386b4e4063436bfc499b0fc07e06c37a.ppt
- Количество слайдов: 8
 Store H H in Materials A Mg. H 2 plex Com es id hydr “hydrogen economy”, in which hydrogen acts as energy carrier along with electricity, is being promoted as an ultimate solution to the world’s energy and environmental problems. A major challenge in commercialization of hydrogen energy is how to safely store the lightest hydrogen to a high energy density required for transportation application. In principle, hydrogen can be stored in gas-, liquid-, and solid-states. In comparison Metal nitride Chemical hydride with the former two forms, the solid state H storage via interaction between special materials and hydrogen possesses significant advantages on energy efficiency and safety issue. Therefore, a “gold rush” flurry of research activity has been directed towards the development of viable hydrogen storage materials for onboard application. Recently, the development of hydrogen storage material was significantly accelerated due to the increasingly strengthened interdisciplinary collaboration and enhanced participation & support of academia, industry and government. As a result, many new research branches associated with the discoveries of novel material structures/systems were established. These progresses, however, have not substantially narrowed the gap between achievable capability and that required for commercial onboard hydrogen application. A long-term high-risk/high pay-off basic research is still required, where the design and discovery of new, higher efficiency hydrogen storage materials is based on better understanding of the chemical and physical processes governing the hydrogen–materials interaction. Our Group Currently, our group focuses on the following hydrogen storage material systems: Our Research Mg-based composites, Complex hydrides, Metal nitrides, Chemical hydride Publications For details of our research, please refer to the latest publications.
Store H H in Materials A Mg. H 2 plex Com es id hydr “hydrogen economy”, in which hydrogen acts as energy carrier along with electricity, is being promoted as an ultimate solution to the world’s energy and environmental problems. A major challenge in commercialization of hydrogen energy is how to safely store the lightest hydrogen to a high energy density required for transportation application. In principle, hydrogen can be stored in gas-, liquid-, and solid-states. In comparison Metal nitride Chemical hydride with the former two forms, the solid state H storage via interaction between special materials and hydrogen possesses significant advantages on energy efficiency and safety issue. Therefore, a “gold rush” flurry of research activity has been directed towards the development of viable hydrogen storage