082e6494eb05e071a4163caaf189df33.ppt
- Количество слайдов: 18

Sterilization Validation & Monitoring System Based on NLPButton Technology Application for Steam Sterilization Presented by OPULUS 2006

What is a NLPButton data-logger? Anatomy of NLPButton data-loggers (actual size) Hygrochrons Thermochrons (temperature/humidity suitable for EO sterilization/Intrinsically safe) (temperature) Li-Battery Microprocessor Capacitive humidity sensor Quartz clock Humidity sensor inlet, covered by Gore-Tex

Content • Steam Sterilization Validation & Monitoring System Components • Regulatory Considerations • Major Performance Characteristics • Critical Functional Characteristics • Documentation Requirements • Support & Maintenance • Cost of Ownership • Benefits to the User

Steam Sterilization Validation & Monitoring System Components • Thermochron data-logger • Certificate of Calibration • Docking Station for Programming/Data download • Integrating Software • SOP (Standard Operating Procedure) • SCORM Compatible Competency Training • IQ-OQ-PQ protocol for compliance verification • 21 CFR Part 11 protocol for compliance verification

Regulatory Considerations • Compliance with US FDA Requirements: – 21 CFR Part 11 Compliance: The Steam Sterilization Validation & Monitoring System is 21 CFR Part 11 Compliant – IQ-OQ-PQ Verification Protocols are Included – 21 CFR Part 11 Verification Protocols are Included – SOP (Standard Operating Procedure) are Included – SOP Competency Training: Web-enabled SCORM (Do. D supported protocol) Compatible SOP Competency Training is Available – Complies with FDA’s “Guidance for the Industry for the Submission Documentation for Sterilization Process Validation in Application for Human and Veterinary Drug Products" http: //www. fda. gov/cder/guidance/cmc 2. pdf • Calibration: each data-logger’s Calibration is Traceable to NIST Reference Standard
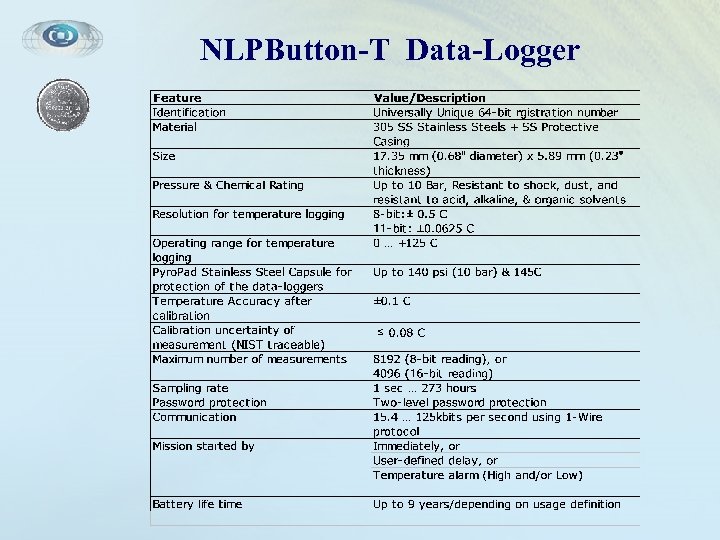
NLPButton-T Data-Logger

Major Performance Characteristics Docking Station for i. Button Programming/Data download • i. Button docking station for parallel programming/data down-load of the data-logger is available in various sizes to hold 8, or 16, or 32, or 50, or 100 i. Buttons • The i. Buttons can be chained through a single Batch No. of multiple docking stations for parallel programming of 1, 000’s of i. Buttons • RS 232, USB, & parallel interfaces are supported
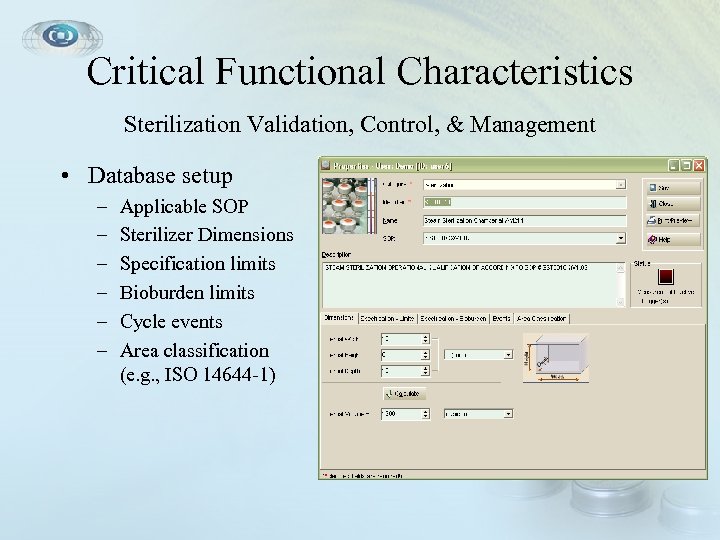
Critical Functional Characteristics Sterilization Validation, Control, & Management • Database setup – – – Applicable SOP Sterilizer Dimensions Specification limits Bioburden limits Cycle events Area classification (e. g. , ISO 14644 -1)
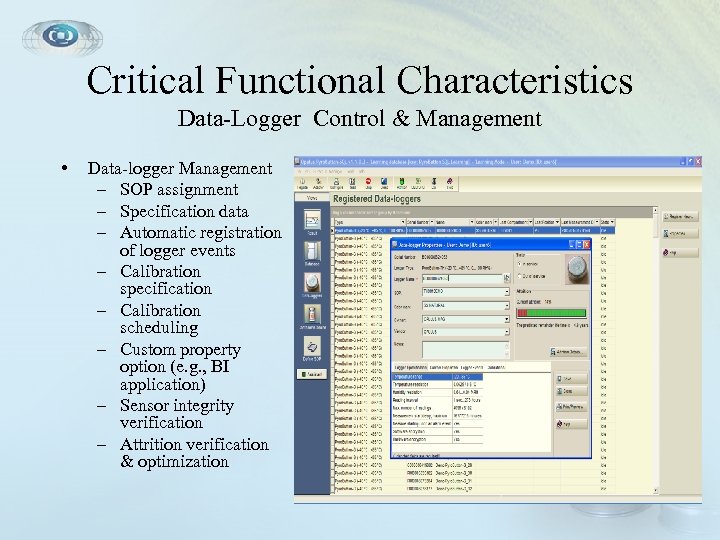
Critical Functional Characteristics Data-Logger Control & Management • Data-logger Management – SOP assignment – Specification data – Automatic registration of logger events – Calibration specification – Calibration scheduling – Custom property option (e. g. , BI application) – Sensor integrity verification – Attrition verification & optimization

