64a14fb975c726f1eb2111c4c75fd82e.ppt
- Количество слайдов: 30
 Story of a curious mole
Story of a curious mole
 Overview In this lesson, you will learn about • Empirical and Molecular Formula
Overview In this lesson, you will learn about • Empirical and Molecular Formula
 and Little Mole Professor Mole
and Little Mole Professor Mole
 How do I know that water is H 2 O and not…er. . H 2 O 2 for example. Because scientists do experiments in the lab in Phew. . Ito determine the order am getting thirsty. I need H 2 O. empirical formula and then derive the molecular formula. See. .
How do I know that water is H 2 O and not…er. . H 2 O 2 for example. Because scientists do experiments in the lab in Phew. . Ito determine the order am getting thirsty. I need H 2 O. empirical formula and then derive the molecular formula. See. .
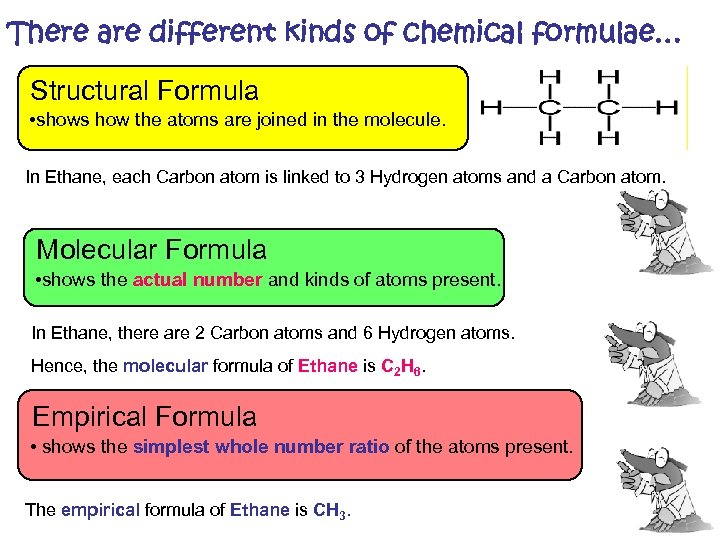 There are different kinds of chemical formulae… Structural Formula • shows how the atoms are joined in the molecule. In Ethane, each Carbon atom is linked to 3 Hydrogen atoms and a Carbon atom. Molecular Formula • shows the actual number and kinds of atoms present. In Ethane, there are 2 Carbon atoms and 6 Hydrogen atoms. Hence, the molecular formula of Ethane is C 2 H 6. Empirical Formula • shows the simplest whole number ratio of the atoms present. The empirical formula of Ethane is CH 3.
There are different kinds of chemical formulae… Structural Formula • shows how the atoms are joined in the molecule. In Ethane, each Carbon atom is linked to 3 Hydrogen atoms and a Carbon atom. Molecular Formula • shows the actual number and kinds of atoms present. In Ethane, there are 2 Carbon atoms and 6 Hydrogen atoms. Hence, the molecular formula of Ethane is C 2 H 6. Empirical Formula • shows the simplest whole number ratio of the atoms present. The empirical formula of Ethane is CH 3.
 Empirical Formula • The empirical formula of a compound shows the simplest whole number ratio of the atoms present. • It is the first step to writing the molecular formula and is used to find the mass of a given compound in the sample. • The empirical formula is determined by experimental results.
Empirical Formula • The empirical formula of a compound shows the simplest whole number ratio of the atoms present. • It is the first step to writing the molecular formula and is used to find the mass of a given compound in the sample. • The empirical formula is determined by experimental results.
 Little Mole has just learnt that magnesium oxide, an ionic compound, is used as refractory material. It’s not easy dude. You’ve gotta do an experiment in the lab first. Come let me show you how. Prof! Teach me how to find the empirical Awesome!of magnesium You are way formula too cool!wanna go school oxide! I and show off to my friends.
