026e8987ff63c0ce5d8c028702e9ee0a.ppt
- Количество слайдов: 31
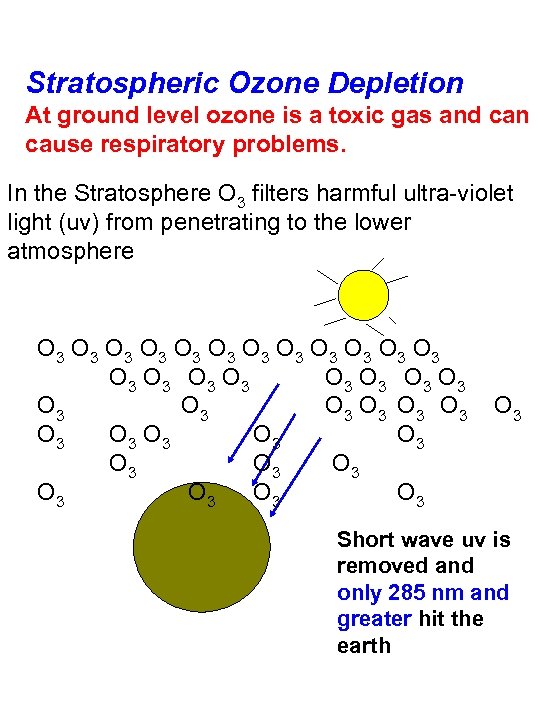 Stratospheric Ozone Depletion At ground level ozone is a toxic gas and can cause respiratory problems. In the Stratosphere O 3 filters harmful ultra-violet light (uv) from penetrating to the lower atmosphere O 3 O 3 O 3 O 3 O 3 O 3 O 3 O 3 O 3 O 3 O 3 Short wave uv is removed and only 285 nm and greater hit the earth
Stratospheric Ozone Depletion At ground level ozone is a toxic gas and can cause respiratory problems. In the Stratosphere O 3 filters harmful ultra-violet light (uv) from penetrating to the lower atmosphere O 3 O 3 O 3 O 3 O 3 O 3 O 3 O 3 O 3 O 3 O 3 Short wave uv is removed and only 285 nm and greater hit the earth
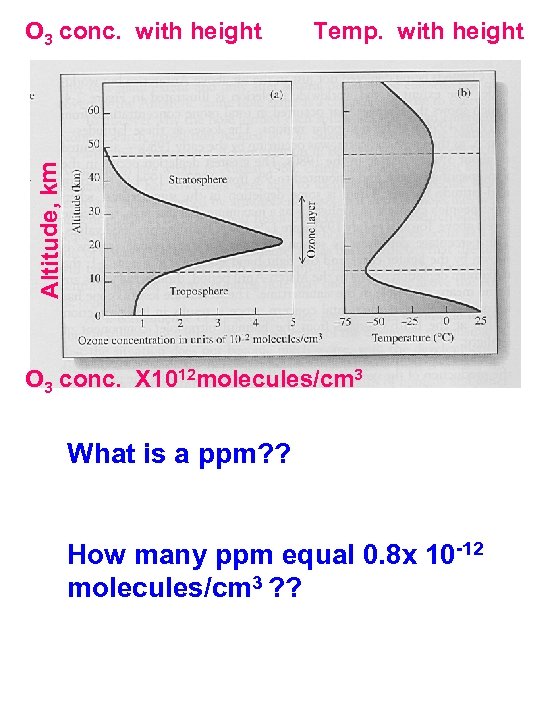 Temp. with height Altitude, km O 3 conc. with height O 3 conc. X 1012 molecules/cm 3 What is a ppm? ? How many ppm equal 0. 8 x 10 -12 molecules/cm 3 ? ?
Temp. with height Altitude, km O 3 conc. with height O 3 conc. X 1012 molecules/cm 3 What is a ppm? ? How many ppm equal 0. 8 x 10 -12 molecules/cm 3 ? ?
 · Stratospheric ozone depletion is of great concern because the ozone layer in the stratosphere prevents 95 -99% of the suns ultraviolet radiation from striking the earth. · If this additional uv light were to strike the earth it would be potentially damaging to life on earth. · Every 1% decrease in the earth’s ozone shield is projected to increases the amount of UV light exposure to the lower atmosphere by 2%. · Recent UV measurements from around the northern hemisphere indicate small UV increases in rural areas and almost no increase in areas near large cities.
· Stratospheric ozone depletion is of great concern because the ozone layer in the stratosphere prevents 95 -99% of the suns ultraviolet radiation from striking the earth. · If this additional uv light were to strike the earth it would be potentially damaging to life on earth. · Every 1% decrease in the earth’s ozone shield is projected to increases the amount of UV light exposure to the lower atmosphere by 2%. · Recent UV measurements from around the northern hemisphere indicate small UV increases in rural areas and almost no increase in areas near large cities.
