Структурная организация белков Белки – это органические высокомолекулярные


Структурная организация белков

Белки – это органические высокомолекулярные азотсодержащие полимерные соединения, мономерами которых являются аминокислоты

Связи в молекулах белков Пептидные Дисульфидные Водородные Ионные Гидрофобные Ван-дер-Ваальсовы
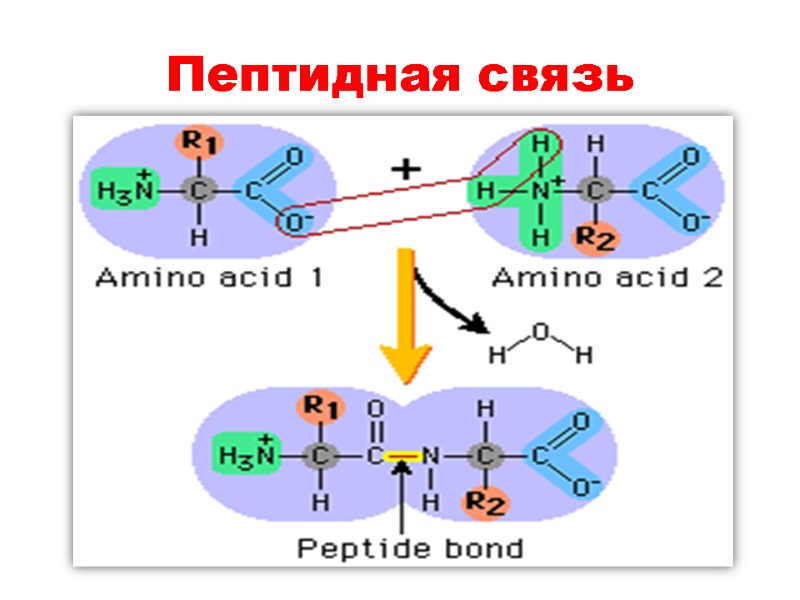
Пептидная связь
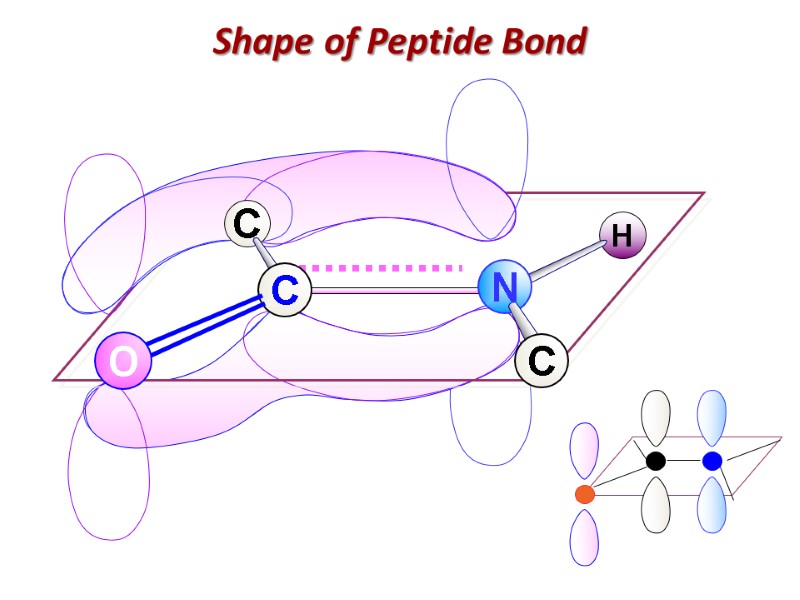
Shape of Peptide Bond

Пептидная связь: Ковалентная, прочная, ригидная Короче чем C-N связь в аминах (0, 132 nm) Все атомы, образующие пептидную связь лежат в одной плоскости (копланарны) Вращение вокруг пептидной связи невозможно

Имеет частично двойной характер Частичное перераспределение двух пар электронов между кислородом карбонильной группы и азотом амидной ведет к тому, что кислород имеет частично отрицательный заряд, а азот – частично положительный, образуя электрический диполь

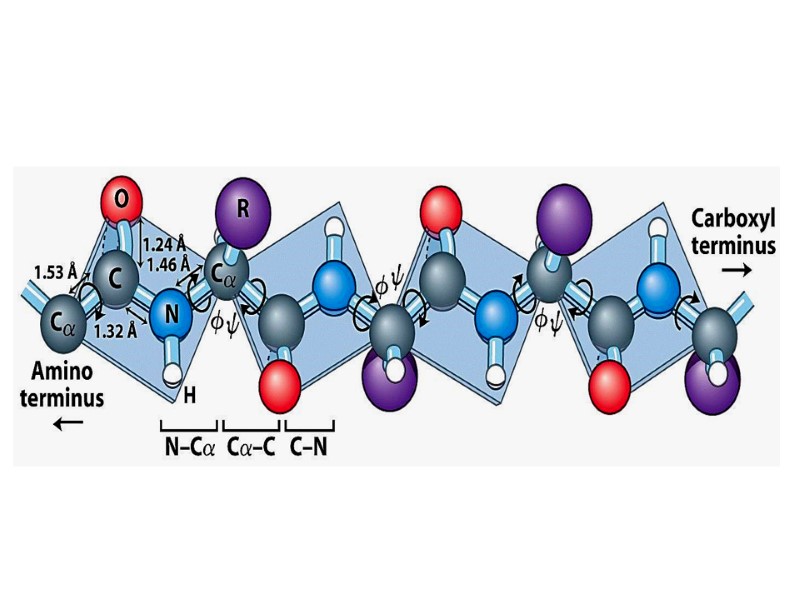
Кислород и азот находятся в транс позиции Пептидная связь образует остов полипептидной цепи Полиептидная цепь, таким образом, может быть представлена серией ригидных плоскостей , совмещенных общей точкой ротации - Cα
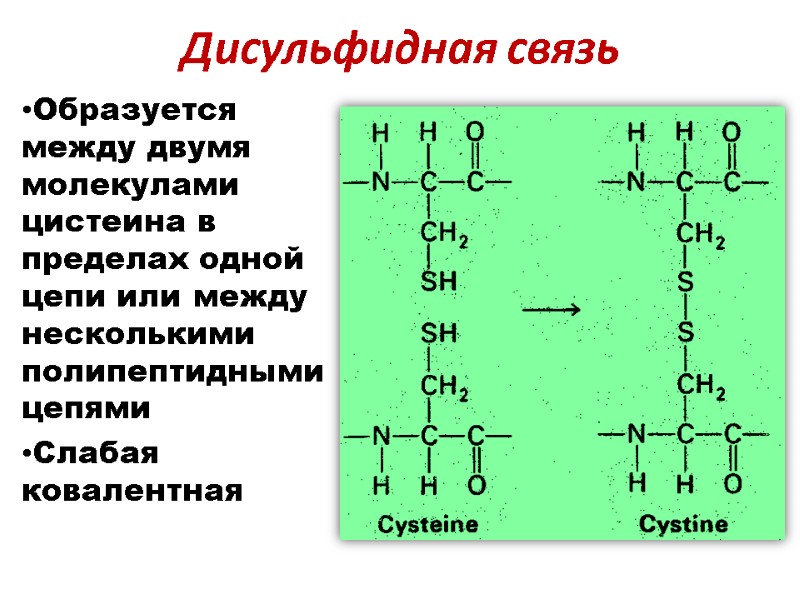
Дисульфидная связь Образуется между двумя молекулами цистеина в пределах одной цепи или между несколькими полипептидными цепями Слабая ковалентная

Нековалентные связи Слабые Обратимые Могут быть иметь отталкивающий или притягивающий характер Вовлечены во взаимодействие внутри одной биомолекулы, между биомолекулами и между биомолекулой и водой
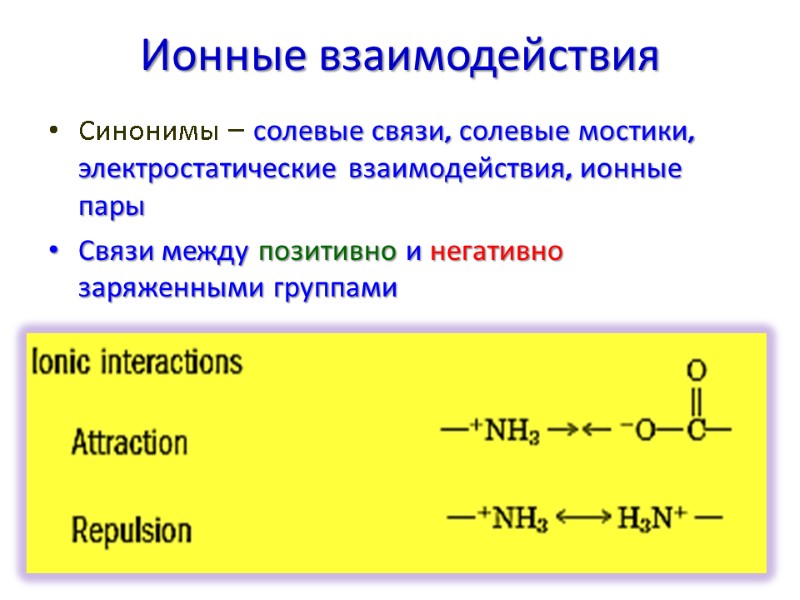
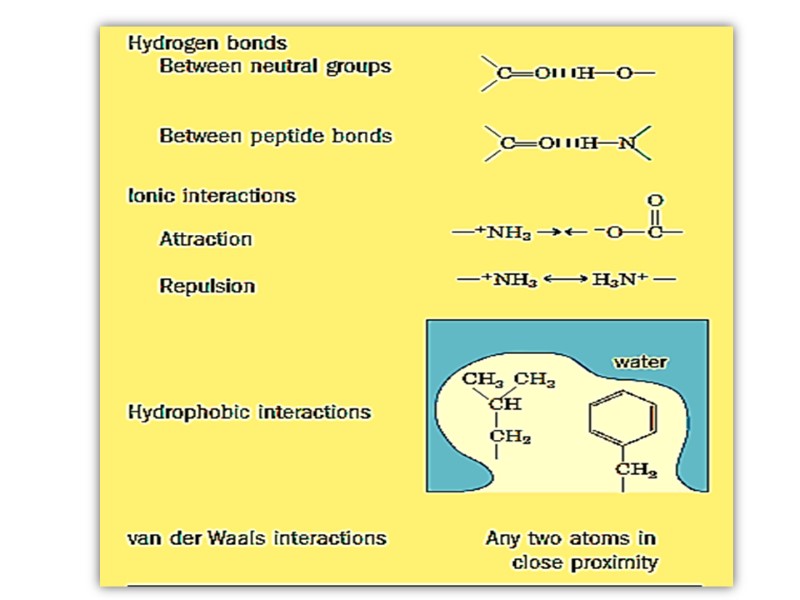
Ионные взаимодействия Синонимы – солевые связи, солевые мостики, электростатические взаимодействия, ионные пары Связи между позитивно и негативно заряженными группами

Водородные связи Формируются между электронегативным атомом (акцептором водорода, обычно кислородом или азотом с неподелённой парой электронов) И атомом водорода, ковалентно связанным с другим электронегативным атомом (донор водорода этой же или другой молекулы)
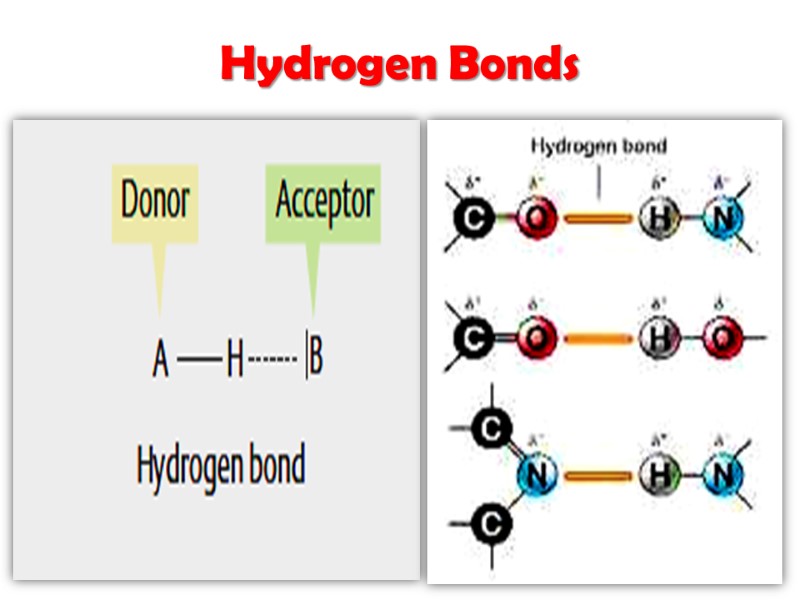
Hydrogen Bonds

Гидрофобные взаимодействия Связи между неполярными группами в молекулах Сила неполярных взаимодействий не связана с каким-то внутренним притяжением между неполярными группами Гидрофобные взаимодействия – это результат наибольшей термодинамической стабильности посредством минимизации числа упорядоченных молекул воды, окружающих гидрофобные области растворенных молекул

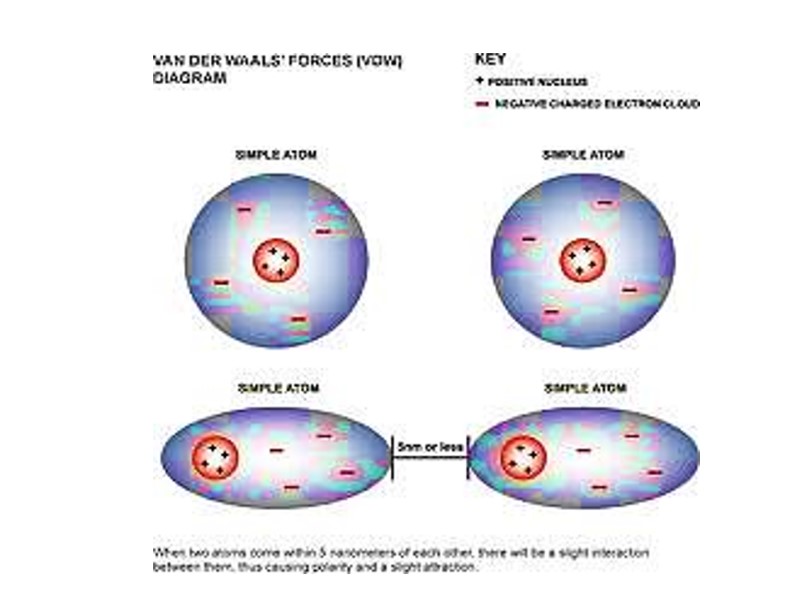
Ван дер Ваальсовы взаимодействия Слабые межатоные притяжения Когда два незаряженных атома приближаются довольно близко друг к другу, их электронные облака взаимодействуют друг с другом Электроны одного из ядер могут создавать временный электрический диполь, который в свою очередь индуцирует временный электрический диполь с противоположным знаком в соседнем атоме
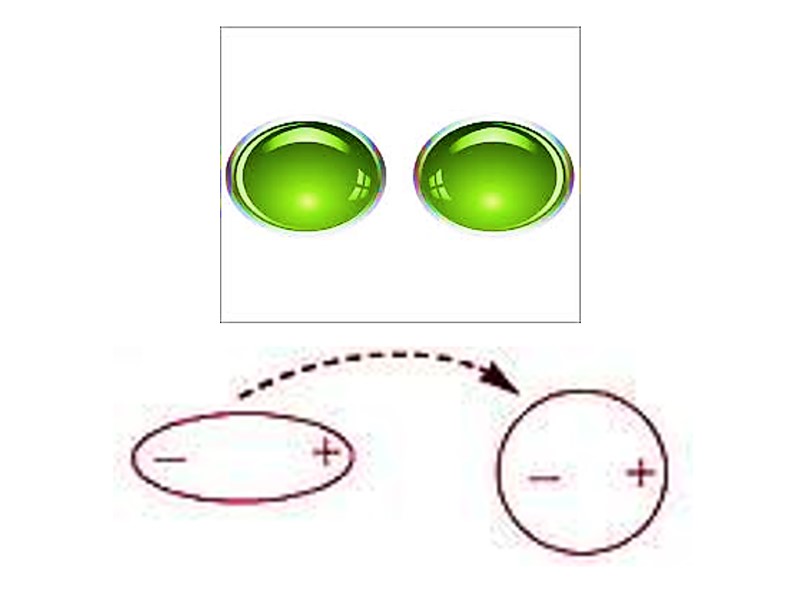

Два диполя слабо притягивают друг друга приближая ядра друг к другу Ядра двигаются друг к другу до тех пор пока их электронные облака не начинают отталкиваться В точке, где сила притяжения точно уравновешивает силы отталкивания, ядра находятся в Ван дер Ваальсовом контакте

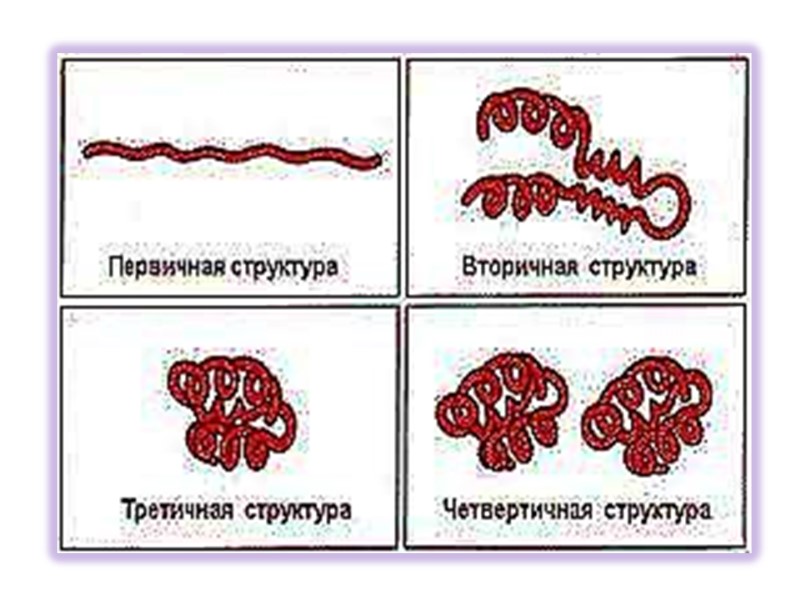
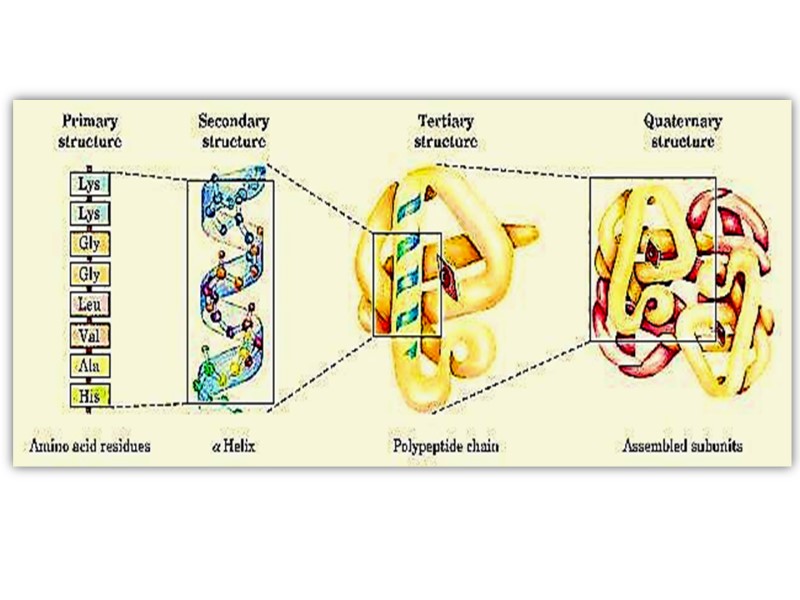
Структуры белков Выделяют 4 уровня структурной организации в белках: Первичная структура: последовательность аминокислот Вторичная структура: модель регулярной укладки Третичная структура: трехмерная укладка в пространстве Четвертичная структура: взаимодействие между отдельными полипептидными цепями
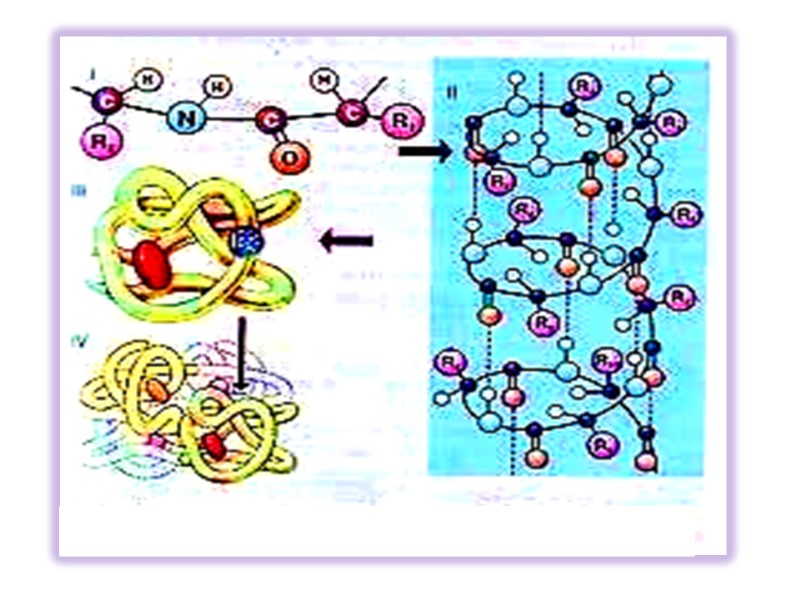
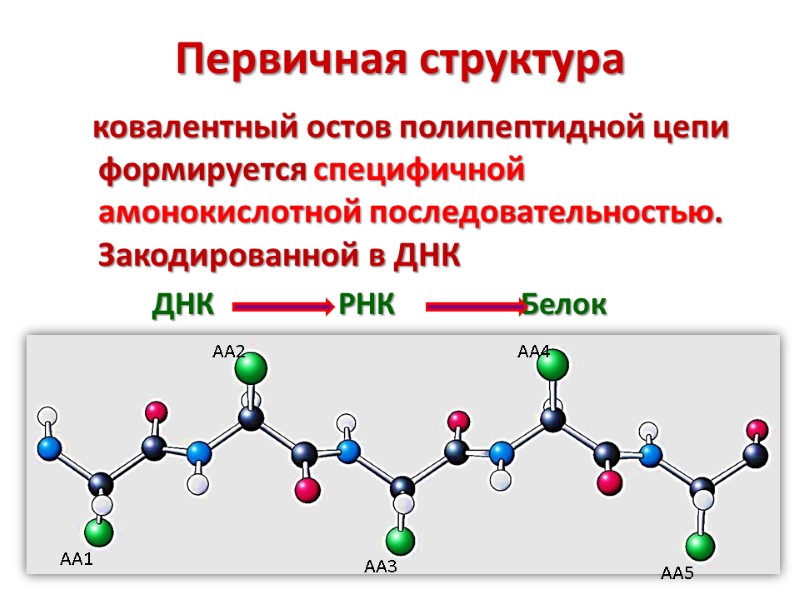
Первичная структура ковалентный остов полипептидной цепи формируется специфичной амонокислотной последовательностью. Закодированной в ДНК ДНК РНК Белок

Аминокислотная последовательность предоставляет важную биохимическую информацию Аминокислотная последовательность белка может проливать свет на трехмерную структуру белка, его функцию, клеточную локализацию, эволюцию

дает возможность реализовать искусственный синтез белка Инсулин был первым белком, первичная структура которого была расшифрована и в 1953 году этот гормон был синтезирован
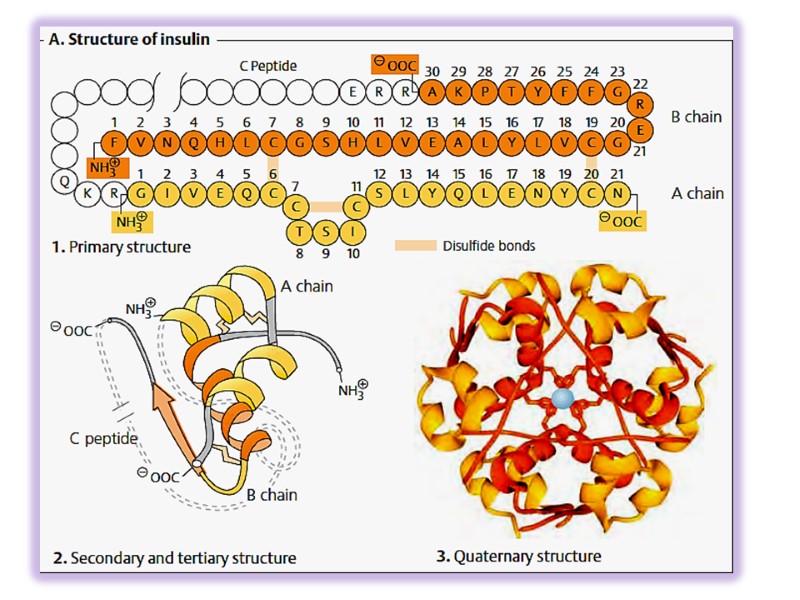

Изменение последовательности аминокислот может привести к потере белком его функций и тяжелым заболеваниям Классический пример – гемоглобинопатии (серповидно-клеточная анемия)
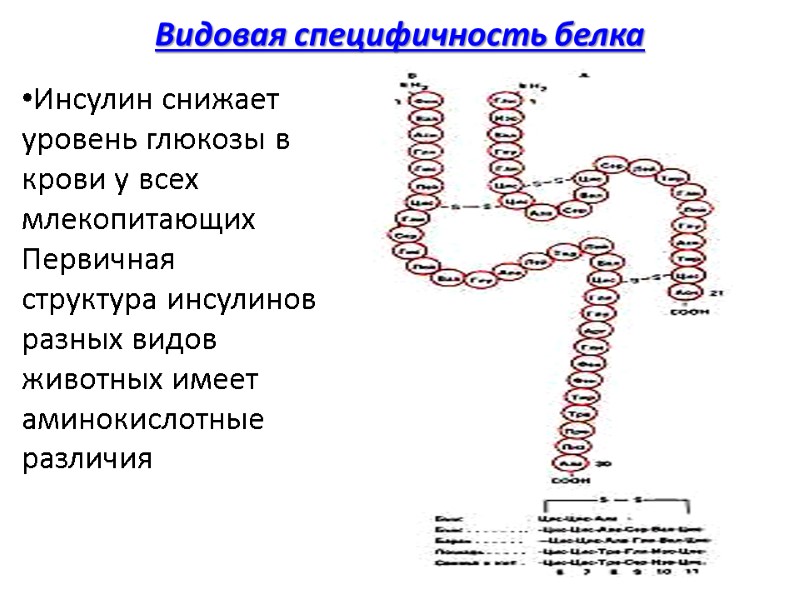
Видовая специфичность белка Инсулин снижает уровень глюкозы в крови у всех млекопитающих Первичная структура инсулинов разных видов животных имеет аминокислотные различия
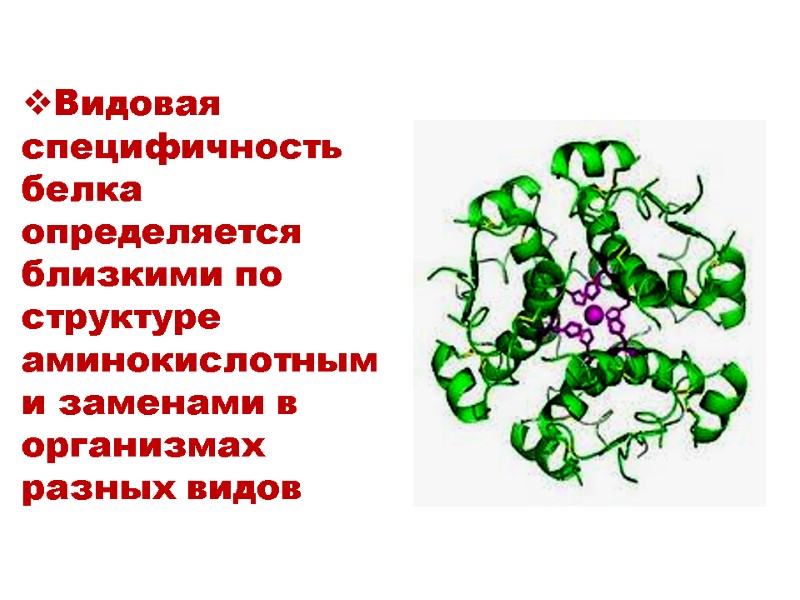
Видовая специфичность белка определяется близкими по структуре аминокислотными заменами в организмах разных видов

Вторичная структура Вторичная структура - это регулярно повторяющаяся форма укладки полипептидной цепи в пространстве Известны два типа вторичной структуры – α- спираль β- складчатая структура
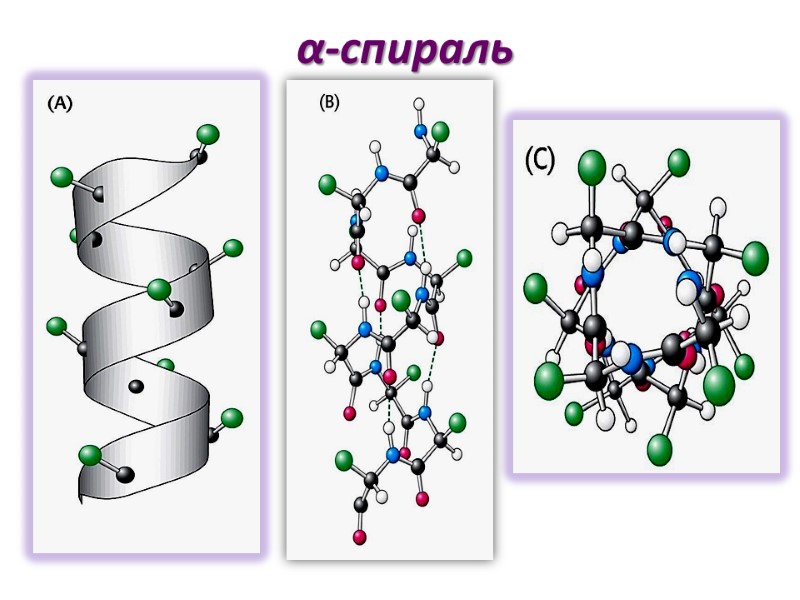
α-спираль