Little Mole has just learnt that magnesium oxide, an ionic compound, is used as refractory material. It’s not easy dude. You’ve gotta do an experiment in the lab first. Come let me show you how. Prof! Teach me how to find the empirical Awesome!of magnesium You are way formula too cool!wanna go school oxide! I and show off to my friends.
 Experiment: Determining formula of Magnesium oxide • To find the formula of magnesium oxide, there are two information we need. – Mass of magnesium in magnesium oxide – Mass of oxygen in magnesium oxide Procedure: • Burn 1. 20 g of Mg coil in a clean, dry crucible until the ribbon become a whitish ash. This is Wicked!=D Hehe. .
Experiment: Determining formula of Magnesium oxide • To find the formula of magnesium oxide, there are two information we need. – Mass of magnesium in magnesium oxide – Mass of oxygen in magnesium oxide Procedure: • Burn 1. 20 g of Mg coil in a clean, dry crucible until the ribbon become a whitish ash. This is Wicked!=D Hehe. .
 Experimental Results • The following masses were recorded. – Mass of crucible + lid = 26. 52 g – Mass of crucible + lid + magnesium = 27. 72 g – Mass of crucible + lid + magnesium oxide = 28. 52 g
Experimental Results • The following masses were recorded. – Mass of crucible + lid = 26. 52 g – Mass of crucible + lid + magnesium = 27. 72 g – Mass of crucible + lid + magnesium oxide = 28. 52 g
 Experimental Results • The following masses were recorded. – Mass of crucible + lid = 26. 52 g – Mass of crucible + lid + magnesium = 27. 72 g – Mass of crucible + lid + magnesium oxide = 28. 52 g I know! So that I know the mass of Magnesium that I used. So 27. 72 – 26. 52 …. means I used 1. 20 g of Magnesium. Think about it, Little Mole. Why did I have to calculate the first two masses?
Experimental Results • The following masses were recorded. – Mass of crucible + lid = 26. 52 g – Mass of crucible + lid + magnesium = 27. 72 g – Mass of crucible + lid + magnesium oxide = 28. 52 g I know! So that I know the mass of Magnesium that I used. So 27. 72 – 26. 52 …. means I used 1. 20 g of Magnesium. Think about it, Little Mole. Why did I have to calculate the first two masses?
 Experimental Results • The following masses were recorded. – Mass of crucible + lid = 26. 52 g – Mass of crucible + lid + magnesium = 27. 72 g – Mass of crucible + lid + magnesium oxide = 28. 52 g Hmm. . You got me there. I dunno. Good! Now, how do I calculate the mass of oxygen used?
Experimental Results • The following masses were recorded. – Mass of crucible + lid = 26. 52 g – Mass of crucible + lid + magnesium = 27. 72 g – Mass of crucible + lid + magnesium oxide = 28. 52 g Hmm. . You got me there. I dunno. Good! Now, how do I calculate the mass of oxygen used?
 Experimental Results • The following masses were recorded. – Mass of crucible + lid = 26. 52 g – Mass of crucible + lid + magnesium = 27. 72 g – Mass of crucible + lid + magnesium oxide = 28. 52 g Ah…I see… The mass of oxygen is the difference of the last two masses! So the amount of oxygen reacted is 28. 52 – 27. 72 g which is 0. 80 g.
Experimental Results • The following masses were recorded. – Mass of crucible + lid = 26. 52 g – Mass of crucible + lid + magnesium = 27. 72 g – Mass of crucible + lid + magnesium oxide = 28. 52 g Ah…I see… The mass of oxygen is the difference of the last two masses! So the amount of oxygen reacted is 28. 52 – 27. 72 g which is 0. 80 g.
