e4b3fdcb2d5bc3d5cce0b2021d29fd4f.ppt
- Количество слайдов: 28

Storage and Record Keeping Requirements for Transfusable Blood Components Created by S. Purcell

Objectives S Transfusable Blood Component Temperature Requirements S Storage and Maintenance of Blood Components S Identifying, Investigating, and Documenting Deviations S Reporting Requirements S Hospital Inspections by Blood Center 2
Why is this important? • Code of Federal Regulations Title 21, Part 600 – Sec. 640. 11 (a). . . the Red Blood Cells shall be placed in storage and maintained at a temperature between 1 and 6 deg. C. – Sec. 640. 25 (a) …Platelets shall be placed in storage at the selected temperature range. If stored at 20 to 24 deg. C, a continuous gentle agitation of the platelet concentrate shall be maintained throughout the storage period. 3

Why do blood product storage deviations need to be reported to the FDA? • Sec. 600. 14 Reporting of biological product deviations by licensed manufacturers. • You must report any event, and information relevant to the event, associated with…storage…if that event… 1. Represents a deviation from current good manufacturing practice, applicable regulations, applicable standards, or established specifications that may affect the safety, purity, or potency of that product. 2. Represents an unexpected or unforeseeable event that may affect the safety, purity, or potency of that product; and 3. Occurs in your facility or another facility under contract with you; and 4. Involves a distributed biological product. 4
Transfusable Blood Component Temperature Requirements • Storage Temperature Requirements – Blood in the body is typically maintained at a temperature of approximately 37 C. – The body has a mix of gases such as oxygen and carbon dioxide along with other elements that are able to maintain the blood at a single constant temperature. – After the blood is collected, there are different storage temperature requirements. Research has established required temperature ranges to maintain the blood’s safety, purity, potency and quality for safe blood transfusions. Blood components have distinct storage temperature requirements due to their varying make-up and purposes as well. – In addition to blood components being maintained at certain temperatures, the blood can only be stored at those temperatures for a fixed period of time. 5
Transfusable Blood Component Temperature Requirements • All blood components have a shelf-life. After the shelf-life has expired the blood components can no longer be safely transfused and they are discarded or used for other purposes. Definitions • Purity – uniform and free from foreign matter. • Potency - the ability of the blood component to produce the desired medical effects. 6
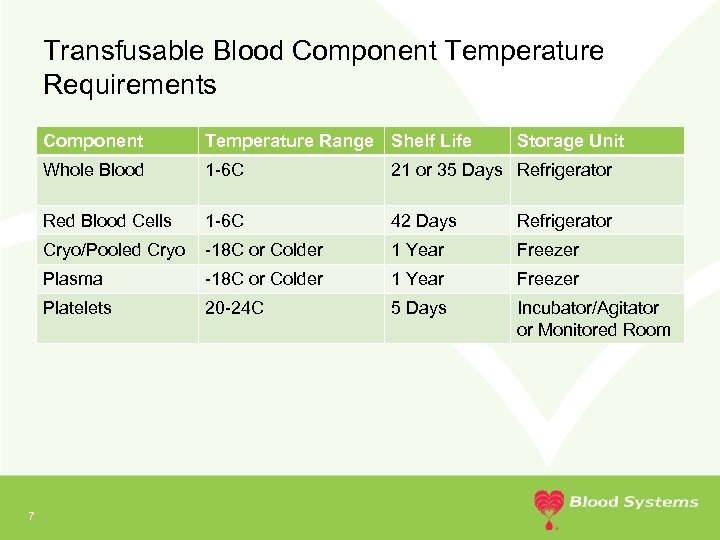
Transfusable Blood Component Temperature Requirements Component Whole Blood 1 -6 C 21 or 35 Days Refrigerator Red Blood Cells 1 -6 C 42 Days Refrigerator Cryo/Pooled Cryo -18 C or Colder 1 Year Freezer Plasma -18 C or Colder 1 Year Freezer Platelets 7 Temperature Range Shelf Life Storage Unit 20 -24 C 5 Days Incubator/Agitator or Monitored Room
Transfusable Blood Component Temperature Requirements • Transportation Temperatures: – Generally the same as storage except for RBCs, which are 1 -10 C – Limited amount of time at 1 -10 C – If a box is used for storage, all storage requirements apply. 8
Storage and Maintenance of Blood Components • Storage unit configuration – Organized in an orderly fashion • • 9 Facilitates proper air circulation and even temperature distribution Facilitates identification of units Facilitates use of short dates first Segregated based on unit status – Blood Type – Quarantine – Autologous – Directed
Storage and Maintenance of Blood Components • Storage unit configuration, continued – Top to bottom • Top: Available general inventory • Above Quarantine, below Available Inventory: Reagents • Bottom: Quarantine and/or biohazardous 10
Storage and Maintenance of Blood Components • Temperature Monitoring and Alarms – Temperatures within the storage units must be monitored at all times. • Automated temperature monitoring systems • Recording charts – Storage units must contain some type of temperature monitoring device (TMD) that constantly records the temperature inside the storage unit. • Small units only require one thermometer. • One thermometer must be in the same solution as the probe for the chart recorder or immediately adjacent – TMD maximum liquid volumes • Refrigerators 250 ml • Platelet incubators 40 ml • Freezers 100 ml 11
Storage and Maintenance of Blood Components Temperature Monitoring and Alarms • – – 12 Recording charts require a daily reading and comparison of the internal TMD with the chart temperature • Refrigerators and Platelet Incubators must be within 1 C of each other • Freezers must be within 2 C of each other • Chart adjustment may be required if the variances are greater Recording charts require: • Date, time, and initials of staff starting chart • Date, time, and initials of staff removing chart • Hospital Name and address • Unique identifier of equipment being monitored • Timely review If the temperature monitoring system ever fails then temperatures must be manually taken and documented on the appropriate Daily Quality Control Record at least every four hours.
Storage and Maintenance of Blood Components Temperature Alarms • All storage units must be equipped with an alarm that is set to trigger if the temperatures are close to drifting out of the acceptable range. • If an alarm sounds staff must respond and investigate immediately. • The alarm reason and investigation must be documented on the appropriate Daily Quality Control Record. • The alarm system must be monitored 24 hours per day. – On site staffing – Remote alarms 13

