86af5b6e31f17eeb492d813c315e3fa5.ppt
- Количество слайдов: 71
 STERILIZATION AND DISINFECTION Meral Sonmezoglu, MD. Professor of Infectious Dıseases 2007
STERILIZATION AND DISINFECTION Meral Sonmezoglu, MD. Professor of Infectious Dıseases 2007
 2007
2007
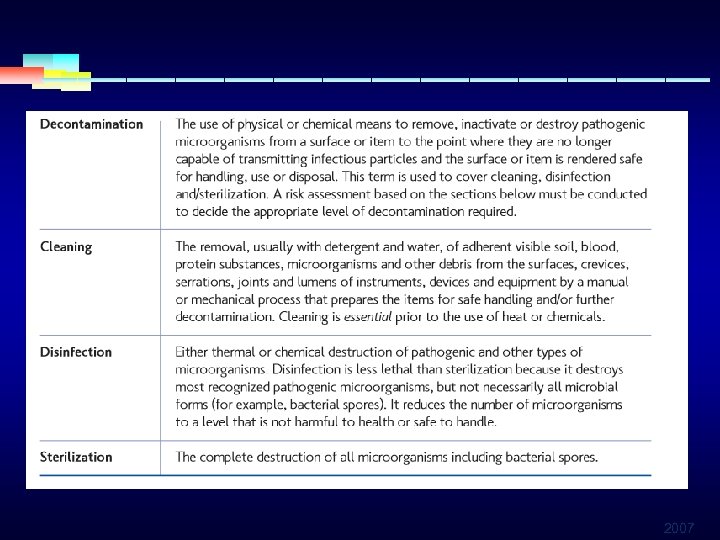
 DEFINITIONS § Decontamination: Removal of disease-producing m. o. to leave an item § Disinfection: Inactivation of disease-producing m. o. (not destroy) § Sterilization: Destruction of all forms of microbial life (bacteria, viruses, spores, fungi) § Sanitation: A process that reduces mo on an inanimate object to a level of below infectious hazard 2007
DEFINITIONS § Decontamination: Removal of disease-producing m. o. to leave an item § Disinfection: Inactivation of disease-producing m. o. (not destroy) § Sterilization: Destruction of all forms of microbial life (bacteria, viruses, spores, fungi) § Sanitation: A process that reduces mo on an inanimate object to a level of below infectious hazard 2007
 2007
2007
 § Disinfection—The process of microbial inactivation that eliminates virtually all recognized pathogenic microorganisms, but not necessarily all microbial forms (e. g. , spores) § Sterilization—The use of physical or chemical procedures to destroy all microbial life, including large numbers of highly resistant bacterial endospores. Procedures include— § Steam sterilization § Heat sterilization § Chemical sterilization 2007
§ Disinfection—The process of microbial inactivation that eliminates virtually all recognized pathogenic microorganisms, but not necessarily all microbial forms (e. g. , spores) § Sterilization—The use of physical or chemical procedures to destroy all microbial life, including large numbers of highly resistant bacterial endospores. Procedures include— § Steam sterilization § Heat sterilization § Chemical sterilization 2007
 Efficacy of Disinfection/Sterilization § Cleaning of the object § Organic and inorganic load present § Type and level of microbial contamination § Concentration of and exposure time to disinfectant/sterilant § Nature of the object § Temperature and relative humidity 2007