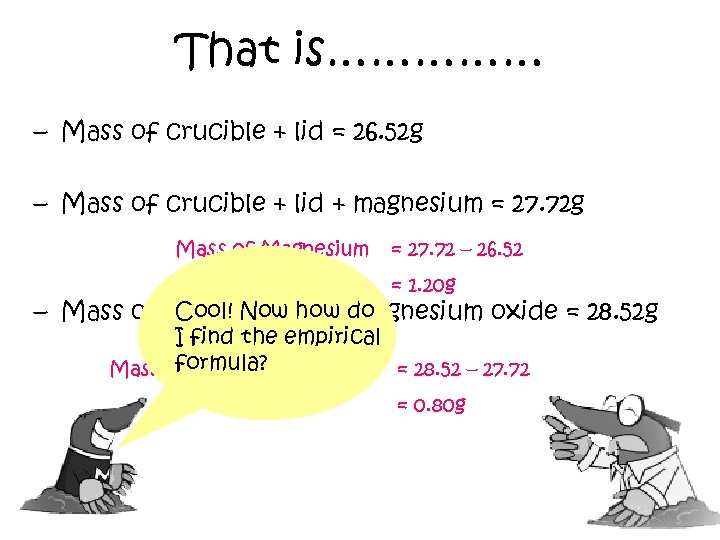 That is…………… – Mass of crucible + lid = 26. 52 g – Mass of crucible + lid + magnesium = 27. 72 g Mass of Magnesium = 27. 72 – 26. 52 = 1. 20 g Cool! Now how magnesium oxide = 28. 52 g – Mass of crucible + lid + do I find the empirical formula? Mass of Oxygen reacted = 28. 52 – 27. 72 = 0. 80 g
That is…………… – Mass of crucible + lid = 26. 52 g – Mass of crucible + lid + magnesium = 27. 72 g Mass of Magnesium = 27. 72 – 26. 52 = 1. 20 g Cool! Now how magnesium oxide = 28. 52 g – Mass of crucible + lid + do I find the empirical formula? Mass of Oxygen reacted = 28. 52 – 27. 72 = 0. 80 g
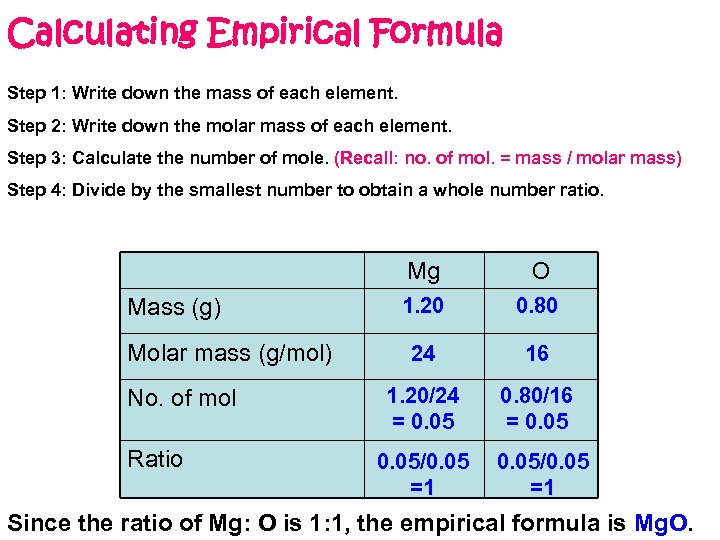 Calculating Empirical Formula Step 1: Write down the mass of each element. Step 2: Write down the molar mass of each element. Step 3: Calculate the number of mole. (Recall: no. of mol. = mass / molar mass) Step 4: Divide by the smallest number to obtain a whole number ratio. Mg Mass (g) Molar mass (g/mol) No. of mol Ratio O 1. 20 0. 80 24 16 1. 20/24 = 0. 05 0. 80/16 = 0. 05/0. 05 =1 Since the ratio of Mg: O is 1: 1, the empirical formula is Mg. O.