Параметры α-спирали α- спираль- правозакрученная (по часовой стрелке) Один оборот включает 3.6 остатков аминокислот Период спирали (расстояние между двумя витками) - 0,54nm
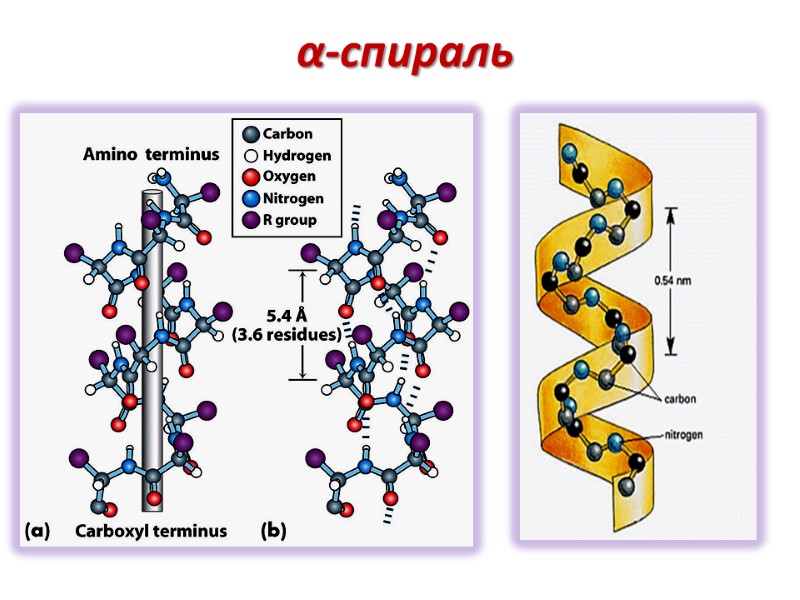
α-cпираль
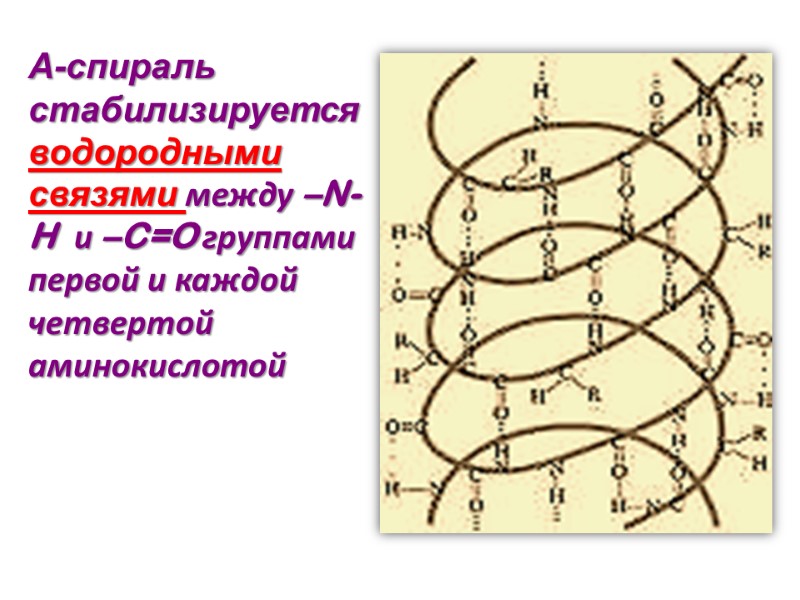
Α-спираль стабилизируется водородными связями между –N-H и –C=O группами первой и каждой четвертой аминокислотой
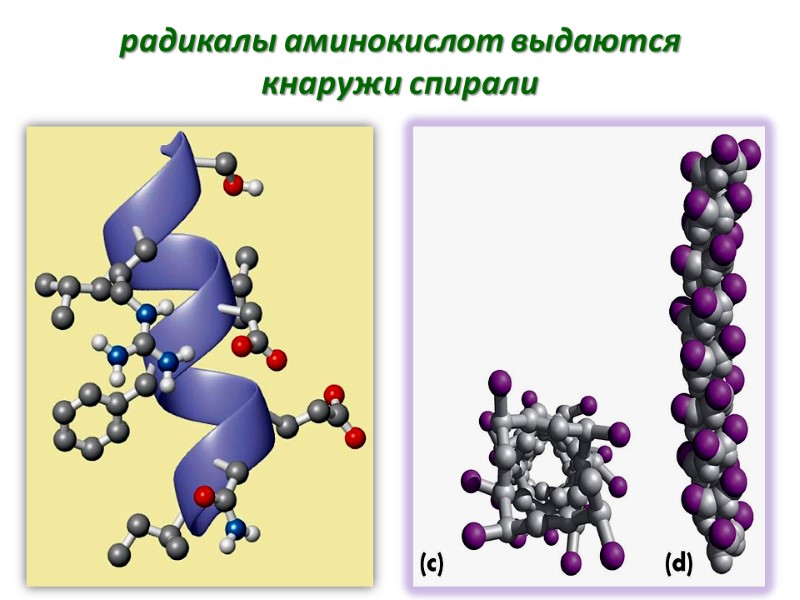
радикалы аминокислот выдаются кнаружи спирали

Длина α-спиральных участков в глобулярных белках варьирует от 0 до 75% (до 100%) общей длинны полипептидной цепи Взаимодействия между боковыми радикалами аминокислот стабилизируют или дестабилизируют эту структуру

Например, большое количество глютаминовой кислоты на каком-то фрагменте полипептидной цепи препятствуют образованию спирали Отрицательно заряженные карбоксильные группы боковых радикалов глютаминовой кислоты отталкиваются настолько сильно, что препятствуют образованию α-спирали
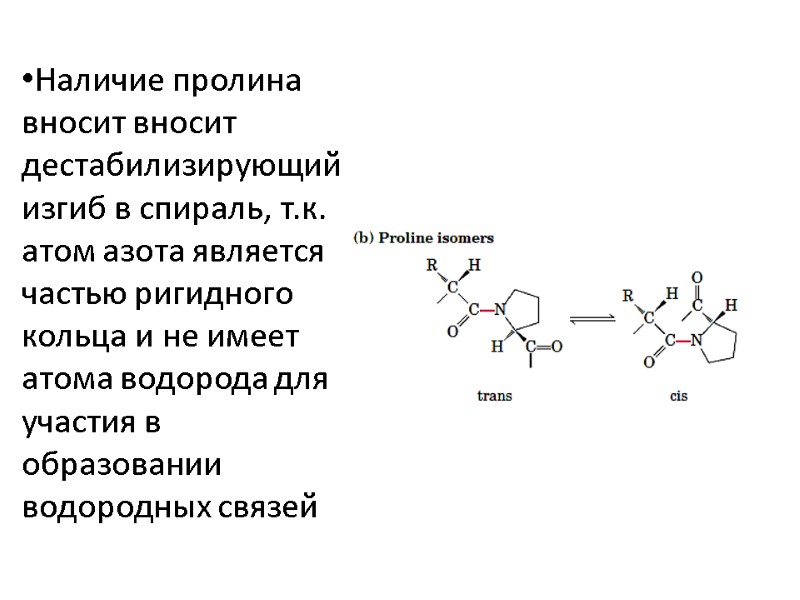
Наличие пролина вносит вносит дестабилизирующий изгиб в спираль, т.к. атом азота является частью ригидного кольца и не имеет атома водорода для участия в образовании водородных связей

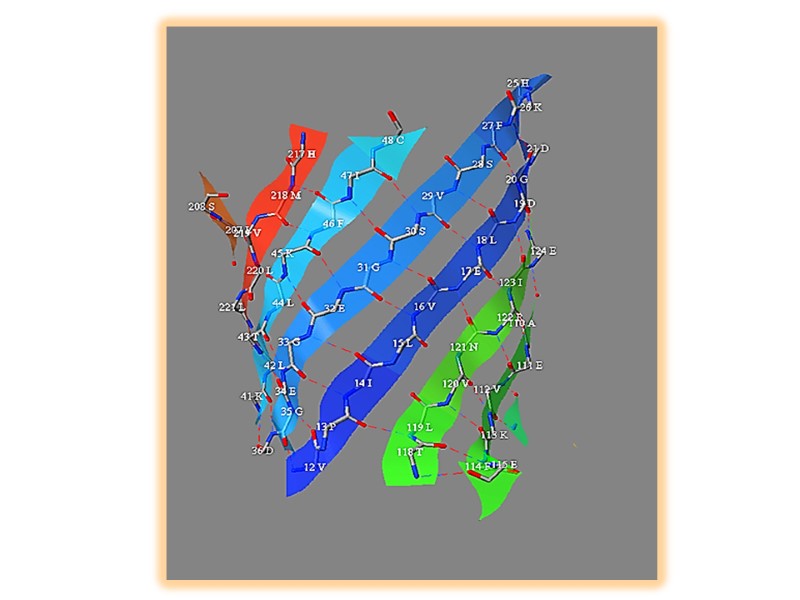
β-складчатая структура Сегменты полипептидной цепи уложены наподобие равномерно сложенных листов бумаги водородные связи формируются между соседними цепями в складчатых структурах Боковые радикалы аминокислот направлены в противоположных направлениях зигзагообразной структуры
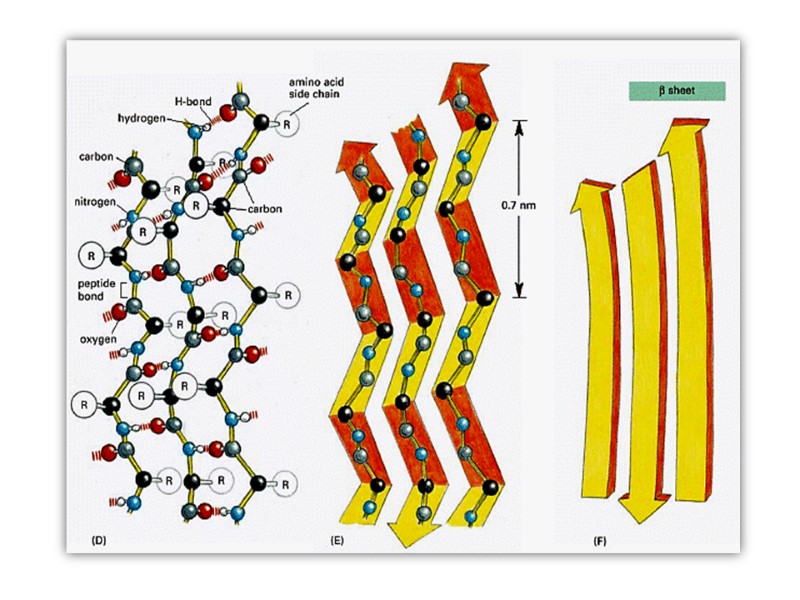

Соседние нити могут быть ориентированы параллельно или антипараллельно
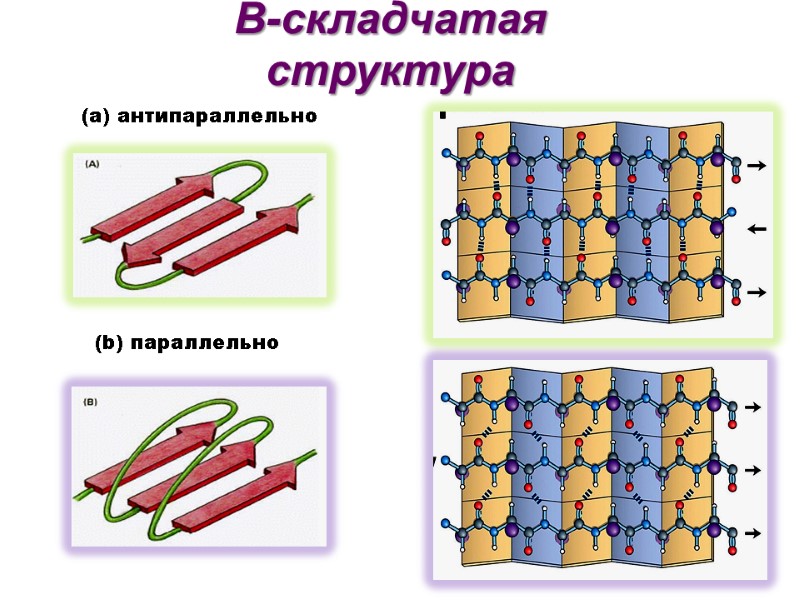
Β-складчатая структура (b) параллельно (а) антипараллельно
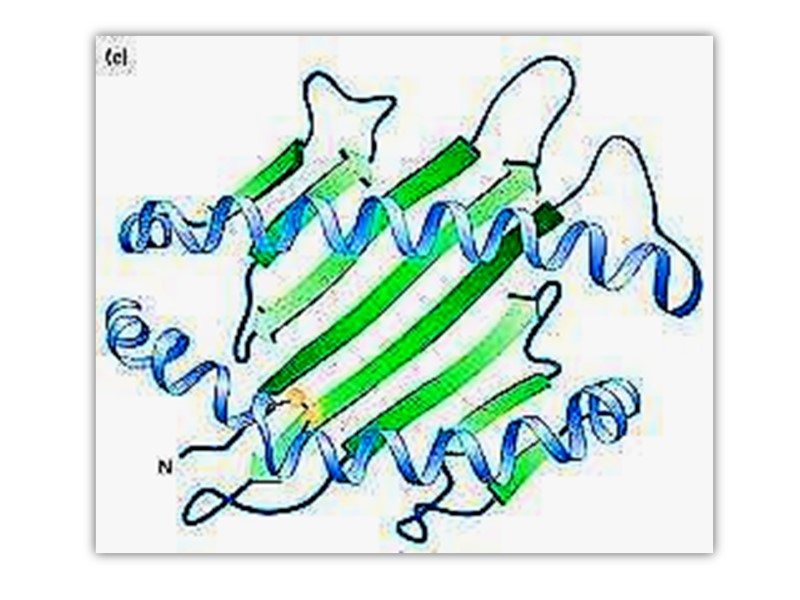

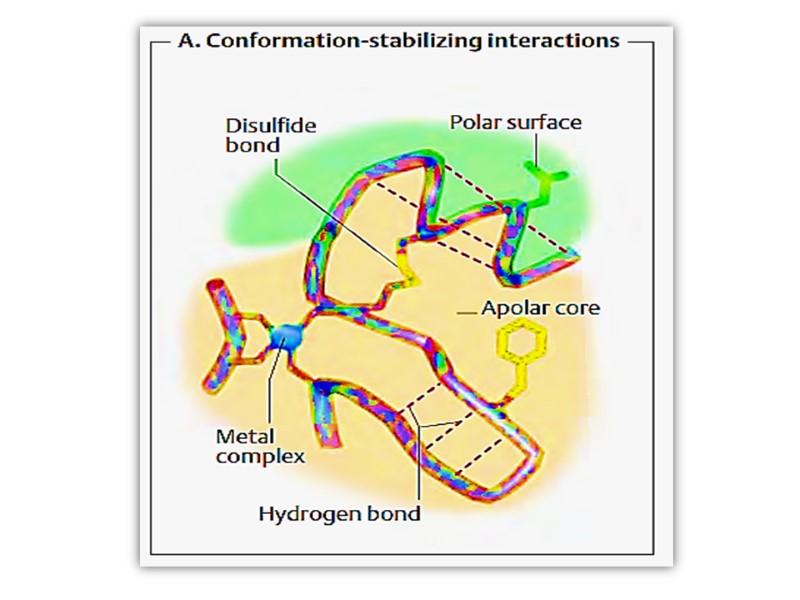
Третичная структура Трехмерное расположение атомов в пространстве представляет собой третичную структуру белка Характерная для каждого белка укладка в пространстве детерминирована аминокислотной последовательностью
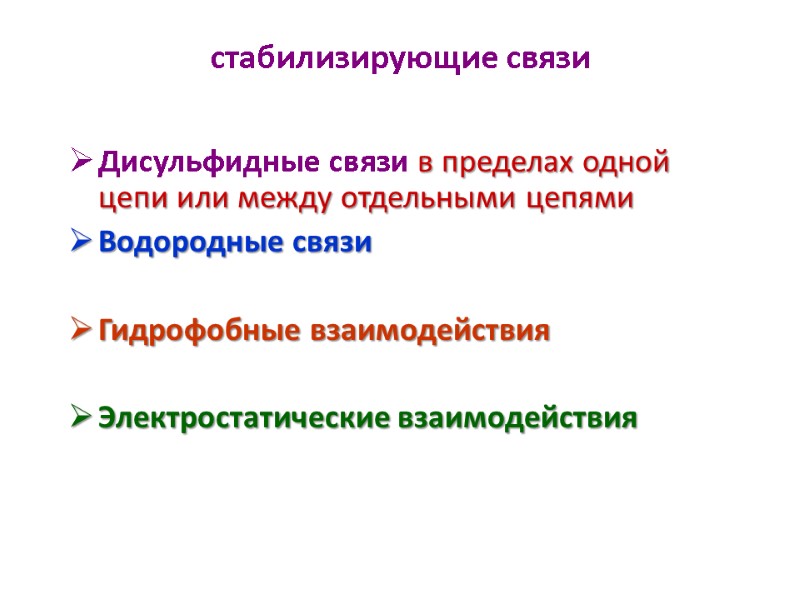
стабилизирующие связи Дисульфидные связи в пределах одной цепи или между отдельными цепями Водородные связи Гидрофобные взаимодействия Электростатические взаимодействия
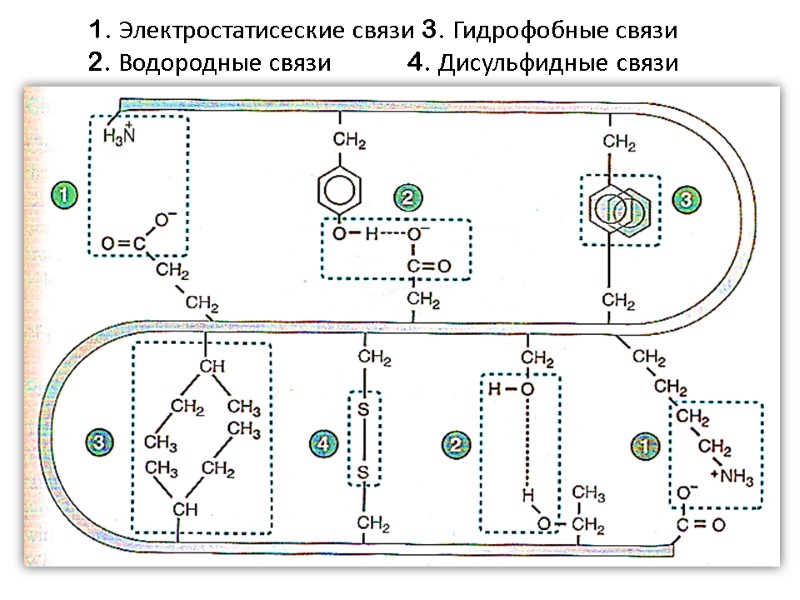
1. Электростатисеские связи 3. Гидрофобные связи 2. Водородные связи 4. Дисульфидные связи

Радикалы неполярных аминокислот чаще располагаются внутри молекулы белка (вдали от молекул воды) Полярные группы – на поверхности, взаимодействуя с диполями воды

Нативное состояние белка В оптимальных условиях белок находится в нативном состоянии (складывается в третичную структуру), что соответствует наиболее термодинамически устойчивому взаиморасположению атомов в молекуле Пространственное расположение атомов в молекуле белка называется конформация

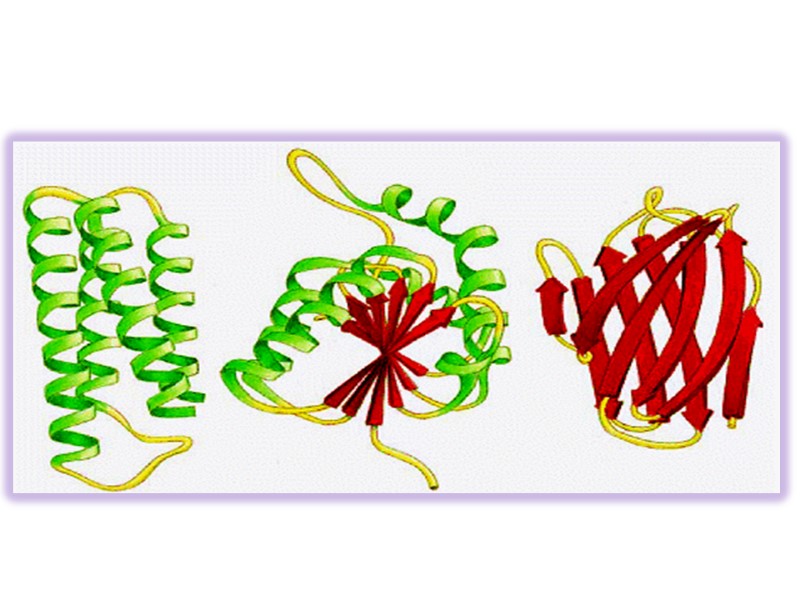
Третичная структура белка может включать различные типы вторичной укладки
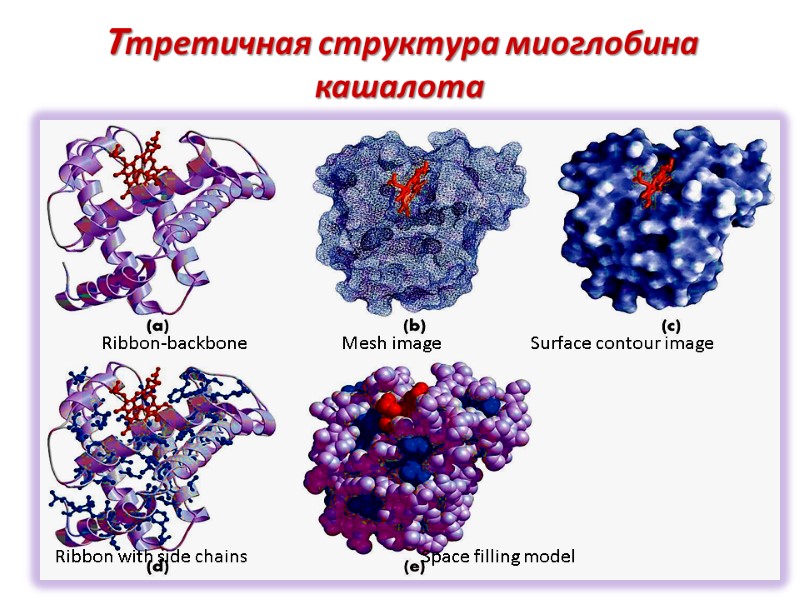
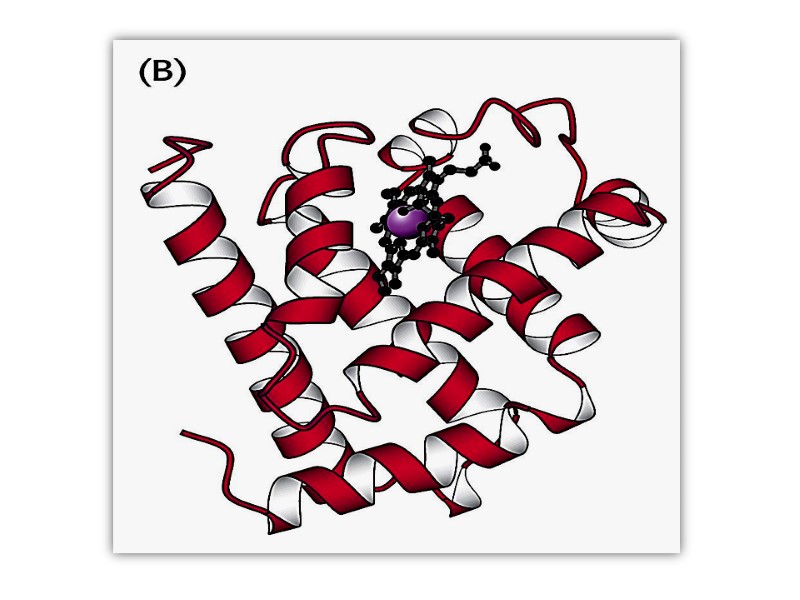
Tтретичная структура миоглобина кашалота Ribbon-backbone Mesh image Surface contour image Ribbon with side chains Space filling model

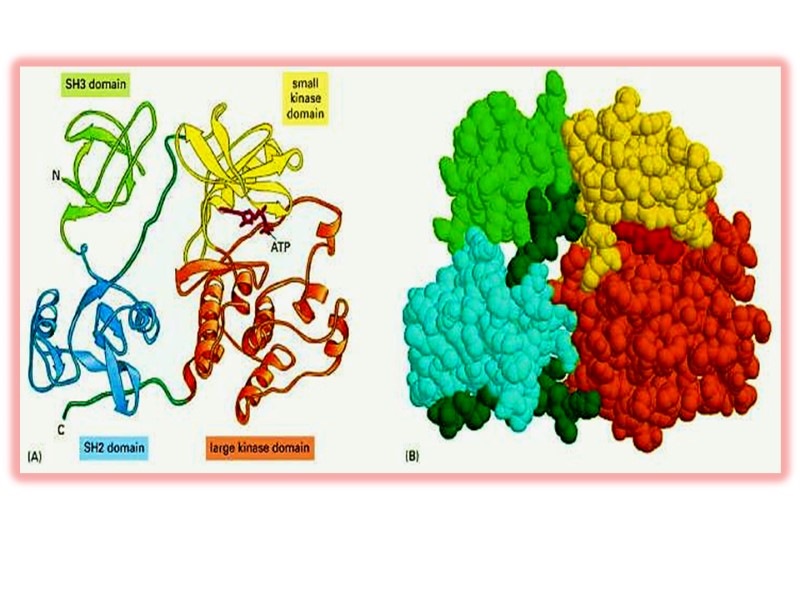
Сегменты одного и того же белка в пределах одной полипептидной цепи могут складываться независимо формируя «домен» Разные домены часто обладают различной функциональной активностью

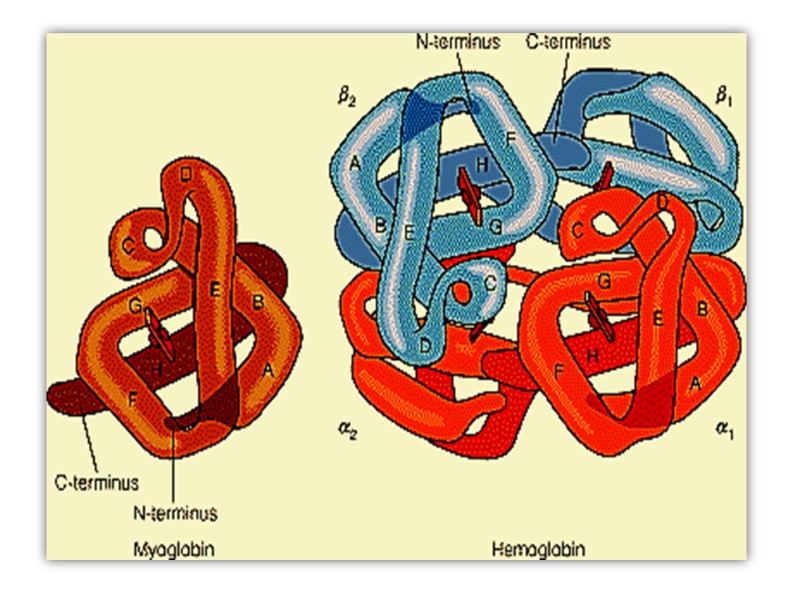
Четвертичная структура белка Некоторые белки включают две и более полипептидные цепи, которые могут быть идентичными и разными. Подобные белки называют олигомерными

Отдельная полипептидная цепь называется протомер Олигомерный белок может быть димером, тетрамером и т.д. Каждый протомер формируется независимо и взаимодействует с другими протомерами формируя целостную структуру Протомеры ассоциируются в соответствии с принципом комплементарности Ассоциация протомеров формирует четвертичную структуру белка
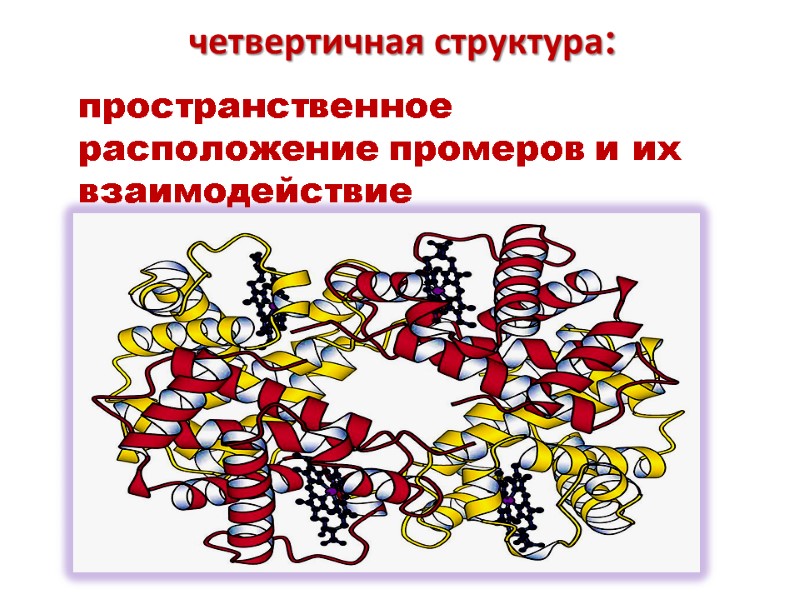
четвертичная структура: пространственное расположение промеров и их взаимодействие

Четвертичная структура стабилизируется слабыми связями: Гидрофобными Электростатическими Водородными

Большинство олигомерных белков состоит из различных функциональных субъединиц Функциональная субъединица может включать один или несколько протомеров и отвечает за функциональную активность белка

В олигомерном белке конформационные изменения в одном протомере вызывают изменения конформаций всех остальных протомеров (функциональных единиц) Это свойство называют кооперативные изменения конформаций протомеров олигомерного белка играет решающую роль в функциональной активности белка
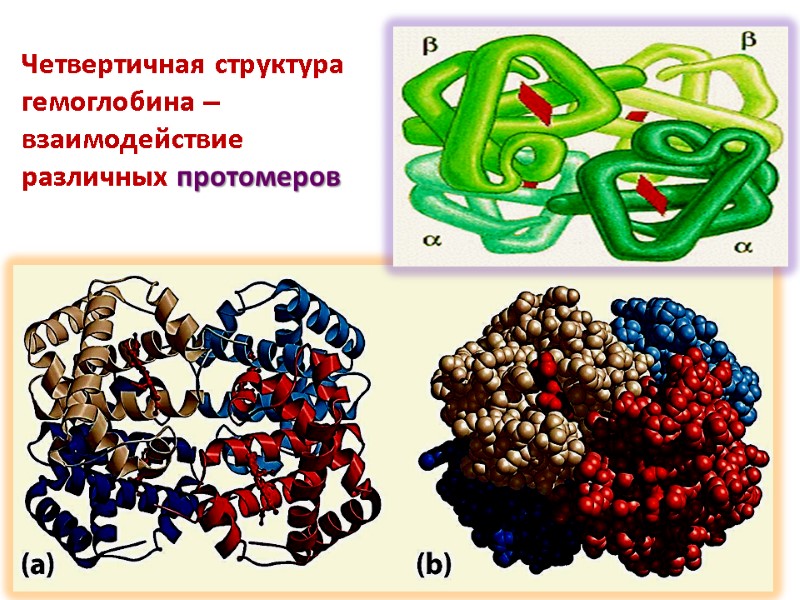
Четвертичная структура гемоглобина – взаимодействие различных протомеров

физико-химические свойства белков

молекулярная масса белков Белки – высокомолекулярные вещества, состоящие из тысяч аминокислот Белки обладают молекулярной массой больше 6000-8000D Пептиды – меньшей Мм В отличие от пептидов белки обладают высшими уровнями структурной организации Пептиды – только первичной структурой

Для определения молекулярной массы используют: Аналитическое ультрацентрифугирование Гель-фильтрационную хроматографию Электрофорез в полиакриламидном геле Электронную микроскопию

Высокая молекулярная масса определяет обуславливает определенные свойства растворов белков Высокая вязкость Медленная диффузия Набухание Низкое осмотическое давление Высокое онкотическое давление
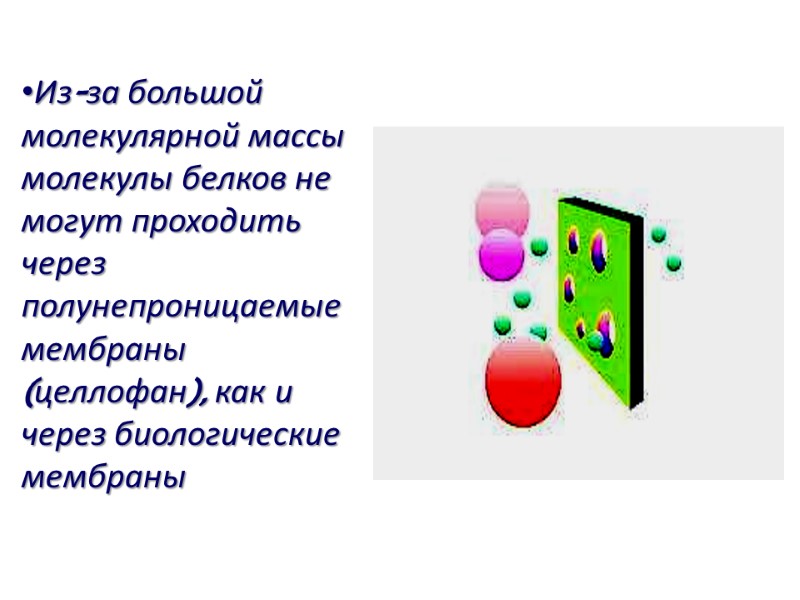
Из-за большой молекулярной массы молекулы белков не могут проходить через полунепроницаемые мембраны (целлофан), как и через биологические мембраны
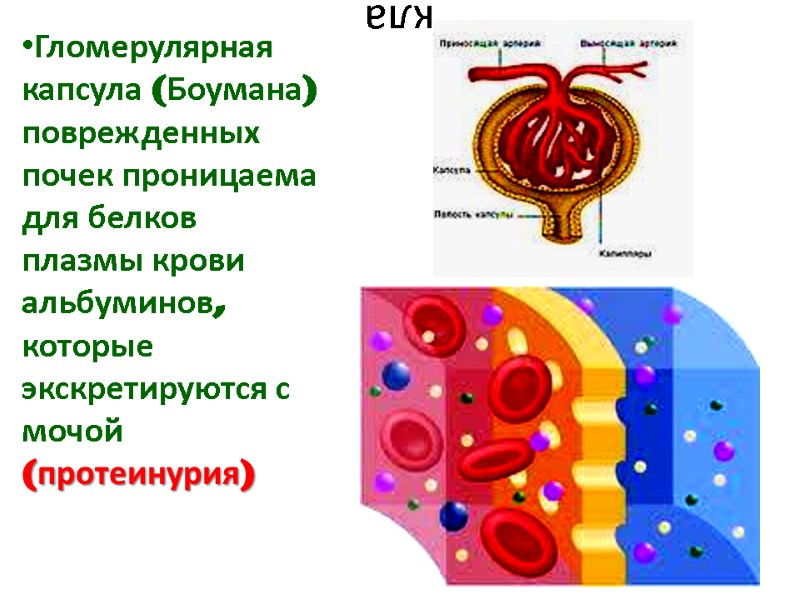
кла Гломерулярная капсула (Боумана) поврежденных почек проницаема для белков плазмы крови альбуминов, которые экскретируются с мочой (протеинурия)