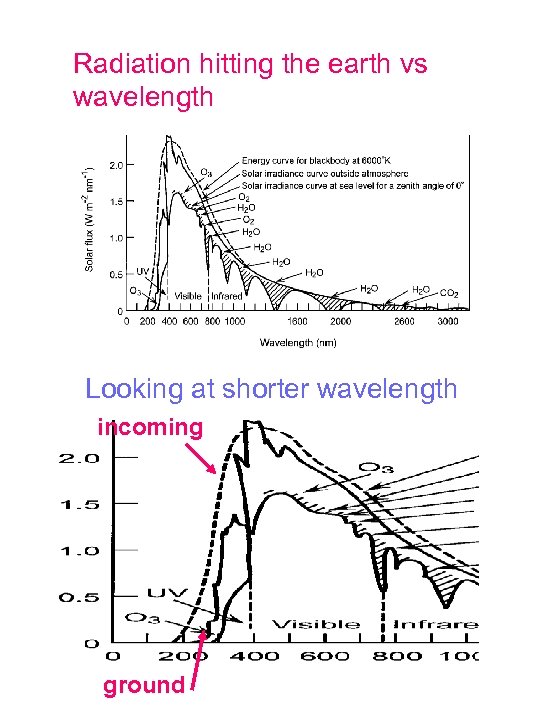 Radiation hitting the earth vs wavelength Looking at shorter wavelength incoming ground
Radiation hitting the earth vs wavelength Looking at shorter wavelength incoming ground
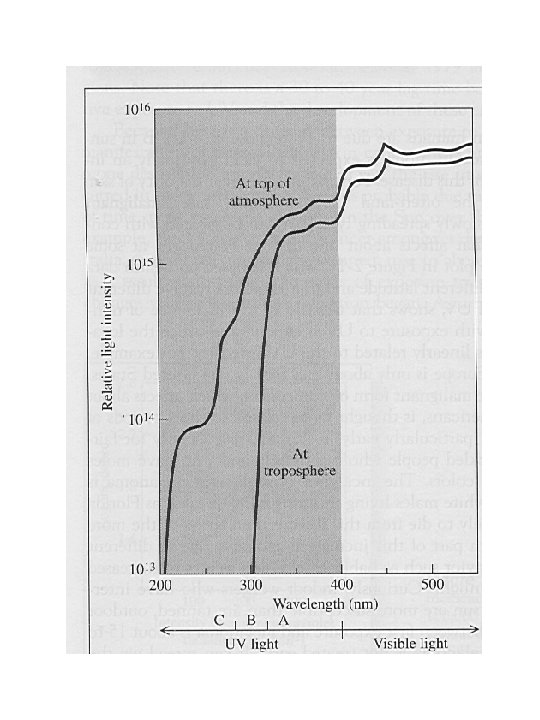
 Solar radiation wave length vs. Intensity UV-A: 315 to 400 nm – (near ultraviolet ranging into the visible~ 7% of the solar flux (which is not particularly harmful to living species) UV-B: 280 to 315 nm (~1. 5% of the total flux) which can be harmful to both plant and animals species, especially after prolonged exposure UV-C: < 280 nm ~5% of the total flux which rapidly damages all types of biota
Solar radiation wave length vs. Intensity UV-A: 315 to 400 nm – (near ultraviolet ranging into the visible~ 7% of the solar flux (which is not particularly harmful to living species) UV-B: 280 to 315 nm (~1. 5% of the total flux) which can be harmful to both plant and animals species, especially after prolonged exposure UV-C: < 280 nm ~5% of the total flux which rapidly damages all types of biota
 A number of consequences can result from increased levels of UV(ultraviolet radiation) striking the earth, including: 1. genetic damage 2. eye damage 3. damage to marine life. 4. increased amounts of photochemical smog.
A number of consequences can result from increased levels of UV(ultraviolet radiation) striking the earth, including: 1. genetic damage 2. eye damage 3. damage to marine life. 4. increased amounts of photochemical smog.
 Eye damage: UV-B (280 -320 nm) can damage the lens, cornea, retina, and conjunctive membrane; A sustained thinning of the ozone layer may cause ~200, 000 new cases of cataracts (clouding of the eye’s lens) per year. Immunosuppression: Exposure to UV-B has been shown to increase the incidence and severity of infectious disease and some cancers. Those infected with HIV tend to experience the onset of AIDs symptoms when exposed to increased UV-B Green plants: Excessive exposure to UVB inhibits the growth process of green plants. This is very important for oceanic algae, which support the food chain in the oceans and consequently land based life which depend on the oceans.
Eye damage: UV-B (280 -320 nm) can damage the lens, cornea, retina, and conjunctive membrane; A sustained thinning of the ozone layer may cause ~200, 000 new cases of cataracts (clouding of the eye’s lens) per year. Immunosuppression: Exposure to UV-B has been shown to increase the incidence and severity of infectious disease and some cancers. Those infected with HIV tend to experience the onset of AIDs symptoms when exposed to increased UV-B Green plants: Excessive exposure to UVB inhibits the growth process of green plants. This is very important for oceanic algae, which support the food chain in the oceans and consequently land based life which depend on the oceans.
 How is O 3 formed in the Stratosphere? The Stratosphere begins about 10 k above the surface of the earth and goes up to 50 k The main gases in the stratosphere, as at the surface, are oxygen and nitrogen uv light of low wave lengths ( high energy) split molecular oxygen (O 2 ) to split oxygen O 2 O. + O. requires 495 k. J mole-1 of heat (enthalpy) What wave length of light can do this? ? Let’s start with hn = E, where h is Planck’s constant and n is the frequency of light and E is the energy associated with one photon.