Calculating Empirical Formula Step 1: Write down the mass of each element. Step 2: Write down the molar mass of each element. Step 3: Calculate the number of mole. (Recall: no. of mol. = mass / molar mass) Step 4: Divide by the smallest number to obtain a whole number ratio. Mg Mass (g) Molar mass (g/mol) No. of mol Ratio O 1. 20 0. 80 24 16 1. 20/24 = 0. 05 0. 80/16 = 0. 05/0. 05 =1 Since the ratio of Mg: O is 1: 1, the empirical formula is Mg. O.
 0. 05 mole of Magnesium combines with 0. 05 mole of Oxygen, right? That means 1 atom of Magnesium combines with 1 atom of Oxygen. Therefore, the empirical formula is Mg. O. I don’tall soit. How I’m This is get cheem. come you around glad I have could go to teach number of moles from me=D to ratio directly?
0. 05 mole of Magnesium combines with 0. 05 mole of Oxygen, right? That means 1 atom of Magnesium combines with 1 atom of Oxygen. Therefore, the empirical formula is Mg. O. I don’tall soit. How I’m This is get cheem. come you around glad I have could go to teach number of moles from me=D to ratio directly?
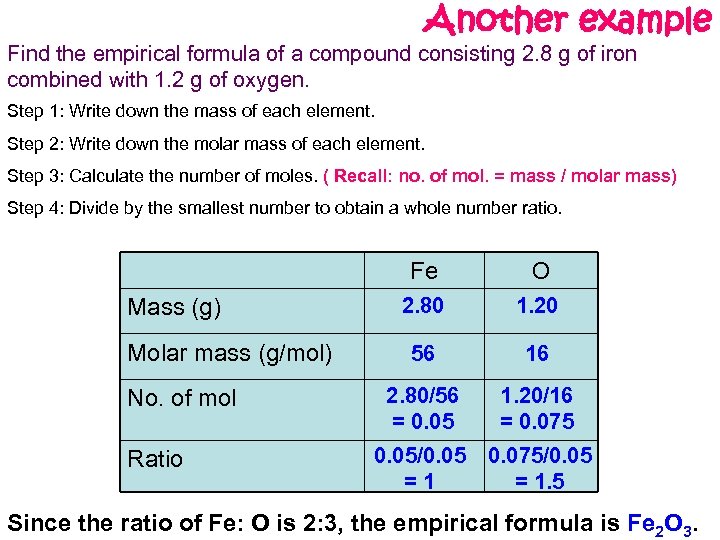 Another example Find the empirical formula of a compound consisting 2. 8 g of iron combined with 1. 2 g of oxygen. Step 1: Write down the mass of each element. Step 2: Write down the molar mass of each element. Step 3: Calculate the number of moles. ( Recall: no. of mol. = mass / molar mass) Step 4: Divide by the smallest number to obtain a whole number ratio. Fe Mass (g) Molar mass (g/mol) No. of mol Ratio O 2. 80 1. 20 56 16 2. 80/56 = 0. 05 1. 20/16 = 0. 075 0. 05/0. 05 =1 0. 075/0. 05 = 1. 5 Since the ratio of Fe: O is 2: 3, the empirical formula is Fe 2 O 3.
Another example Find the empirical formula of a compound consisting 2. 8 g of iron combined with 1. 2 g of oxygen. Step 1: Write down the mass of each element. Step 2: Write down the molar mass of each element. Step 3: Calculate the number of moles. ( Recall: no. of mol. = mass / molar mass) Step 4: Divide by the smallest number to obtain a whole number ratio. Fe Mass (g) Molar mass (g/mol) No. of mol Ratio O 2. 80 1. 20 56 16 2. 80/56 = 0. 05 1. 20/16 = 0. 075 0. 05/0. 05 =1 0. 075/0. 05 = 1. 5 Since the ratio of Fe: O is 2: 3, the empirical formula is Fe 2 O 3.