форма молекул белков В соответствии с наличием уровней высокой организации белки классифицируются на две большие группы: фибриллярные белки полипептидные цепи которых располагаются в виде длинных нитей или листов (отношение длины к ширине больше 10) глобулярные белки свернутые в виде сфер или глобул (дл/ш< 10)
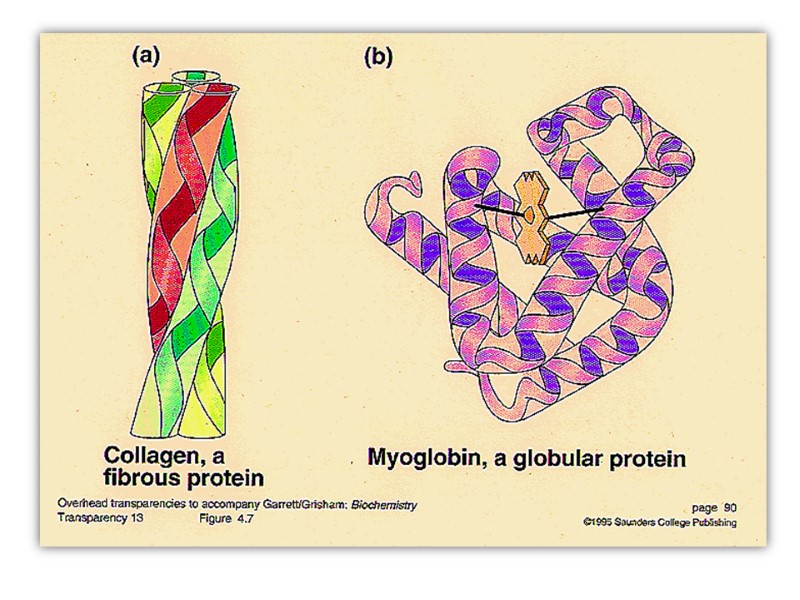
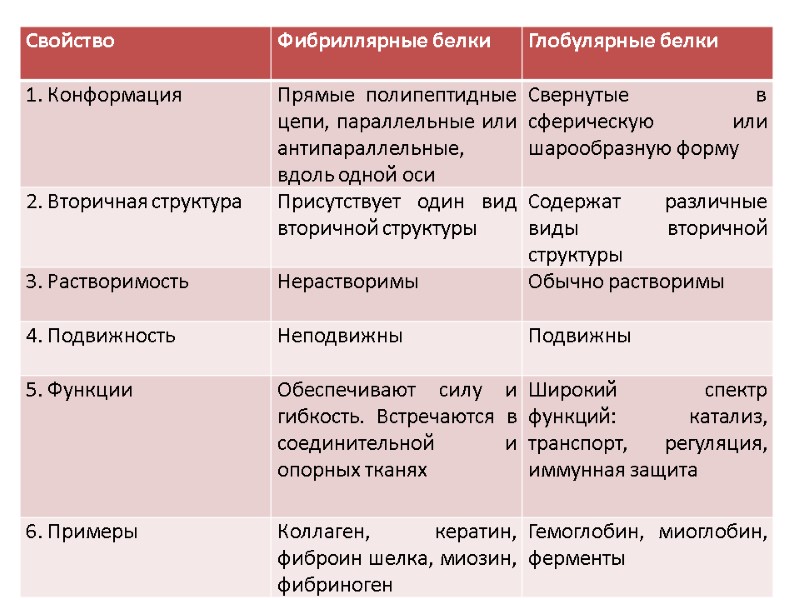

Растворимость белков Растворимость белков зависит от количества гидрофобных остатков, размеров, формы белковой молекулы и суммарного заряда белки: Растворимы в дистиллированной воде (альбумины) Нерастворимы в дистиллированной воде, но растворимы в слабо солевых растворах (5-10% NaCl, KCl; глобулины) Нерастворимые (коллаген, эластин) растворы белков – истинные растворы

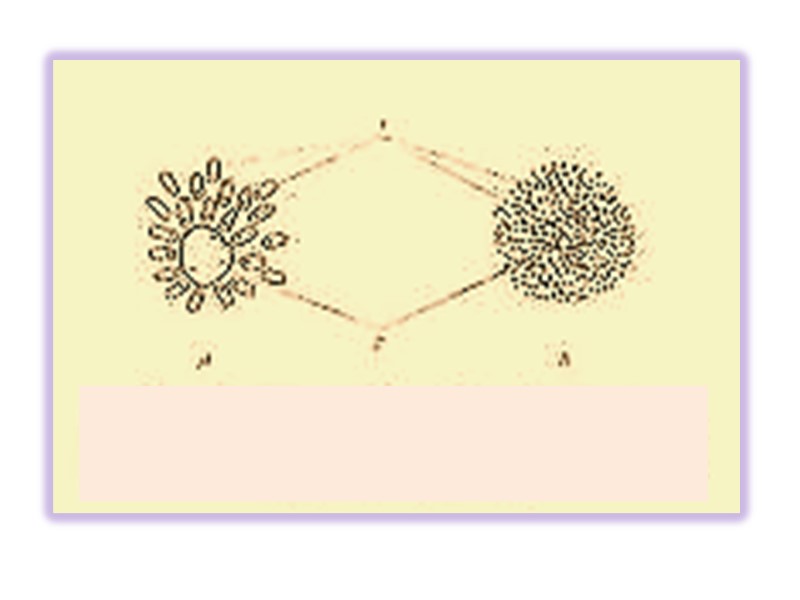
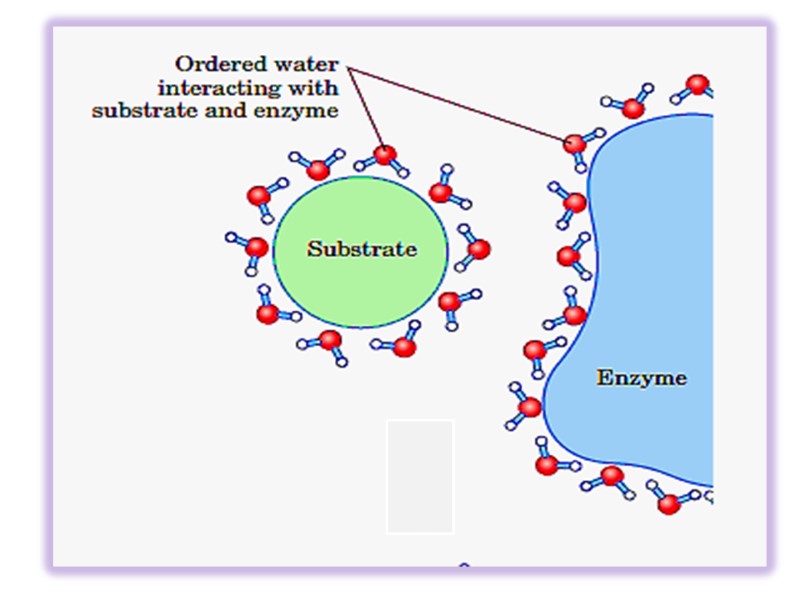
Факторы стабильности белковых растворов І. Образование гидратной оболочки Гидрофобные радикалы аминокислот стремятся сгруппироваться внутри молекулы белка как можно дальше от молекул воды Полярные группы обычно образуют водородные связи с диполями воды (растворимы)
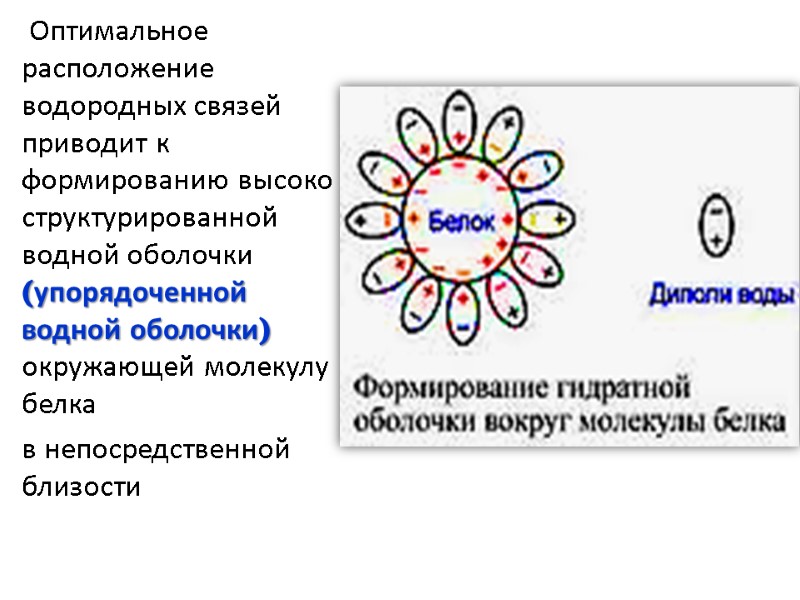
Оптимальное расположение водородных связей приводит к формированию высоко структурированной водной оболочки (упорядоченной водной оболочки) окружающей молекулу белка в непосредственной близости

ІІ. Образование заряда Белки это амфотерные соединения благодаря концевым амино- и карбоксильным группам и диссоциирующим боковым радикалам (лиз, арг, гис, асп, глю) В зависимости от рН среды и количества кислотных или основных групп молекулы белка несут положительный или отрицательный заряд, вызывающий электростатическое отталкивание
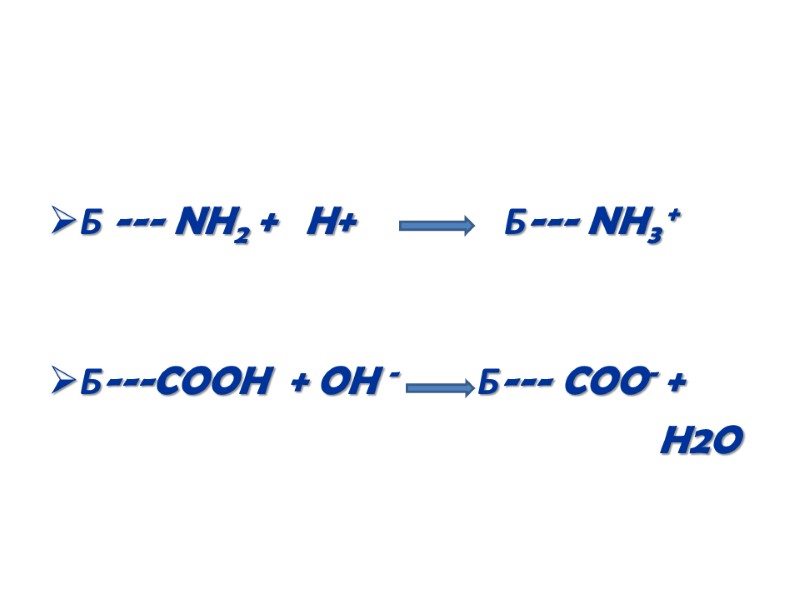
Б --- NH2 + H+ Б--- NH3 + Б---COOH + OH - Б--- COO- + H2O

Осаждение белка из раствора Для седиментации белка используют следующие техники: Дегидратация Изоэлектрическое осаждение Денатурация

Дегидратация проводится а. добавлением водоотнимающих веществ: ацетон, эфир и др.

б. высаливанием добавление растворов солей высокой концентрации Например, сульфат аммония (NH4)2SO4

SO4 2- притягивают диполи воды из раствора и из водной оболочки окружающей молекулу белка Белок, лишенный водной оболочки, осаждается Различные белки осаждаются различными концентрациями солей, чем пользуются для разделения белков


Изоэлектрическое осаждение Изменяя рН раствора возможно уравнять количество положительно и отрицательно заряженных групп, при этом заряд молекулы будет равен нулю Белок находится в изоэлектрическом состоянии, когда его заряд равен нулю Определенная pH при которой белок имеет нулевой заряд называется изоэлектрической точкой (pI).

Изоэлектрическая точка(pI) большинства животных белков находится в диапазоне 5,5 – 7,0, что указывает на преобладание кислых аминокислот рl альбуминов- 4,7 рl глобулинов~ 7,0 Стабильность белков в изоэлектрическом состоянии снижается и они осаждаются из раствора
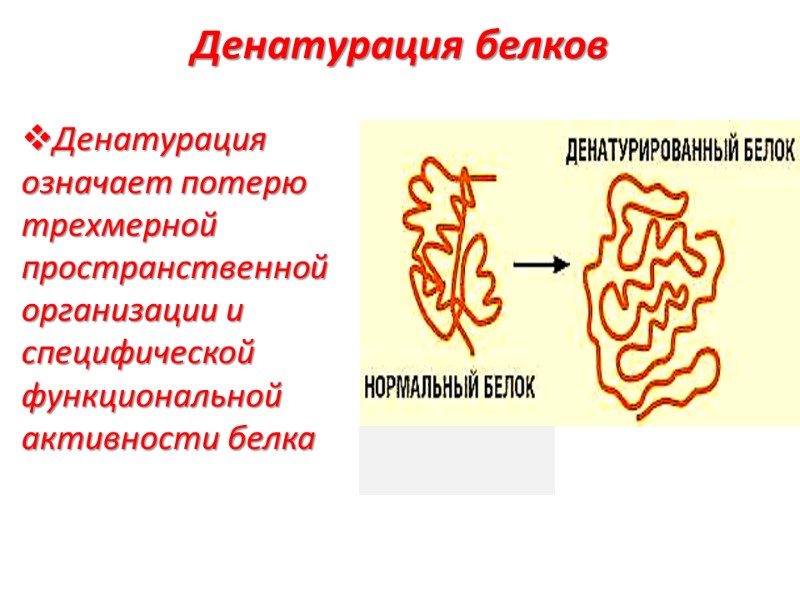
Денатурация белков Денатурация означает потерю трехмерной пространственной организации и специфической функциональной активности белка

Денатурированный белок теряет все высшие уровни организации кроме первичной

Разрушение уникальной нативной конформации сопровождается потерей специфических свойств: растворимости, электрофоретической подвижности, функциональной активности

Нативные молекулы белка раскручиваются, образуя случайные и неупорядоченные структуры Денатурация связана, главным образом, с разрывом слабых связей и дисульфидных мостиков

Факторы вызывающие денатурацию Физические: Температура (воздействует на слабые взаимодействия, преимущественно на водородные связи) Давление Вибрация УФЛ

Химические: Органические и минеральные кислоты Основания Соли тяжелых металлов Мочевина, детергенты

Денатурация может быть обратимой Ренатурация возможна и белок восстановит свою нативную конформацию и биологическую активность, если будет помещен в условия, в которых эта конформация стабильна
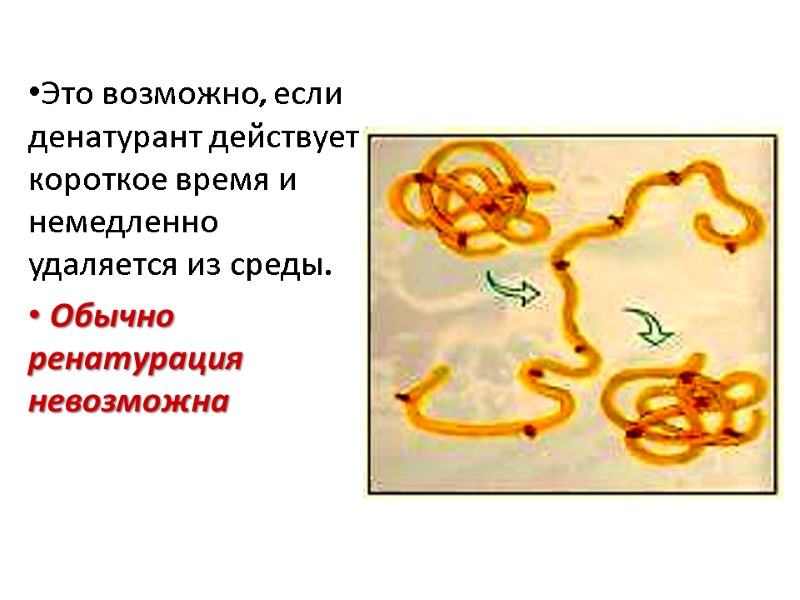
Это возможно, если денатурант действует короткое время и немедленно удаляется из среды. Обычно ренатурация невозможна

В живой клетке белки денатурируют с медленной скоростью В клетках присутствуют специальные белки, функция которых состоит в восстановлении нативной структуры частично денатурированных белков Это шапероны

Использование денатурации Определение наличия белка в растворе Измерение концентрации белка Удаление белка из раствора

Стерилизация (высокая температура, УФЛ, раствор фенола)

При отравлении солями тяжелых металлов Используют сырой яичный белок – денатурируя в жкт он препятствует всасыванию тяжелых металлов

денатурированные белки легче перевариваются

Оптические свойства растворов белка Поглощение ультрафиолетового цвета Раствор белка поглощает свет 280 nm благодаря присутствию ароматических аминокислот Рефракция (преломление)пучка света Рассеивание пучка света

Открытие белка в растворах І. Цветные реакции : биуретовая, ксантопротеиновая, Фоля и др. ІІ. Реакции осаждения: денатурация, высаливание

определение концентрации белка По количеству азота в данном белке (N=16%) Основанные на оптических свойствах: рефрактометрия нефелометрия спектрофотометрия колориметрия

Protein classification Hemoglobin, myoglobin. Collagen
Classification of proteins There are two main groups of proteins depend on chemical structure: simple and conjugated. Simple proteins are composed of amino acids only and hydrolyzed to the corresponding amino acids.

Conjugated proteins contain non-protein component besides amino acids which is referred to as prosthetic group. Choloprotein(conjugated protein) = apoprotein (protein part) + prosthetic group (non-protein part).

Simple proteins are divided into several subgroups in dependence on amino acid composition and physico-chemical properties: albumins globulins protamines histones prolamines glutelins Proteinoids (collagen, elastin, keratin)
The subdivision of conjugated proteins is based on the chemical nature of prosthetic group. Accordingly they are chromoproteins (containing color part) nucleoproteins (nucleic acids) glycoproteins (carbohydrate) lipoproeins (lipid) phosphoprotiens (phosphate) metalloproteins (metal)
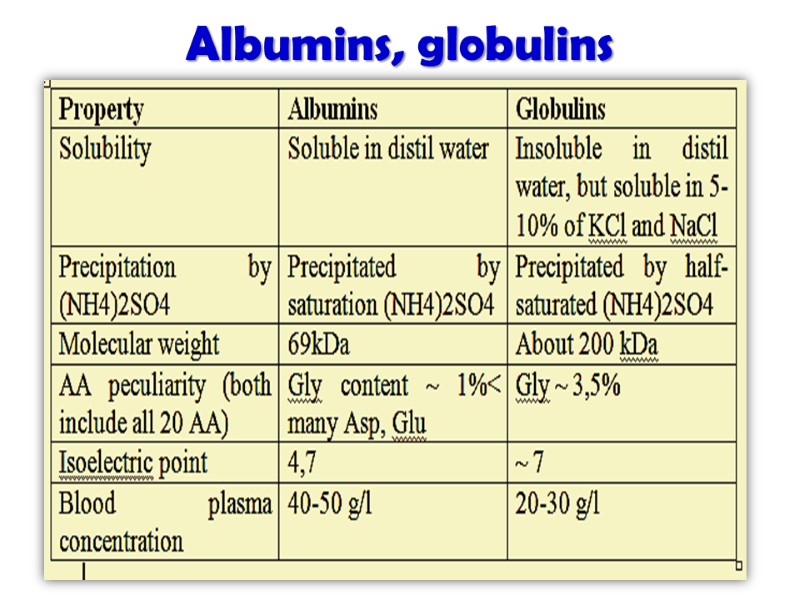
Albumins, globulins
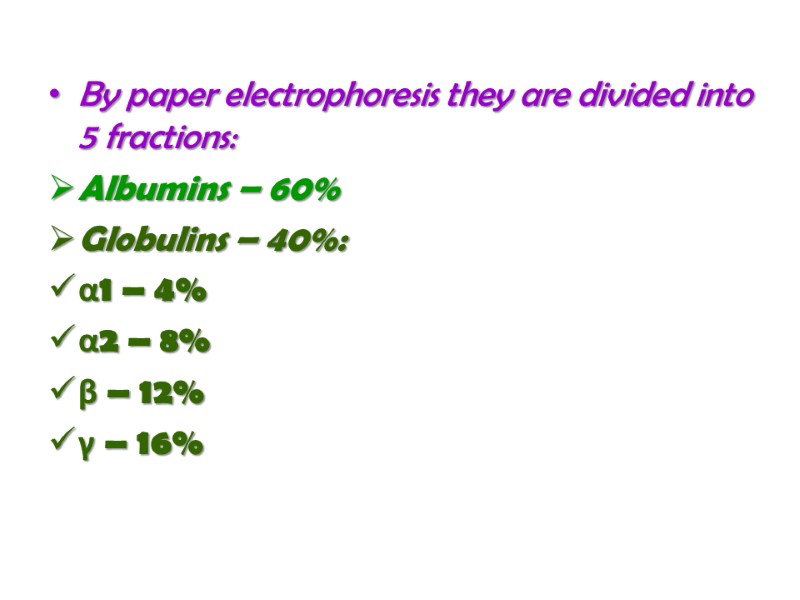
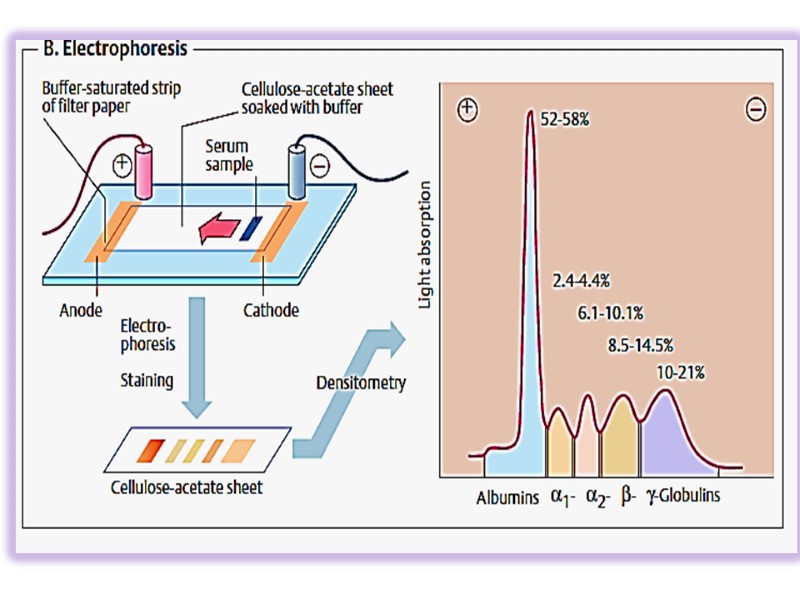
By paper electrophoresis they are divided into 5 fractions: Albumins – 60% Globulins – 40%: α1 – 4% α2 – 8% β – 12% γ – 16%
Albumins fulfill many functions: supporting oncotic pressure, transport of small molecules and ions (fatty acids, bilirubin, Ca2+, Cu2+, some drugs) Globulins take part in transport functions (hormones, vitamins, ions), protective function.
Protamines, histones These proteins contain many basic amino acids (Arg and Lys) that determines their properties. Protamins include 60-85% of Arg, histones – 20-30% of Arg+Lys. Isoelectric point lies in the alkaline region. At pH 7,4 they bear positive charge They have low molecular weight (histones ~ 11-22 kGa). They are readily soluble in water.
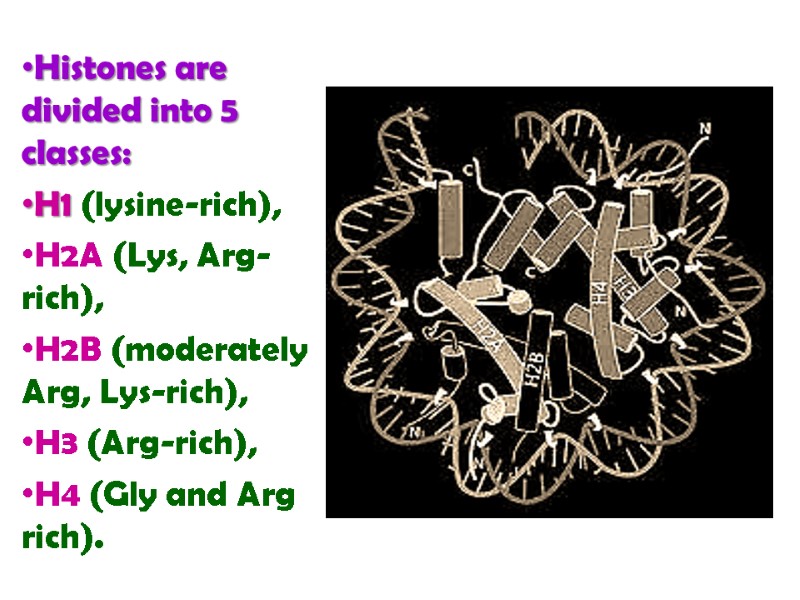
Histones are divided into 5 classes: H1 (lysine-rich), H2A (Lys, Arg-rich), H2B (moderately Arg, Lys-rich), H3 (Arg-rich), H4 (Gly and Arg rich).
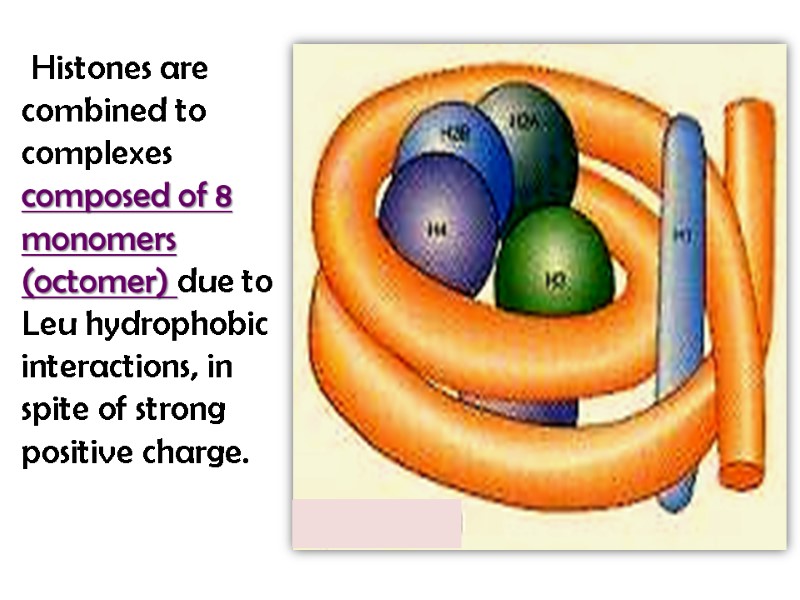
Histones are combined to complexes composed of 8 monomers (octomer) due to Leu hydrophobic interactions, in spite of strong positive charge.
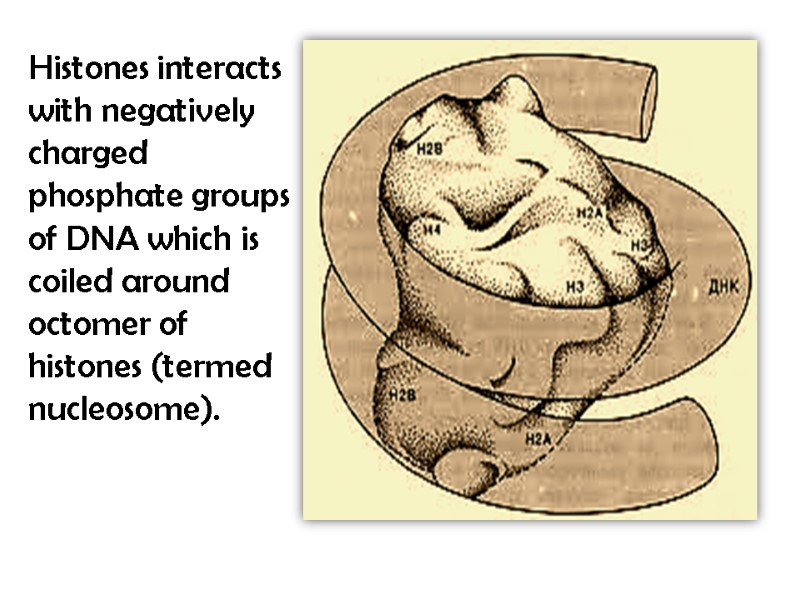
Histones interacts with negatively charged phosphate groups of DNA which is coiled around octomer of histones (termed nucleosome).
Nucleus of a each cell includes about 60 mln molecules of each class of histones. Amino groups of Arg and Lys residues and terminus can be modified with acetic group, phosphate, and methyl. This may change the charge of molecule and can lead to conformational changes and influence interaction between DNA and histones.

Prolamines, glutelins These are plant proteins containing many Glu and Pro but not enough essential AA.
Prolamines contain 20-25% of Glu, 10-15% of Pro. Prolamines and glutelins are insoluble in water, but soluble in 60-80% ethanol solution while other proteins precipitate under these conditions.

They are chiefly found in cereal seeds as major constituents of gluten.
Nucleoproteins Nucleoproteins are composed of protein part and nucleic acids: deoxyribonucleoproteins and ribonucleoproteins. DNP and RNP are found in nuclei, RNP – in cytoplasm, DNP occurs in mitochondria.

DNA is a data bank for storing the hereditary information.
RNA serves for transmission of the genetic information.

