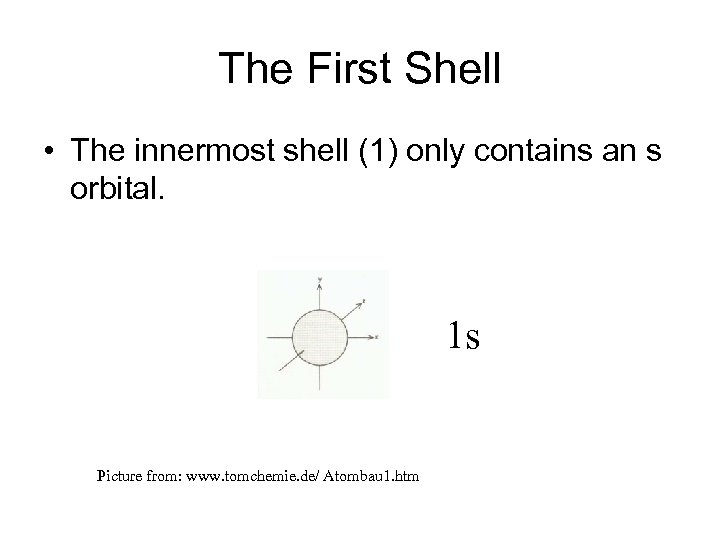
The First Shell • The innermost shell (1) only contains an s orbital. 1 s Picture from: www. tomchemie. de/ Atombau 1. htm
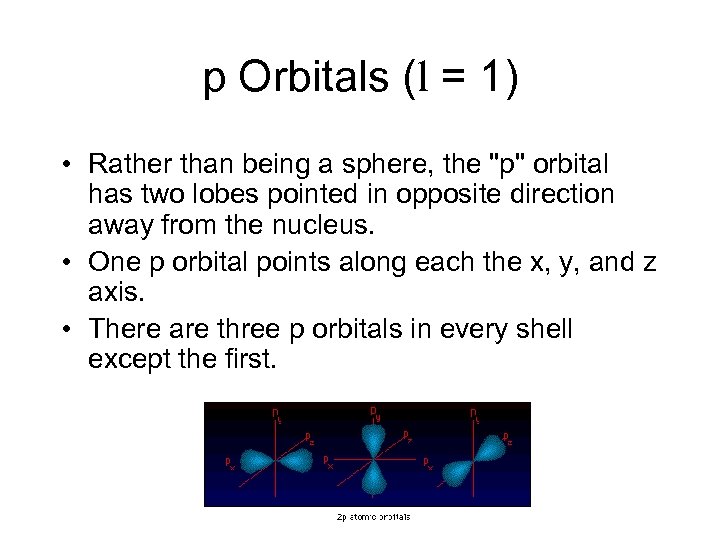
p Orbitals (l = 1) • Rather than being a sphere, the "p" orbital has two lobes pointed in opposite direction away from the nucleus. • One p orbital points along each the x, y, and z axis. • There are three p orbitals in every shell except the first.