 That’s right! You also have Little Mole, have you to include anything about noticedworkings for the final step for number of moles of the way we present our each element and their answers? simplest ratio. Hmm. . You always present it in a table…. . and include units of g for mass and g/mol for molar mass.
That’s right! You also have Little Mole, have you to include anything about noticedworkings for the final step for number of moles of the way we present our each element and their answers? simplest ratio. Hmm. . You always present it in a table…. . and include units of g for mass and g/mol for molar mass.
 Find the empirical formula of a compound consisting of 2. 8 g of When your iron combined with 1. 2 g of oxygen. See. Whatnumber’s close to happens you a whole number. If you have You multiply when I 1. 9 forratios of 1. 5 ratio multiply 2 to that and have example, then you will round up to 2. Likewise, if of 1: 1. 5? it becomes 2: 3 1. 25 get 1. 001, then you round you in order to get a right? down to 1. It all comes with whole number. practice, my child. Wait! I am When do I round do is Ahh. . I see. . All this I Err. . . ok. . So how up or confused. . You said the down then? make sense. startingof Fe: O is 1: 1. 5 know when to multiply? ratio to right? How come it became Fe 2 O 3?
Find the empirical formula of a compound consisting of 2. 8 g of When your iron combined with 1. 2 g of oxygen. See. Whatnumber’s close to happens you a whole number. If you have You multiply when I 1. 9 forratios of 1. 5 ratio multiply 2 to that and have example, then you will round up to 2. Likewise, if of 1: 1. 5? it becomes 2: 3 1. 25 get 1. 001, then you round you in order to get a right? down to 1. It all comes with whole number. practice, my child. Wait! I am When do I round do is Ahh. . I see. . All this I Err. . . ok. . So how up or confused. . You said the down then? make sense. startingof Fe: O is 1: 1. 5 know when to multiply? ratio to right? How come it became Fe 2 O 3?
 An oxide of sulfur contains 40% sulfur and 60% oxygen. Find its empirical formula. 60 g! You rock *Gulps* Huh? ? ! How am I Professor Mole! doing supposed to start. What Err. . . 40 g? would I do without this question? you? And when you have Simple. Assume that you 60% oxygen, how much have 100 g of the sample. oxygen do you have in So if you have 40% 100 g of themuch sulfur, how sample? do you have in 100 g?
An oxide of sulfur contains 40% sulfur and 60% oxygen. Find its empirical formula. 60 g! You rock *Gulps* Huh? ? ! How am I Professor Mole! doing supposed to start. What Err. . . 40 g? would I do without this question? you? And when you have Simple. Assume that you 60% oxygen, how much have 100 g of the sample. oxygen do you have in So if you have 40% 100 g of themuch sulfur, how sample? do you have in 100 g?
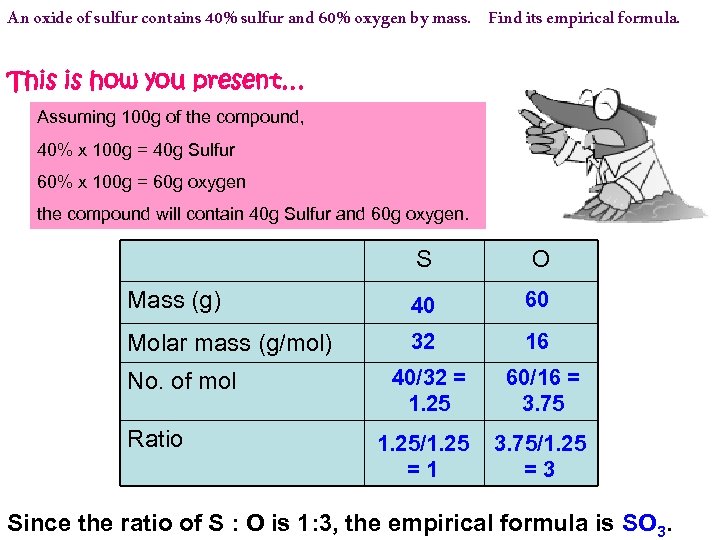 An oxide of sulfur contains 40% sulfur and 60% oxygen by mass. Find its empirical formula. This is how you present… Assuming 100 g of the compound, 40% x 100 g = 40 g Sulfur 60% x 100 g = 60 g oxygen the compound will contain 40 g Sulfur and 60 g oxygen. S O Mass (g) 40 60 Molar mass (g/mol) 32 16 40/32 = 1. 25 60/16 = 3. 75 1. 25/1. 25 =1 3. 75/1. 25 =3 No. of mol Ratio Since the ratio of S : O is 1: 3, the empirical formula is SO 3.