Efficacy of Disinfection/Sterilization § Cleaning of the object § Organic and inorganic load present § Type and level of microbial contamination § Concentration of and exposure time to disinfectant/sterilant § Nature of the object § Temperature and relative humidity 2007
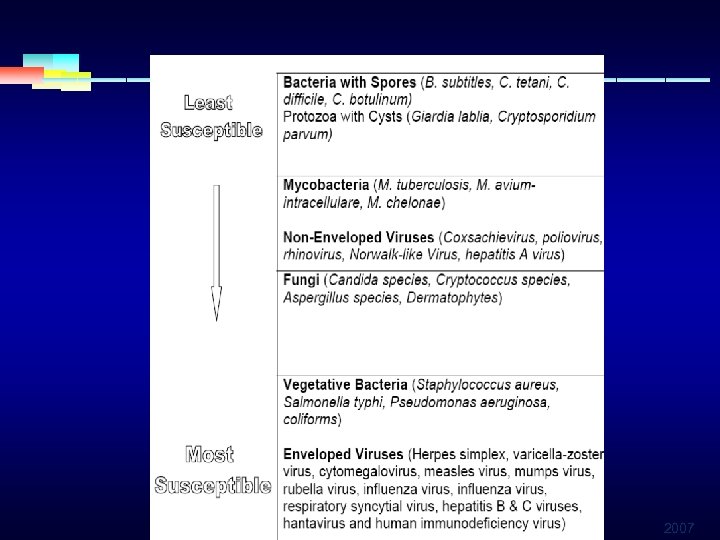
 Decreasing Order of Resistance of Microorganisms to Disinfectants/Sterilants Prions Spores Mycobacteria Non-Enveloped Viruses Fungi Bacteria Enveloped Viruses 2007
Decreasing Order of Resistance of Microorganisms to Disinfectants/Sterilants Prions Spores Mycobacteria Non-Enveloped Viruses Fungi Bacteria Enveloped Viruses 2007
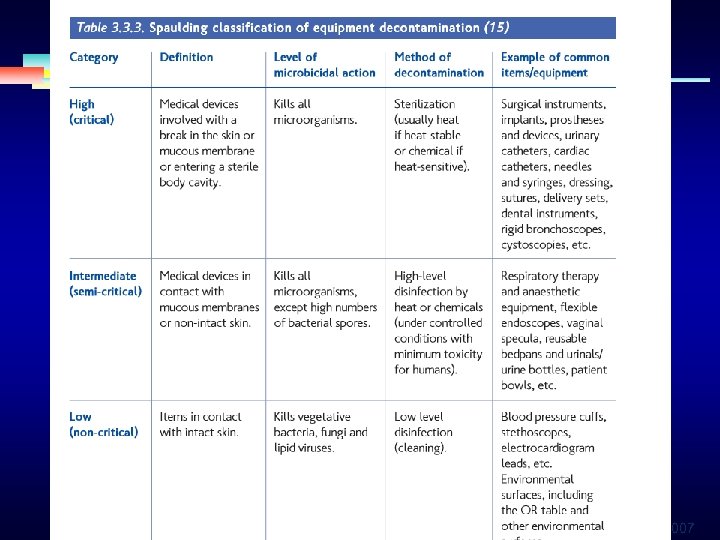
 DISINFECTION AND STERILIZATION • Over 45 years ago, Earle H. Spaulding devised a rational approach to disinfection and sterilization of patient care items or equipment. • The nature of disinfection , instruments and items for patient care were divided into 3 categories § 2007
DISINFECTION AND STERILIZATION • Over 45 years ago, Earle H. Spaulding devised a rational approach to disinfection and sterilization of patient care items or equipment. • The nature of disinfection , instruments and items for patient care were divided into 3 categories § 2007
 2007
2007
 Disinfection and Sterilization An object will be disinfected depended on the object’s intended use. CRITICAL - objects which enter normally sterile tissue or the vascular system or through which blood flows should be sterile. SEMICRITICAL - objects that touch mucous membranes or skin that is not intact require a disinfection process (highlevel disinfection[HLD]) that kills all microorganisms but high numbers of bacterial spores. NONCRITICAL -objects that touch only intact skin require low-level disinfection. 2007