Lipoproteins Lipoproteins are the complexes of protein and different lipids which are held by non-covalent forces. Lipoproteins include fats, fatty acids, phospholipids, cholesterol.
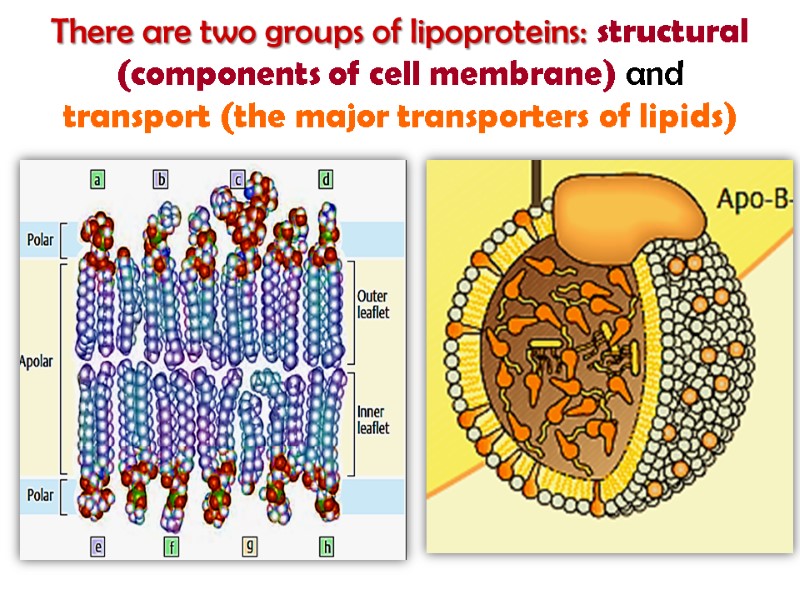
There are two groups of lipoproteins: structural (components of cell membrane) and transport (the major transporters of lipids)

Lipoproteins participate in structural complex organization of myelin sheath, nervous tissue, photoreceptor, electron-transport system. It is known lipoprotein of lung tissue thromboplastic protein; lipovitellin of hen’s egg yolk, phospholipoproteins of milk.
Glycoprotiens Glycoprotiens are the class of conjugated proteins with carbohydrate moieties covalently bound to the peptide chains by serine, threonine, tyrosine hydroxyl groups. The carbohydrate units may include a single sugar and more than 12 to 15 residues: monosaccharides, uronic acids, neuraminic acids, sialic acids.
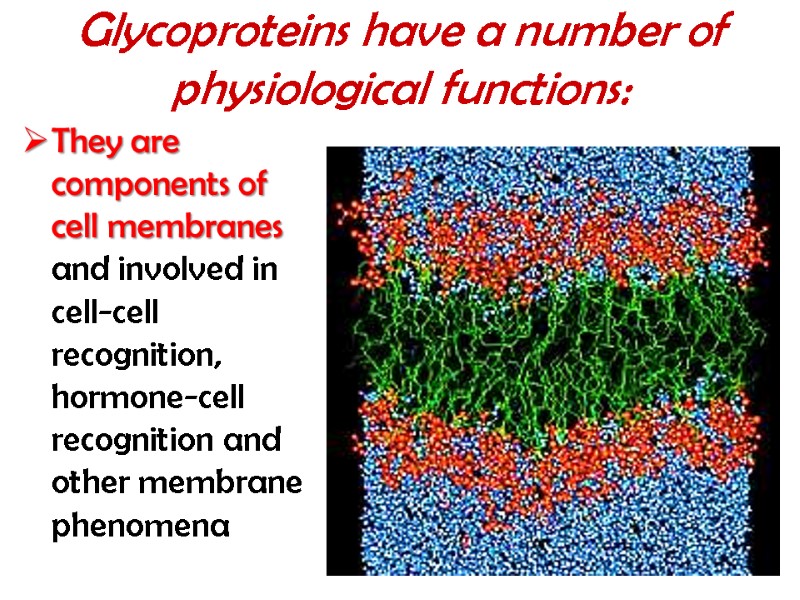
Glycoproteins have a number of physiological functions: They are components of cell membranes and involved in cell-cell recognition, hormone-cell recognition and other membrane phenomena
They are components of mucus secreted by specialized epithelial cells; they serve in the protection of tissues that line the ducts of the body. They are hormones: TTH(thyrotropic hormone), GT(gonadotropins) They are transport proteins: transferrin (iron transport), ceruloplasmin(cooper transport) They are found in connective tissue and serve to hold all components of extracellular matrix (fibronectin)

Phosphoproteins Phosphoprotiens include phosphoric acids. Phosphoric acid residue is joined covalently via hydroxyl group of serine and threonine residues. Phosphoproteins take a special place both owing to specific structural organization and their wide functional involvement into the metabolism. A number of key enzymes control the intracellular metabolism in both phosphorylated and dephosphorylated form.
Phosphoproteins contain an organically bound labile phosphate which is absolutely essential for the performance of a number of biological functions. Phosphoprotiens are abundant in the central nervous system (CNS).

Milk protein – caseinogen (1% of phosphoric acid) Hen’s egg yolk protein – vitellin, vitellinin; ovalbumin hen’s egg white
Metalloprotiens Metalloprotiens contain one or more metal ions. Ferritin is a high-molecular water-soluble protein (900 000 Da) with the iron concentration of 17-23%. It is localized mainly in the spleen, liver, bone marrow and serves for the storage of iron in the organism.
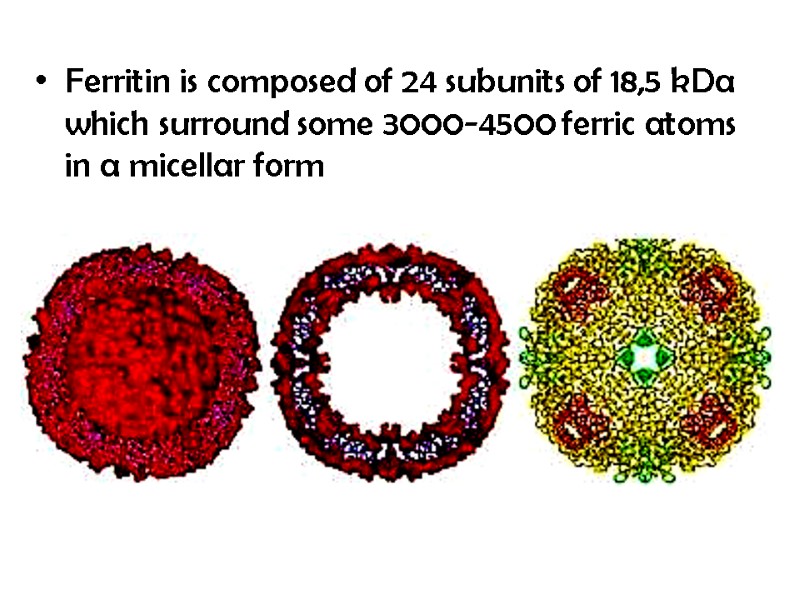
Ferritin is composed of 24 subunits of 18,5 kDa which surround some 3000-4500 ferric atoms in a micellar form
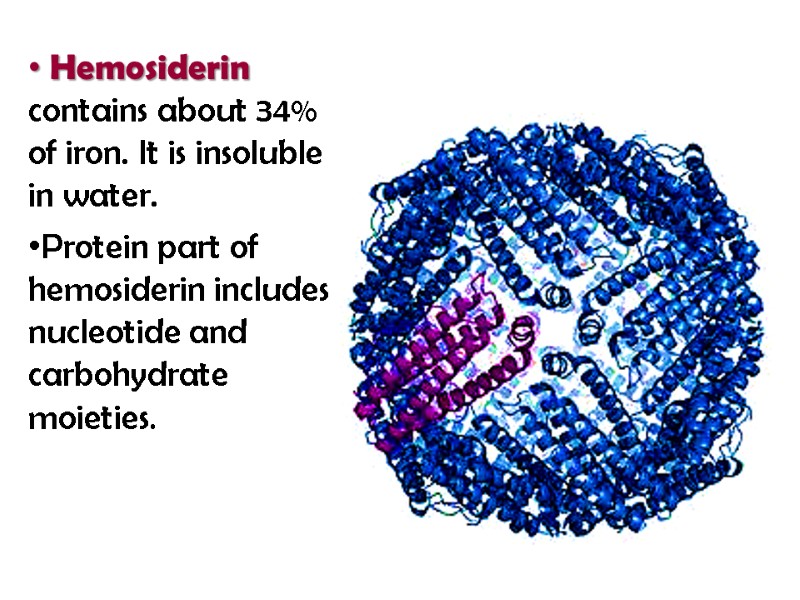
Hemosiderin contains about 34% of iron. It is insoluble in water. Protein part of hemosiderin includes nucleotide and carbohydrate moieties.
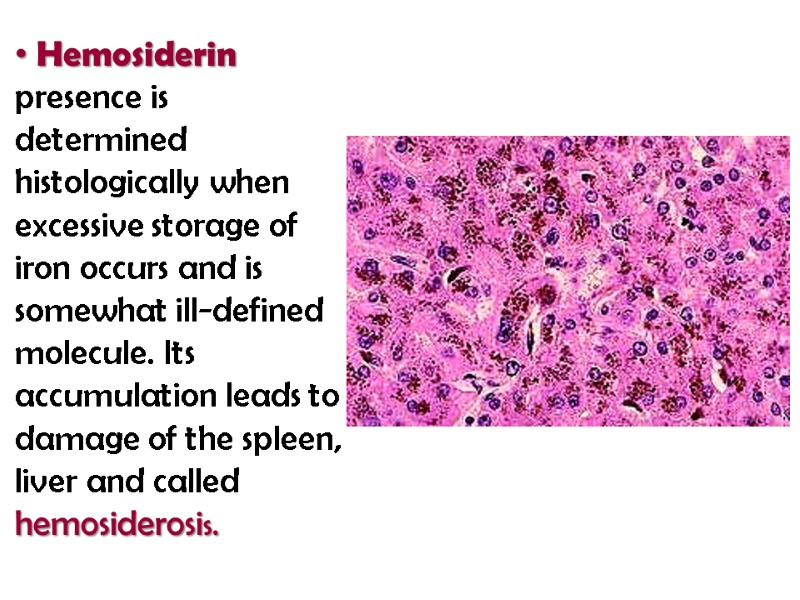
Hemosiderin presence is determined histologically when excessive storage of iron occurs and is somewhat ill-defined molecule. Its accumulation leads to damage of the spleen, liver and called hemosiderosis.
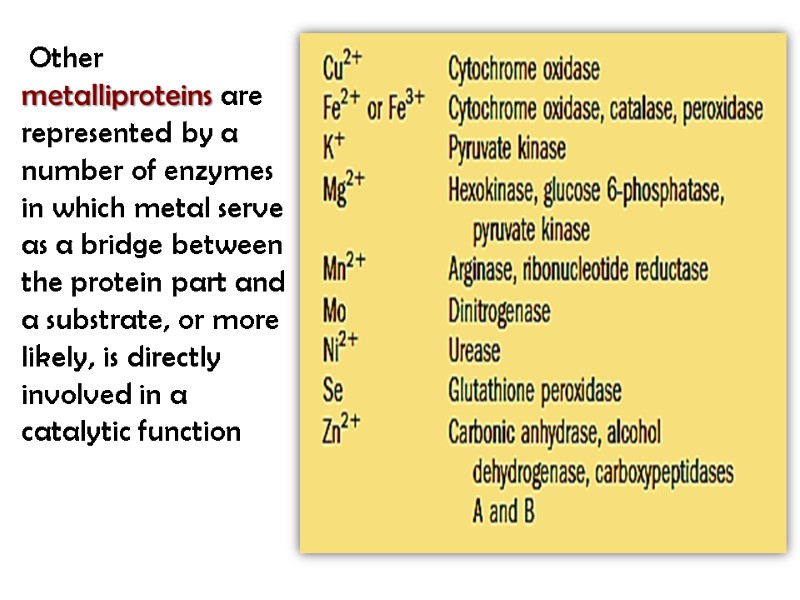
Other metalliproteins are represented by a number of enzymes in which metal serve as a bridge between the protein part and a substrate, or more likely, is directly involved in a catalytic function
Chromoproteins Chromoproteins contain a colored non-protein part (from Greek “chroma” – dye) Chromoproteins possess a number of unique biological properties: they are involved in the fundamental process of vital activity such as photosynthesis, respiration of cells and whole organism, transport of oxygen and carbon dioxide, oxidation-reduction reactions, light and color perceptibility and others.
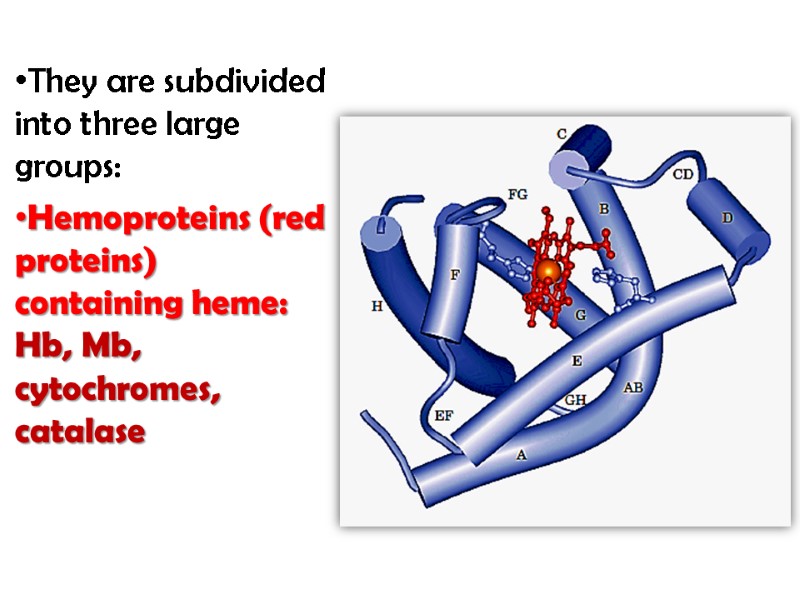
They are subdivided into three large groups: Hemoproteins (red proteins) containing heme: Hb, Mb, cytochromes, catalase
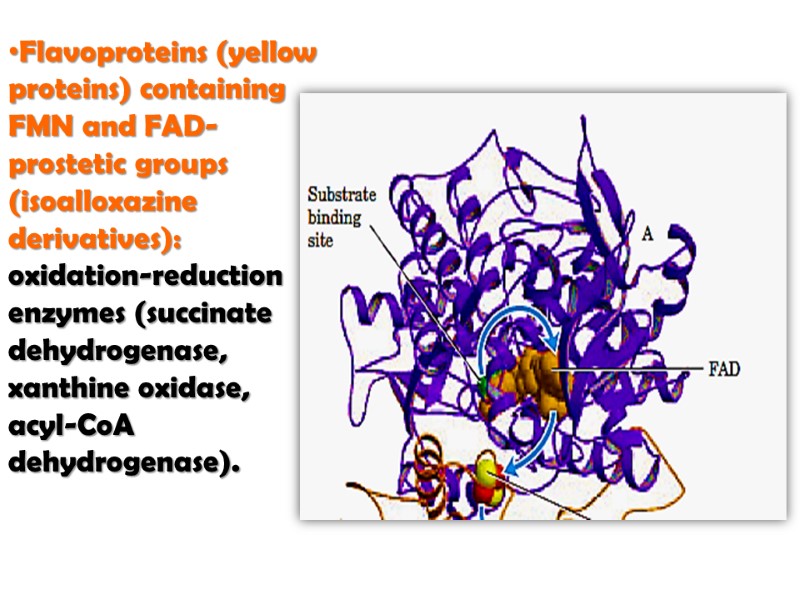
Flavoproteins (yellow proteins) containing FMN and FAD-prostetic groups (isoalloxazine derivatives): oxidation-reduction enzymes (succinate dehydrogenase, xanthine oxidase, acyl-CoA dehydrogenase).
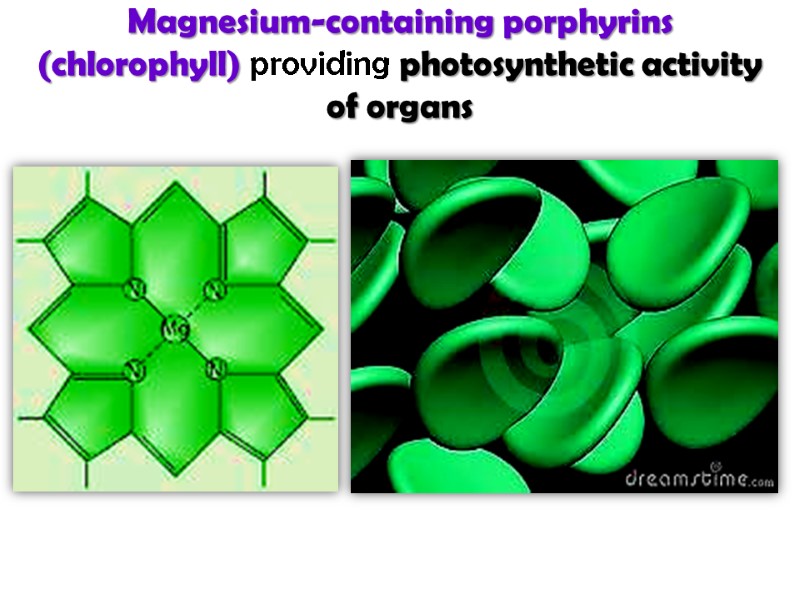
Magnesium-containing porphyrins (chlorophyll) providing photosynthetic activity of organs

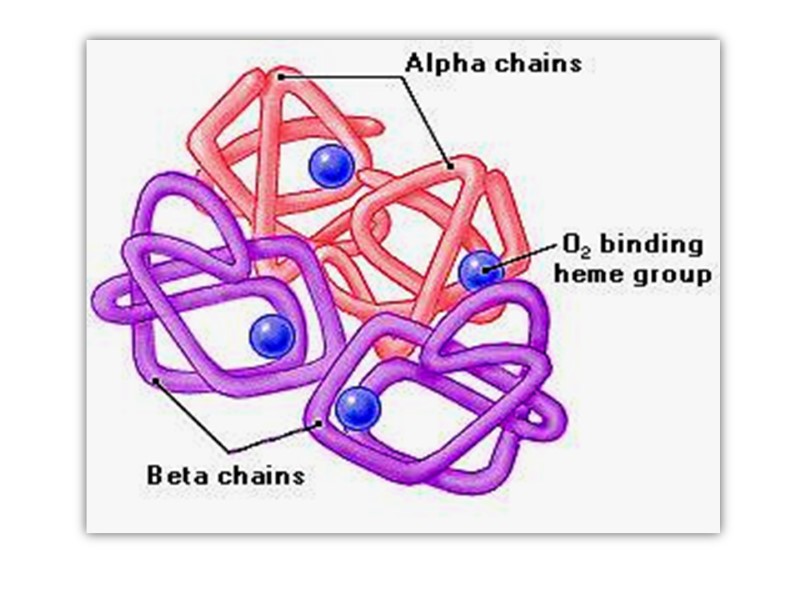
Hemoglobin Hemoglobin (Mr 64,500; abbreviated Hb(αα β β)) is roughly spherical, with a diameter of nearly 5.5 nm), a water soluble globular protein which is composed of Heme and Globin. :
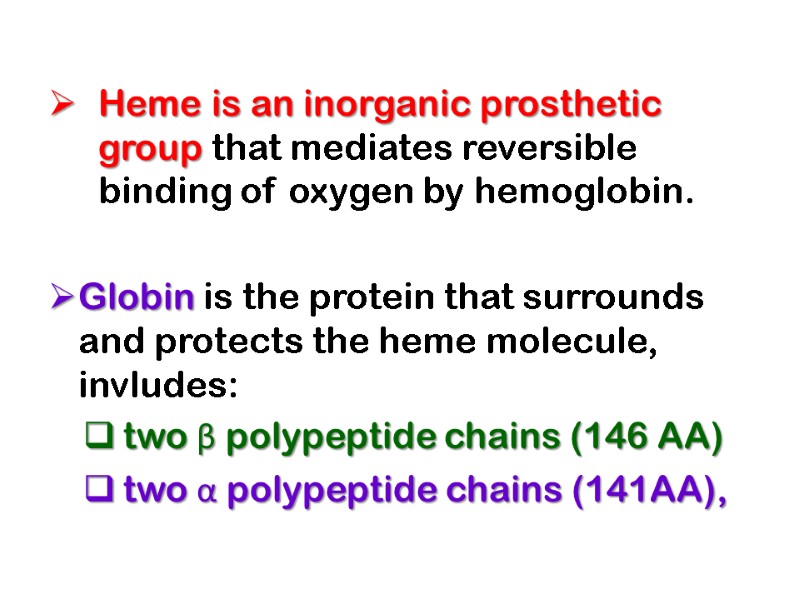
Heme is an inorganic prosthetic group that mediates reversible binding of oxygen by hemoglobin. Globin is the protein that surrounds and protects the heme molecule, invludes: two β polypeptide chains (146 AA) two α polypeptide chains (141AA),
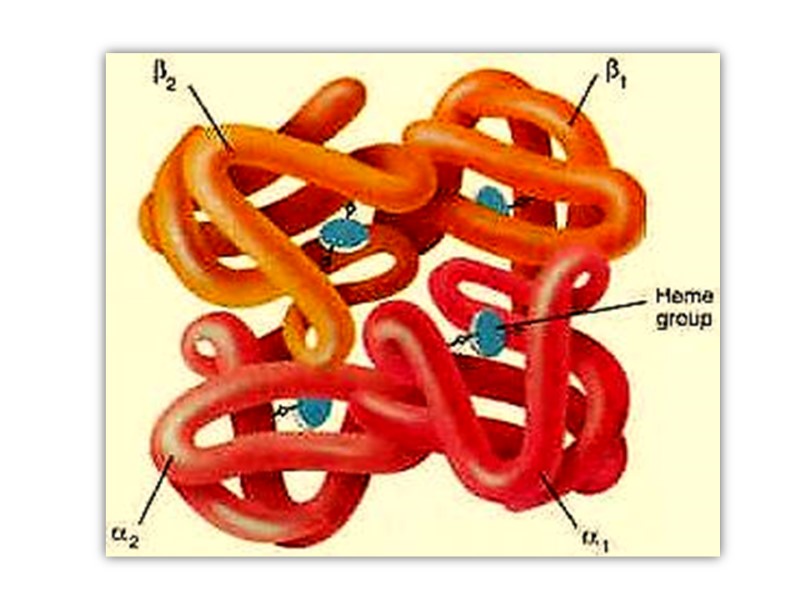
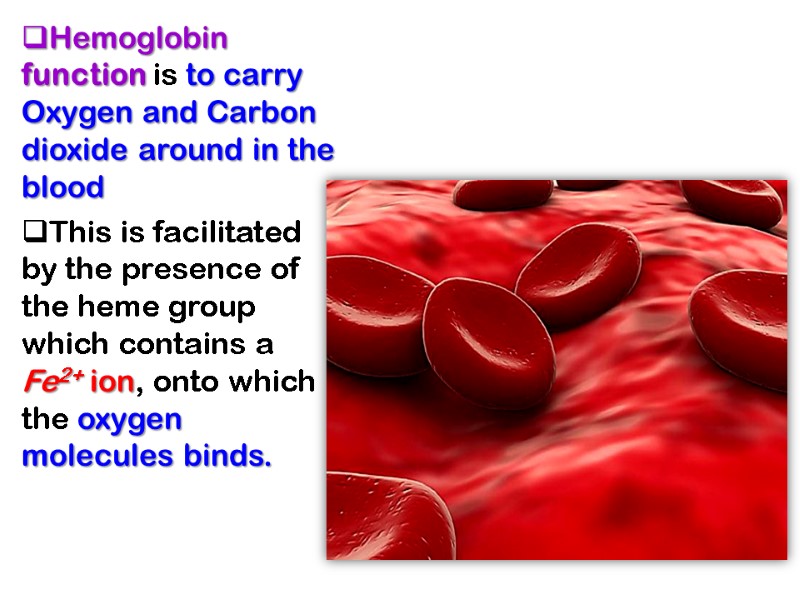
Hemoglobin function is to carry Oxygen and Carbon dioxide around in the blood This is facilitated by the presence of the heme group which contains a Fe2+ ion, onto which the oxygen molecules binds.
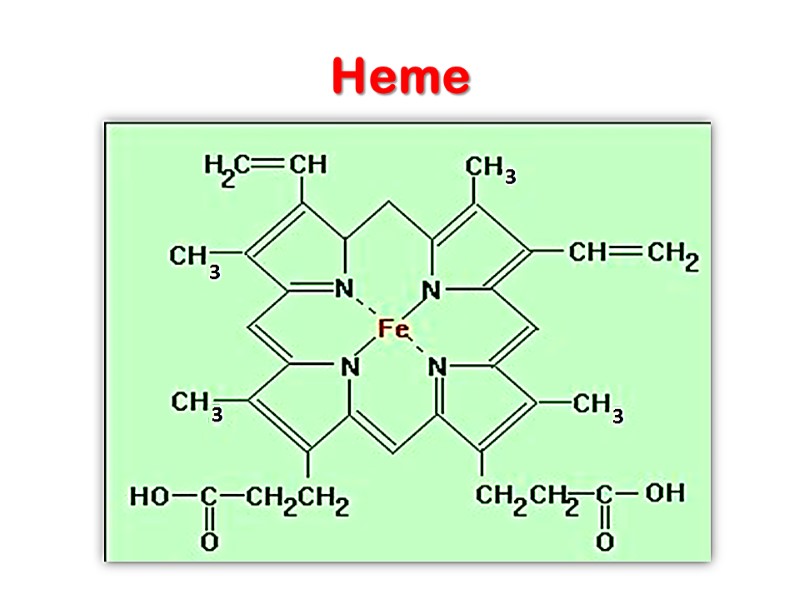
Heme

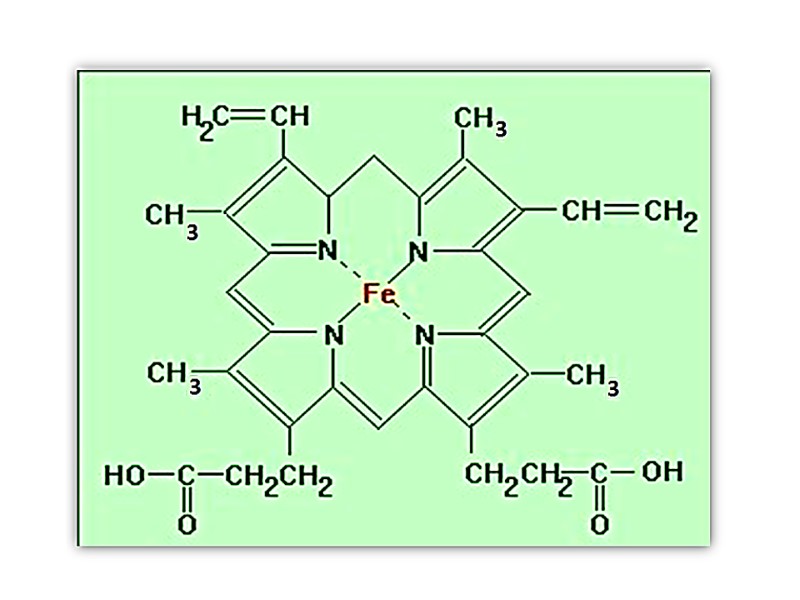
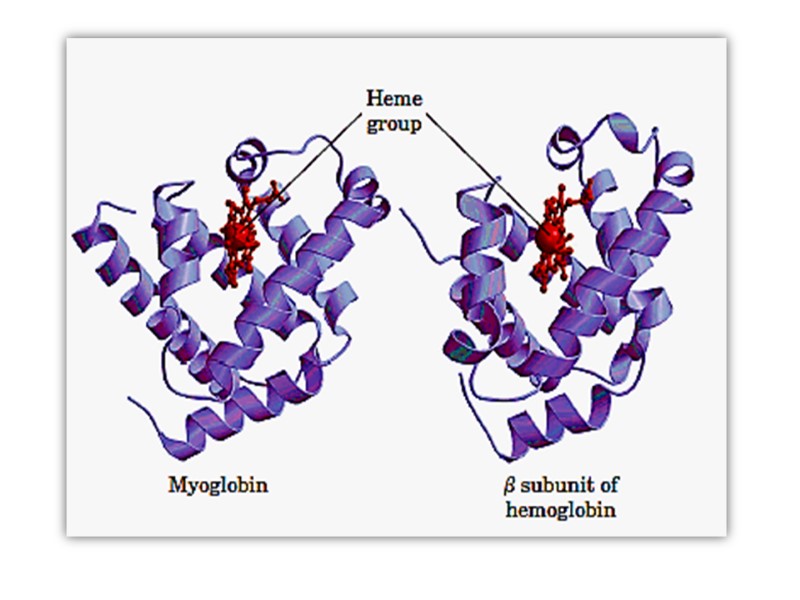
The heme group is present in myoglobin, hemoglobin, and many other proteins, designated heme proteins. Heme consists of a complex organic ring structure, protoporphyrin IX, to which is bound an iron atom in its ferrous (Fe2+ ) state.

Porphyrins, of which protoporphyrin IX is only one example, consist of four pyrrole rings linked by methene bridges, with substitutions at one or more of the positions denoted X.
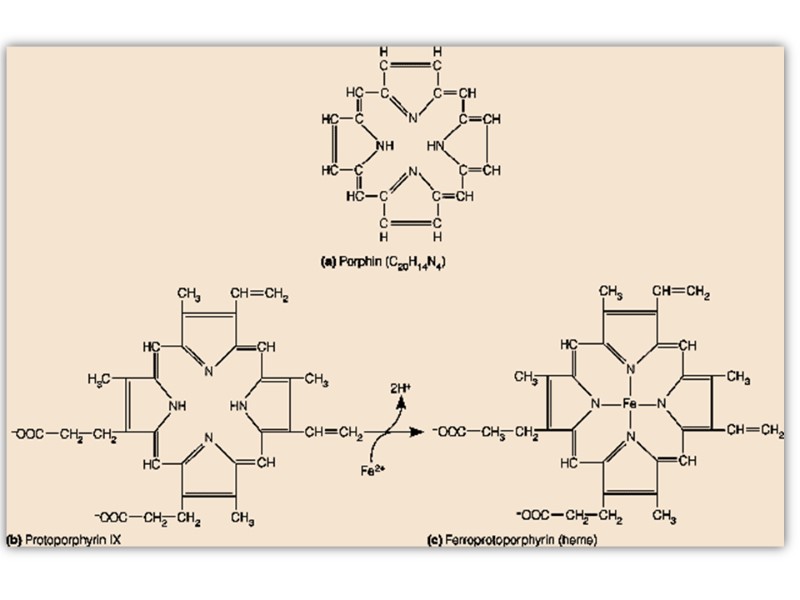
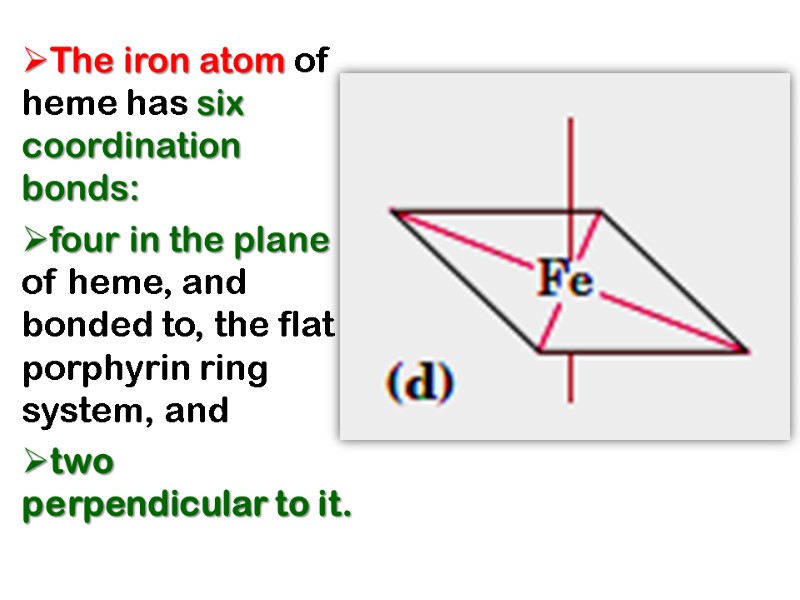
The iron atom of heme has six coordination bonds: four in the plane of heme, and bonded to, the flat porphyrin ring system, and two perpendicular to it.
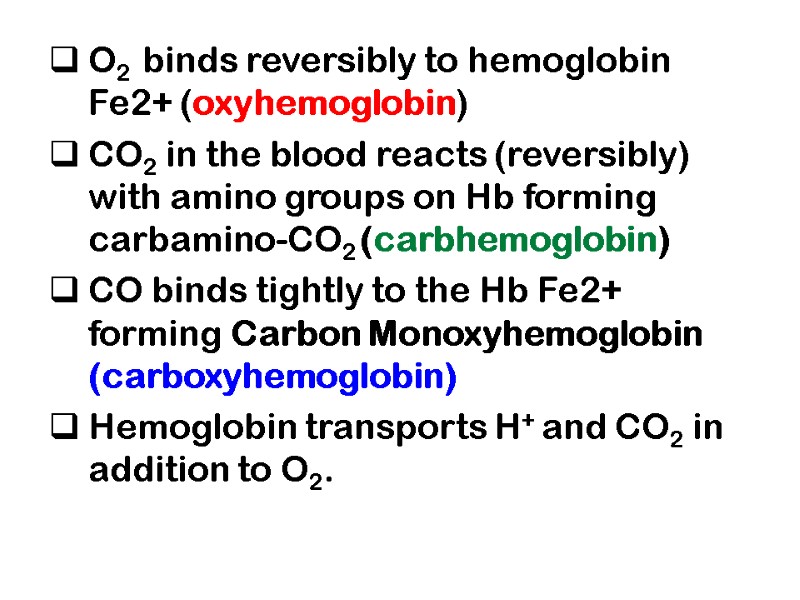
O2 binds reversibly to hemoglobin Fe2+ (oxyhemoglobin) CO2 in the blood reacts (reversibly) with amino groups on Hb forming carbamino-CO2 (carbhemoglobin) CO binds tightly to the Hb Fe2+ forming Carbon Monoxyhemoglobin (carboxyhemoglobin) Hemoglobin transports H+ and CO2 in addition to O2.