An oxide of sulfur contains 40% sulfur and 60% oxygen by mass. Find its empirical formula. This is how you present… Assuming 100 g of the compound, 40% x 100 g = 40 g Sulfur 60% x 100 g = 60 g oxygen the compound will contain 40 g Sulfur and 60 g oxygen. S O Mass (g) 40 60 Molar mass (g/mol) 32 16 40/32 = 1. 25 60/16 = 3. 75 1. 25/1. 25 =1 3. 75/1. 25 =3 No. of mol Ratio Since the ratio of S : O is 1: 3, the empirical formula is SO 3.
 Let’s look next at: Molecular Formula
Let’s look next at: Molecular Formula
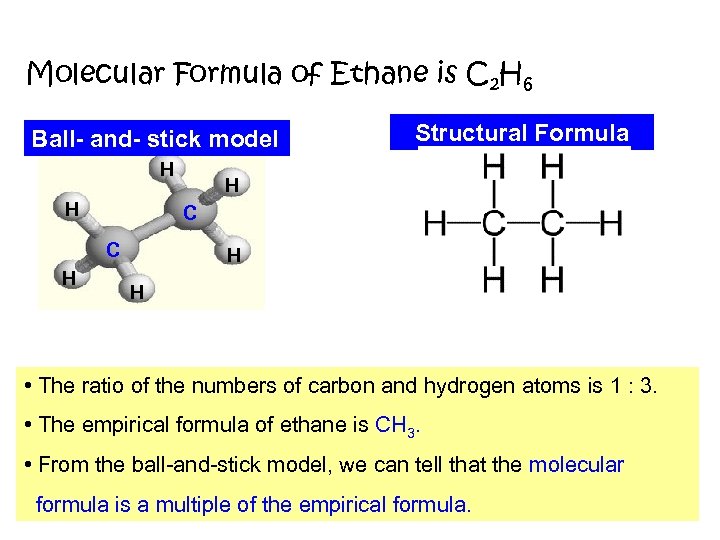 Molecular Formula of Ethane is C 2 H 6 Ball- and- stick model H H H C C H Structural Formula H H • The ratio of the numbers of carbon and hydrogen atoms is 1 : 3. • The empirical formula of ethane is CH 3. • From the ball-and-stick model, we can tell that the molecular formula is a multiple of the empirical formula.
Molecular Formula of Ethane is C 2 H 6 Ball- and- stick model H H H C C H Structural Formula H H • The ratio of the numbers of carbon and hydrogen atoms is 1 : 3. • The empirical formula of ethane is CH 3. • From the ball-and-stick model, we can tell that the molecular formula is a multiple of the empirical formula.