Disinfection and Sterilization An object will be disinfected depended on the object’s intended use. CRITICAL - objects which enter normally sterile tissue or the vascular system or through which blood flows should be sterile. SEMICRITICAL - objects that touch mucous membranes or skin that is not intact require a disinfection process (highlevel disinfection[HLD]) that kills all microorganisms but high numbers of bacterial spores. NONCRITICAL -objects that touch only intact skin require low-level disinfection. 2007
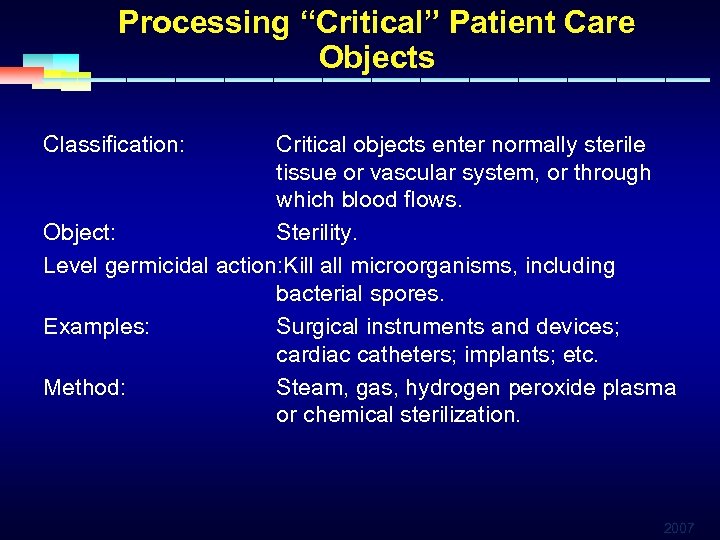 Processing “Critical” Patient Care Objects Classification: Critical objects enter normally sterile tissue or vascular system, or through which blood flows. Object: Sterility. Level germicidal action: Kill all microorganisms, including bacterial spores. Examples: Surgical instruments and devices; cardiac catheters; implants; etc. Method: Steam, gas, hydrogen peroxide plasma or chemical sterilization. 2007
Processing “Critical” Patient Care Objects Classification: Critical objects enter normally sterile tissue or vascular system, or through which blood flows. Object: Sterility. Level germicidal action: Kill all microorganisms, including bacterial spores. Examples: Surgical instruments and devices; cardiac catheters; implants; etc. Method: Steam, gas, hydrogen peroxide plasma or chemical sterilization. 2007
 Critical Objects § Surgical instruments § Cardiac catheters § Implants 2007
Critical Objects § Surgical instruments § Cardiac catheters § Implants 2007
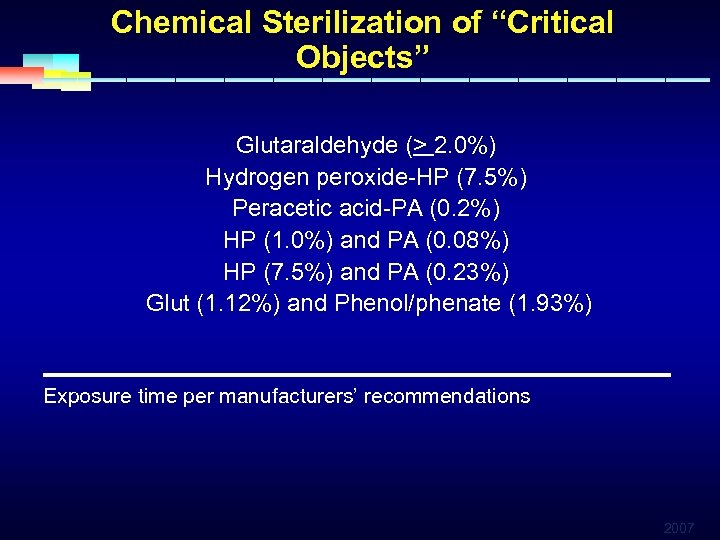 Chemical Sterilization of “Critical Objects” Glutaraldehyde (> 2. 0%) Hydrogen peroxide-HP (7. 5%) Peracetic acid-PA (0. 2%) HP (1. 0%) and PA (0. 08%) HP (7. 5%) and PA (0. 23%) Glut (1. 12%) and Phenol/phenate (1. 93%) ________________________ Exposure time per manufacturers’ recommendations 2007
Chemical Sterilization of “Critical Objects” Glutaraldehyde (> 2. 0%) Hydrogen peroxide-HP (7. 5%) Peracetic acid-PA (0. 2%) HP (1. 0%) and PA (0. 08%) HP (7. 5%) and PA (0. 23%) Glut (1. 12%) and Phenol/phenate (1. 93%) ________________________ Exposure time per manufacturers’ recommendations 2007
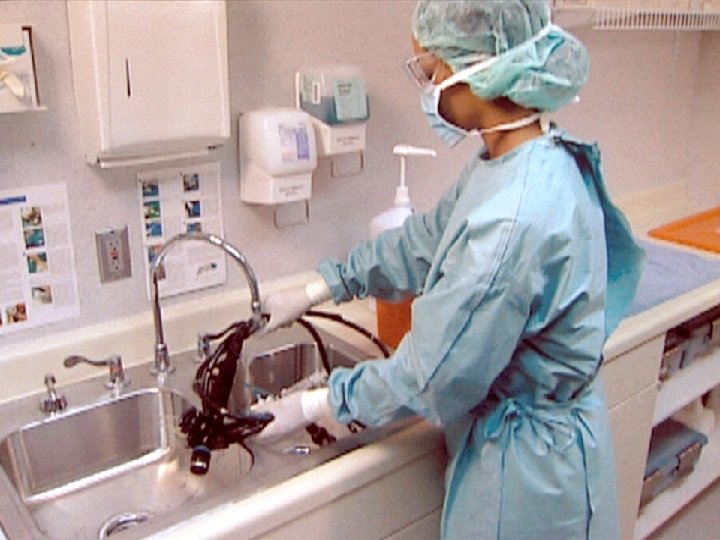 2007
2007