Myoglobin Has a Single Binding Site for Oxygen Myoglobin (Mr 16,700; abbreviated Mb) is a relatively simple oxygen-binding protein found in almost all mammals, primarily in muscle tissue. It facilitates oxygen diffusion in muscle.

Myoglobin is particularly abundant in the muscles of diving mammals such as seals and whales, where it has an oxygen-storage function for prolonged excursions undersea.
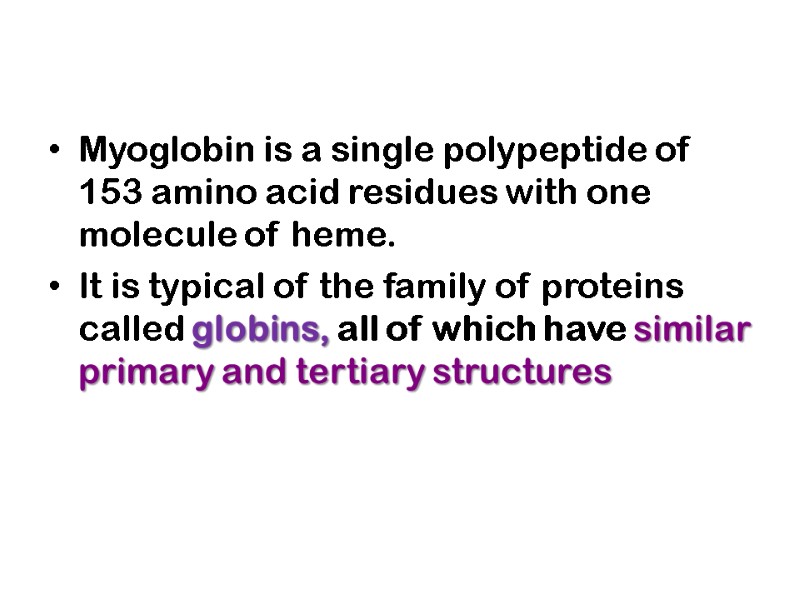
Myoglobin is a single polypeptide of 153 amino acid residues with one molecule of heme. It is typical of the family of proteins called globins, all of which have similar primary and tertiary structures
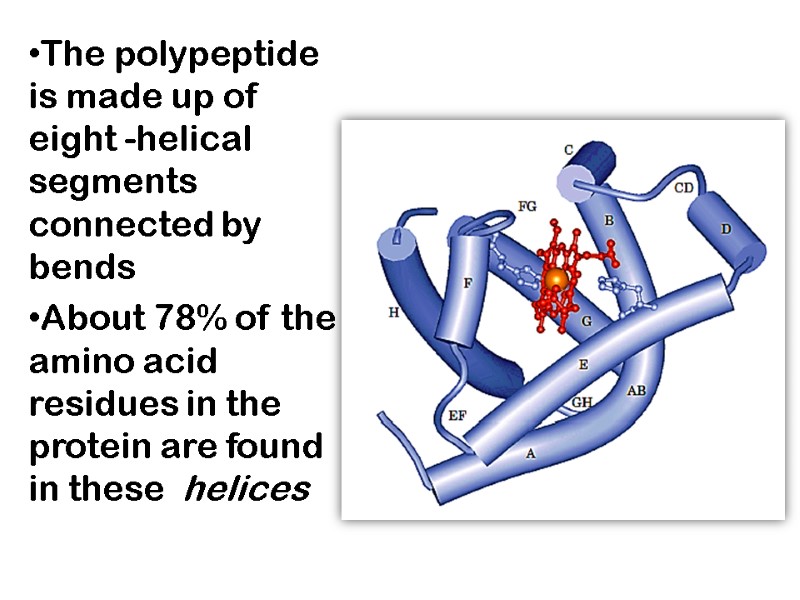
The polypeptide is made up of eight -helical segments connected by bends About 78% of the amino acid residues in the protein are found in these helices

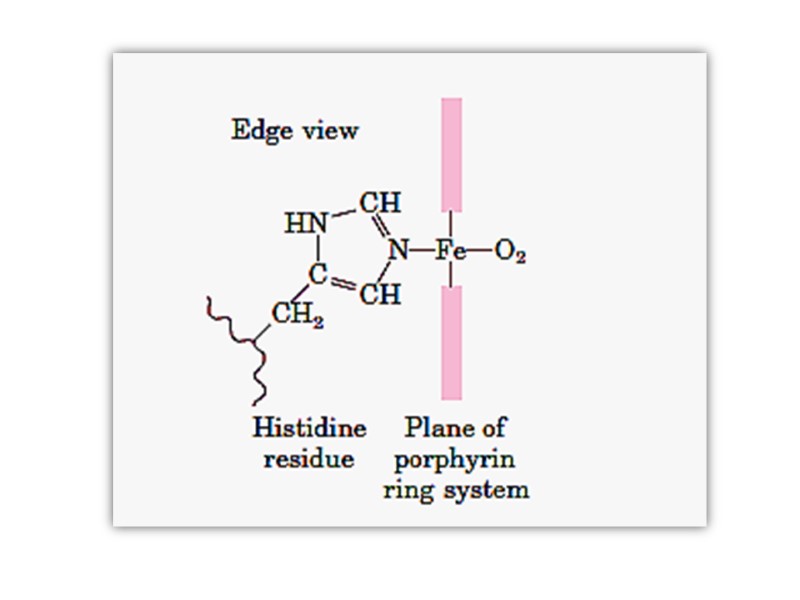
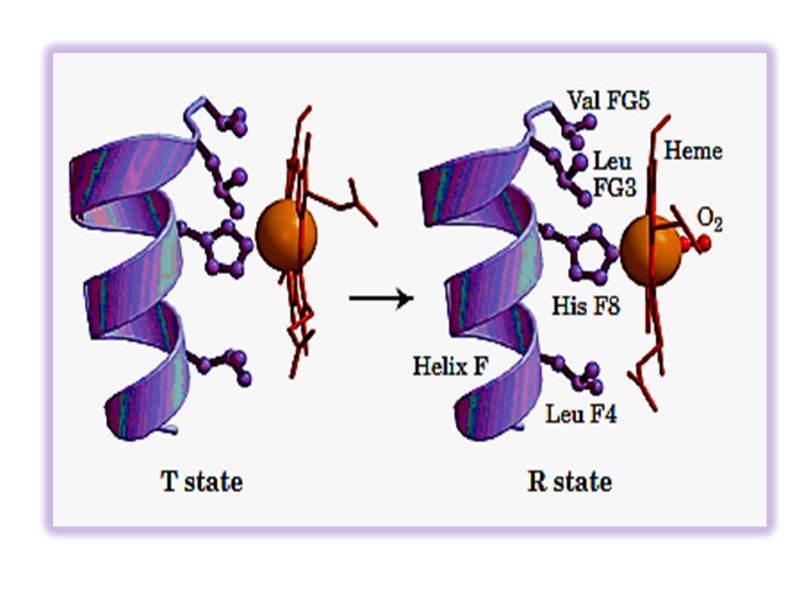
Oxygenation of myoglobin and hemoglobin chain(ether α- or β- )is accompanies by movement of the iron atom consequently movement of His F8 and residues covalently linked to the His F8 , towards the plane of the ring. This motion brings about a new conformation of proteins of the protein.

Conformational Changes in Hemoglobin upon binding of O2 The heme iron atom in deoxyHb is slightly out of the plane of the porphryin ring and bound to a histidine imidazole side chain. When oxygen binds to the heme on the side opposite the histidine, the iron atom’s electron cloud becomes slightly smaller, and the iron moves into the plane of the porphryin ring, also pulling the histidine side chain in the same direction
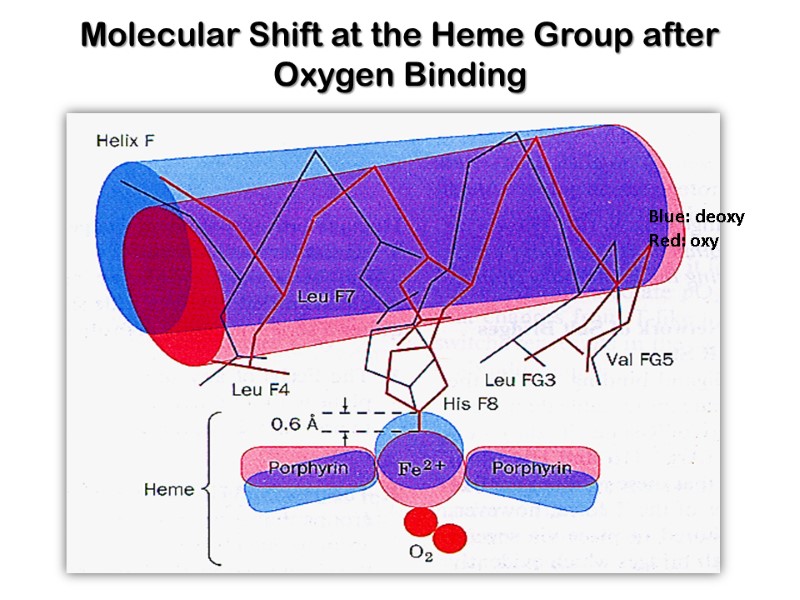
Molecular Shift at the Heme Group after Oxygen Binding Blue: deoxy Red: oxy
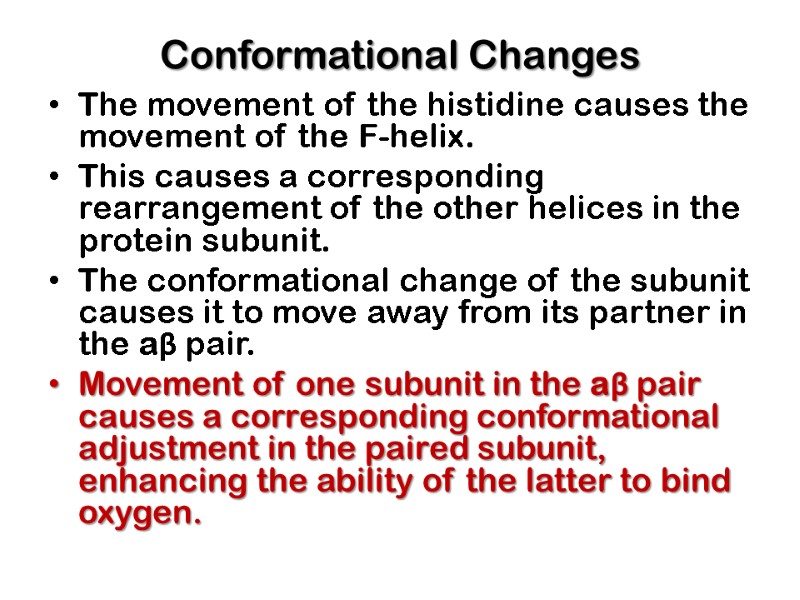
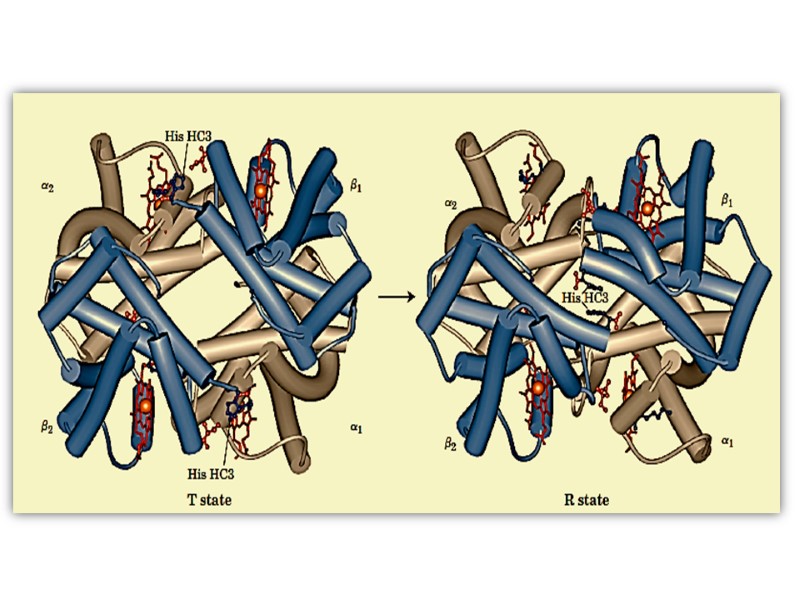
Conformational Changes The movement of the histidine causes the movement of the F-helix. This causes a corresponding rearrangement of the other helices in the protein subunit. The conformational change of the subunit causes it to move away from its partner in the aβ pair. Movement of one subunit in the aβ pair causes a corresponding conformational adjustment in the paired subunit, enhancing the ability of the latter to bind oxygen.
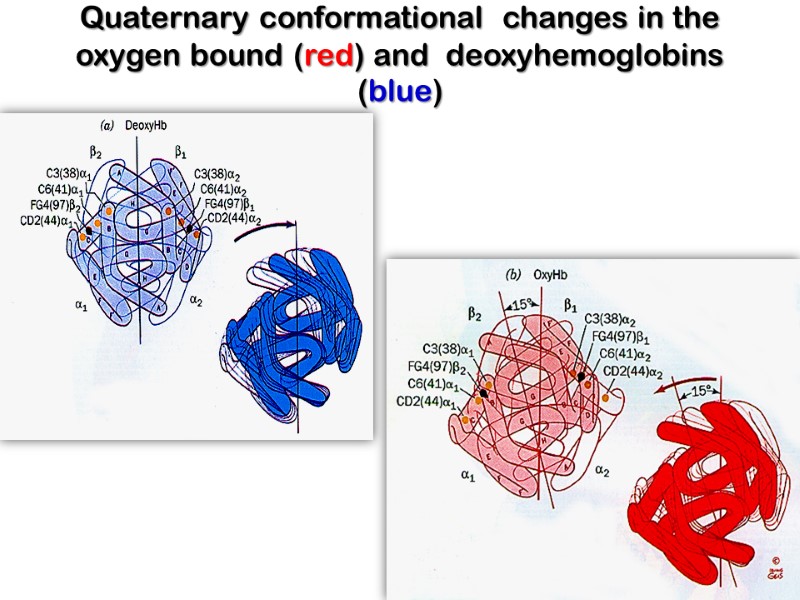
Quaternary conformational changes in the oxygen bound (red) and deoxyhemoglobins (blue)

Hemoglobin Binds Oxygen Cooperatively Thus, filling the first oxygen binding site in hemoglobin increases the affinity of the remaining sites for oxygen. Conversely, losing an oxygen from hemoglobin makes it easier for the protein to lose its remaining oxygen molecules.
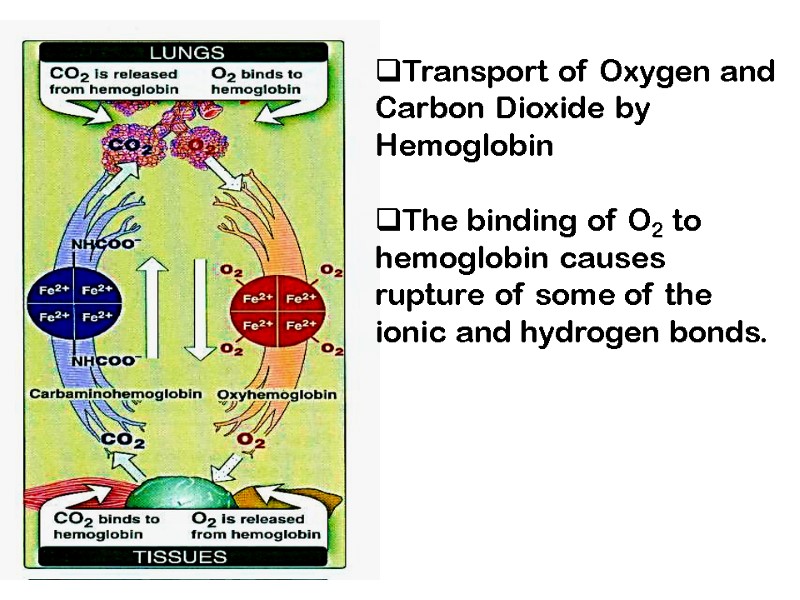
Transport of Oxygen and Carbon Dioxide by Hemoglobin The binding of O2 to hemoglobin causes rupture of some of the ionic and hydrogen bonds.
The tertiary configuration of low affinity, deoxygenated hemoglobin (Hb) is known as the taut (T) state. Conversely, the quaternary structure of the fully oxygenated high affinity form of hemoglobin (HbO2) is known as the relaxed (R) state.
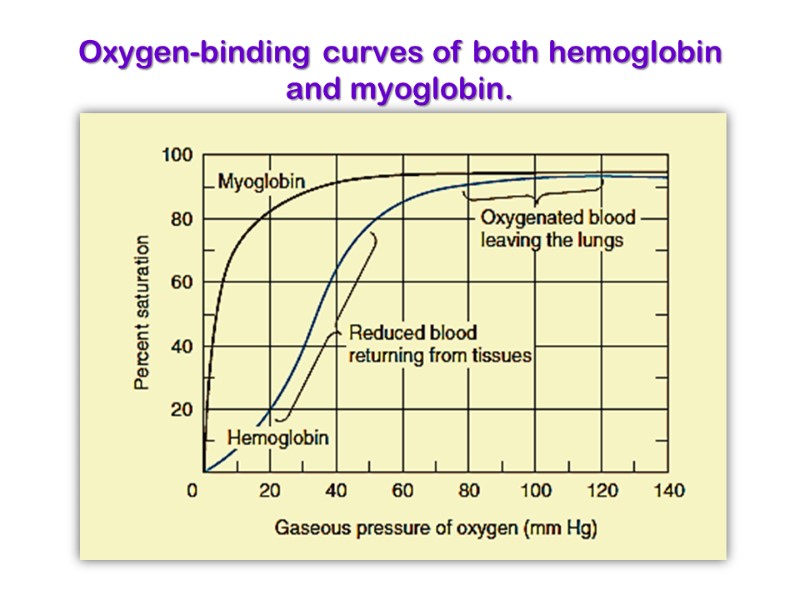
Oxygen-binding curves of both hemoglobin and myoglobin.

The myoglobin curve is hyperbolic while that of hemoglobin is sigmoidal. The latter indicates cooperativity as mentioned above. Myoglobin, with its hyperbolic binding curve for oxygen, is relatively insensitive to small changes in the concentration of dissolved oxygen and so functions well as an oxygen-storage protein. The affinity for oxygen by Myoglobin 25 times fold
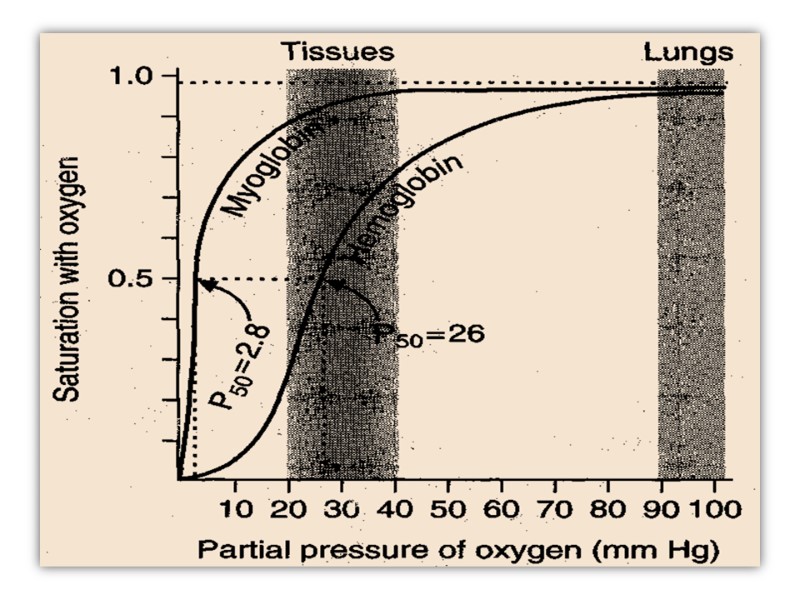

Hemoglobin, with its multiple subunits and O2-binding sites, is better suited to oxygen transport. Interactions between the subunits of a multimeric protein can permit a highly sensitive response to small changes in ligand concentration.
Interactions among the subunits in hemoglobin cause conformational changes that alter the affinity of the protein for oxygen. The modulation of oxygen binding allows the O2-transport protein to respond to changes in oxygen demand by tissues.

Hemoglobin must bind oxygen efficiently in the lungs, where the pO2 is about 13.3 kPa (90-100 mmHg), and release oxygen in the tissues, where the pO2 is about 4 kPa(20-40 mmHg). Myoglobin, or any protein that binds oxygen with a hyperbolic binding curve, would be ill-suited to this function

A protein that bound O2 with high affinity would bind it efficiently in the lungs but would not release much of it in the tissues. If the protein bound oxygen with a sufficiently low affinity to release it in the tissues, it would not pick up much oxygen in the lungs.
Hemoglobin solves the problem by undergoing a transition from a low-affinity state (the T state) to a high-affinity state (the R state) as more O2 molecules are bound. As a result, hemoglobin has a hybrid S-shaped, or sigmoid, binding curve for oxygen.
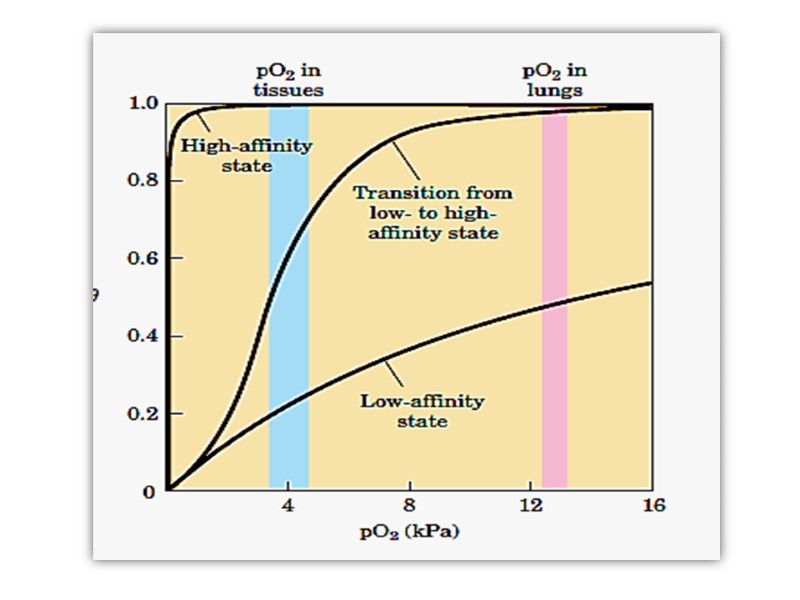

A single-subunit protein with a single ligand-binding site cannot produce a sigmoid binding curve— even if binding elicits a conformational change— because each molecule of ligand binds independently and cannot affect the binding of another molecule In contrast, O2 binding to individual subunits of hemoglobin can alter the affinity for O2 in adjacent subunits.

The first molecule of O2 that interacts with deoxyhemoglobin binds weakly, because it binds to a subunit in the T state. Its binding, however, leads to conformational changes that are communicated to adjacent subunits, making it easier for additional molecules of O2 to bind.

In effect, the T n R transition occurs more readily in the second subunit once O2 is bound to the first subunit. The last (fourth) O2 molecule binds to a heme in a subunit that is already in the R state, and hence it binds with much higher affinity than the first molecule
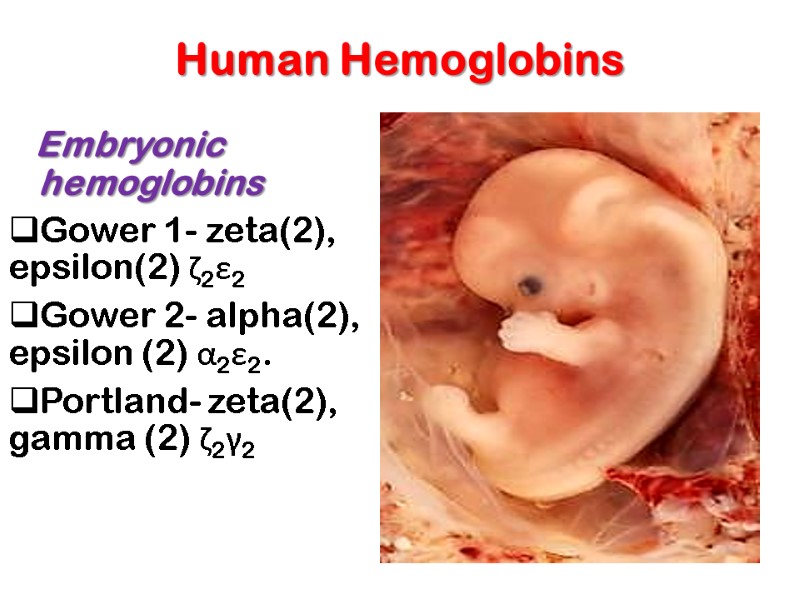
Human Hemoglobins Embryonic hemoglobins Gower 1- zeta(2), epsilon(2) ζ2ε2 Gower 2- alpha(2), epsilon (2) α2ε2. Portland- zeta(2), gamma (2) ζ2γ2
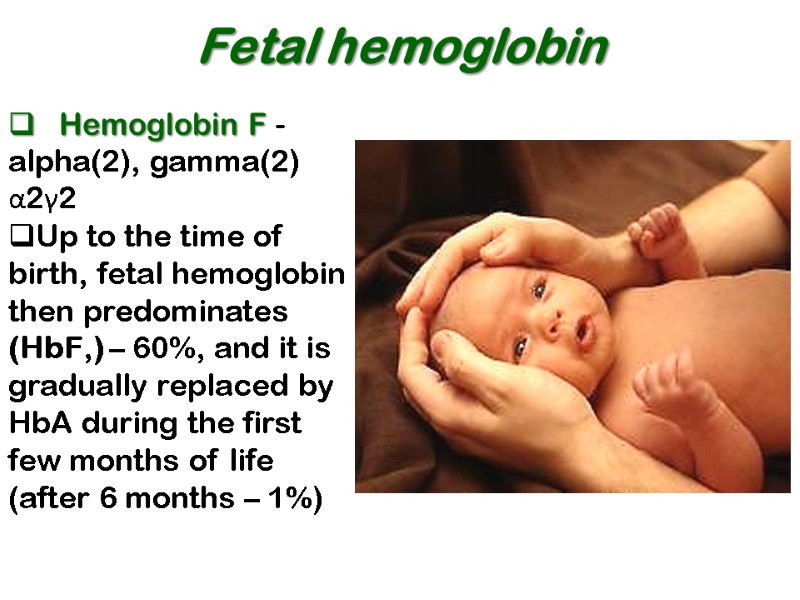
Fetal hemoglobin Hemoglobin F - alpha(2), gamma(2) α2γ2 Up to the time of birth, fetal hemoglobin then predominates (HbF,) – 60%, and it is gradually replaced by HbA during the first few months of life (after 6 months – 1%)
Embryonic and fetal hemoglobins have higher O2 affinities than HbA, as they have to take up oxygen from the maternal circulation.
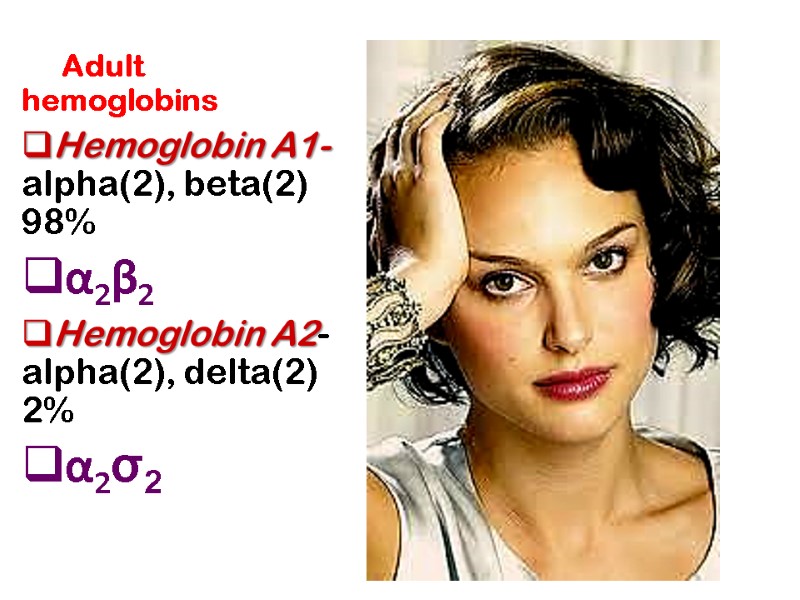
Adult hemoglobins Hemoglobin A1- alpha(2), beta(2) 98% α2β2 Hemoglobin A2- alpha(2), delta(2) 2% α2σ2
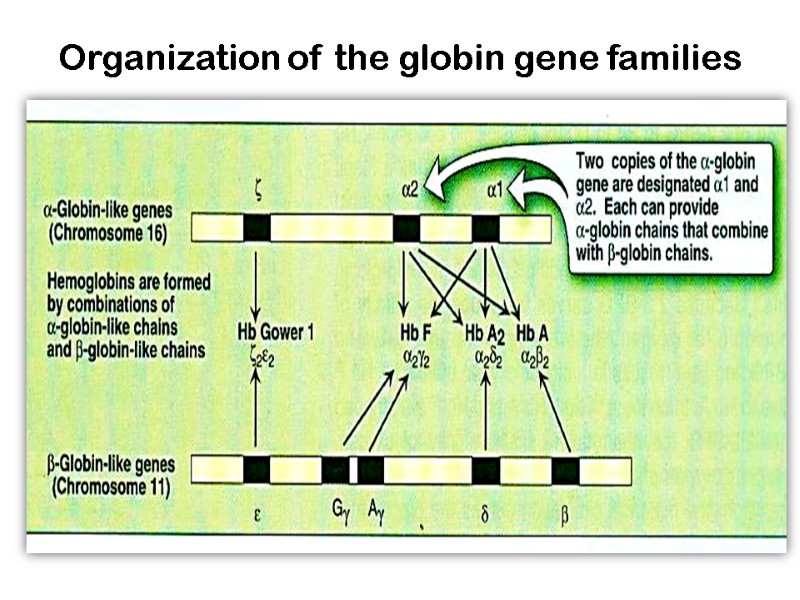
Organization of the globin gene families
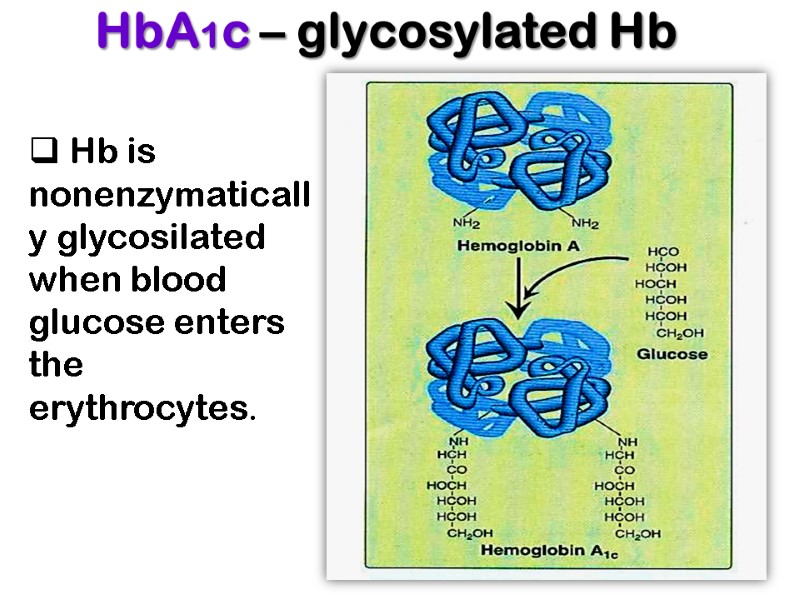
HbA1c – glycosylated Hb Hb is nonenzymatically glycosilated when blood glucose enters the erythrocytes.