How is O 3 formed in the Stratosphere? The Stratosphere begins about 10 k above the surface of the earth and goes up to 50 k The main gases in the stratosphere, as at the surface, are oxygen and nitrogen uv light of low wave lengths ( high energy) split molecular oxygen (O 2 ) to split oxygen O 2 O. + O. requires 495 k. J mole-1 of heat (enthalpy) What wave length of light can do this? ? Let’s start with hn = E, where h is Planck’s constant and n is the frequency of light and E is the energy associated with one photon.
 And, n l = c where c is the speed of light and l is the wave length of light Combining we can solve for the wave length that will break apart oxygen at an enthalpy of 495, 000 J mole-1 l= h c/ E If the value of Planck’s constant is 6. 62 10 -34 joules sec c = 2. 9979 x 108 m sec-1 l= h c/ E = 241 nm can you verify this calculation? Hint energy E is for one photon? ?
And, n l = c where c is the speed of light and l is the wave length of light Combining we can solve for the wave length that will break apart oxygen at an enthalpy of 495, 000 J mole-1 l= h c/ E If the value of Planck’s constant is 6. 62 10 -34 joules sec c = 2. 9979 x 108 m sec-1 l= h c/ E = 241 nm can you verify this calculation? Hint energy E is for one photon? ?
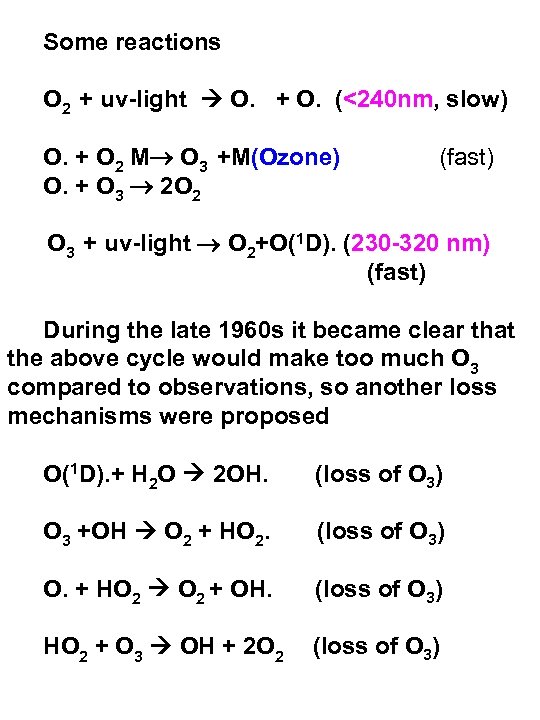 Some reactions O 2 + uv-light O. + O. (<240 nm, slow) O. + O 2 M O 3 +M(Ozone) O. + O 3 2 O 2 (fast) O 3 + uv-light O 2+O(1 D). (230 -320 nm) (fast) During the late 1960 s it became clear that the above cycle would make too much O 3 compared to observations, so another loss mechanisms were proposed O(1 D). + H 2 O 2 OH. (loss of O 3) O 3 +OH O 2 + HO 2. (loss of O 3) O. + HO 2 + OH. (loss of O 3) HO 2 + O 3 OH + 2 O 2 (loss of O 3)
Some reactions O 2 + uv-light O. + O. (<240 nm, slow) O. + O 2 M O 3 +M(Ozone) O. + O 3 2 O 2 (fast) O 3 + uv-light O 2+O(1 D). (230 -320 nm) (fast) During the late 1960 s it became clear that the above cycle would make too much O 3 compared to observations, so another loss mechanisms were proposed O(1 D). + H 2 O 2 OH. (loss of O 3) O 3 +OH O 2 + HO 2. (loss of O 3) O. + HO 2 + OH. (loss of O 3) HO 2 + O 3 OH + 2 O 2 (loss of O 3)
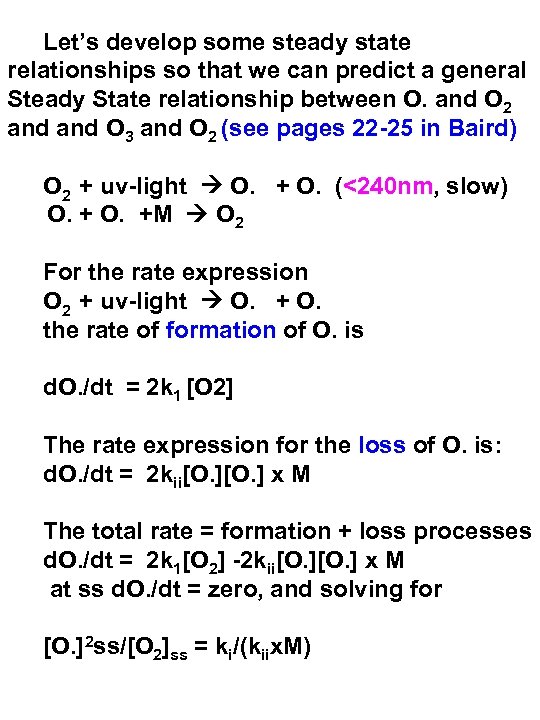 Let’s develop some steady state relationships so that we can predict a general Steady State relationship between O. and O 2 and O 3 and O 2 (see pages 22 -25 in Baird) O 2 + uv-light O. + O. (<240 nm, slow) O. + O. +M O 2 For the rate expression O 2 + uv-light O. + O. the rate of formation of O. is d. O. /dt = 2 k 1 [O 2] The rate expression for the loss of O. is: d. O. /dt = 2 kii[O. ] x M The total rate = formation + loss processes d. O. /dt = 2 k 1[O 2] -2 kii[O. ] x M at ss d. O. /dt = zero, and solving for [O. ]2 ss/[O 2]ss = ki/(kiix. M)