Critical Functional Characteristics Cycle Specification with Load Assignment • Cycle Specification

Critical Functional Characteristics Logger Positional Assignment • Logger Positional Specifiation

Critical Functional Characteristics Logging Data Security
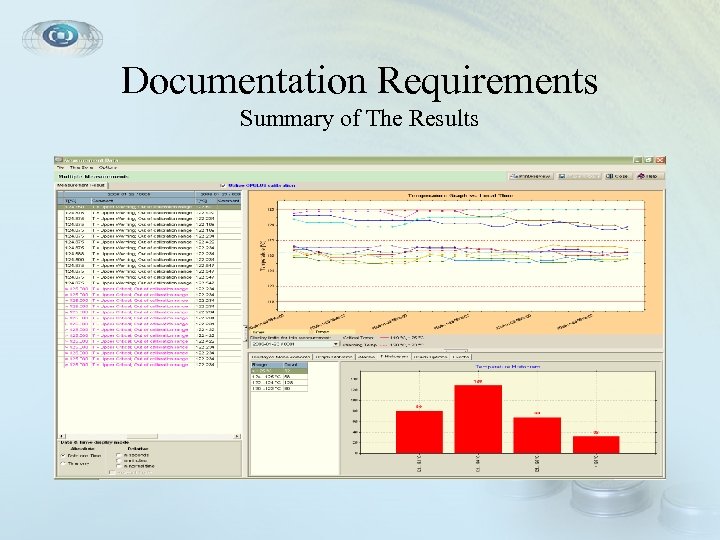
Documentation Requirements Summary of The Results
Documentation Requirements SQC/SPC

Documentation Requirements Temperature Control Chart Characteristics Including Load Analysis in Selected Sterilizer
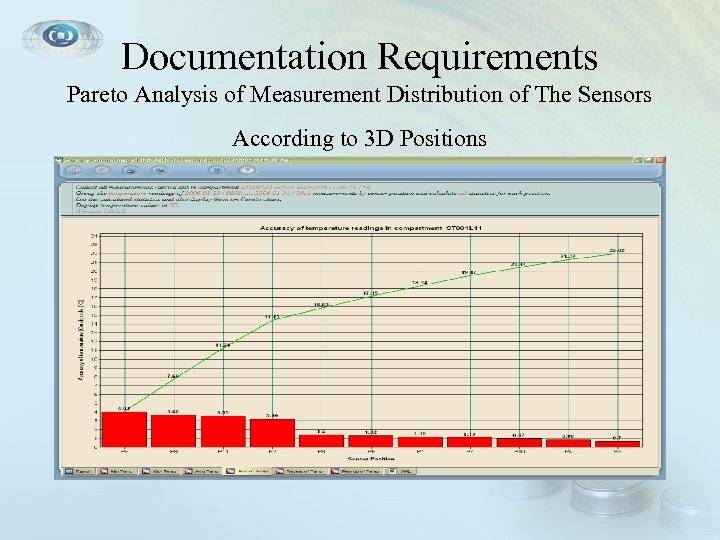
Documentation Requirements Pareto Analysis of Measurement Distribution of The Sensors According to 3 D Positions

Support & Maintenance • 24/7 On-line Web Access for Interactive Support • NIST Compliant Calibration Service • IQ-OQ-PQ protocol verification – On-site or Web-supported • 21 CFR Part 11 compliance verification – On-site or Web-supported via Intelligent Decision Tree • SOP templates – 24/7 On-line Web access • SCORM – 24/7 On-line Web, desktop, or enterprise training

Benefits to the User • Low Purchase Cost • Best in Class Price to Performance Ratio • Total Solution with Full Regulatory Compliance – – Certificate of Calibration SOP SCORM Compatible Training Complies with FDA’s “Guidance for the Industry for the Submission Documentation for Sterilization Process Validation in Application for Human and Veterinary Drug Products“ • Ensures Best Sterilization Practices
082e6494eb05e071a4163caaf189df33.ppt