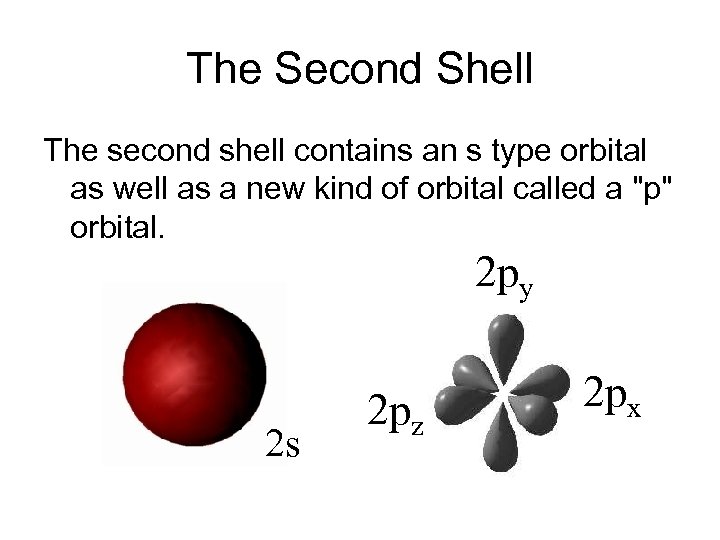
The Second Shell The second shell contains an s type orbital as well as a new kind of orbital called a "p" orbital. 2 py 2 s 2 pz 2 px
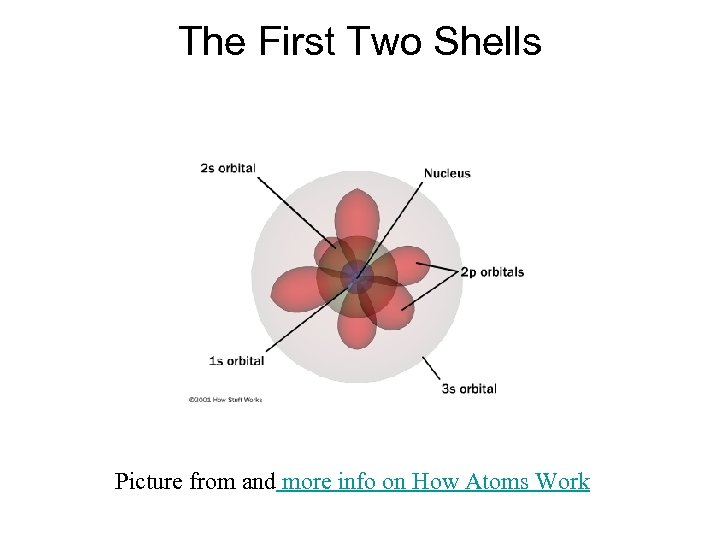
The First Two Shells Picture from and more info on How Atoms Work
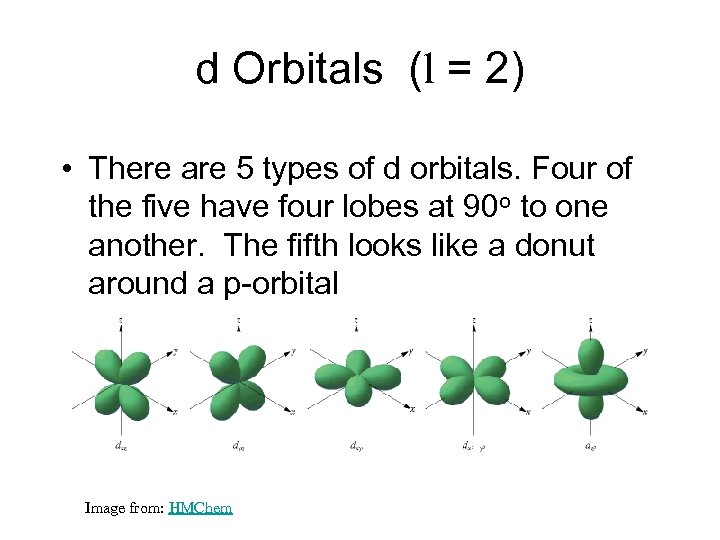
d Orbitals (l = 2) • There are 5 types of d orbitals. Four of the five have four lobes at 90 o to one another. The fifth looks like a donut around a p-orbital Image from: HMChem
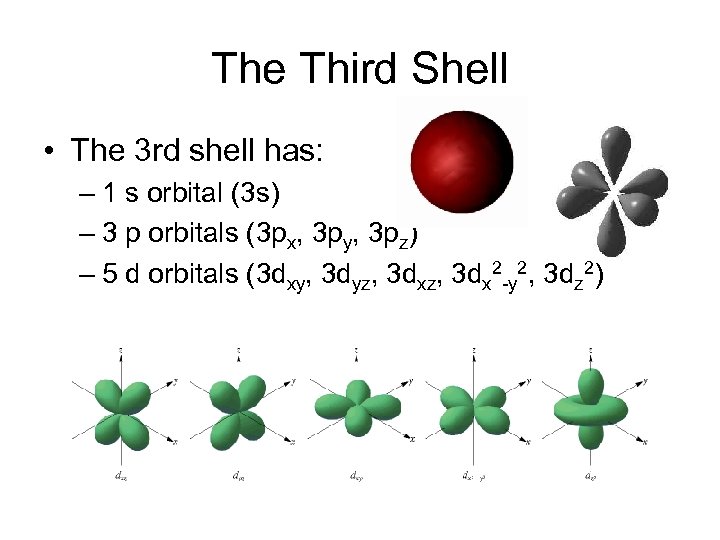
The Third Shell • The 3 rd shell has: – 1 s orbital (3 s) – 3 p orbitals (3 px, 3 py, 3 pz) – 5 d orbitals (3 dxy, 3 dyz, 3 dx 2 -y 2, 3 dz 2)