Let’s develop some steady state relationships so that we can predict a general Steady State relationship between O. and O 2 and O 3 and O 2 (see pages 22 -25 in Baird) O 2 + uv-light O. + O. (<240 nm, slow) O. + O. +M O 2 For the rate expression O 2 + uv-light O. + O. the rate of formation of O. is d. O. /dt = 2 k 1 [O 2] The rate expression for the loss of O. is: d. O. /dt = 2 kii[O. ] x M The total rate = formation + loss processes d. O. /dt = 2 k 1[O 2] -2 kii[O. ] x M at ss d. O. /dt = zero, and solving for [O. ]2 ss/[O 2]ss = ki/(kiix. M)
![[O. ]2 ss/[O 2]ss = ki/(kiix. M) What this says is that the ratio [O. ]2 ss/[O 2]ss = ki/(kiix. M) What this says is that the ratio](https://present5.com/presentation/026e8987ff63c0ce5d8c028702e9ee0a/image-13.jpg) [O. ]2 ss/[O 2]ss = ki/(kiix. M) What this says is that the ratio of O atoms to oxygen molecules (O 2) varies indirectly with M. M represents both oxygen and nitrogen molecules. So as we increase in altitude the ratio of [O. ]2 ss/[O 2]ss increases. If we go back to the basic reactions that form O 3 in the stratosphere O 2 + uv-light O. + O. k 1 O. + O 2 M O 3 +M(Ozone) O. + O 3 2 O 2 k 2 k 3 O 3 + uv-light O 2+O(1 D). ) k 4 And proceed through similar arguments to that we used for d. O. /dt (page 23 Baird) for d. O 3/dt [O 3] ss/[O 2]ss = M 1/2 (k 1 k 2/k 3 k 4)1/2 what does this say happens to O 3 with z?
[O. ]2 ss/[O 2]ss = ki/(kiix. M) What this says is that the ratio of O atoms to oxygen molecules (O 2) varies indirectly with M. M represents both oxygen and nitrogen molecules. So as we increase in altitude the ratio of [O. ]2 ss/[O 2]ss increases. If we go back to the basic reactions that form O 3 in the stratosphere O 2 + uv-light O. + O. k 1 O. + O 2 M O 3 +M(Ozone) O. + O 3 2 O 2 k 2 k 3 O 3 + uv-light O 2+O(1 D). ) k 4 And proceed through similar arguments to that we used for d. O. /dt (page 23 Baird) for d. O 3/dt [O 3] ss/[O 2]ss = M 1/2 (k 1 k 2/k 3 k 4)1/2 what does this say happens to O 3 with z?
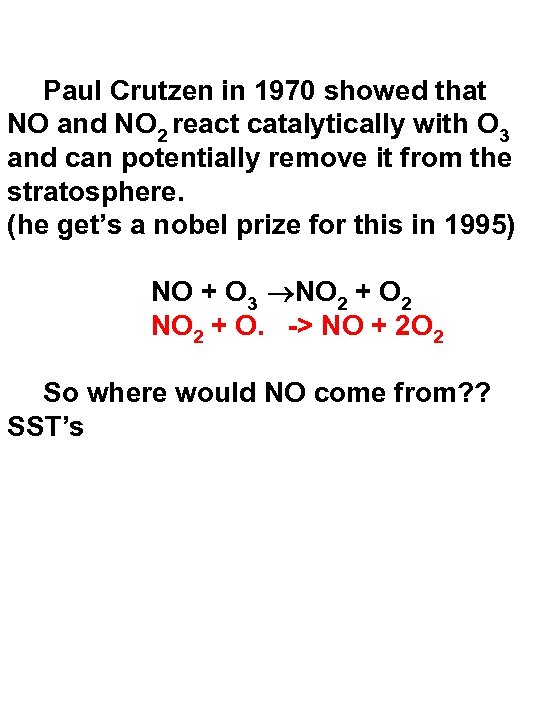 Paul Crutzen in 1970 showed that NO and NO 2 react catalytically with O 3 and can potentially remove it from the stratosphere. (he get’s a nobel prize for this in 1995) NO + O 3 NO 2 + O 2 NO 2 + O. -> NO + 2 O 2 So where would NO come from? ? SST’s