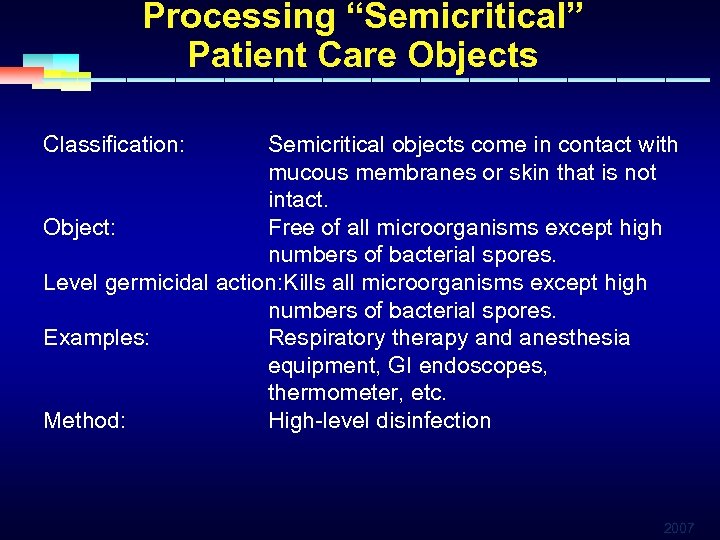 Processing “Semicritical” Patient Care Objects Classification: Semicritical objects come in contact with mucous membranes or skin that is not intact. Object: Free of all microorganisms except high numbers of bacterial spores. Level germicidal action: Kills all microorganisms except high numbers of bacterial spores. Examples: Respiratory therapy and anesthesia equipment, GI endoscopes, thermometer, etc. Method: High-level disinfection 2007
Processing “Semicritical” Patient Care Objects Classification: Semicritical objects come in contact with mucous membranes or skin that is not intact. Object: Free of all microorganisms except high numbers of bacterial spores. Level germicidal action: Kills all microorganisms except high numbers of bacterial spores. Examples: Respiratory therapy and anesthesia equipment, GI endoscopes, thermometer, etc. Method: High-level disinfection 2007
 Semicritical Items § Endoscopes § Respiratory therapy equipment § Anesthesia equipment § Endocavitary probes § Tonometers § Diaphragm fitting rings 2007
Semicritical Items § Endoscopes § Respiratory therapy equipment § Anesthesia equipment § Endocavitary probes § Tonometers § Diaphragm fitting rings 2007
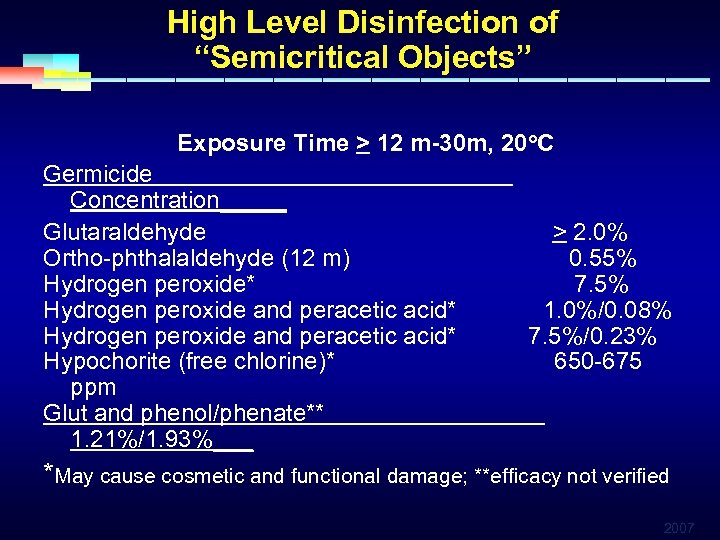 High Level Disinfection of “Semicritical Objects” Exposure Time > 12 m-30 m, 20 o. C Germicide Concentration_____ Glutaraldehyde > 2. 0% Ortho-phthalaldehyde (12 m) 0. 55% Hydrogen peroxide* 7. 5% Hydrogen peroxide and peracetic acid* 1. 0%/0. 08% Hydrogen peroxide and peracetic acid* 7. 5%/0. 23% Hypochorite (free chlorine)* 650 -675 ppm Glut and phenol/phenate** 1. 21%/1. 93%___ *May cause cosmetic and functional damage; **efficacy not verified 2007
High Level Disinfection of “Semicritical Objects” Exposure Time > 12 m-30 m, 20 o. C Germicide Concentration_____ Glutaraldehyde > 2. 0% Ortho-phthalaldehyde (12 m) 0. 55% Hydrogen peroxide* 7. 5% Hydrogen peroxide and peracetic acid* 1. 0%/0. 08% Hydrogen peroxide and peracetic acid* 7. 5%/0. 23% Hypochorite (free chlorine)* 650 -675 ppm Glut and phenol/phenate** 1. 21%/1. 93%___ *May cause cosmetic and functional damage; **efficacy not verified 2007