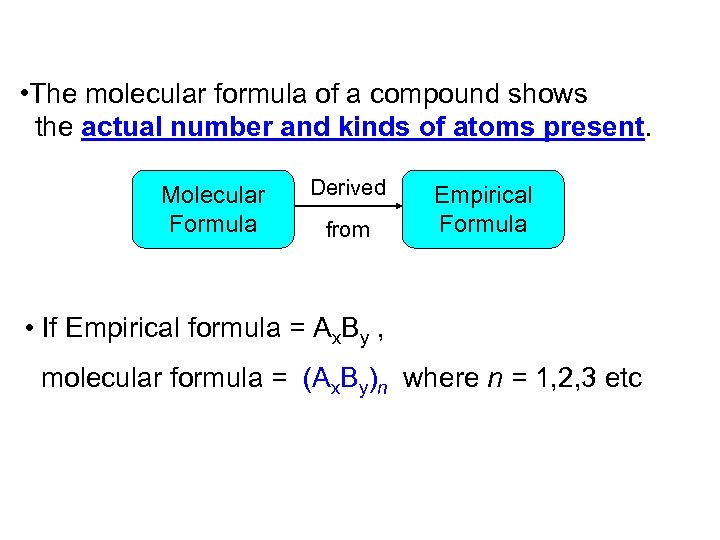 • The molecular formula of a compound shows the actual number and kinds of atoms present. Molecular Formula Derived from Empirical Formula • If Empirical formula = Ax. By , molecular formula = (Ax. By)n where n = 1, 2, 3 etc
• The molecular formula of a compound shows the actual number and kinds of atoms present. Molecular Formula Derived from Empirical Formula • If Empirical formula = Ax. By , molecular formula = (Ax. By)n where n = 1, 2, 3 etc
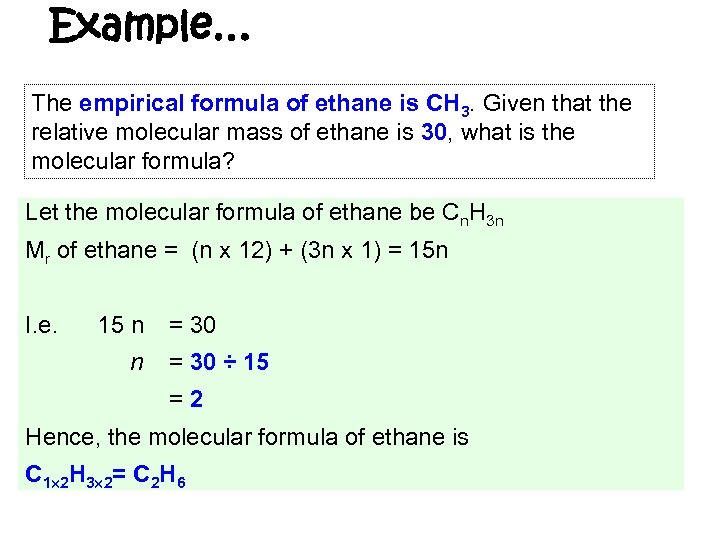 Example… The empirical formula of ethane is CH 3. Given that the relative molecular mass of ethane is 30, what is the molecular formula? Let the molecular formula of ethane be Cn. H 3 n Mr of ethane = (n x 12) + (3 n x 1) = 15 n I. e. 15 n n = 30 ÷ 15 =2 Hence, the molecular formula of ethane is C 1 2 H 3 2= C 2 H 6
Example… The empirical formula of ethane is CH 3. Given that the relative molecular mass of ethane is 30, what is the molecular formula? Let the molecular formula of ethane be Cn. H 3 n Mr of ethane = (n x 12) + (3 n x 1) = 15 n I. e. 15 n n = 30 ÷ 15 =2 Hence, the molecular formula of ethane is C 1 2 H 3 2= C 2 H 6
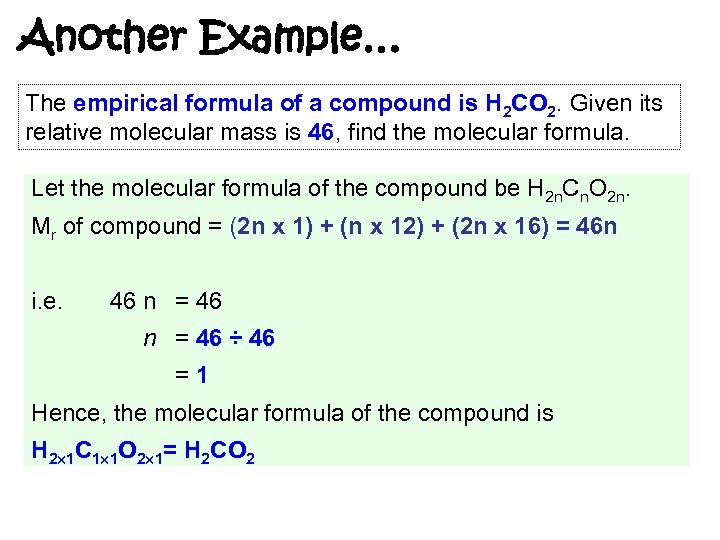 Another Example… The empirical formula of a compound is H 2 CO 2. Given its relative molecular mass is 46, find the molecular formula. Let the molecular formula of the compound be H 2 n. Cn. O 2 n. Mr of compound = (2 n x 1) + (n x 12) + (2 n x 16) = 46 n i. e. 46 n = 46 ÷ 46 =1 Hence, the molecular formula of the compound is H 2 1 C 1 1 O 2 1= H 2 CO 2
Another Example… The empirical formula of a compound is H 2 CO 2. Given its relative molecular mass is 46, find the molecular formula. Let the molecular formula of the compound be H 2 n. Cn. O 2 n. Mr of compound = (2 n x 1) + (n x 12) + (2 n x 16) = 46 n i. e. 46 n = 46 ÷ 46 =1 Hence, the molecular formula of the compound is H 2 1 C 1 1 O 2 1= H 2 CO 2
 Think Time! Is this true? “The empirical formula is always simpler than the molecular formula of a compound”. NO! In compounds such as Mg. O and Na. Cl, the empirical formula is the same as the molecular formula. Empirical formula of an ionic compound is also its molecular formula.