Paul Crutzen in 1970 showed that NO and NO 2 react catalytically with O 3 and can potentially remove it from the stratosphere. (he get’s a nobel prize for this in 1995) NO + O 3 NO 2 + O 2 NO 2 + O. -> NO + 2 O 2 So where would NO come from? ? SST’s
 Chloro-fluro carbons (CFCs like CCl 3 F) can also destroy Ozone 1. Freons are inert compounds that are very stable in the lower atmosphere. Light of high enough energy does not penetrate into the troposphere. 2. Freons do not react with OH. Radicals which remove most other organic gases in the atmosphere. OH. + H 2 C=CH 2 products CCl 3 F + OH. does not happen 3. Given a very long life in the troposphere, because nothing removes them, they can ultimately mix up into the stratosphere 4. In the stratosphere, the uv light is not filtered even at low wavelengths 5. Some of this low wave length radiation can photolyze freons (Molina and Rowland)
Chloro-fluro carbons (CFCs like CCl 3 F) can also destroy Ozone 1. Freons are inert compounds that are very stable in the lower atmosphere. Light of high enough energy does not penetrate into the troposphere. 2. Freons do not react with OH. Radicals which remove most other organic gases in the atmosphere. OH. + H 2 C=CH 2 products CCl 3 F + OH. does not happen 3. Given a very long life in the troposphere, because nothing removes them, they can ultimately mix up into the stratosphere 4. In the stratosphere, the uv light is not filtered even at low wavelengths 5. Some of this low wave length radiation can photolyze freons (Molina and Rowland)
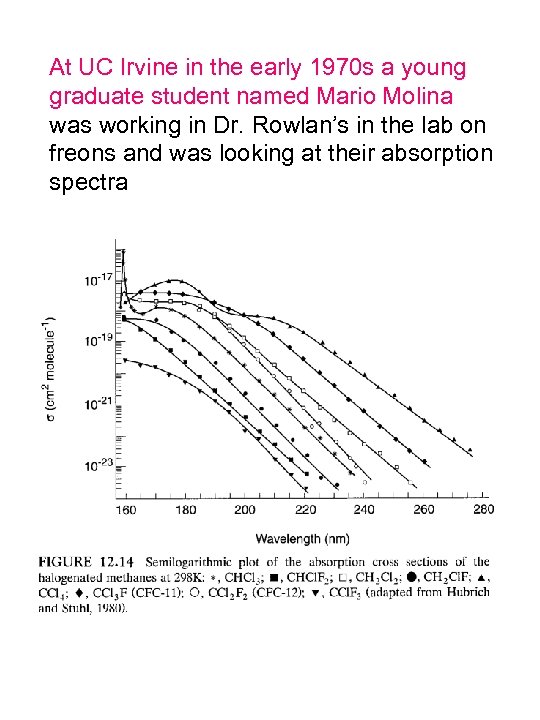 At UC Irvine in the early 1970 s a young graduate student named Mario Molina was working in Dr. Rowlan’s in the lab on freons and was looking at their absorption spectra
At UC Irvine in the early 1970 s a young graduate student named Mario Molina was working in Dr. Rowlan’s in the lab on freons and was looking at their absorption spectra
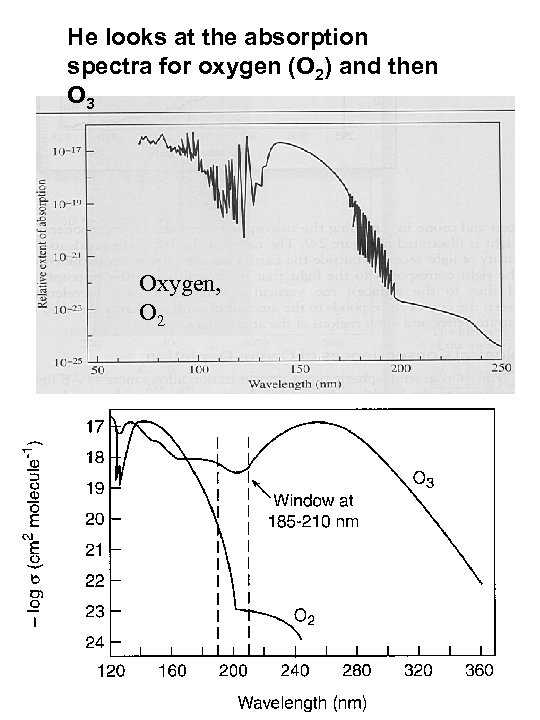 He looks at the absorption spectra for oxygen (O 2) and then O 3 Oxygen, O 2
He looks at the absorption spectra for oxygen (O 2) and then O 3 Oxygen, O 2
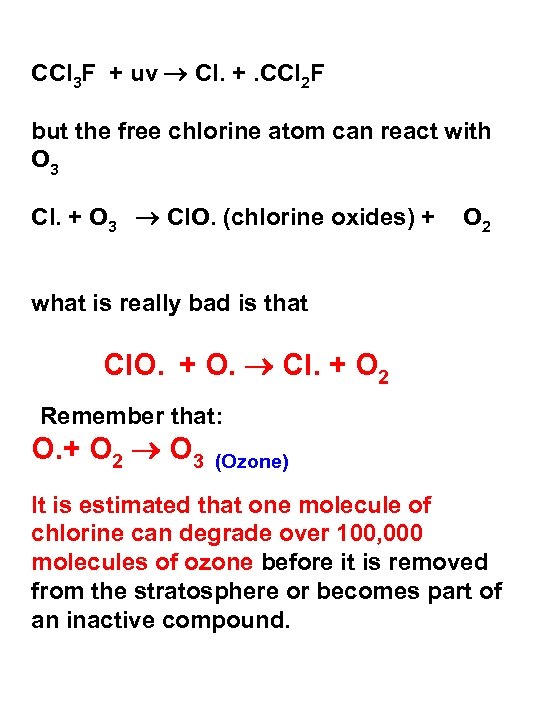 CCl 3 F + uv Cl. +. CCl 2 F but the free chlorine atom can react with O 3 Cl. + O 3 Cl. O. (chlorine oxides) + O 2 what is really bad is that Cl. O. + O. Cl. + O 2 Remember that: O. + O 2 O 3 (Ozone) It is estimated that one molecule of chlorine can degrade over 100, 000 molecules of ozone before it is removed from the stratosphere or becomes part of an inactive compound.