materials for onboard application. Recently, the development of hydrogen storage material was significantly accelerated due to the increasingly strengthened interdisciplinary collaboration and enhanced participation & support of academia, industry and government. As a result, many new research branches associated with the discoveries of novel material structures/systems were established. These progresses, however, have not substantially narrowed the gap between achievable capability and that required for commercial onboard hydrogen application. A long-term high-risk/high pay-off basic research is still required, where the design and discovery of new, higher efficiency hydrogen storage materials is based on better understanding of the chemical and physical processes governing the hydrogen–materials interaction. Our Group Currently, our group focuses on the following hydrogen storage material systems: Our Research Mg-based composites, Complex hydrides, Metal nitrides, Chemical hydride Publications For details of our research, please refer to the latest publications.
 Our Research Group Mg. H 2 plex Com es id hydr Metal nitride Dr. Ping Wang, Group Leader Chemical hydride Mr. Xiang-Dong Kang Ms. Hong Liu Mr. Lai-Peng Ma Ph. D student Our Group Our Research Publications Mr. Zhan-Zhao Fang Master student Go back
Our Research Group Mg. H 2 plex Com es id hydr Metal nitride Dr. Ping Wang, Group Leader Chemical hydride Mr. Xiang-Dong Kang Ms. Hong Liu Mr. Lai-Peng Ma Ph. D student Our Group Our Research Publications Mr. Zhan-Zhao Fang Master student Go back
 Dr. Ping Wang Contact Information Mg. H 2 plex Com es id hydr Position: Associate Professor, Institute of Metal Research, Chinese Academy of Sciences Address: 72 # Wenhua Road, Shenyang 110016, P. R. China Tel: (+86) 24 2397 1622 Fax: (+86) 24 2389 1320 E-Mail: pingwang@imr. ac. cn Metal nitride Education and Employment Chemical hydride 2004 - Associate Professor, Institute of Metal Research, Chinese Academy of Sciences 2002 -2004 Guest Researcher, Chemistry Department, Hawaii University, USA 2002 Guest Researcher, Department of Physics, National University of Singapore, Singapore 2001 -2002 Guest Researcher, Faculty of integrated Arts & Sciences, Hiroshima University, Japan 1998 -2001 Institute of Metal Research, CAS, Materials Science, Ph. D degree 1992 -1995 Northeast University, China, Metallurgical Physichemsitry, MA degree 1988 -1992 Northeast University, China, Metallurgical Physichemsitry, BA degree Ten Representative Publications 10. Exploration of the nature of active Ti-species in metallic Ti-doped Na. Al. H 4 P. Wang, X. D. Kang and H. M. Cheng, J. Phys. Chem. B, 109 (2005) 20131 -20136. Our Group Our Research Publications 9. Direct formation of Na 3 Al. H 6 by mechanical milling Na. H/Al with Ti. F 3 P. Wang, X. D. Kang and H. M. Cheng, Appl. Phys. Lett. , 87 (2005) 071911. 8. Improved hydrogen storage property of Ti. F 3 -doped Na. Al. H 4 P. Wang, X. D. Kang and H. M. Cheng, Chem. Phys. Chem, 6 (2005) 2488 -2451. 7. KH+Ti co-doped Na. Al. H 4 for high-capacity hydrogen storage Go back
