c0cb5eaf39593acd3f273973f469e2bf.ppt
- Количество слайдов: 38

Stoichiometry By: Ms. Michelle Buroker Scott High School Taylor Mill, Kentucky Go Eagles! Let’s Get Started!

A Brief Introduction…. Stoichiometry is the mathematics of chemical reactions. This is a tutorial meant to introduce you to the basics so that you will be able to calculate with confidence! There is a brief quiz at the end of the lesson, you need to take it, print out the certificate at the end, and bring it to me for credit. Here We Go!

Have you ever wondered when you were watching fireworks exactly how they make that happen? It’s Stoichiometry! Stoichiometry is the study of quantitative relationships between amounts of reactants used and products formed by a chemical reaction. The purpose of this learning module is for you to interactively learn Stoichiometry: mathematical operations which are very important to scientists. When you follow the lessons laid out in this assignment, hopefully you will gain an understanding of the process by which you can solve questions such as: if I start with so many grams of reactants, how many grams of product will I get? Main Menu
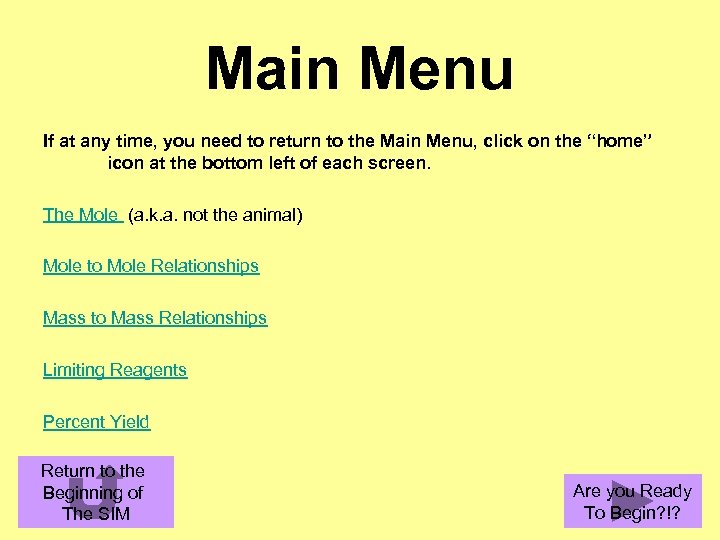
Main Menu If at any time, you need to return to the Main Menu, click on the “home” icon at the bottom left of each screen. The Mole (a. k. a. not the animal) Mole to Mole Relationships Mass to Mass Relationships Limiting Reagents Percent Yield Return to the Beginning of The SIM Are you Ready To Begin? !?

The Mole Click Here To Watch a Video about The Mole The SI base unit used to measure the amount of a substance! One mole of anything is 6. 02 x 1023 particles … – Remember that a particle can be: atoms, molecules, or formula units Ex: 1 mole of C = 6. 02 x 1023 atoms 1 mole of Na. Cl = 6. 02 x 1023 formula units 1 mole of H 2 O = 6. 02 x 1023 molecules The mass of any pure substance is equal to it’s molar mass - Remember that the molar mass of any element is equal to its atomic weight and the molar mass of any molecule or formula unit is equal to the sum of the atomic weight’s of the individual atoms - Ex: 1 mole of Ca weighs 40. 08 g 1 mole of H 2 O weighs 18. 0 g Conversion Factors

Conversion Factors • The following are the conversion factors you need to “do the math: ” 1. ) To go from moles to grams: number of moles given X atomic weight or molar mass 1 1 mole 2. ) To go from grams to moles: number of grams given X 1 mole 1 atomic weight or molar mass Let’s Try Some Practice Problems

Conversion Factors • The following are the conversion factors you need to “do the math: ” 1. ) To go from moles to grams: number of moles given X atomic weight or molar mass 1 1 mole 2. ) To go from grams to moles: number of grams given X 1 mole 1 atomic weight or molar mass Let’s Try Some Practice Problems

Let’s Practice!!!! #1: How much does 5 moles of Ag. NO 3 weight? * Click on your choice! A. 169. 91 g B. 849. 40 g C. 339. 76 g

Try Again … • Take a look at the conversation factor page again … use those to help you. Conversation Factor Page Back to the Problem

Try Again … • Take a look at the conversation factor page again … use those to help you. Conversation Factor Page Back to the Problem