CCl 3 F + uv Cl. +. CCl 2 F but the free chlorine atom can react with O 3 Cl. + O 3 Cl. O. (chlorine oxides) + O 2 what is really bad is that Cl. O. + O. Cl. + O 2 Remember that: O. + O 2 O 3 (Ozone) It is estimated that one molecule of chlorine can degrade over 100, 000 molecules of ozone before it is removed from the stratosphere or becomes part of an inactive compound.
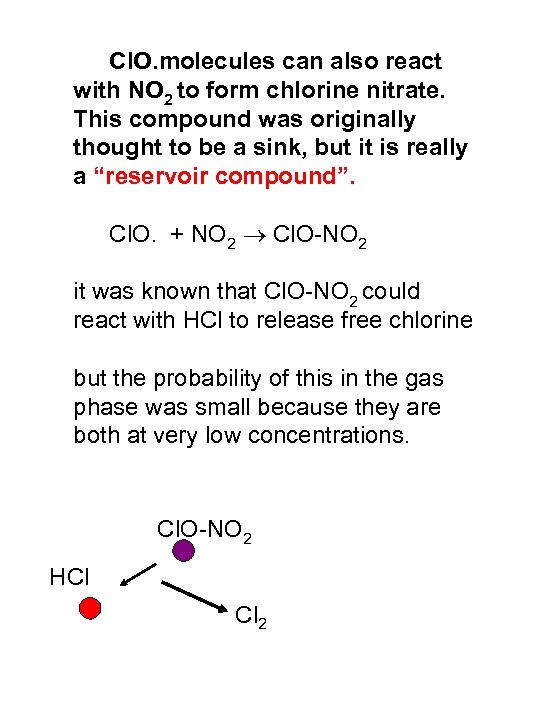 Cl. O. molecules can also react with NO 2 to form chlorine nitrate. This compound was originally thought to be a sink, but it is really a “reservoir compound”. Cl. O. + NO 2 Cl. O-NO 2 it was known that Cl. O-NO 2 could react with HCl to release free chlorine but the probability of this in the gas phase was small because they are both at very low concentrations. Cl. O-NO 2 HCl Cl 2
Cl. O. molecules can also react with NO 2 to form chlorine nitrate. This compound was originally thought to be a sink, but it is really a “reservoir compound”. Cl. O. + NO 2 Cl. O-NO 2 it was known that Cl. O-NO 2 could react with HCl to release free chlorine but the probability of this in the gas phase was small because they are both at very low concentrations. Cl. O-NO 2 HCl Cl 2
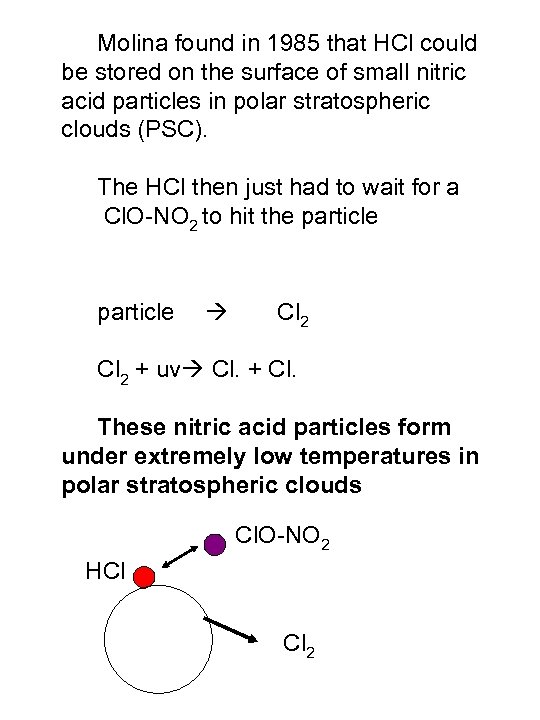 Molina found in 1985 that HCl could be stored on the surface of small nitric acid particles in polar stratospheric clouds (PSC). The HCl then just had to wait for a Cl. O-NO 2 to hit the particle Cl 2 + uv Cl. + Cl. These nitric acid particles form under extremely low temperatures in polar stratospheric clouds Cl. O-NO 2 HCl Cl 2
Molina found in 1985 that HCl could be stored on the surface of small nitric acid particles in polar stratospheric clouds (PSC). The HCl then just had to wait for a Cl. O-NO 2 to hit the particle Cl 2 + uv Cl. + Cl. These nitric acid particles form under extremely low temperatures in polar stratospheric clouds Cl. O-NO 2 HCl Cl 2
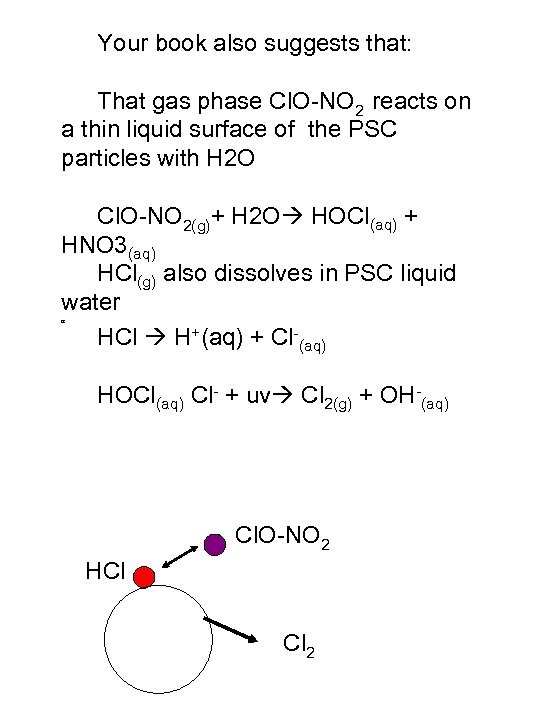 Your book also suggests that: That gas phase Cl. O-NO 2 reacts on a thin liquid surface of the PSC particles with H 2 O Cl. O-NO 2(g)+ H 2 O HOCl(aq) + HNO 3(aq) HCl(g) also dissolves in PSC liquid water HCl H+(aq) + Cl-(aq) HOCl(aq) Cl- + uv Cl 2(g) + OH-(aq) H Cl. O-NO 2 HCl Cl 2