The fraction of hemoglobin glycosylated normally about 5% and is proportionate to blood glucose concentration. Measurement of HbA1c provides information useful for the management of diabetes mellitus.
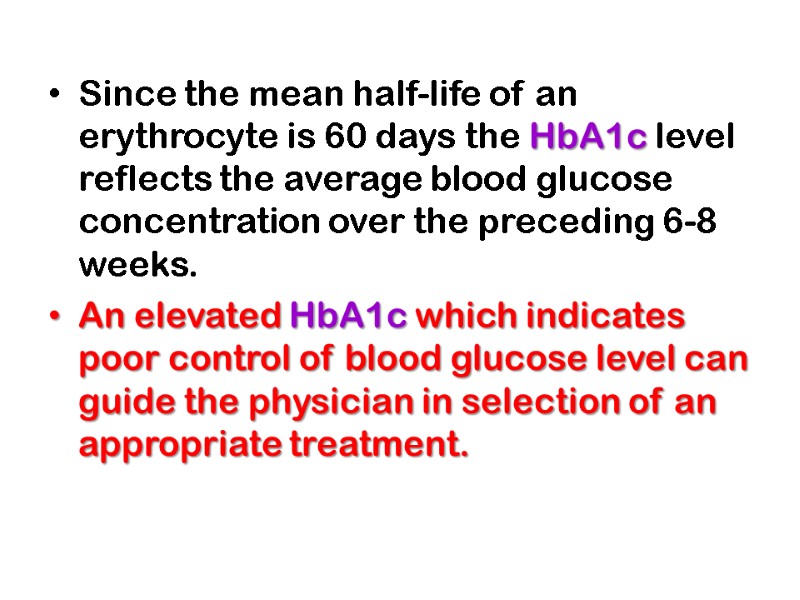
Since the mean half-life of an erythrocyte is 60 days the HbA1c level reflects the average blood glucose concentration over the preceding 6-8 weeks. An elevated HbA1c which indicates poor control of blood glucose level can guide the physician in selection of an appropriate treatment.
Thalassemias The genetic defects known as thalassemias result from the partial or total absence of one or more α or β chains of hemoglobin. Over 750 different mutations have been identified, but only three are common. This group of hereditary hemolytic diseases caused by faulty hemoglobin synthesis, widespread in Mediterranean, African, and Asian countries

Either the α chain (alpha thalassemias) or β chain (beta thalassemias) can be affected. A superscript indicates whether a subunit is completely absent (α0 or β0) or whether its synthesis is reduced (α+ or β+). Apart from bone marrow transplantation, treatment is symptomatic.

Reduced synthesis of one of the globin chains can cause the formation of abnormal hemoglobin molecules, thus causing anemia, the characteristic presenting symptom of the thalassemias.

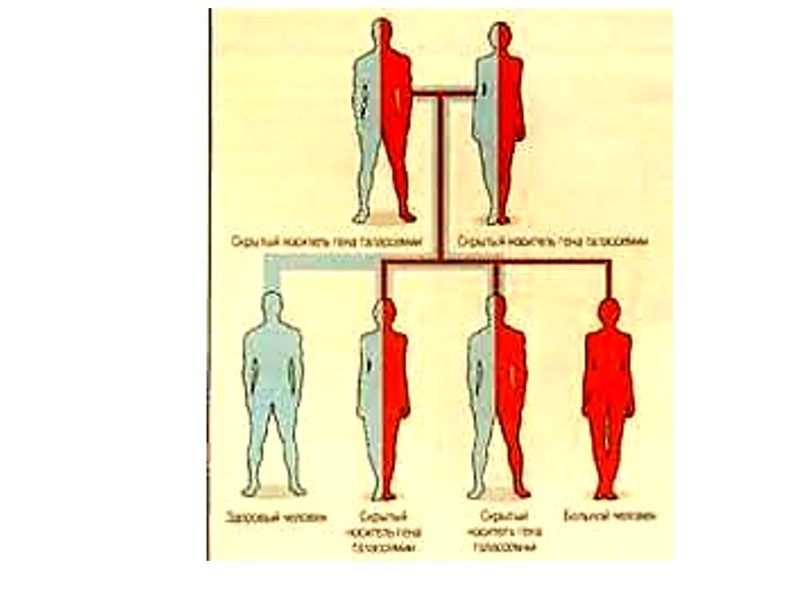
alpha thalassemias 1.The loss of one gene diminishes the production of the alpha protein only slightly. This condition is so close to normal that it can be detected only by specialized laboratory techniques. A person with this condition is called a "silent carrier" because of the difficulty in detection.

2.The loss of two globin genes (two-gene deletion alpha thalassemia) produces a condition with small red blood cells, and at most a mild anemia. People with this condition look and feel normal. However, the condition can be detected by routine blood testing.
3. The loss of three alpha genes produces a serious hematological problem (three-gene deletion alpha thalassemia). Patients with this condition have a severe anemia, and often require blood transfusions to survive. The severe imbalance between the alpha chain production (now powered by one gene, instead of four) and beta chain production (which is normal) causes an accumulation of beta chains inside the red blood cells.
Normally, beta chains pair only with alpha chains. With three-gene deletion alpha thalassemia, however, beta chains begin to associate in groups of four, producing an abnormal hemoglobin, called “Hemoglobin H“ (ββββ). The condition is called “Hemoglobin H disease".
Hemoglobin H has two problems. First it does not carry oxygen properly, making it functionally useless to the cell. Second, hemoglobin H protein damages the membrane that surrounds the red cell, accelerating cell destruction.

The combination of the very low production of alpha chains and destruction of red cells in hemoglobin H disease produces a severe, life-threatening anemia. Untreated, most patients die in childhood or early adolescence.

4. The loss of all four alpha genes produces a condition that is incompatible with life. The gamma chains produced during fetal life associate in groups of four to form an abnormal hemoglobin called “Hemoglobin Barts". This condition clinically is called “Hydrops fetalis” Most people with four-gene deletion alpha thalassemia die in utero or shortly after birth.

beta thalassemias The fact that there are only two genes for the beta chain of hemoglobin makes beta thalassemia a bit simpler to understand than alpha thalassemia. Unlike alpha thalassemia, beta thalassemia rarely arises from the complete loss of a beta globin gene. The beta globin gene is present, but produces little beta globin protein. The degree of suppression varies.

In some cases, the affected gene makes essentially no beta globin protein (beta-0-thalassemia). In other cases, the production of beta chain protein is lower than normal, but not zero (beta-(+)-thalassemia). The severity of beta thalassemia depends in part on the type of beta thalassemic genes that a person has inherited.

1. one-gene beta thalassemia has one beta globin gene that is normal, and a second, affected gene with a variably reduced production of beta globin. The degree of imbalance with the alpha globin depends on the residual production capacity of the defective beta globin gene. Even when the affected gene produces no beta chain, the condition is mild since one beta gene functions normally.
The red cells are small and a mild anemia may exist. People with the condition generally have no symptoms. The condition can be detected by a routine laboratory blood evaluation. The one-gene beta thalassemia and the two-gene alpha thalassemia are very similar, from a clinical point of view. Each results in small red cells and a mild anemia.
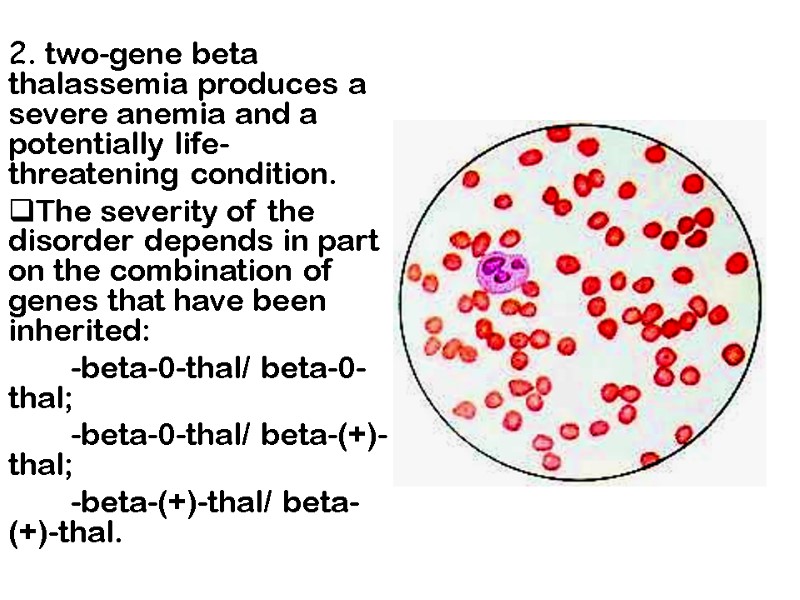
2. two-gene beta thalassemia produces a severe anemia and a potentially life-threatening condition. The severity of the disorder depends in part on the combination of genes that have been inherited: -beta-0-thal/ beta-0-thal; -beta-0-thal/ beta-(+)-thal; -beta-(+)-thal/ beta-(+)-thal.

Hemoglobinopathies The reason of hemoglobinopathies is the alteration in AA sequence. Classical example is sickle cell anemia
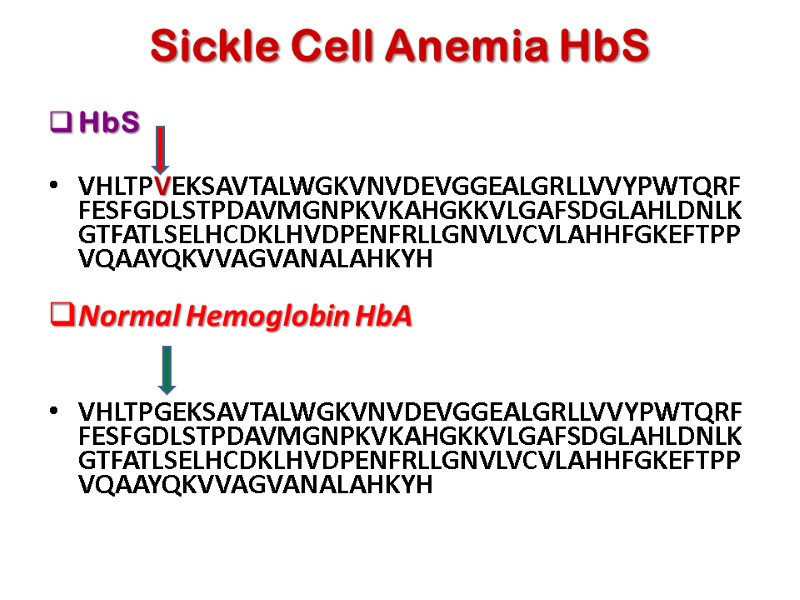
Sickle Cell Anemia HbS HbS VHLTPVEKSAVTALWGKVNVDEVGGEALGRLLVVYPWTQRFFESFGDLSTPDAVMGNPKVKAHGKKVLGAFSDGLAHLDNLKGTFATLSELHCDKLHVDPENFRLLGNVLVCVLAHHFGKEFTPPVQAAYQKVVAGVANALAHKYH Normal Hemoglobin HbA VHLTPGEKSAVTALWGKVNVDEVGGEALGRLLVVYPWTQRFFESFGDLSTPDAVMGNPKVKAHGKKVLGAFSDGLAHLDNLKGTFATLSELHCDKLHVDPENFRLLGNVLVCVLAHHFGKEFTPPVQAAYQKVVAGVANALAHKYH
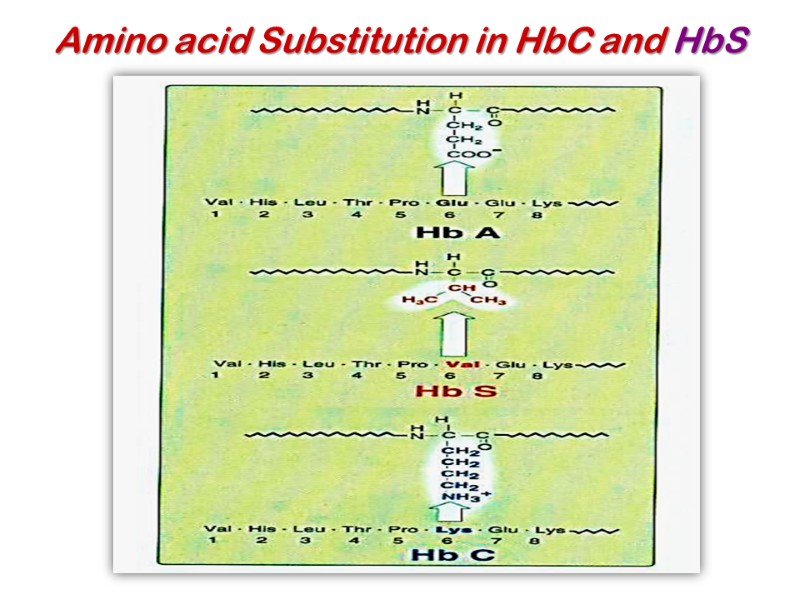
Amino acid Substitution in HbC and HbS

In HbS, the nonpolar amino acid valine has replaced the polar surface residue Glu6 of the β subunit, generating a hydrophobic “sticky patch” on the surface of the β subunit of both oxyHbS and deoxyHbS. Both HbA and HbS contain a complementary sticky patch on their surfaces that is exposed only in the deoxygenated, T state. Thus, at low PO2, deoxyHbS can polymerize to form long, insoluble fibers.
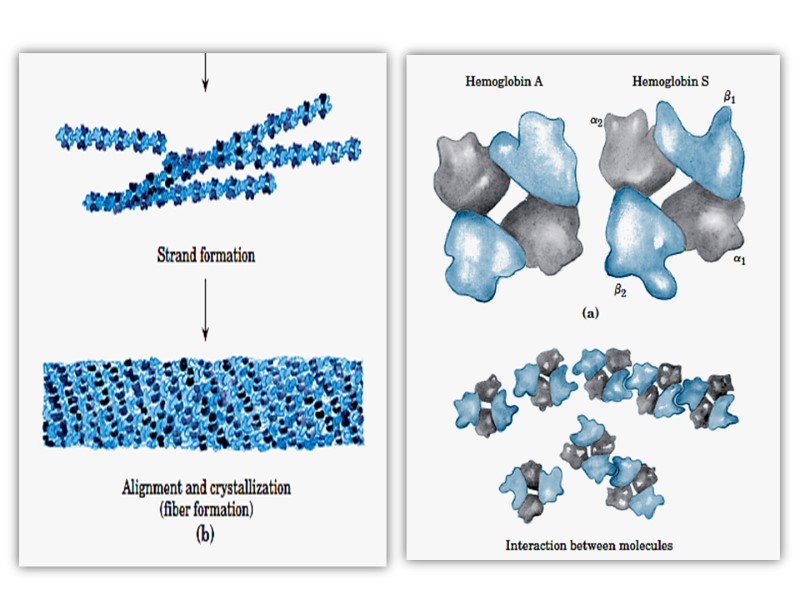
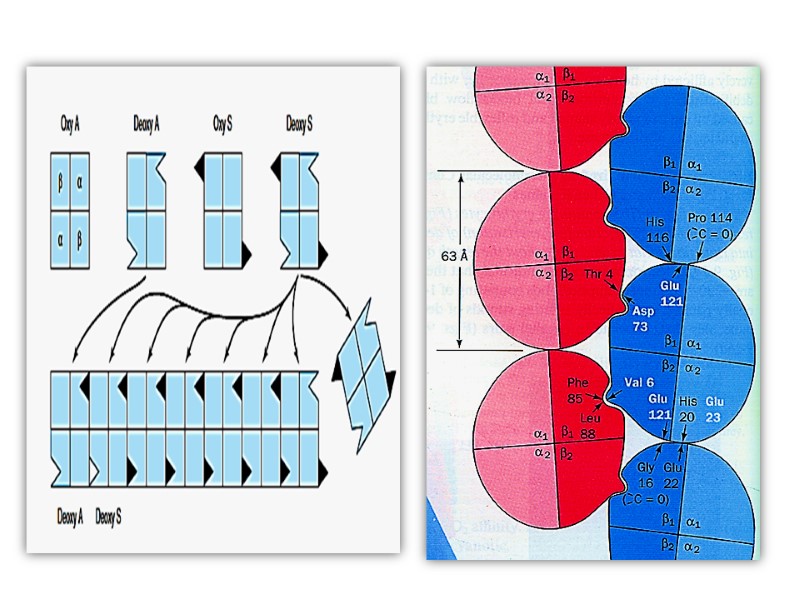

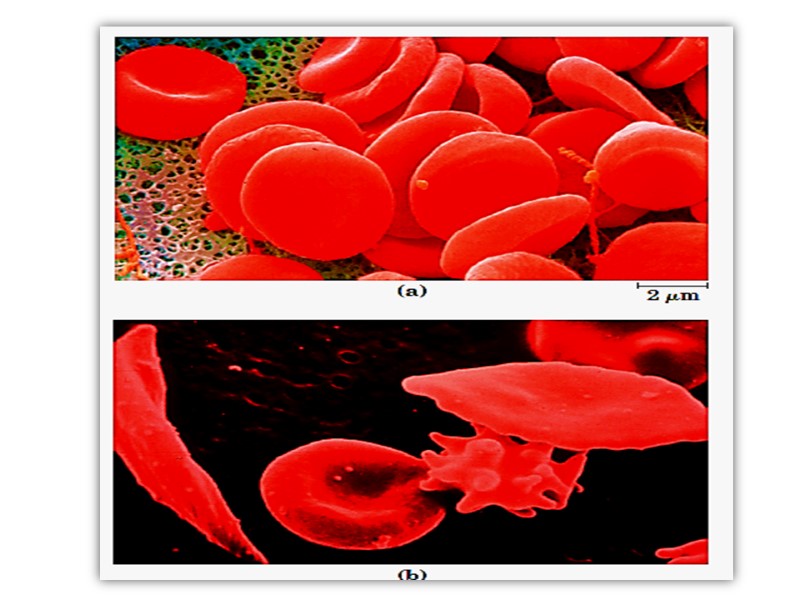
Binding of deoxy- HbA terminates fiber polymerization, since HbA lacks the second sticky patch necessary to bind another Hb molecule. These twisted helical fibers distort the erythrocyte into a characteristic sickle shape, rendering it vulnerable to lysis in the interstices of the splenic sinusoids. They also cause multiple secondary clinical effects. A low PO2 such as that at high altitudes exacerbates the tendency to polymerize.
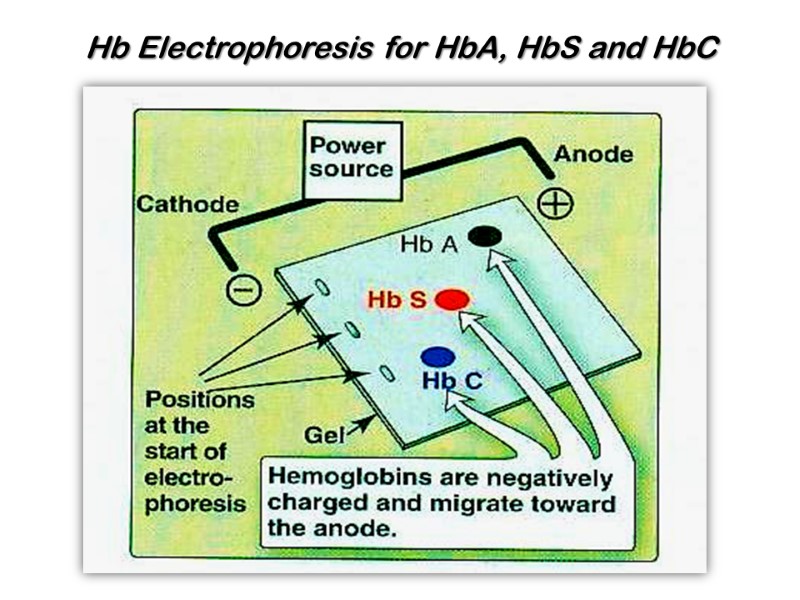
Hb Electrophoresis for HbA, HbS and HbC

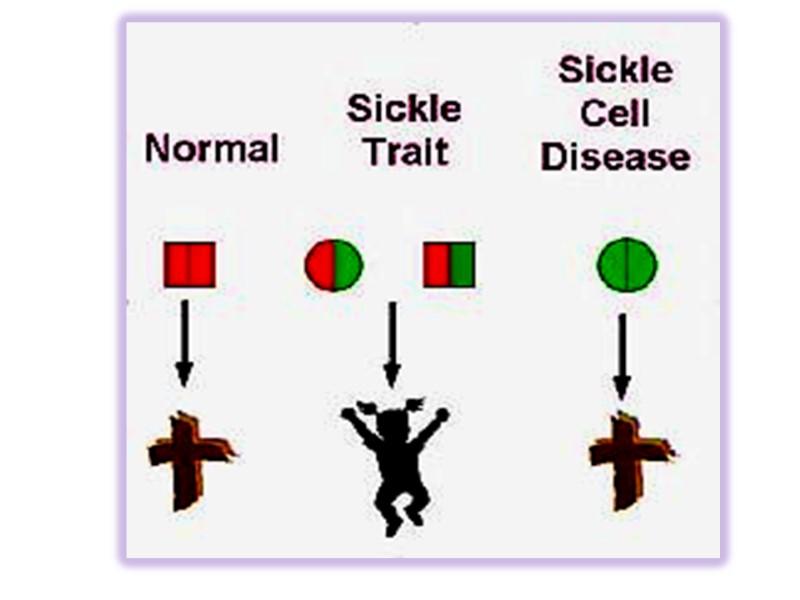
Sickle-cell anemia, as we have noted, occurs in individuals homozygous for the sickle-cell allele of the gene encoding the subunit of hemoglobin. Individuals who receive the sickle-cell allele from only one parent and are thus heterozygous experience a milder condition called sickle-cell trait; only about 1% of their erythrocytes become sickled on deoxygenation. These individuals may live completely normal lives if they avoid vigorous exercise or other stresses on the circulatory system.

Sickle-cell anemia is a life-threatening and painful disease. People with sickle-cell anemia suffer from repeated crises brought on by physical exertion. They become weak, dizzy, and short of breath, and they also experience heart murmurs and an increased pulse rate.
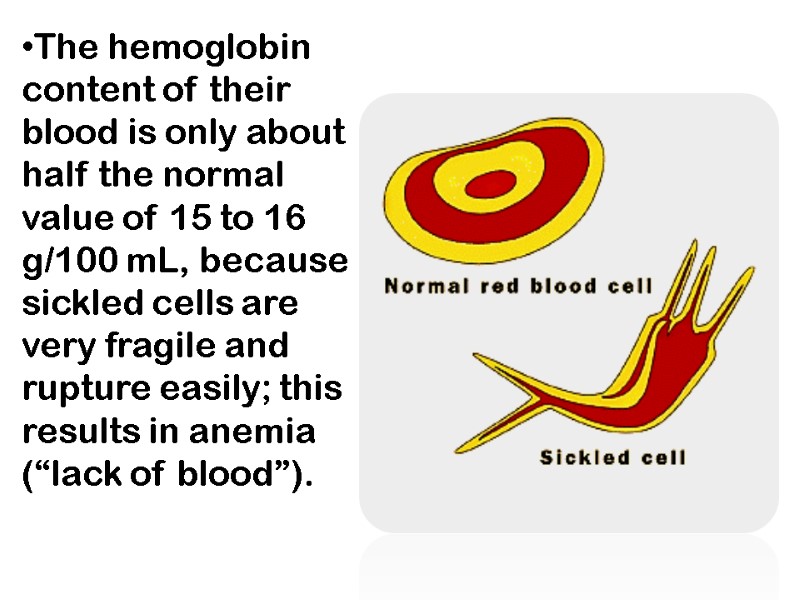
The hemoglobin content of their blood is only about half the normal value of 15 to 16 g/100 mL, because sickled cells are very fragile and rupture easily; this results in anemia (“lack of blood”).
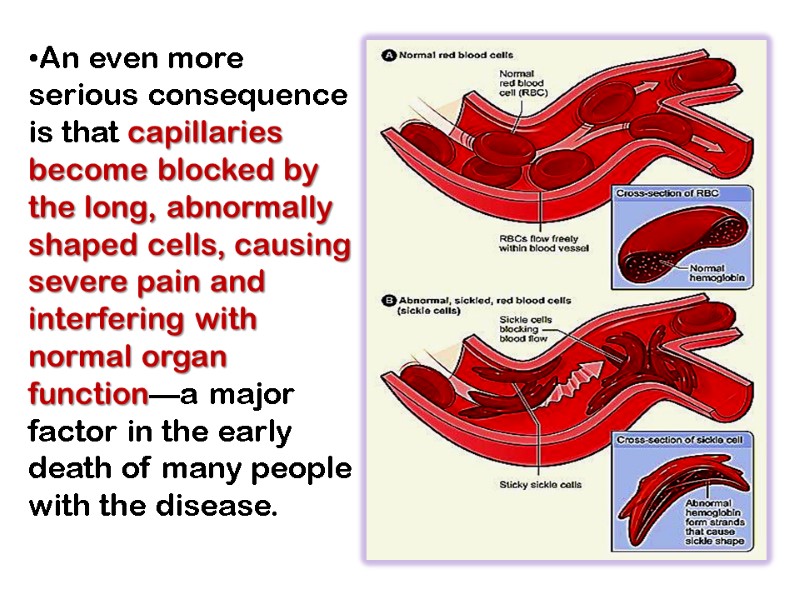
An even more serious consequence is that capillaries become blocked by the long, abnormally shaped cells, causing severe pain and interfering with normal organ function—a major factor in the early death of many people with the disease.
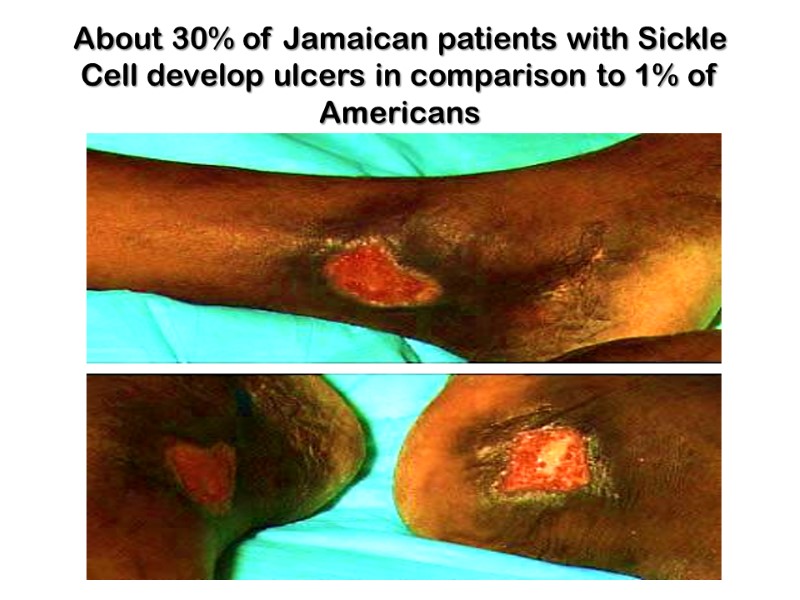
About 30% of Jamaican patients with Sickle Cell develop ulcers in comparison to 1% of Americans
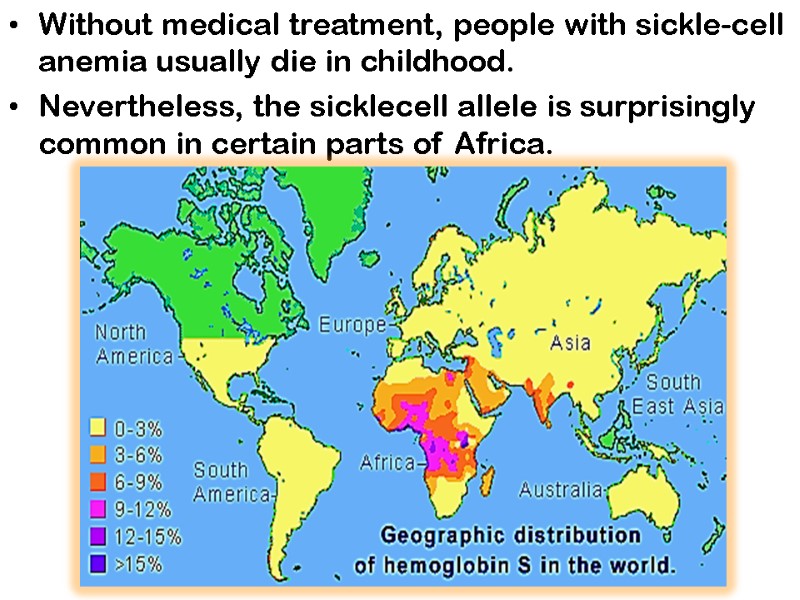
Without medical treatment, people with sickle-cell anemia usually die in childhood. Nevertheless, the sicklecell allele is surprisingly common in certain parts of Africa.

Investigation into the persistence of an allele that is so obviously deleterious in homozygous individuals led to the finding that in heterozygous individuals, the allele confers a small but significant resistance to lethal forms of malaria.

Natural selection has resulted in an allele population that balances the deleterious effects of the homozygous condition against the resistance to malaria afforded by the heterozygous condition. Even heterozygous carriers can experience sickle cell symptoms after vigorous exercise or unpressurized travel at high altitudes.

Sickle cell Trait are resistant to Malaria, theories… The carriers of Sickle Cell have some abnormal Hemoglobin, and when they come in contact with the Malaria parasite they become sickled. Then those cells go through the spleen, which eliminates the cells because of their sickle shape, so the Malaria would be eliminated as well. The Sickle Cell trait causes the malaria to stay in the body for an extended period of time, so it is able to build up a defense to it.

Because oxygen concentration is low in the spleen, and because infected cells often get trapped in the spleen, it is possible that they are destroyed in the spleen 4. The Malaria parasite produces an acid when it is inside of the red blood cells. This causes the red blood cells to polymerize, and the cells will sickle. These sickled cells are then destroyed when the blood cells go through the spleen.
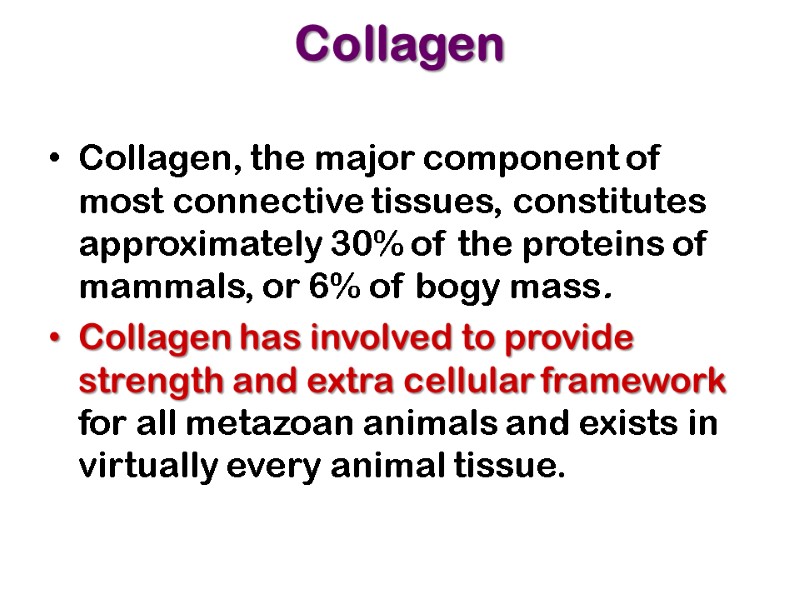
Collagen Collagen, the major component of most connective tissues, constitutes approximately 30% of the proteins of mammals, or 6% of bogy mass. Collagen has involved to provide strength and extra cellular framework for all metazoan animals and exists in virtually every animal tissue.