Let’s Practice!!! #2. How many moles are 250 g of Na. Cl? *Click on your choice! A. 58. 45 moles B. 1. 46 x 104 moles C. 4. 28 moles

Mole to Mole Relationships Now that you have mastered basic conversions between moles and grams … you’re ready to move on to calculations within chemical reactions! The mole is how we relate reactants to other reactants … products to other products … or reactants to products. It’s called the Mole Ratio! Say What? ? ?
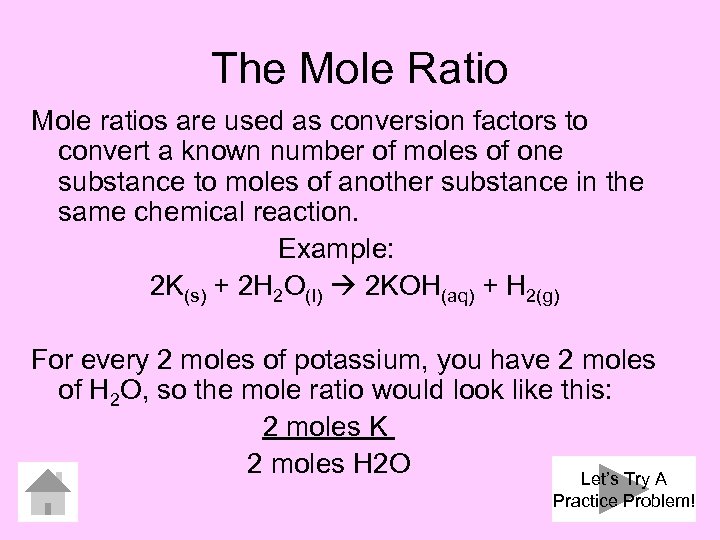
The Mole Ratio Mole ratios are used as conversion factors to convert a known number of moles of one substance to moles of another substance in the same chemical reaction. Example: 2 K(s) + 2 H 2 O(l) 2 KOH(aq) + H 2(g) For every 2 moles of potassium, you have 2 moles of H 2 O, so the mole ratio would look like this: 2 moles K 2 moles H 2 O Let’s Try A Practice Problem!

Let’s Practice! One disadvantage to burning propane (C 3 H 8) is that carbon dioxide (CO 2) is one of the products. The release of carbon dioxide increases the growing concentration of CO 2 in the atmosphere. How many moles of carbon dioxide are produced when 10. 0 moles of propane are burned in excess oxygen in a gas grill? Having Trouble Getting Started and Need a Hint … Check Your Answer

Here’s a Hint …. You need to write a balanced chemical equation before you can solve this problem! Hopefully, you recognize this as a combustion reaction. C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O On To The Answer!
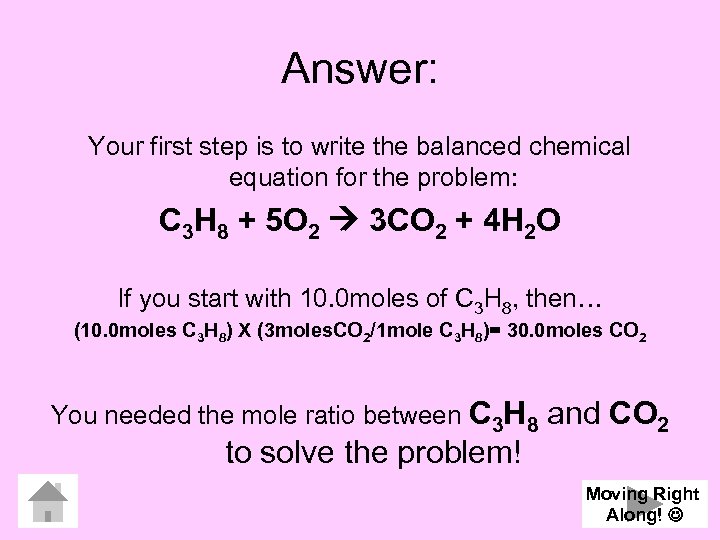
Answer: Your first step is to write the balanced chemical equation for the problem: C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O If you start with 10. 0 moles of C 3 H 8, then… (10. 0 moles C 3 H 8) X (3 moles. CO 2/1 mole C 3 H 8)= 30. 0 moles CO 2 You needed the mole ratio between C 3 H 8 to solve the problem! and CO 2 Moving Right Along!
Mass to Mass Relationships • The coefficients of a balanced equation give the relative amounts (in moles) of reactants and products. Calculations to find the masses of materials involved in reactions are called Mass -Mass Problems. Okay … but how do I actually solve a problem?