Your book also suggests that: That gas phase Cl. O-NO 2 reacts on a thin liquid surface of the PSC particles with H 2 O Cl. O-NO 2(g)+ H 2 O HOCl(aq) + HNO 3(aq) HCl(g) also dissolves in PSC liquid water HCl H+(aq) + Cl-(aq) HOCl(aq) Cl- + uv Cl 2(g) + OH-(aq) H Cl. O-NO 2 HCl Cl 2
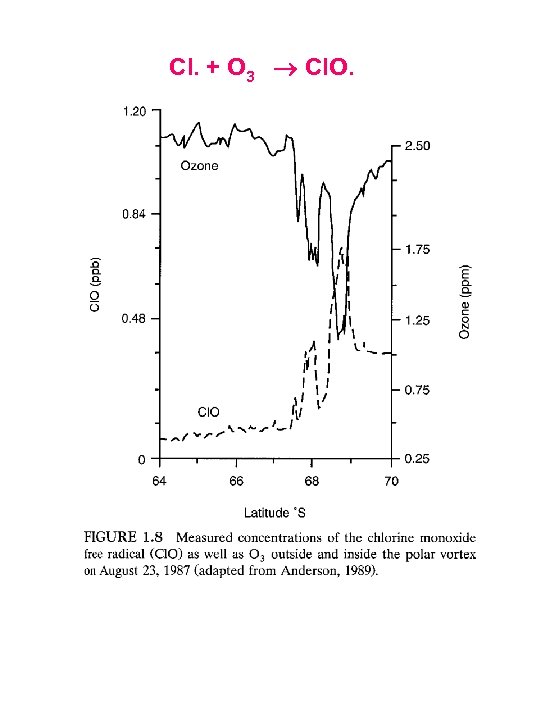 Cl. + O 3 Cl. O.
Cl. + O 3 Cl. O.
 Fluorine and Bromine are also potentially important, but their potency depends on the stability of the receiver compounds. Hydrogen fluoride, HF, is so very stable that fluorocarbons have relatively no known impact on ozone. Bromine reservoirs, such as HBr and Br. ONO 2, are much more easily broken up by sunlight, causing bromine to be from 10 to 100 times more effective than chlorine at destroying ozone. From 30 -60% of bromocarbons released to the atmosphere are man-made (methyl bromide fumigants and halon fire extinguishers) and both compounds will soon be restricted by international agreement.
Fluorine and Bromine are also potentially important, but their potency depends on the stability of the receiver compounds. Hydrogen fluoride, HF, is so very stable that fluorocarbons have relatively no known impact on ozone. Bromine reservoirs, such as HBr and Br. ONO 2, are much more easily broken up by sunlight, causing bromine to be from 10 to 100 times more effective than chlorine at destroying ozone. From 30 -60% of bromocarbons released to the atmosphere are man-made (methyl bromide fumigants and halon fire extinguishers) and both compounds will soon be restricted by international agreement.
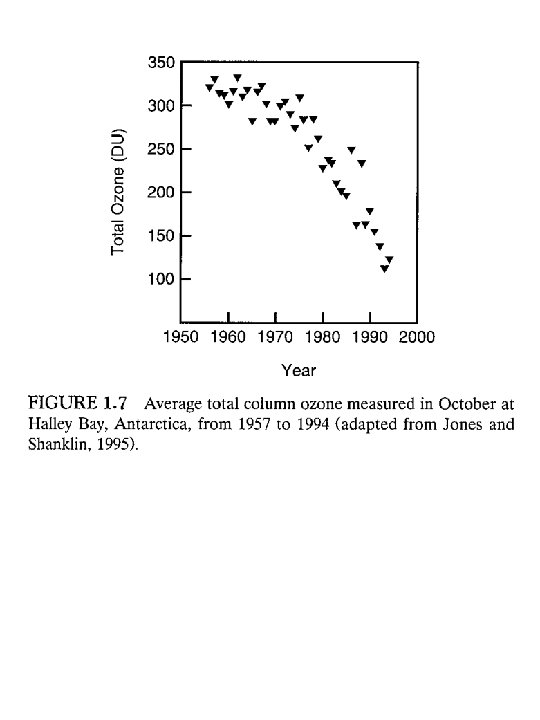
 In 1985 the world was shocked when the first decrease in stratospheric O 3 was reported. Joseph Farmon and his colleagues found that over an 18 year study that the stratospheric O 3 over Antarctica (Halley’s bay) had decreased by 35% in 1984 compared to 1957 -1960 It went from 300 to less than 200 Dobson units (DU) A Dobson unit (DU) translates the O 3 aloft into an amount that would cover the entire surface of the earth in pure ozone. The units are: matm cm If 100 DU were brought to the earth’s surface it would form a layer 1 millimeter thick.