Dr. Ping Wang Contact Information Mg. H 2 plex Com es id hydr Position: Associate Professor, Institute of Metal Research, Chinese Academy of Sciences Address: 72 # Wenhua Road, Shenyang 110016, P. R. China Tel: (+86) 24 2397 1622 Fax: (+86) 24 2389 1320 E-Mail: pingwang@imr. ac. cn Metal nitride Education and Employment Chemical hydride 2004 - Associate Professor, Institute of Metal Research, Chinese Academy of Sciences 2002 -2004 Guest Researcher, Chemistry Department, Hawaii University, USA 2002 Guest Researcher, Department of Physics, National University of Singapore, Singapore 2001 -2002 Guest Researcher, Faculty of integrated Arts & Sciences, Hiroshima University, Japan 1998 -2001 Institute of Metal Research, CAS, Materials Science, Ph. D degree 1992 -1995 Northeast University, China, Metallurgical Physichemsitry, MA degree 1988 -1992 Northeast University, China, Metallurgical Physichemsitry, BA degree Ten Representative Publications 10. Exploration of the nature of active Ti-species in metallic Ti-doped Na. Al. H 4 P. Wang, X. D. Kang and H. M. Cheng, J. Phys. Chem. B, 109 (2005) 20131 -20136. Our Group Our Research Publications 9. Direct formation of Na 3 Al. H 6 by mechanical milling Na. H/Al with Ti. F 3 P. Wang, X. D. Kang and H. M. Cheng, Appl. Phys. Lett. , 87 (2005) 071911. 8. Improved hydrogen storage property of Ti. F 3 -doped Na. Al. H 4 P. Wang, X. D. Kang and H. M. Cheng, Chem. Phys. Chem, 6 (2005) 2488 -2451. 7. KH+Ti co-doped Na. Al. H 4 for high-capacity hydrogen storage Go back
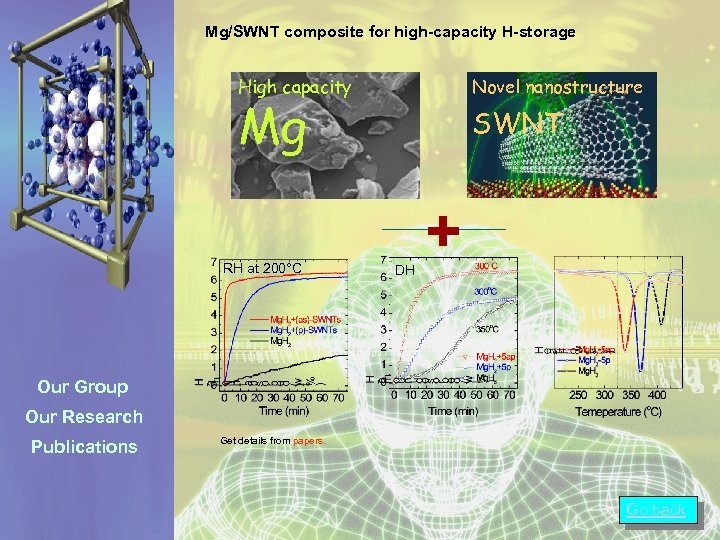 Mg/SWNT composite for high-capacity H-storage Metal hydride High capacity Novel nanostructure Mg RH at 200°C SWNT DH + Our Group Our Research Publications Get details from papers. Go back
Mg/SWNT composite for high-capacity H-storage Metal hydride High capacity Novel nanostructure Mg RH at 200°C SWNT DH + Our Group Our Research Publications Get details from papers. Go back
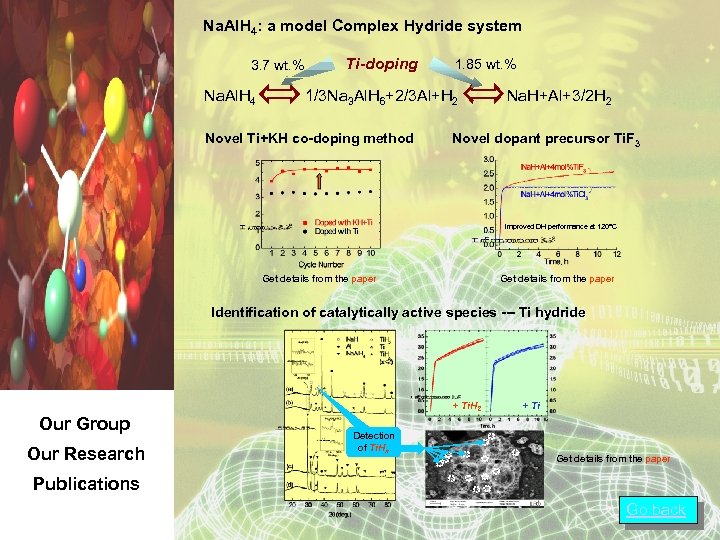 Na. Al. H 4: a model Complex Hydride system 3. 7 wt. % Na. Al. H 4 Ti-doping 1. 85 wt. % 1/3 Na 3 Al. H 6+2/3 Al+H 2 Novel Ti+KH co-doping method Na. H+Al+3/2 H 2 Novel dopant precursor Ti. F 3 Improved DH performance at 120°C Get details from the paper Identification of catalytically active species --- Ti hydride + Ti. H 2 Our Group Our Research Detection of Ti. Hx + Ti Get details from the paper Publications Go back