The Pathway … • There is a step- by- step method to solving mass- mass problems; the following steps must be followed! Grams Reactant Moles Product Grams Product Convert Grams to Moles Mole Ratio Convert Moles to grams *Note: If you begin with grams of Product, the process is simply done in reverse! Let’s Look at an Example
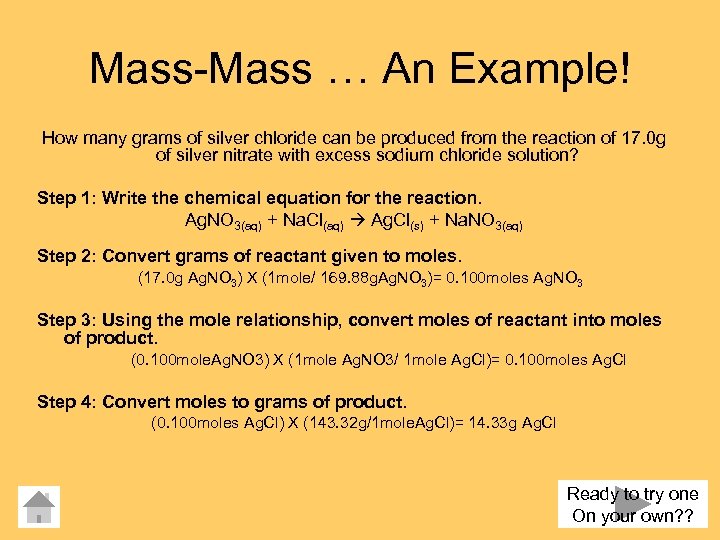
Mass-Mass … An Example! How many grams of silver chloride can be produced from the reaction of 17. 0 g of silver nitrate with excess sodium chloride solution? Step 1: Write the chemical equation for the reaction. Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(s) + Na. NO 3(aq) Step 2: Convert grams of reactant given to moles. (17. 0 g Ag. NO 3) X (1 mole/ 169. 88 g. Ag. NO 3)= 0. 100 moles Ag. NO 3 Step 3: Using the mole relationship, convert moles of reactant into moles of product. (0. 100 mole. Ag. NO 3) X (1 mole Ag. NO 3/ 1 mole Ag. Cl)= 0. 100 moles Ag. Cl Step 4: Convert moles to grams of product. (0. 100 moles Ag. Cl) X (143. 32 g/1 mole. Ag. Cl)= 14. 33 g Ag. Cl Ready to try one On your own? ?

Hmmm … Let’s See How You Do On Your Own. Ammonium nitrate (NH 4 NO 3), an important fertilizer, produces N 2 O gas and H 2 O when it decomposes. Determine the mass of water produced from the decomposition of 25. 0 g of solid ammonium nitrate. Try it on your own before checking the answer! Answer

The Origins of Fertilizer? ? ? Fertilizer originated from a process known as the Haber Process, developed during WW I in Germany. (If you’re interested in reading a bit more about the Haber Process … click the link for some brief info) … Before you see the answer, follow these steps to solve the problem… Step 1: Write the balanced chemical equation for the reaction. Step 2: Convert grams of reactant given to moles. Step 3: Using the mole relationship, convert moles of reactant into moles of product. Step 4: Convert moles to grams of product. Now Take a Look at the Answer

Okay … This Time it Really is The Answer! NH 4 NO 3 N 2 O + 2 H 2 O Convert grams of NH 4 NO 3 to moles of NH 4 NO 3. (25. 0 g. NH 4 NO 3/1) X (1 mol NH 4 NO 3/ 80. 06 g. NH 4 NO 3 )= 0. 312 mol NH 4 NO 3 Convert moles of NH 4 NO 3 to moles of H 2 O. (0. 312 mol NH 4 NO 3 /1) X (2 mole H 2 O/1 mole NH 4 NO 3)= 0. 624 mole H 2 O Covert moles of H 2 O to grams of H 2 O. (0. 624 mol. H 2 O/1) X (18. 0 g H 2 O/ 1 mole H 2 O)= 11. 24 g H 2 O

Why do reactions stop? The answer is … limiting reagents! If you put 10 boys and 6 girls in a room and asked them to pair up: one boy to one girl, who will be left without a partner? The boys … there will be four boys left with no partner. The girls are the limiting factor, in other words, they control how many pairs will be formed. Keep Moving!