Fundamental quantity of collagen is concentrated in mineralized tissues and skin, about 10% - in stroma of internal organs. It is found in connective tissue such as tendons, cartilage, the organic matrix of bone, and the cornea of the eye.
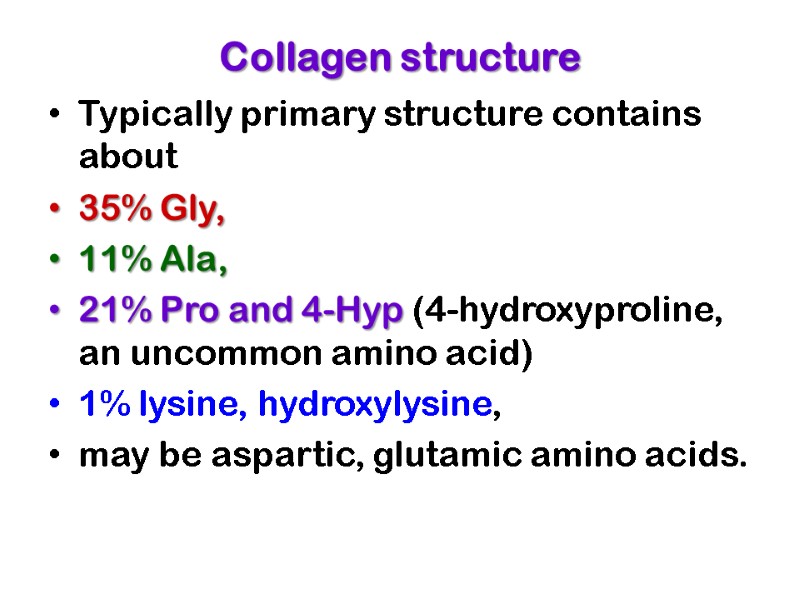
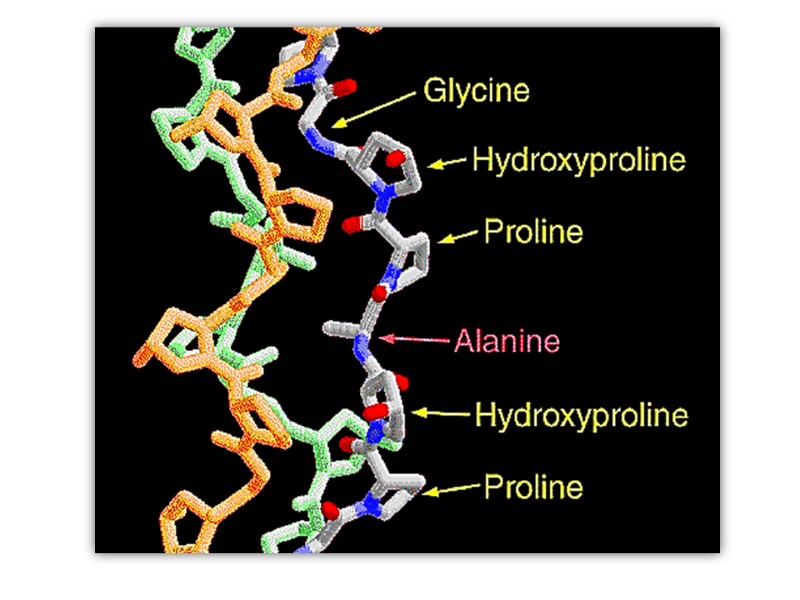
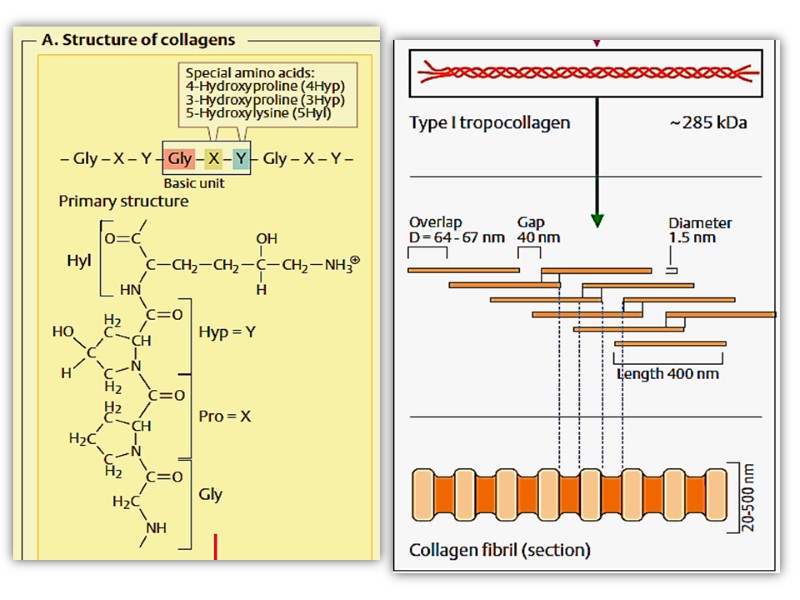
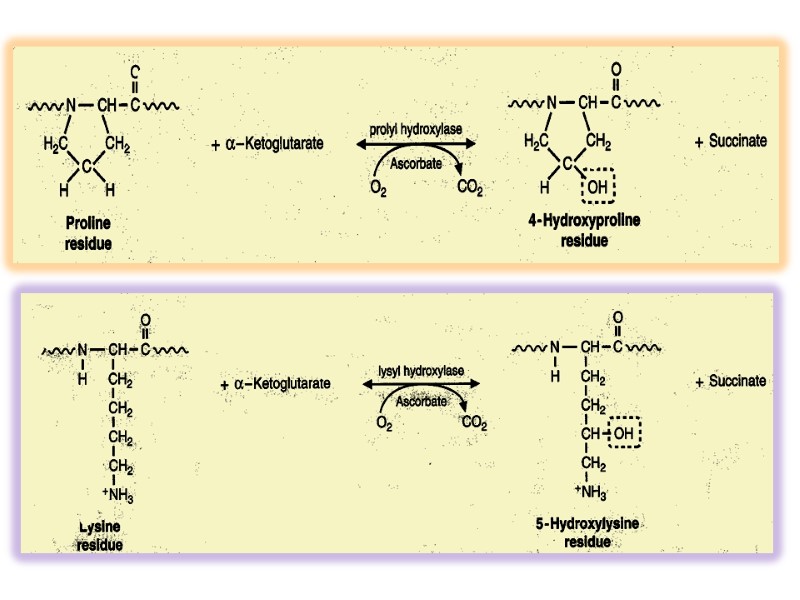
Collagen structure Typically primary structure contains about 35% Gly, 11% Ala, 21% Pro and 4-Hyp (4-hydroxyproline, an uncommon amino acid) 1% lysine, hydroxylysine, may be aspartic, glutamic amino acids.
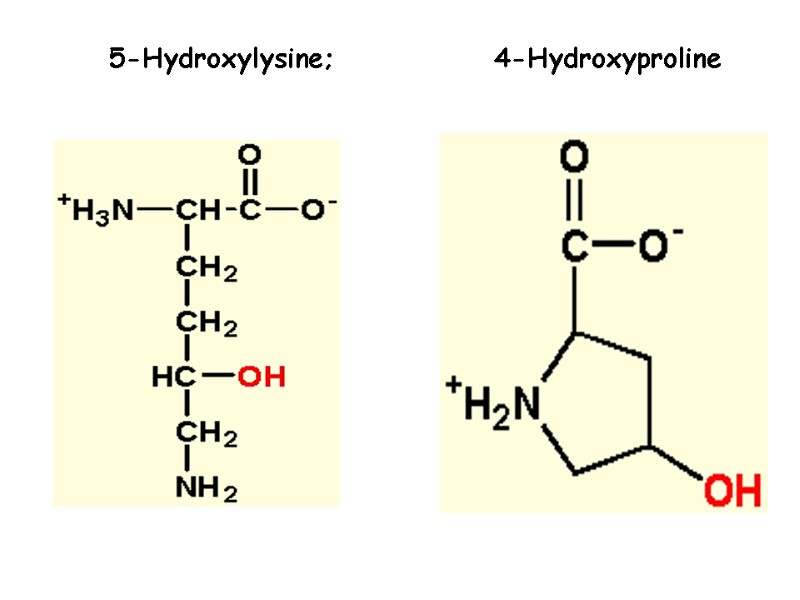
5-Hydroxylysine; 4-Hydroxyproline
These hydroxylation reactions require molecular oxygen and the reducing agent Vitamin C (Ascorbic Acid)

The food product gelatin is derived from collagen; It has little nutritional value as a protein, because collagen is extremely low in many amino acids that are essential in the human diet.
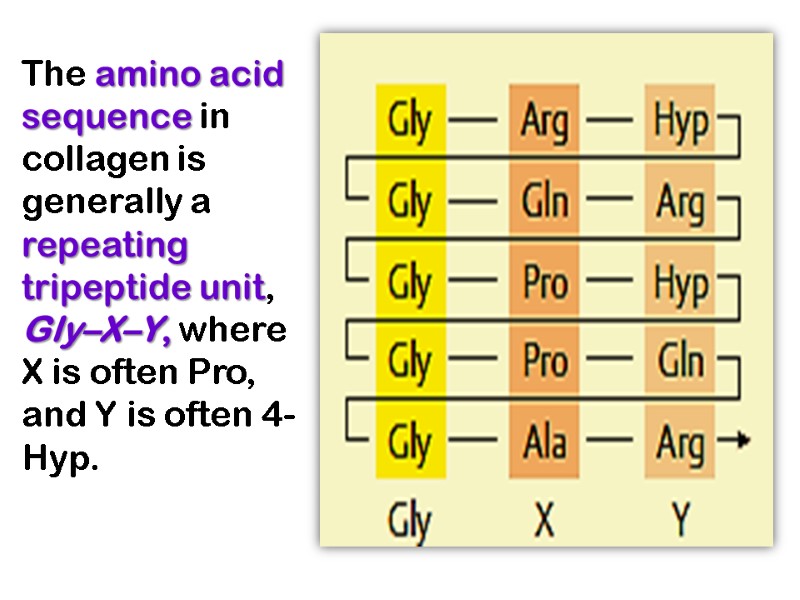
The amino acid sequence in collagen is generally a repeating tripeptide unit, Gly–X–Y, where X is often Pro, and Y is often 4-Hyp.

Each chain includes 1000 amino acid residues. The collagen helix (one peptide chain) is a unique secondary structure quite distinct from the α- helix. It is left-handed and has three amino acid residues per turn.
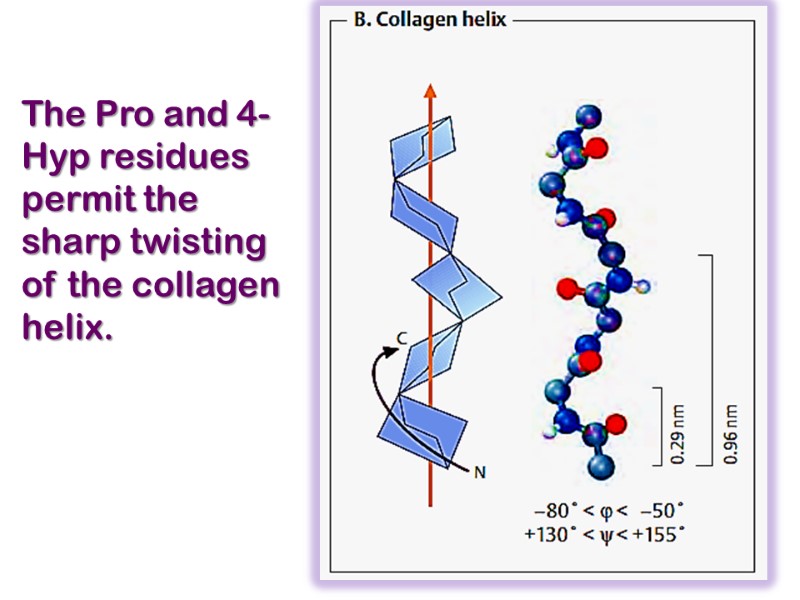
The Pro and 4-Hyp residues permit the sharp twisting of the collagen helix.

Collagen is also a coiled coil, but one with distinct tertiary and quaternary structures: three separate polypeptides, called α- chains (not to be confused with α- helices), are supertwisted about each other. Hydrogen bonds form between these coils, which are around 1000 amino acids in length, which gives the structure strength.

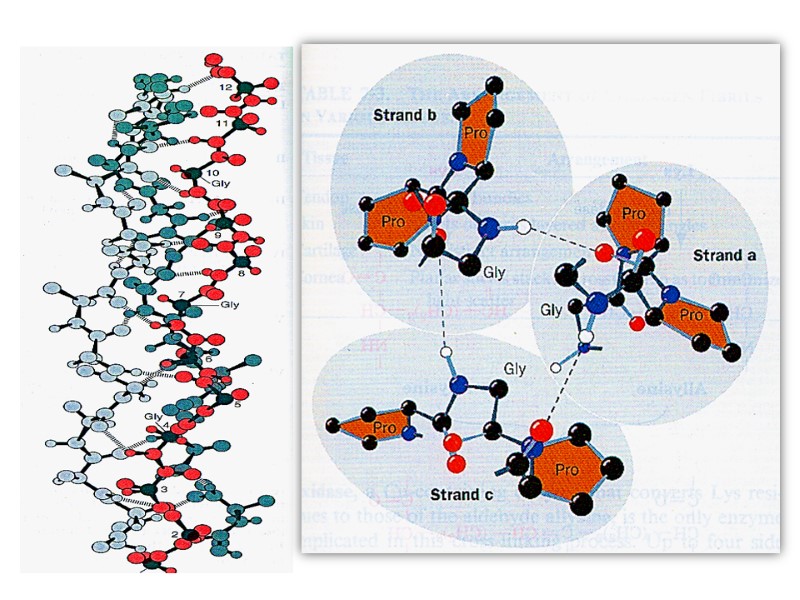
The amino acid sequence and the supertwisted quaternary structure of collagen allow a very close packing of its three polypeptides. Only Gly residues can be accommodated at the very tight junctions between the individual α- chains.
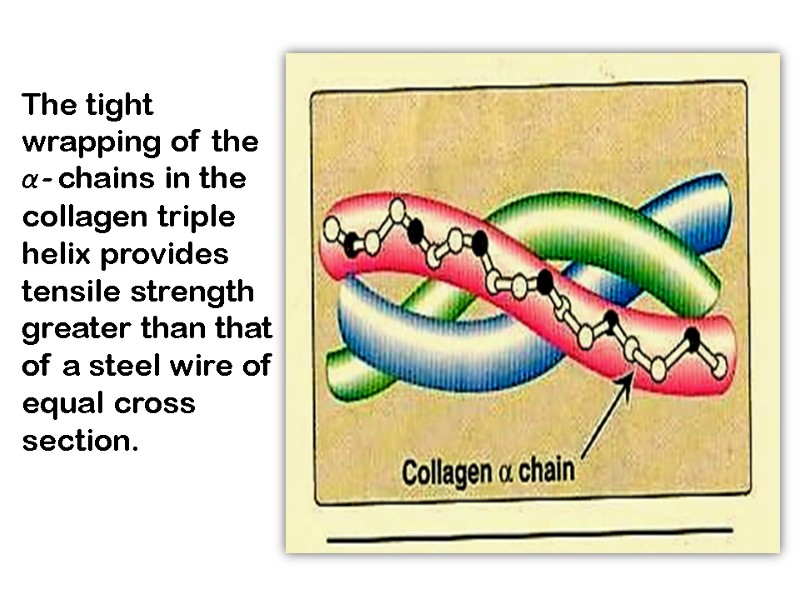
The tight wrapping of the α- chains in the collagen triple helix provides tensile strength greater than that of a steel wire of equal cross section.
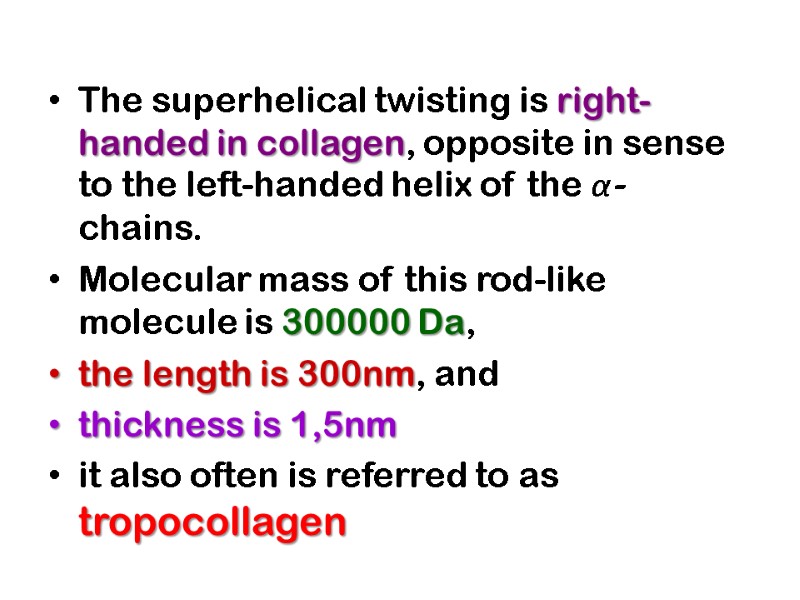
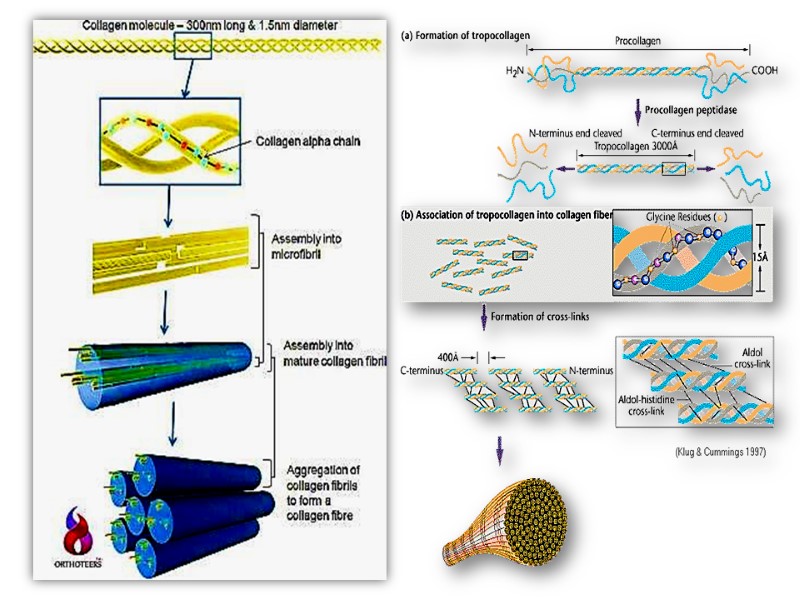
The superhelical twisting is right-handed in collagen, opposite in sense to the left-handed helix of the α- chains. Molecular mass of this rod-like molecule is 300000 Da, the length is 300nm, and thickness is 1,5nm it also often is referred to as tropocollagen
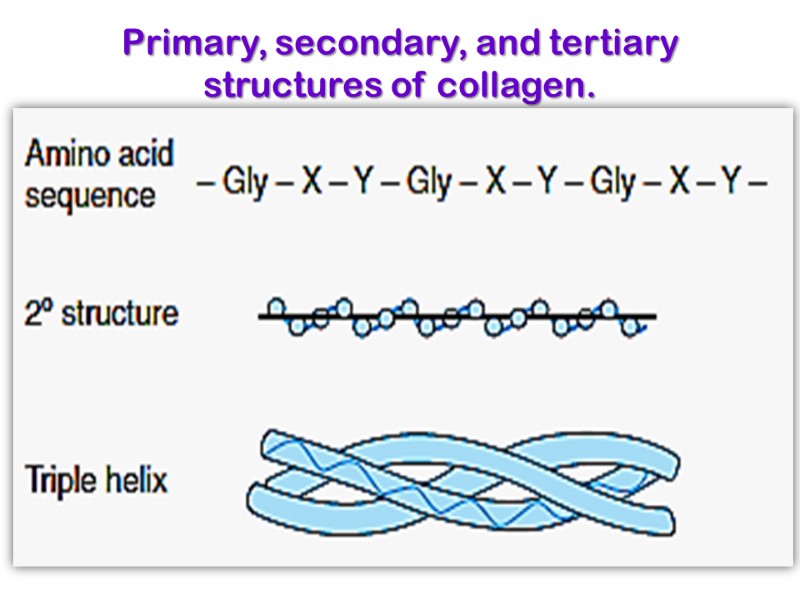
Primary, secondary, and tertiary structures of collagen.
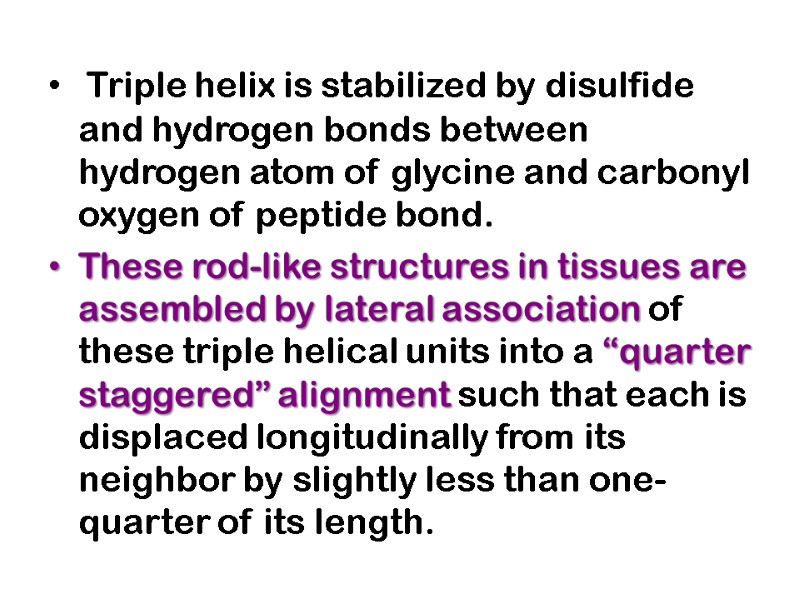
Triple helix is stabilized by disulfide and hydrogen bonds between hydrogen atom of glycine and carbonyl oxygen of peptide bond. These rod-like structures in tissues are assembled by lateral association of these triple helical units into a “quarter staggered” alignment such that each is displaced longitudinally from its neighbor by slightly less than one-quarter of its length.
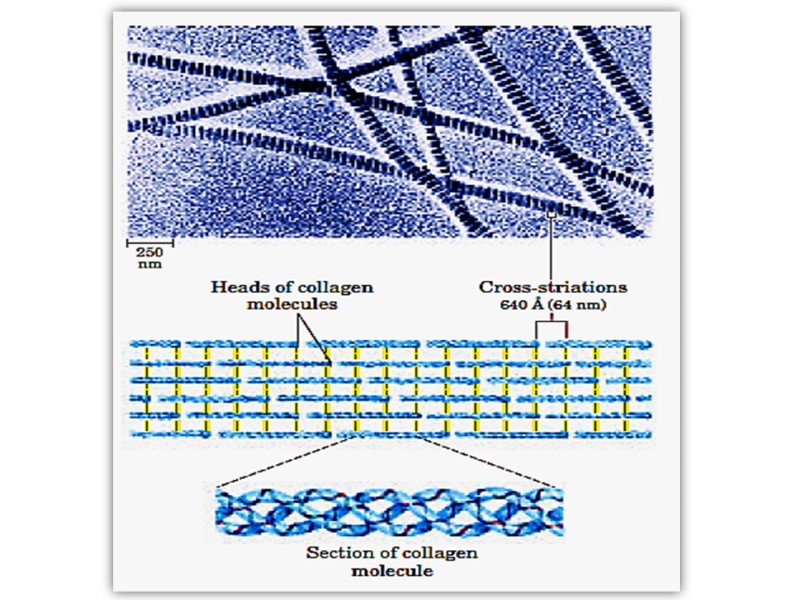
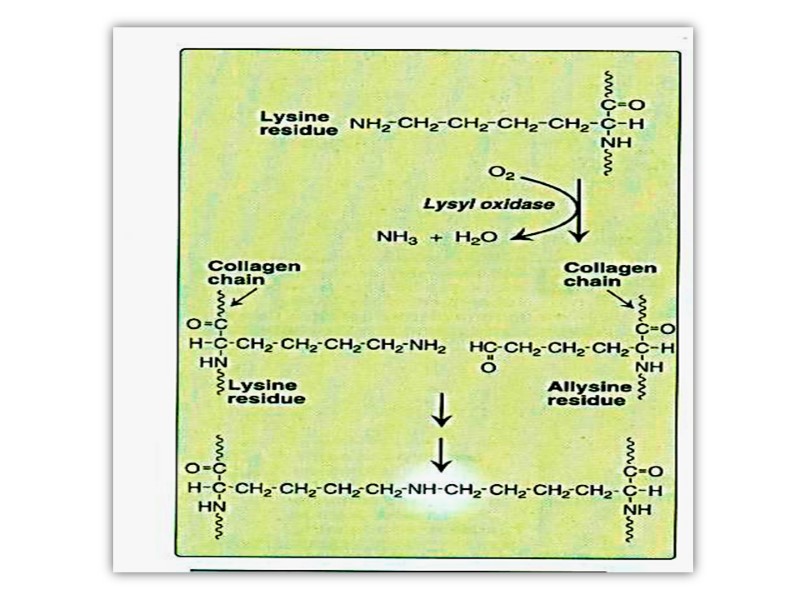
The α- chains of collagen molecules and the collagen molecules of fibrils are cross-linked by unusual types of covalent bonds involving Lys, HyLys (5-hydroxylysine); or His residues that are present at a few of the X and Y positions in collagens.

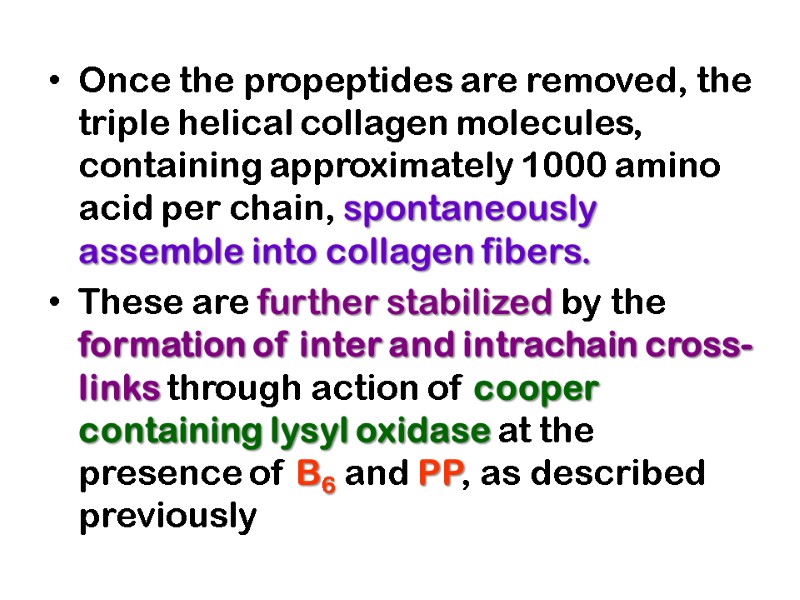
These cross-links form through the action of lysyl oxidase, a cooper-dependant enzyme (active at the presence of vitamin PP and B6) that oxidatively deaminates the ε-amino groups of certain lysine and hydroxylysine residues yielding reactive aldehydes.

Such aldehydes can form aldol condensation products with either lysine- or hydroxylysine-derived aldehydes or form Schiff bases with the ε-amino groups of unoxidized lysines or hydroxylysines.

These reactions after further chemical rearrangements result in stable covalent cross links that are important for tensile strength of the fibers. These links create uncommon amino acid residues such as dehydrohydroxylysinonorleucine.
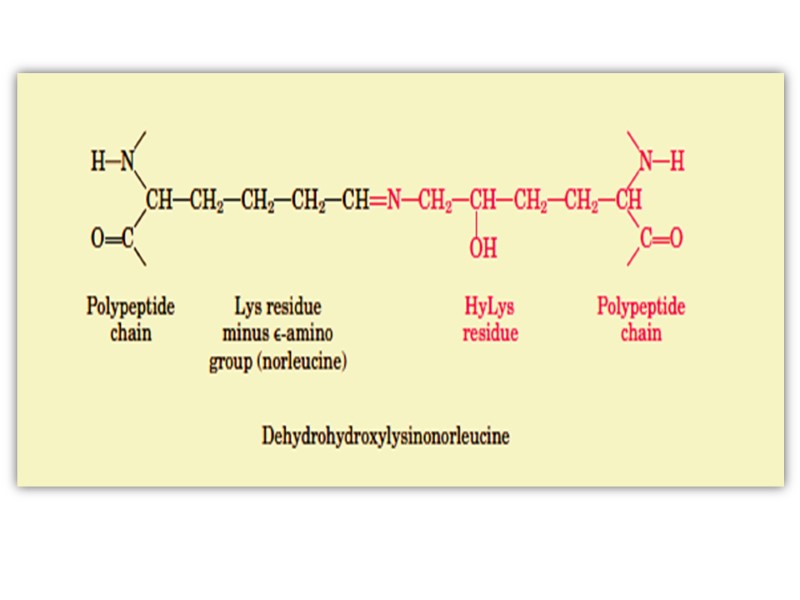

Multiple twisting provides high tensile strength of collagen fibers. Collagen is generally glycosylated, glucose, galactose or lactose is linked to hydroxylysine residues by o-glycosidic bonds.

Known 19 types of collagen differ by degree of glycosilation and, hydroxylation and distribution in organism. Several collagen types do not form fibrils in tissues, type 1, 2, 3,5,11 form fibrils.

The most spread collagen type1, enter in the most connective tissues, bones, tendons, and cornea. Type 2 is spread in cartilage, vitreous humor. Type 4 collagen is an important component of basement membranes where it forms a mesh-like network. Type 7 includes anchoring fibrils participating in joining of epidermis to derma.

Collagen metabolism. Collagen metabolism intensity vary depend on animal, tissue type, age, character of diet, pathology presence. In adulthood collagen renovation (renewal) occurs slowly, the rates of synthesis and degradation is balanced. Half-life period is measured by weeks and months. The most active renovation occurs under 20 years old

Metabolism becomes also more active in some infectious disorders, in collagenoses and hyperparathyroidism. In childhood the synthesis predominate over the degradation and the common mass of collagen is increased mostly at the expense of the osseous tissue.
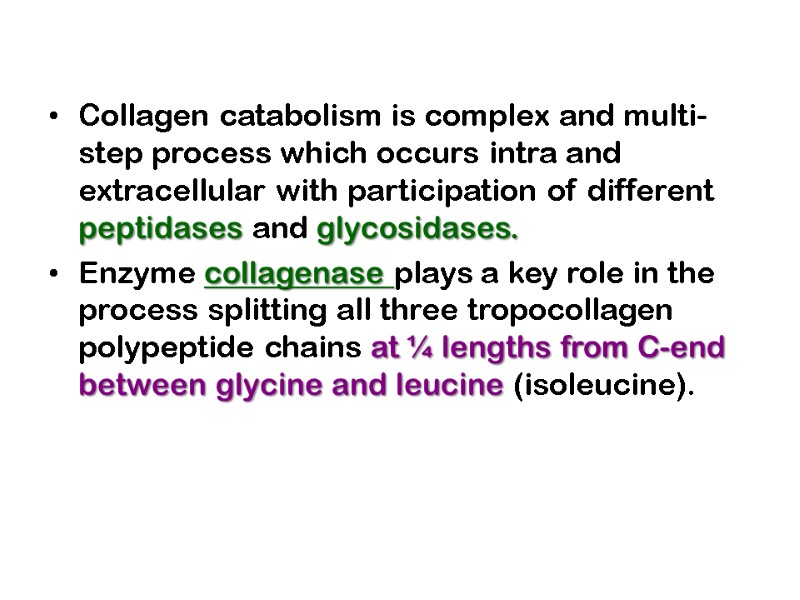
Collagen catabolism is complex and multi-step process which occurs intra and extracellular with participation of different peptidases and glycosidases. Enzyme collagenase plays a key role in the process splitting all three tropocollagen polypeptide chains at ¼ lengths from C-end between glycine and leucine (isoleucine).