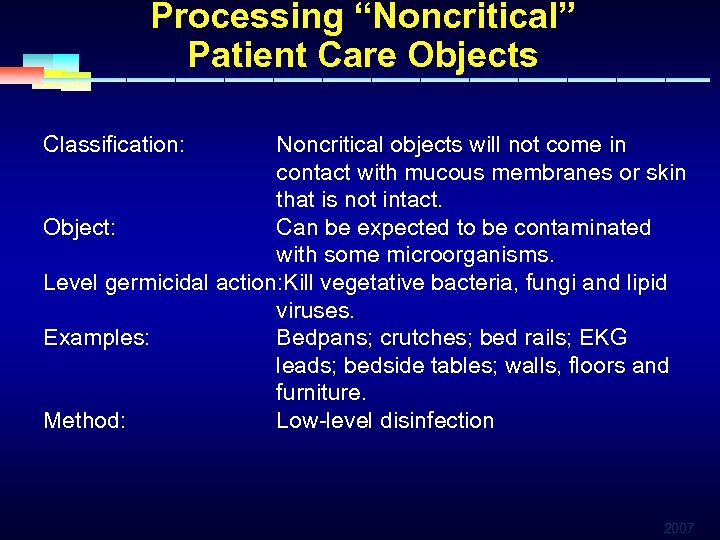 Processing “Noncritical” Patient Care Objects Classification: Noncritical objects will not come in contact with mucous membranes or skin that is not intact. Object: Can be expected to be contaminated with some microorganisms. Level germicidal action: Kill vegetative bacteria, fungi and lipid viruses. Examples: Bedpans; crutches; bed rails; EKG leads; bedside tables; walls, floors and furniture. Method: Low-level disinfection 2007
Processing “Noncritical” Patient Care Objects Classification: Noncritical objects will not come in contact with mucous membranes or skin that is not intact. Object: Can be expected to be contaminated with some microorganisms. Level germicidal action: Kill vegetative bacteria, fungi and lipid viruses. Examples: Bedpans; crutches; bed rails; EKG leads; bedside tables; walls, floors and furniture. Method: Low-level disinfection 2007
 Low-Level Disinfection for “Noncritical” Objects Exposure time > 1 min Germicide Use Concentration Ethyl or isopropyl alcohol 70 -90% Chlorine 100 ppm (1: 500 dilution) Phenolic UD Iodophor UD Quaternary ammonium UD ___________________ UD=Manufacturer’s recommended use dilution 2007
Low-Level Disinfection for “Noncritical” Objects Exposure time > 1 min Germicide Use Concentration Ethyl or isopropyl alcohol 70 -90% Chlorine 100 ppm (1: 500 dilution) Phenolic UD Iodophor UD Quaternary ammonium UD ___________________ UD=Manufacturer’s recommended use dilution 2007
 Disinfectants for Surface Disinfection § Noncritical Surfaces Ø Medical equipment surfaces (BP cuff, stethoscopes) § § § Ø May frequently become contaminated with patient material Repeatedly touched by health care personnel Disinfectant/detergent should be used Housekeeping surfaces (bed rails, bedside tables) § § May play a theoretical but less significant role in diseases transmission Disinfectants/detergents may be used (II) and detergents (non-patient care areas) 2007