Limiting Reagents and Reactions Just like the previous example, in the lab, reactions are limited by the reactant present in the LEAST amount. So, to solve limiting reagent problems, you have to first find out which reactant is the limiting reagent!!! Hint … convert grams of reactant to moles! Let’s Take a Look At an Example!

Air Bags …. Watch Air Bag Deployment The reaction between solid sodium and iron (III) oxide is one in a series of reactions that inflates an automobile airbag. 6 Na(s) + Fe 2 O 3(s) 3 Na 2 O(s) + 2 Fe(s) If 100. 0 g Na and 100. 0 g Fe 2 O 3 are used in this reaction, determine: a. the limiting reagent b. the excess reagent c. the mass of solid iron produced d. the amount of excess reaction remaining after the reaction is complete. Take a look at How to work it out!

The Solution … 6 Na(s) + Fe 2 O 3(s) 3 Na 2 O(s) + 2 Fe(s) 1. ) Determine the Limiting Reagent by Converting grams of product into moles of product. (100. 0 g. Na/1) X (1 mol Na/ 23. 0 g. Na)= 4. 35 mol Na (100. 0 g. Fe 2 O 3/1) X (1 mol. Fe 2 O 3/159. 7 g. Fe 2 O 3)= 0. 6262 mol Fe 2 O 3 is present in the least amount, therefore, it is the limiting reagent. The excess reactant would be Na. 2. ) Determine the amount of solid Fe produced by converting moles of the limiting reagent into grams of product. (0. 6262 mol Fe 2 O 3/1) X (2 mol Fe/1 mol Fe 2 O 3) X (55. 85 g Fe/1 mol Fe)= 69. 95 g Fe 3. ) To determine the amount of excess reactant left, convert moles of limiting reagent to grams of excess reactant and subtract that number from what you started with. (0. 6262 mol Fe 2 O 3/1) X (6 mol Na/1 mol Fe 2 O 3) X (23. 0 g Na/ 1 mol Na)= 86. 41 g Na 100. 0 g Na Started with – 86. 41 g Na used= 13. 59 g Na left in excess Try a Problem on Your Own Now …

Time to Try a Problem on Your Own! Good Luck If 4. 44 g of calcium oxide are mixed with 7. 77 g of water, how many grams of calcium hydroxide will form? Try working it out on your own before looking at the answer!!! Do You Need A Hint? Answer

Need a Hint? You need to write a balanced chemical equation to solve this problem! Hopefully you recognized this as a synthesis reaction Ca. O + H 2 O Ca(OH)2 On To The Answer!
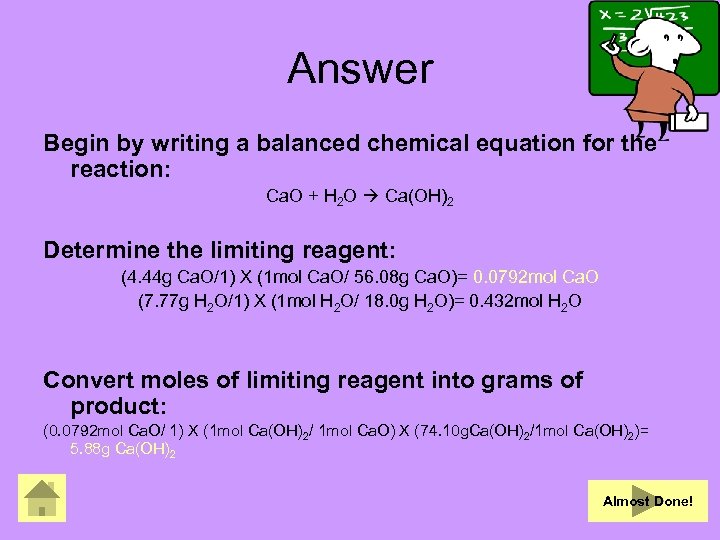
Answer Begin by writing a balanced chemical equation for the reaction: Ca. O + H 2 O Ca(OH)2 Determine the limiting reagent: (4. 44 g Ca. O/1) X (1 mol Ca. O/ 56. 08 g Ca. O)= 0. 0792 mol Ca. O (7. 77 g H 2 O/1) X (1 mol H 2 O/ 18. 0 g H 2 O)= 0. 432 mol H 2 O Convert moles of limiting reagent into grams of product: (0. 0792 mol Ca. O/ 1) X (1 mol Ca(OH)2/ 1 mol Ca. O) X (74. 10 g. Ca(OH)2/1 mol Ca(OH)2)= 5. 88 g Ca(OH)2 Almost Done!