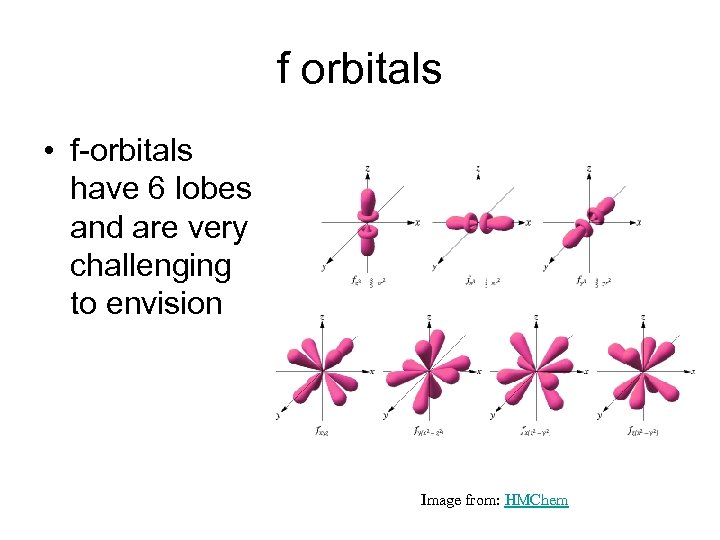
f orbitals • f-orbitals have 6 lobes and are very challenging to envision Image from: HMChem

The Fourth Shell • The 4 th shell has: – 1 s orbital (4 s) – 3 p orbitals (4 px, 4 py, 4 pz) – 5 d orbitals (4 dxy, 4 dyz, 4 dx 2 -y 2, 4 dz 2) – 7 f orbitals • How many electrons do each of the first four shells hold?
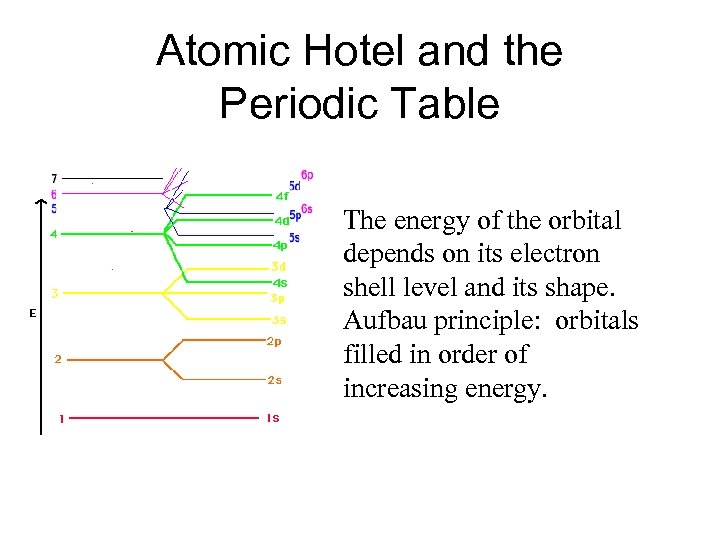
Atomic Hotel and the Periodic Table The energy of the orbital depends on its electron shell level and its shape. Aufbau principle: orbitals filled in order of increasing energy.