Formed fragments are water-soluble and hydrolysed further by different proteases. Degraded products (amino acids, peptides) are excreted with urine. Hydroxyproline and proline are markers of connective tissues: 15-20 mg of hydroxyproline in adulthood and 200 mg in childhood are excreted per 24 hours.

In some connective tissue disorders hydroxyproline excreation is increased due to higher collagen degradation. It is noted in hyperfunction of parathyroid glands, fractures of tubular osseous, some bone tumors, paradontitis, and hereditary hyperhydroxyprolinemia. The last disorder is caused by inherited insufficiency of hydroxyprolinoxidase which must oxidize the part of hydroxyproline in health.
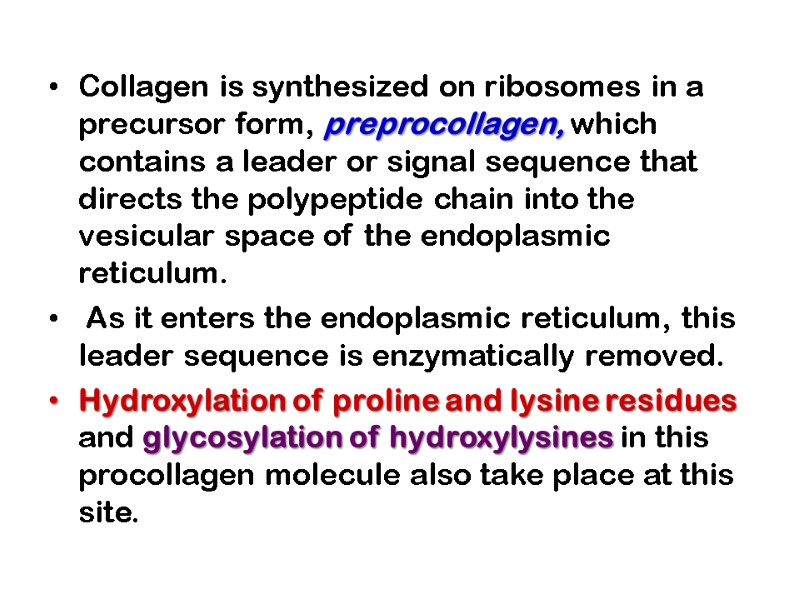
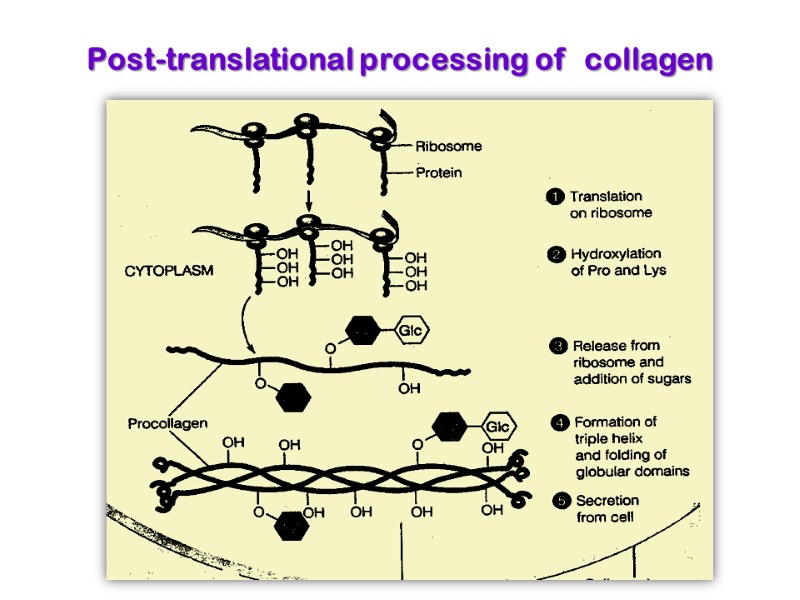
Collagen is synthesized on ribosomes in a precursor form, preprocollagen, which contains a leader or signal sequence that directs the polypeptide chain into the vesicular space of the endoplasmic reticulum. As it enters the endoplasmic reticulum, this leader sequence is enzymatically removed. Hydroxylation of proline and lysine residues and glycosylation of hydroxylysines in this procollagen molecule also take place at this site.

Enzymes are prolyl hydroxylase and lysyl hydroxylase, whose Cofactores are ascorbic acid (vitamin C) and α-ketoglutarate, molecular O2, Fe2+ Prolyl hydroxylase and lysyl hydroxylase include Fe2+ in active site.
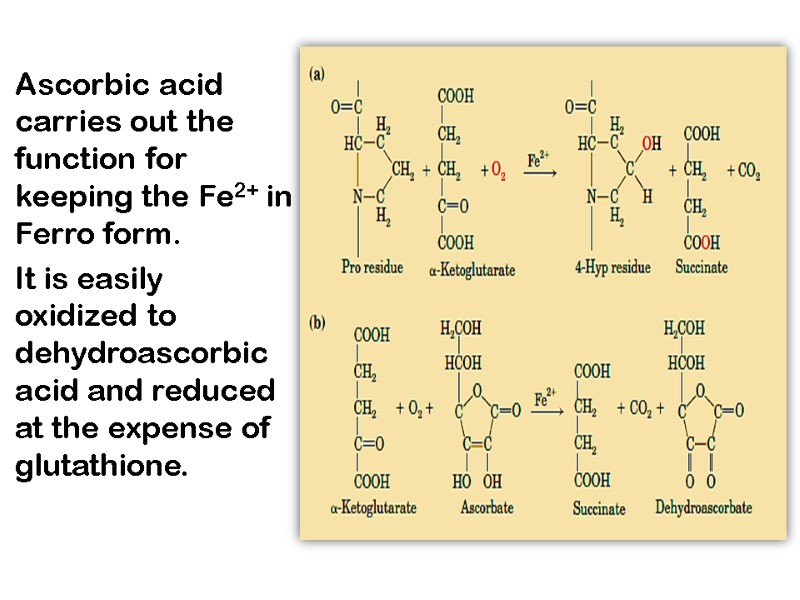
Ascorbic acid carries out the function for keeping the Fe2+ in Ferro form. It is easily oxidized to dehydroascorbic acid and reduced at the expense of glutathione.
The procollagen molecule contains polypeptide extensions (extension peptides) of 20-35 kDa at both its amino and carboxyl terminal ends, neither of which is present in mature collagen.

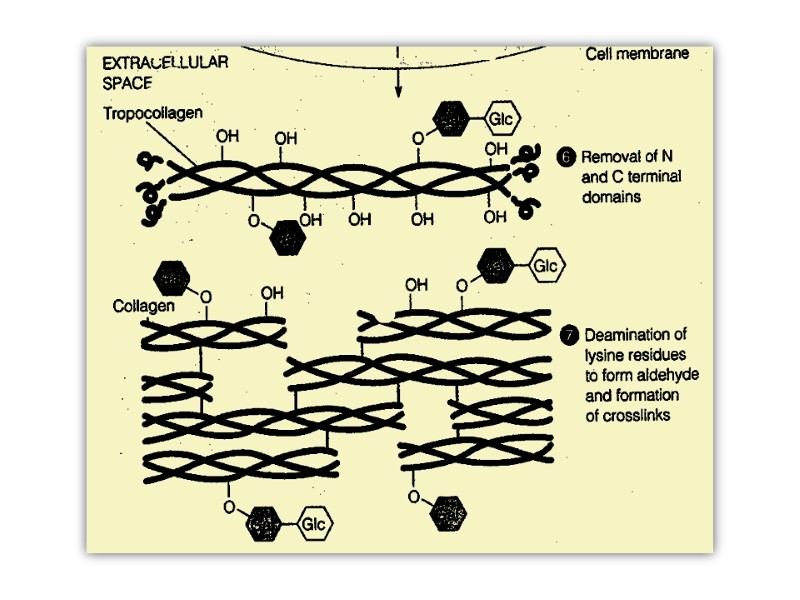
Following secretion from the cell by way of the Golgi apparatus, extracellular enzymes called procollagen aminoproteinase and procollagen carboxyprotienase remove the extension peptides at the amino and carboxyl terminal ends, respectively. Cleavage of these propeptides may occur within crypts or folds in the cell membrane.
Once the propeptides are removed, the triple helical collagen molecules, containing approximately 1000 amino acid per chain, spontaneously assemble into collagen fibers. These are further stabilized by the formation of inter and intrachain cross-links through action of cooper containing lysyl oxidase at the presence of B6 and PP, as described previously
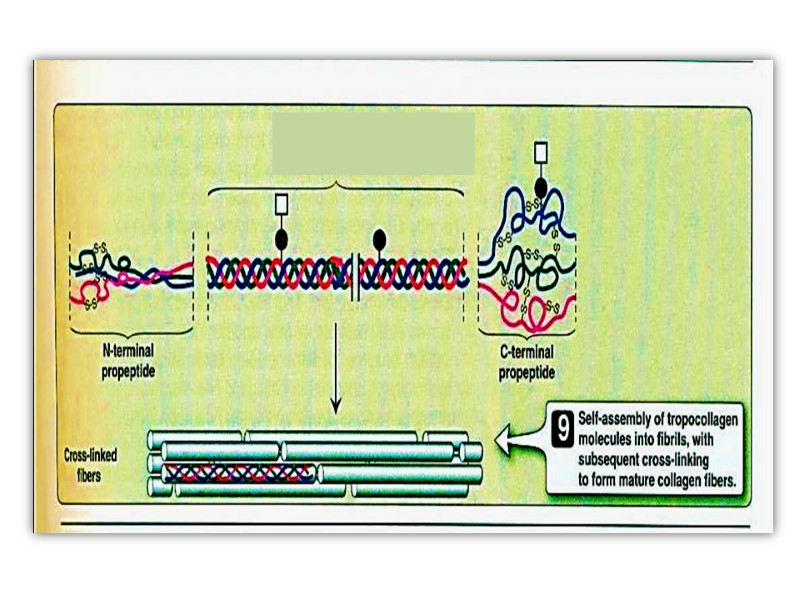
Post-translational processing of collagen
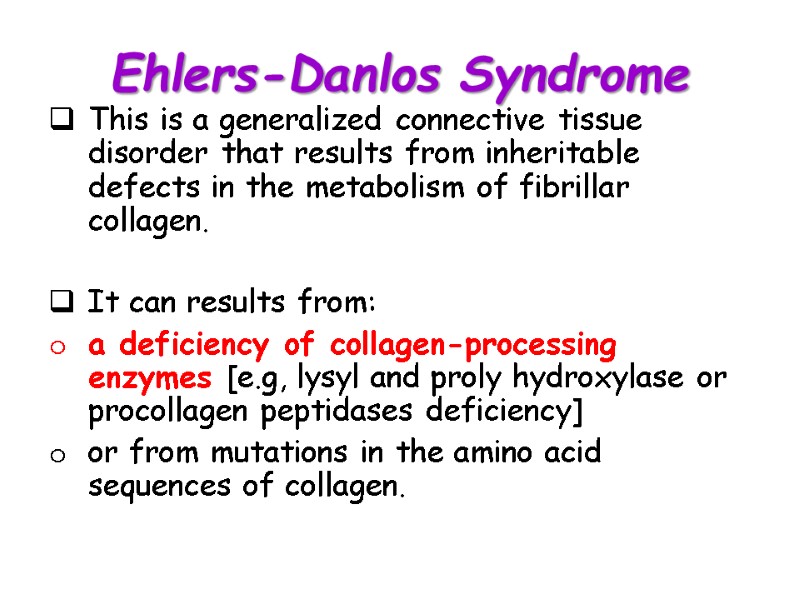
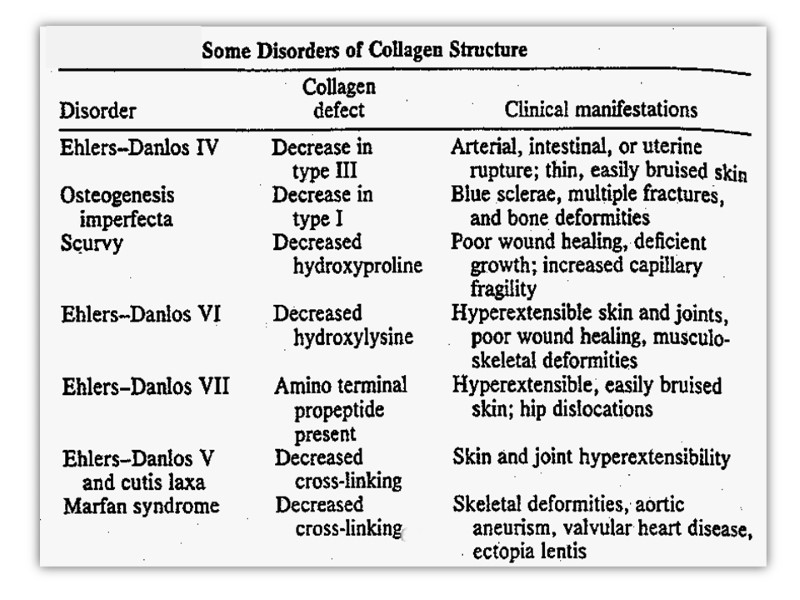
Ehlers-Danlos Syndrome This is a generalized connective tissue disorder that results from inheritable defects in the metabolism of fibrillar collagen. It can results from: a deficiency of collagen-processing enzymes [e.g, lysyl and proly hydroxylase or procollagen peptidases deficiency] or from mutations in the amino acid sequences of collagen.
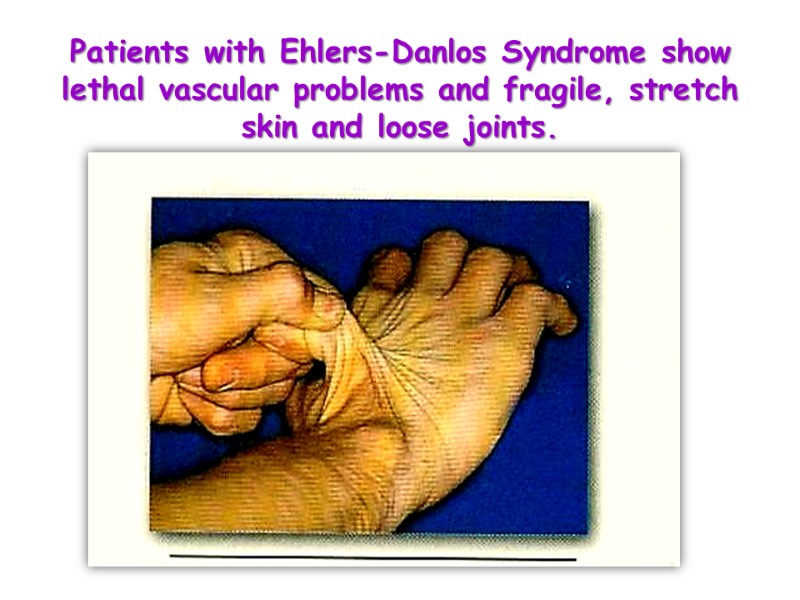
Patients with Ehlers-Danlos Syndrome show lethal vascular problems and fragile, stretch skin and loose joints.
Osteogenesis imperfecta Osteogenesis imperfecta can be due to decreased production of α-chains or due to abnormal α-chains due to mutations. The most common mutations cause the replacement of Glycine residues (in –Gly–X–Y–) by amino amino acid with bulky side chains. The resultant structurally abnormal pro – α-chains prevent the formation of the required triple-helical conformation
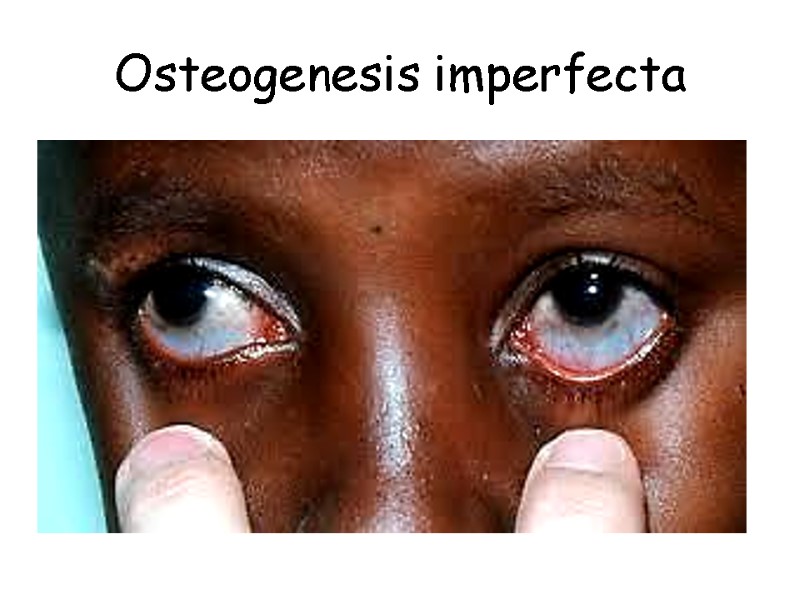
Osteogenesis imperfecta

Elastin Elastin is a connective tissue protein that is responsible for properties of extensibility and elastic recoil in tissues.
Although not as widespread as collagen, elastin is present in large amounts, particularly in tissues that require these physical properties, eg, lung, large arterial blood vessels, and some elastic ligaments.
Smaller quantities of elastin are also found in skin, ear, cartilage, and several other tissues.
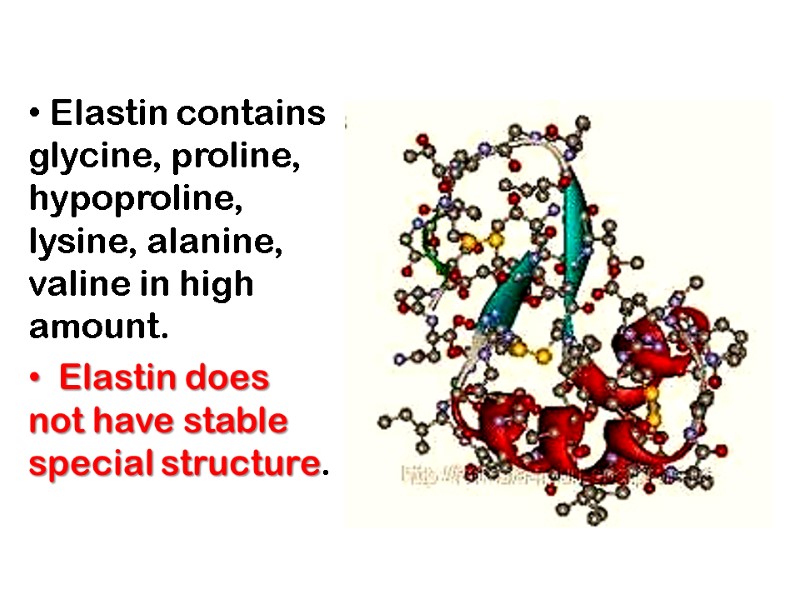
Elastin contains glycine, proline, hypoproline, lysine, alanine, valine in high amount. Elastin does not have stable special structure.
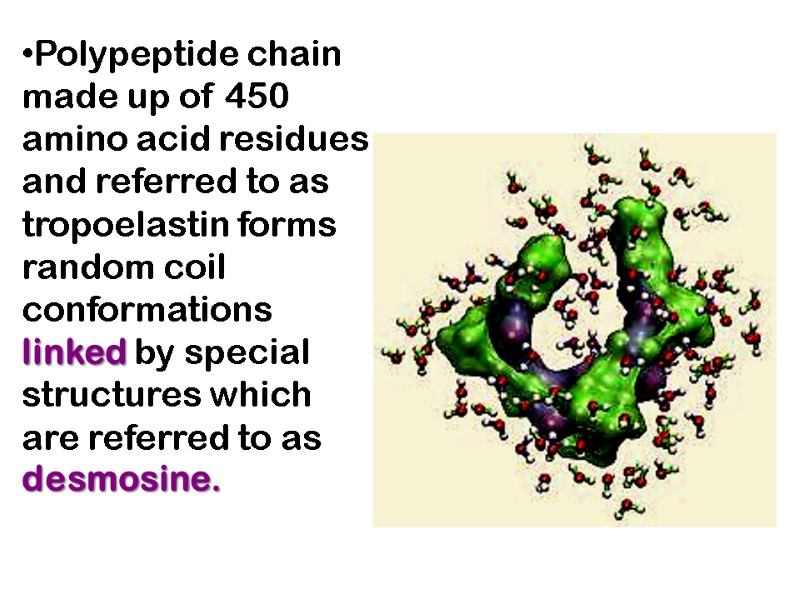
Polypeptide chain made up of 450 amino acid residues and referred to as tropoelastin forms random coil conformations linked by special structures which are referred to as desmosine.
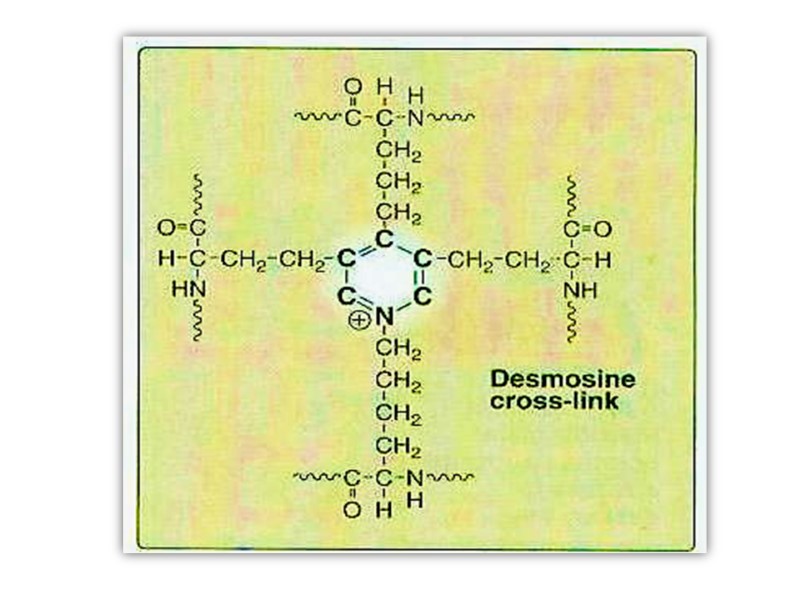

Desmosine has pyridine ring formed by 2-3-4 lysine residues. This special structure gives elastin the ability to stretch and subsequently recoil in two perpendicular directions during the performance of its physiological functions. Elastin is highly insoluble and extremely stable and has a very low turnover rate.
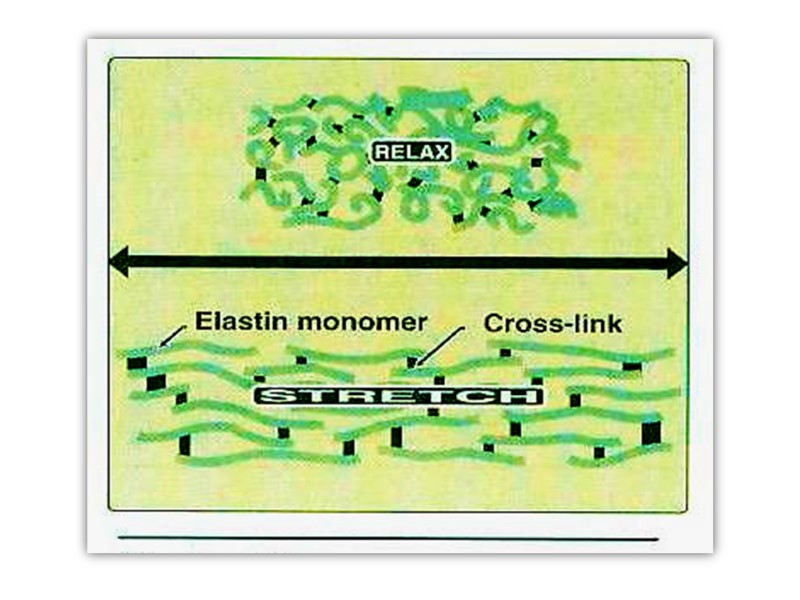

Elastin is synthesized as a soluble monomer of 70 kDa called “tropoelastin”. Some of the prolines in tropoelastin are hydroxylated to hydroxyproline by prolyl hydroxylase, though hydroxylysine and glycosylated hydroxylysine are not present.

Unlike collagen, tropoelastine is not synthesized in a proform with extension peptides. Furthermore, elastin does not contain repeat Gly-X-Y sequences, triple helical structure, or carbohydrate moieties.
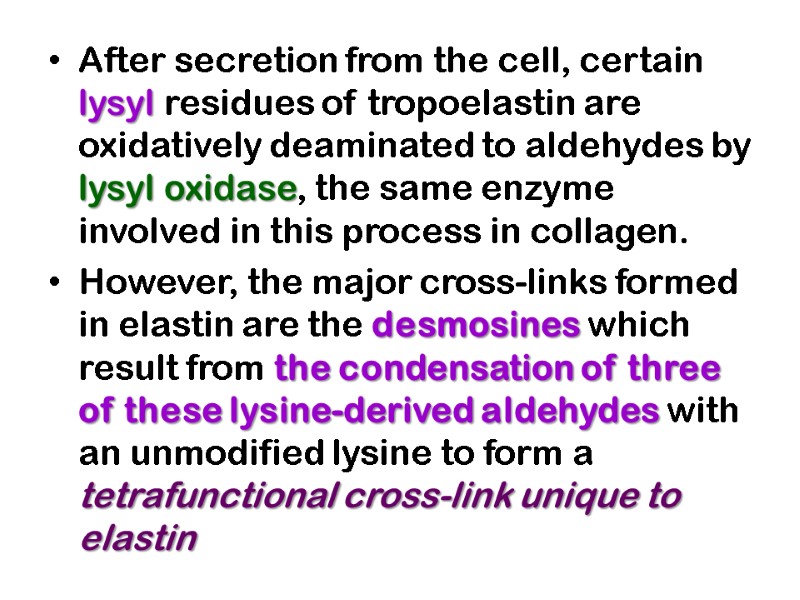
After secretion from the cell, certain lysyl residues of tropoelastin are oxidatively deaminated to aldehydes by lysyl oxidase, the same enzyme involved in this process in collagen. However, the major cross-links formed in elastin are the desmosines which result from the condensation of three of these lysine-derived aldehydes with an unmodified lysine to form a tetrafunctional cross-link unique to elastin
A number of skin diseases (eg, sclerodermia) are associated with accumulation of elastin.
Fragmentation or, alternatively, a decrease of elastin is found in conditions such as pulmonary emphysema, cutis laxa, and aging of the skin.

By aging elasticity of tissues is diminished because the amount of cross-links is decreased and tissues become more sensitive to mechanical affects.

Keratins Keratins constitute almost the entire dry weight of hair, wool, nails, claws quills, horns, hooks and much of the outer layer of skin.



α-keratin is rich in hydrophobic residues of Ala, Val, Leu, Ile, Met and Phe, many Cys. Keratins constitute a large family with about 30 members. Type I (acidic) 40-50 kDa Type II (basic) 50-70 kDa
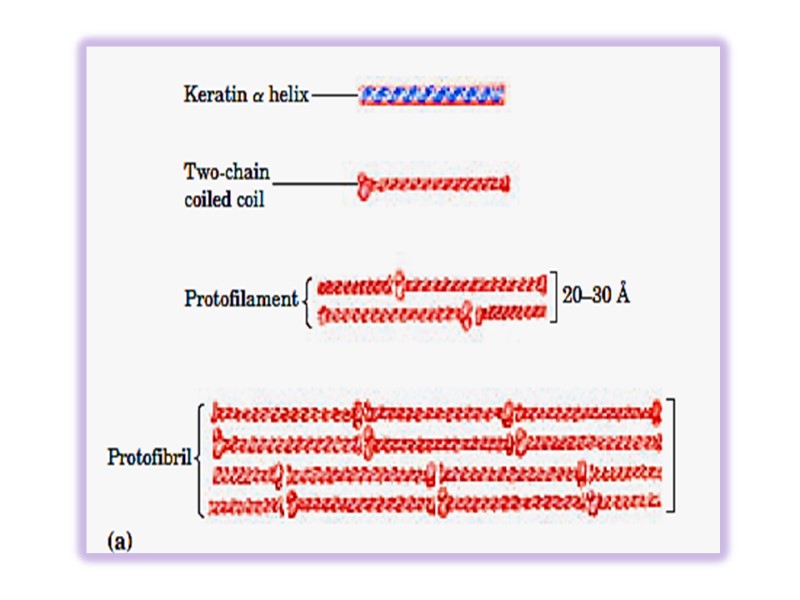

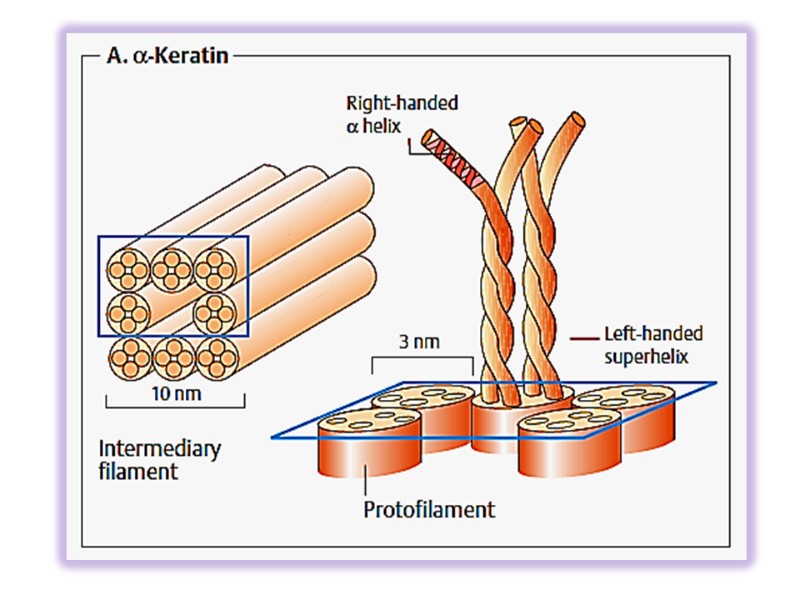
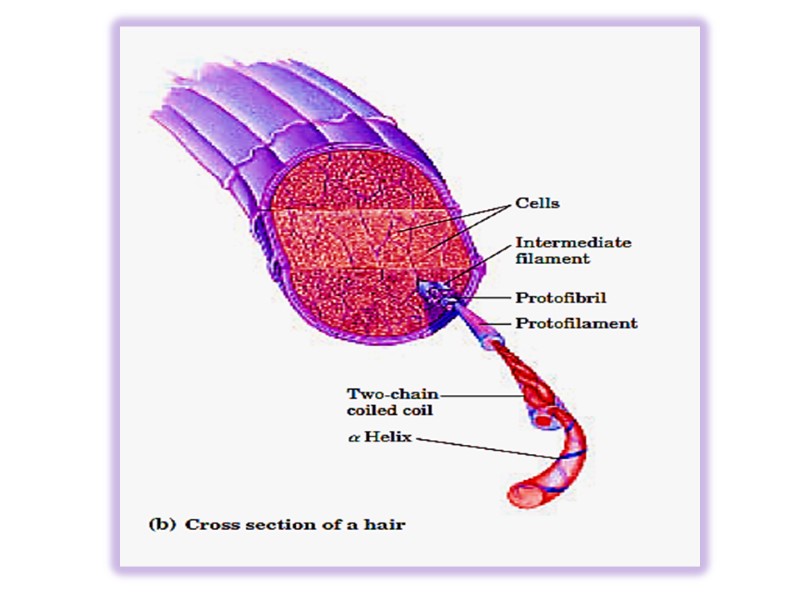
Keratin helix is right-handed α- helix. Two α – helixes from different class oriented in parallel manner are wrapped about each other to form a super twisted collided coil (protofibril). Many coiled coils are assembled into large supermolecular complexes and their arrangement forms the intermediate filament of hair. Microfibril → macrifibril → filament of hair
8036-1.__protein_structural_organization.ppt
- Количество слайдов: 302