Storage and Maintenance of Blood Components Unacceptable Storage Conditions – Outside of the acceptable temperature range – Unacceptable variance between a TMD and the recording chart – The recording chart stops recording – These require immediate correction and investigation • Was the door shut all the way? • Has it been opened frequently? • Is there power? • Are the probes in the solution? • Is the recording chart recording accurately? • 14 If the problem cannot be resolved within 30 minutes of the last acceptable reading, components must be moved to an alternate location
Storage and Maintenance of Blood Components • If relocation is required – Use another monitored unit with the same temperature range if available. OR – Pack in UBS shipping containers (or other local blood center approved shipper). OR – Move to another location that maintains the same temperature range. AND – Record the temperature at least every 4 hours. • Document all actions taken. 15
Storage and Maintenance of Blood Components • Maintenance of storage units – Routine preventative maintenance keep equipment operating efficiently – Review all operator’s manuals upon receipt of new equipment • Learn how to operate the equipment • Identify recommended maintenance and testing schedules – Follow manufacturers recommendations 16
Storage and Maintenance of Blood Components Reading Temperatures • • • 17 Record temperatures to the nearest increment – If the device reads in whole numbers, record temperatures in whole numbers – Round up if in the middle of two markings Calibrate thermometers – Annually for most – Electronic thermometers may require more frequent calibration. • Refer to certificate and operators manual – Indicate next calibration due date on thermometer Recording charts – Determine increments of recording chart – Read from the center (usually the highest temperature) outward (usually the lowest temperature) – Compare daily against closest thermometer – Verify date, time, and temperature increments before reading temperature