Think Time! Is this true? “The empirical formula is always simpler than the molecular formula of a compound”. NO! In compounds such as Mg. O and Na. Cl, the empirical formula is the same as the molecular formula. Empirical formula of an ionic compound is also its molecular formula.
 Think Time! Is this true? “The empirical formula for different compounds are different. ” NO! The empirical formula of ethane (C 2 H 4) and propane (C 3 H 6) are the same.
Think Time! Is this true? “The empirical formula for different compounds are different. ” NO! The empirical formula of ethane (C 2 H 4) and propane (C 3 H 6) are the same.
 But what’s the point of Then the empirical knowingthe empirical formula is worked out? formula? To useful formula of It is find thein Organic That’s right! My mere the new substance, a Chemistry. Everyday, a presence must be making sample is analyzed to new smarter. Then you compound is obtain the mass eitherout the Mr or or work discovered and percentage composition madethethe lab. then in molecular of each element in the formula. compound
But what’s the point of Then the empirical knowingthe empirical formula is worked out? formula? To useful formula of It is find thein Organic That’s right! My mere the new substance, a Chemistry. Everyday, a presence must be making sample is analyzed to new smarter. Then you compound is obtain the mass eitherout the Mr or or work discovered and percentage composition madethethe lab. then in molecular of each element in the formula. compound
 Certificate of Completion This is to certify that Little Mole has successfully completed the e-learning module on empirical & molecular formula. Presented by Professor Mole
Certificate of Completion This is to certify that Little Mole has successfully completed the e-learning module on empirical & molecular formula. Presented by Professor Mole
 Prof, it looks like I’m done with learning empirical formula. Yup. You’ve successfully graduated. Don’t forget to practise though. Do the questions in Worksheet 2 and the handout.
Prof, it looks like I’m done with learning empirical formula. Yup. You’ve successfully graduated. Don’t forget to practise though. Do the questions in Worksheet 2 and the handout.