Na. Al. H 4: a model Complex Hydride system 3. 7 wt. % Na. Al. H 4 Ti-doping 1. 85 wt. % 1/3 Na 3 Al. H 6+2/3 Al+H 2 Novel Ti+KH co-doping method Na. H+Al+3/2 H 2 Novel dopant precursor Ti. F 3 Improved DH performance at 120°C Get details from the paper Identification of catalytically active species --- Ti hydride + Ti. H 2 Our Group Our Research Detection of Ti. Hx + Ti Get details from the paper Publications Go back
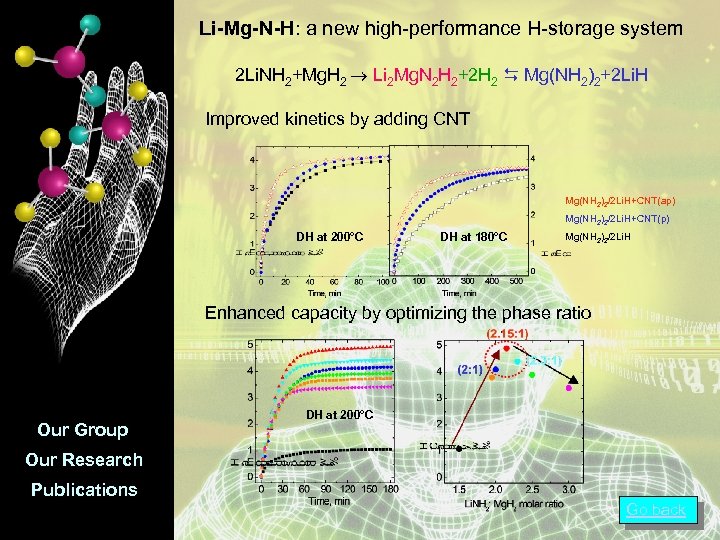 Li-Mg-N-H: a new high-performance H-storage system 2 Li. NH 2+Mg. H 2 Li 2 Mg. N 2 H 2+2 H 2 Mg(NH 2)2+2 Li. H Improved kinetics by adding CNT Mg(NH 2)2/2 Li. H+CNT(ap) Mg(NH 2)2/2 Li. H+CNT(p) DH at 200°C DH at 180°C Mg(NH 2)2/2 Li. H Enhanced capacity by optimizing the phase ratio Our Group DH at 200°C Our Research Publications Go back
Li-Mg-N-H: a new high-performance H-storage system 2 Li. NH 2+Mg. H 2 Li 2 Mg. N 2 H 2+2 H 2 Mg(NH 2)2+2 Li. H Improved kinetics by adding CNT Mg(NH 2)2/2 Li. H+CNT(ap) Mg(NH 2)2/2 Li. H+CNT(p) DH at 200°C DH at 180°C Mg(NH 2)2/2 Li. H Enhanced capacity by optimizing the phase ratio Our Group DH at 200°C Our Research Publications Go back
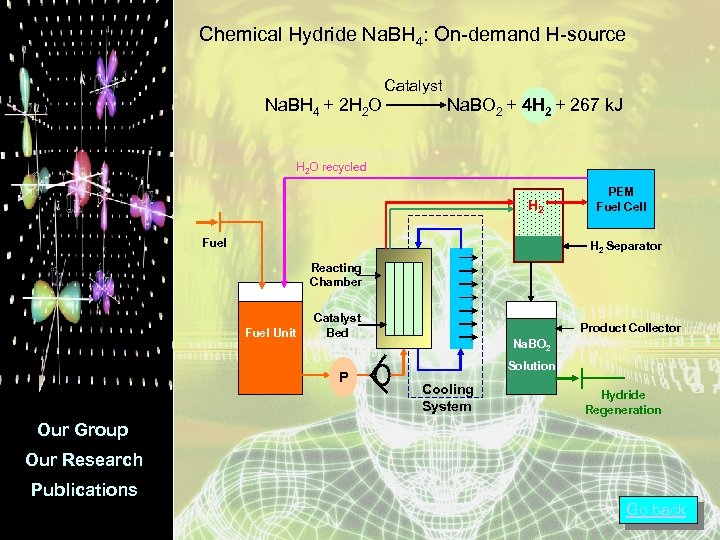 Chemical Hydride Na. BH 4: On-demand H-source Catalyst Na. BH 4 + 2 H 2 O Na. BO 2 + 4 H 2 + 267 k. J H 2 O recycled H 2 Fuel PEM Fuel Cell H 2 Separator Reacting Chamber Fuel Unit Catalyst Bed P Product Collector Na. BO 2 Solution Cooling System Hydride Regeneration Our Group Our Research Publications Go back
Chemical Hydride Na. BH 4: On-demand H-source Catalyst Na. BH 4 + 2 H 2 O Na. BO 2 + 4 H 2 + 267 k. J H 2 O recycled H 2 Fuel PEM Fuel Cell H 2 Separator Reacting Chamber Fuel Unit Catalyst Bed P Product Collector Na. BO 2 Solution Cooling System Hydride Regeneration Our Group Our Research Publications Go back
 Latest Publications Mg. H 2 plex Com es id hydr 10. Hydrogen storage properties of Mg. H 2/SWNT composite prepared by ball milling C. Z. Wu, P. Wang, X. D. Yao, C. Liu, D. M. Chen, G. Q. Lu, and H. M. Cheng, J. Alloys Compd. , (2006) online published. 9. Effect of carbon/noncarbon addition on hydrogen storage behaviors of magnesium hydride C. Z. Wu, P. Wang, X. D. Yao, C. Liu, D. M. Chen, G. Q. Lu, and H. M. Cheng, J. Alloys Compd. , (2006) online published. 8. Structure and hydrogen storage property of ball-milled Li. NH 2/Mg. H 2 mixture Y. Chen, C. Z. Wu, P. Wang, H. M. Cheng, Inter. J. Hydrogen Energy, (2006) online-published. Metal nitride Chemical hydride 7. Catalytic effect of Al 3 Ti on the reversible dehydrogenation of Na. Al. H 4 X. D. Kang, P. Wang, X. P. Song, X. D. Yao, G. Q. Lu and H. M. Cheng, J. Alloys Compd. , (2006) online-published. 6. Dependence of H-storage performance on preparation conditions in Ti. F 3 doped Na. Al. H 4 P. Wang, X. D. Kang and H. M. Cheng, J. Alloy compd. , (2006) online-published. 