Identifying, Investigating, and Documenting Deviations Respond appropriately to potential and actual temperature deviations. • Respond to warnings to prevent temperature deviations. • Properly handle products if they need to be moved. • Appropriately document activities, temperatures and comments. – Document on affected record or record location of documentation on record – It is not sufficient to attach records. You must explain the situation. 18

Identifying, Investigating, and Documenting Deviations • The majority of common errors generally fall into one of the following four categories: – Failure to respond to warnings or alarms – Untimely relocation of products – Failure to document activities, temperatures and comments – Lack of timely notification to the blood center 19

Identifying, Investigating, and Documenting Deviations • Problems causing temperature deviations and/or excursions – There is no documentation or insufficient documentation regarding what happened and what steps were taken when a temperature excursion occurred – Warnings are not addressed before temperature exceeds the acceptable limits – Equipment going through its normal defrost cycle along with a high amount of traffic causing a temperature excursion – A storage door being open too long or didn’t close completely – Equipment is under repair yet it is still being used to store product 20
Identifying, Investigating, and Documenting Deviations • Contributing Factors – Staff believe it is acceptable for temperatures to be out of range as long as the temperature is “close” to the acceptable range. • It is never acceptable for temperature excursions beyond the component limits. – The rules for rounding temperature measurements are difficult to interpret. • When necessary, temperatures must be rounded up. – Some product storage units have a combination of alarm delays and alarm set points that allow the unit to go out of the acceptable range without the alarm sounding. • Alarms must sound prior to temperature excursions beyond acceptable limits. – There is confusion about the length of time products can be exposed to temperatures beyond the acceptable range for storage. • Components must not be out of storage greater than 30 minutes. 21

Identifying, Investigating, and Documenting Deviations • What is an Alarm Signal? – Alarm – An electronic or mechanical device that serves to warn of danger or by means of a sound or signal. – How does this relate to what you do? • An alarm signal = a warning = an opportunity to prevent a deviation. • If corrective action will not or cannot prevent a deviation, the alarm allows you time to react such as; moving the product to alternate storage. • 22 Remember: Alarms = potential/actual deviation = more documentation of: – Temperature and times – Individual(s) involved – Actions taken – When, where, how products are moved

Identifying, Investigating, and Documenting Deviations Posted instructions for corrective action: • Is it current? • Does it present easy to follow instructions so staff can respond appropriately? • Is it visible? Adequate documentation of an event must include: • Date • Time of all steps taken • Initials for employee(s) involved • Duration • Action Taken • Identification for; storage, thermometers, etc. 23
Reporting Requirements • Important numbers: – 30 • The number of minutes a component may be out of controlled storage. OR • The number of minutes a component may be exposed to unacceptable temperatures. • NOTE: The amount of time blood components are out of appropriate storage should be minimized. – 4 • The maximum acceptable hours between manual temperature recordings. • Usually satisfied through the use of a chart recorder or automated monitoring system. • If those are not available, manual temperatures are required. 24

Reporting Requirements • Dates and Times of all actions taken and observations made: • • • What time was the problem identified? How long did it occur? Were there components stored within at the time? What time were they moved? What were the temperatures of both the pre and post movement storage chambers? • Do you have a backup system that has been validated and QC is current? • What units were involved? The blood center must be notified prior to returning or transferring any components that fall outside acceptable limits. – The documentation will be evaluated and you will be informed whether the units are acceptable for return. What are your options if you cannot return? Consult your Medical Director regarding the use of the components at your facility. 25
Hospital Inspections by Blood Center • What are we assessing? We use three forms to inspect all storage areas. You can find each form (BS 983 A, BS 983 B, BS 983 C) on the United Blood Services website. Use the forms to prepare prior to your inspection. 26
Hospital Inspections by Blood Center • What are the possible outcomes? • No findings • Findings requiring a written response within 30 days • Suspension of return privileges • Recall of affected components requiring reporting to the FDA 27

Thank you Questions? Please contact your local blood center
e4b3fdcb2d5bc3d5cce0b2021d29fd4f.ppt