Pauli Exclusion Principle: at most two electrons can be assigned to any one atomic orbital and these two electrons must have opposite spins Hunds rule: Electrons pair only after each orbital in a set of the same shaped orbitals at the same energy level is occupied by a single electron
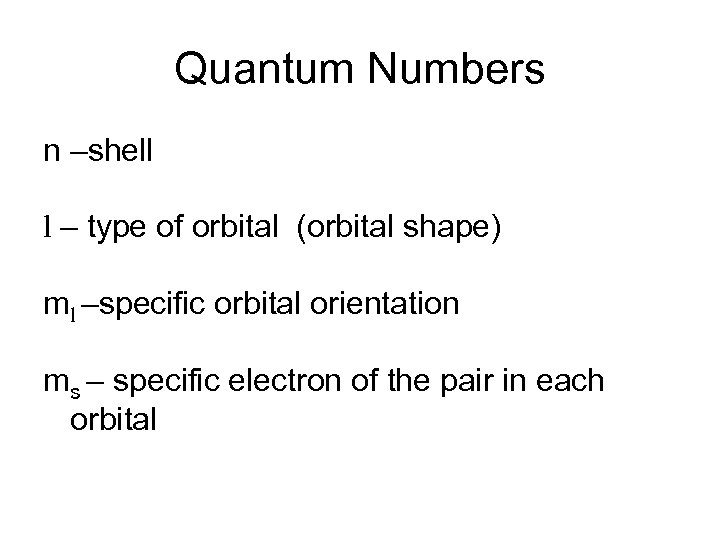
Quantum Numbers n –shell l – type of orbital (orbital shape) ml –specific orbital orientation ms – specific electron of the pair in each orbital
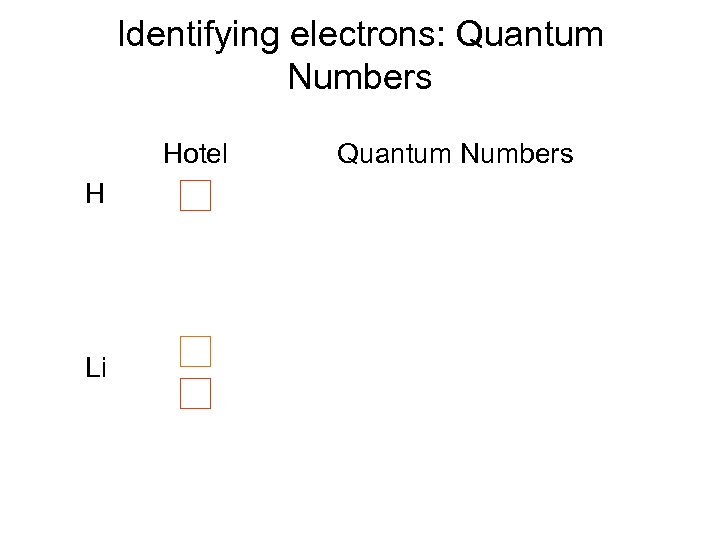
Identifying electrons: Quantum Numbers Hotel H Li Quantum Numbers
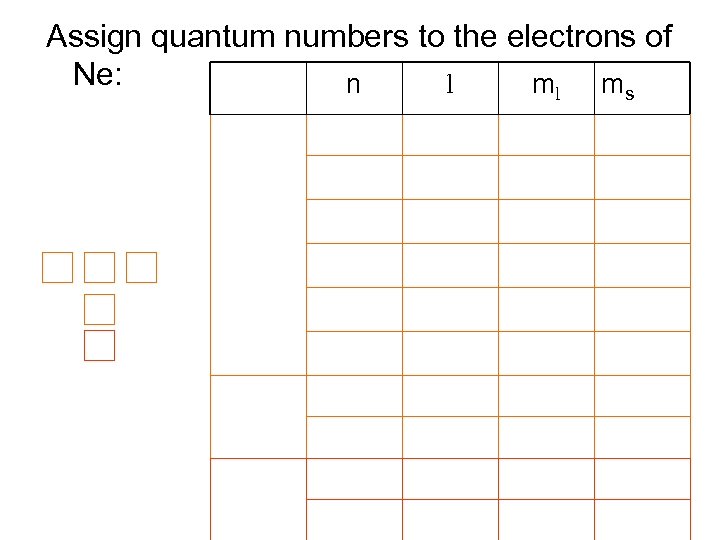
Assign quantum numbers to the electrons of Ne: l n ml ms
2f77ed526b4c0967100b376d773e9827.ppt