5. Effects of SWNT and metallic catalyst on hydrogen absorption/desorption performance of Mg. H 2 C. Z. Wu, P. Wang, X. D. Yao, C. Liu, D. M. Chen, G. Q. Lu, and H. M. Cheng, J. Phys Chem B, 109 (2005) 22217 -22221. 4. Exploration of the nature of active Ti-species in metallic Ti-doped Na. Al. H 4 P. Wang, X. D. Kang and H. M. Cheng, J. Phys. Chem. B, 109 (2005) 20131 -20136. 3. Direct formation of Na 3 Al. H 6 by mechanical milling Na. H/Al with Ti. F 3 P. Wang, X. D. Kang and H. M. Cheng, Appl. Phys. Lett. , 87 (2005) 071911. 2. Improved hydrogen storage property of Ti. F 3 -doped Na. Al. H 4 P. Wang, X. D. Kang and H. M. Cheng, Chem. Phys. Chem, 6 (2005) 2488 -2451. Our Group 1. KH+Ti co-doped Na. Al. H 4 for high-capacity hydrogen storage P. Wang, X. D. Kang and H. M. Cheng, J. Appl. Phys. , 98 (2005) 074905. Our Research Publications Go back
Latest Publications Mg. H 2 plex Com es id hydr 10. Hydrogen storage properties of Mg. H 2/SWNT composite prepared by ball milling C. Z. Wu, P. Wang, X. D. Yao, C. Liu, D. M. Chen, G. Q. Lu, and H. M. Cheng, J. Alloys Compd. , (2006) online published. 9. Effect of carbon/noncarbon addition on hydrogen storage behaviors of magnesium hydride C. Z. Wu, P. Wang, X. D. Yao, C. Liu, D. M. Chen, G. Q. Lu, and H. M. Cheng, J. Alloys Compd. , (2006) online published. 8. Structure and hydrogen storage property of ball-milled Li. NH 2/Mg. H 2 mixture Y. Chen, C. Z. Wu, P. Wang, H. M. Cheng, Inter. J. Hydrogen Energy, (2006) online-published. Metal nitride Chemical hydride 7. Catalytic effect of Al 3 Ti on the reversible dehydrogenation of Na. Al. H 4 X. D. Kang, P. Wang, X. P. Song, X. D. Yao, G. Q. Lu and H. M. Cheng, J. Alloys Compd. , (2006) online-published. 6. Dependence of H-storage performance on preparation conditions in Ti. F 3 doped Na. Al. H 4 P. Wang, X. D. Kang and H. M. Cheng, J. Alloy compd. , (2006) online-published. 5. Effects of SWNT and metallic catalyst on hydrogen absorption/desorption performance of Mg. H 2 C. Z. Wu, P. Wang, X. D. Yao, C. Liu, D. M. Chen, G. Q. Lu, and H. M. Cheng, J. Phys Chem B, 109 (2005) 22217 -22221. 4. Exploration of the nature of active Ti-species in metallic Ti-doped Na. Al. H 4 P. Wang, X. D. Kang and H. M. Cheng, J. Phys. Chem. B, 109 (2005) 20131 -20136. 3. Direct formation of Na 3 Al. H 6 by mechanical milling Na. H/Al with Ti. F 3 P. Wang, X. D. Kang and H. M. Cheng, Appl. Phys. Lett. , 87 (2005) 071911. 2. Improved hydrogen storage property of Ti. F 3 -doped Na. Al. H 4 P. Wang, X. D. Kang and H. M. Cheng, Chem. Phys. Chem, 6 (2005) 2488 -2451. Our Group 1. KH+Ti co-doped Na. Al. H 4 for high-capacity hydrogen storage P. Wang, X. D. Kang and H. M. Cheng, J. Appl. Phys. , 98 (2005) 074905. Our Research Publications Go back