How accurate Was my Experiment? To answer this question … you need to know about the percent yield. Percent yield is essentially how much product you got from a chemical reaction vs. how much you should have gotten back according to your Stoichiometry calculation. The Formula

Percent Yield = Actual Yield X 100 Theoretical Yield Actual Yield= What you get from the experiment or the “experimental value” Theoretical Yield= How much product the stoichiometric calculation says you should get, based on how much reactant you started with. Let’s see how to use This equation …

Here’s An Example …. When potassium chromate (K 2 Cr. O 4) is added to a solution containing 0. 500 g silver nitrate (Ag. NO 3), solid silver chromate (Ag 2 Cr. O 4) is formed. A. ) Determine theoretical yield of silver chromate precipitate. B. ) Determine the percent yield if 0. 455 g silver chromate was actually recovered. *If you follow the steps … find theoretical yield first, then the calculation becomes quite simple. Click Here if you Want to see the Problem Worked Out

Answer to The Example …. 1. ) As always, begin by writing a balanced chemical equation for the reaction: K 2 Cr. O 4 + 2 Ag. NO 3 Ag 2 Cr. O 4 + 2 KNO 3 2. ) Figure out theoretical yield by converting 0. 500 g Ag. NO 3 to grams of Ag 2 Cr. O 4. (0. 500 g. Ag. NO 3/1) X (1 mol Ag. NO 3/169. 91 g Ag. NO 3)= 0. 00294 mol Ag. NO 3 (0. 00294 mol Ag. NO 3/1) X (1 mol Ag 2 Cr. O 4/ 2 mol Ag. NO 3)= 0. 00147 mol Ag 2 Cr. O 4 (0. 00147 mol Ag 2 Cr. O 4/1) X (331. 8 g. Ag 2 Cr. O 4/ 1 mol Ag 2 Cr. O 4)= 0. 488 g Ag 2 Cr. O 4 3. ) Now that you have theoretical yield, 0. 488 g Ag 2 Cr. O 4, you can use the actual yield of 0. 455 g Ag 2 Cr. O 4 and find the percent yield: (0. 455 g. Ag 2 Cr. O 4/ 0. 488 g Ag 2 Cr. O 4) X 100= 93. 2% Yeah!!!

Great Job!!!!!!! Finally … you have completed this learning module over Stoichiometry. My hope for you is that you learned something! The last leg of your journey is to complete a questionnaire over this Learning Module. You need to Print out the page, answer the questions, and then turn the sheet into me for credit. Click Here to Proceed to the Evaluation

The Evaluation You must take this evaluation in order to receive the extra credit for completing this Learning Module. Follow the evaluation link, print the page, answer the questions, and turn it into me! The End … Thanks for Stopping By The Evaluation

Correct! Great Job!!! Moving On!

Way To Go!!!!!! Ready for What’s Next? ? ?
References Thank You and I hope you enjoyed the Experience! Unless designated otherwise, all pictures are from clip art. Slide 1 Scientist Picture: (http: //istockphoto. com/imageindex/310/7/310758/The_Mad_Scientist. html) Eagle Picture: (http: //www. scott. kenton. kyschools. us/default 1. htm) Slide 5 Mole Picture: (http: //www. ringwood. hants. sch. uk/school 2/_Subjects/sci. Chemistry_ks 3/Spencer. New. Si te/page_7 E_acids. html) Resource Book Used: Dingrando, Laurel, Kathleen V. Gregg, Nicholas Hainen, Phillip Lampe, Cynthia Roepcke, Dingrando, Hainen, Roepcke, and Cheryl Wistrom. Chemistry: Matter and Change. 1 st ed. Vol. 1. Columbus: Wistrom. Glencoe/ Mc. Graw Hill, 2002. Return to the Beginning of The SIM Press Escape to End the Learning Module.
c0cb5eaf39593acd3f273973f469e2bf.ppt