In 1985 the world was shocked when the first decrease in stratospheric O 3 was reported. Joseph Farmon and his colleagues found that over an 18 year study that the stratospheric O 3 over Antarctica (Halley’s bay) had decreased by 35% in 1984 compared to 1957 -1960 It went from 300 to less than 200 Dobson units (DU) A Dobson unit (DU) translates the O 3 aloft into an amount that would cover the entire surface of the earth in pure ozone. The units are: matm cm If 100 DU were brought to the earth’s surface it would form a layer 1 millimeter thick.
 1. AS a consequence of these observations The nations of the world under United Nations Environmental Program (UNEP) agreed to the Montreal Protocol (1987) calling for a 50% cutback in yearly CFC production by the end of the century. 2. It was strengthened in 1990 in London calling for a complete ban by the year 2000. 3 In Copenhagen in 1992 to a complete ban on production on CFC production and use by 1996. So has this made a difference? ?
1. AS a consequence of these observations The nations of the world under United Nations Environmental Program (UNEP) agreed to the Montreal Protocol (1987) calling for a 50% cutback in yearly CFC production by the end of the century. 2. It was strengthened in 1990 in London calling for a complete ban by the year 2000. 3 In Copenhagen in 1992 to a complete ban on production on CFC production and use by 1996. So has this made a difference? ?
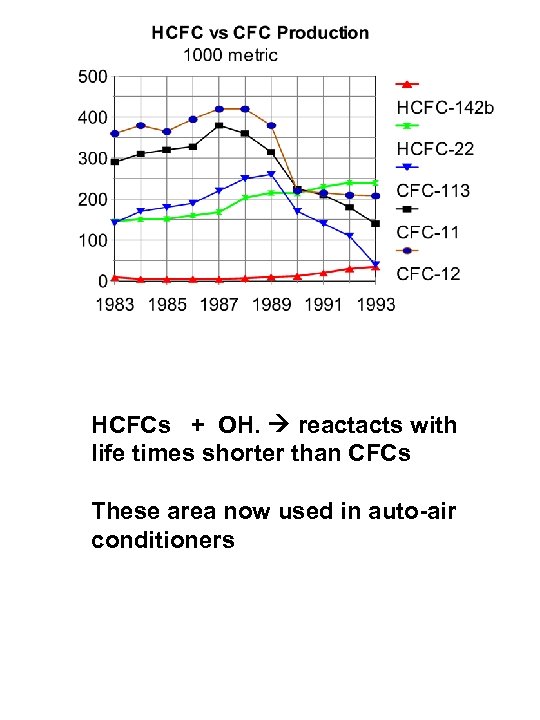 HCFCs + OH. reactacts with life times shorter than CFCs These area now used in auto-air conditioners
HCFCs + OH. reactacts with life times shorter than CFCs These area now used in auto-air conditioners
 In 1998 the ozone hole in the south and north poles have increased!!! This has been associated with the lowest stratospheric temperatures in two decades and raise concerns that the O 3 hole may not “heal” as fast as predicted. It is possible that “green house” gases that heat the troposphere, may be cooling the stratosphere. Depletion of O 3 also adds to cooling. According to NASA satellite measurements the O 3 hole grew to 27. 3 million km 2, up from a previous high of 26 million km 2. Also the O 3 concentration in the worst section of the hole “bottomed out at 90 Dobson units, or 1/3 of what it should be.
In 1998 the ozone hole in the south and north poles have increased!!! This has been associated with the lowest stratospheric temperatures in two decades and raise concerns that the O 3 hole may not “heal” as fast as predicted. It is possible that “green house” gases that heat the troposphere, may be cooling the stratosphere. Depletion of O 3 also adds to cooling. According to NASA satellite measurements the O 3 hole grew to 27. 3 million km 2, up from a previous high of 26 million km 2. Also the O 3 concentration in the worst section of the hole “bottomed out at 90 Dobson units, or 1/3 of what it should be.
 The loss of O 3 and greenhouse gases can not explain all of the observed stratospheric cooling. Typically natural disturbances rippling up to the stratosphere send pressure disturbances rippling up to the stratosphere. These planetary waves warm the polar stratosphere and slow O 3 destruction. In recent years, few planetary waves have buffeted the Arctic and Antarctica during the critical season of springtime O 3 loss. Current computer models are at odds on the relationship between planetary wave frequency and greenhouse warming.
The loss of O 3 and greenhouse gases can not explain all of the observed stratospheric cooling. Typically natural disturbances rippling up to the stratosphere send pressure disturbances rippling up to the stratosphere. These planetary waves warm the polar stratosphere and slow O 3 destruction. In recent years, few planetary waves have buffeted the Arctic and Antarctica during the critical season of springtime O 3 loss. Current computer models are at odds on the relationship between planetary wave frequency and greenhouse warming.