Disinfectants for Surface Disinfection § Noncritical Surfaces Ø Medical equipment surfaces (BP cuff, stethoscopes) § § § Ø May frequently become contaminated with patient material Repeatedly touched by health care personnel Disinfectant/detergent should be used Housekeeping surfaces (bed rails, bedside tables) § § May play a theoretical but less significant role in diseases transmission Disinfectants/detergents may be used (II) and detergents (non-patient care areas) 2007
 Sterilization and Disinfection of Patient Care Items
Sterilization and Disinfection of Patient Care Items
 Critical Instruments § § § Penetrate mucous membranes or contact bone, the bloodstream, or other normally sterile tissues (of the mouth) Heat sterilize between uses or use sterile single-use, disposable devices Examples include surgical instruments, scalpel blades, periodontal scalers, and surgical dental burs 2007
Critical Instruments § § § Penetrate mucous membranes or contact bone, the bloodstream, or other normally sterile tissues (of the mouth) Heat sterilize between uses or use sterile single-use, disposable devices Examples include surgical instruments, scalpel blades, periodontal scalers, and surgical dental burs 2007
 Semi-critical Instruments § § § Contact mucous membranes but do not penetrate soft tissue Heat sterilize or high-level disinfect Examples: Dental mouth mirrors, amalgam condensers, and dental handpieces 2007
Semi-critical Instruments § § § Contact mucous membranes but do not penetrate soft tissue Heat sterilize or high-level disinfect Examples: Dental mouth mirrors, amalgam condensers, and dental handpieces 2007
 Noncritical Instruments and Devices § § § Contact intact skin Clean and disinfect using a low to intermediate level disinfectant Examples: X-ray heads, facebows, pulse oximeter, blood pressure cuff 2007
Noncritical Instruments and Devices § § § Contact intact skin Clean and disinfect using a low to intermediate level disinfectant Examples: X-ray heads, facebows, pulse oximeter, blood pressure cuff 2007
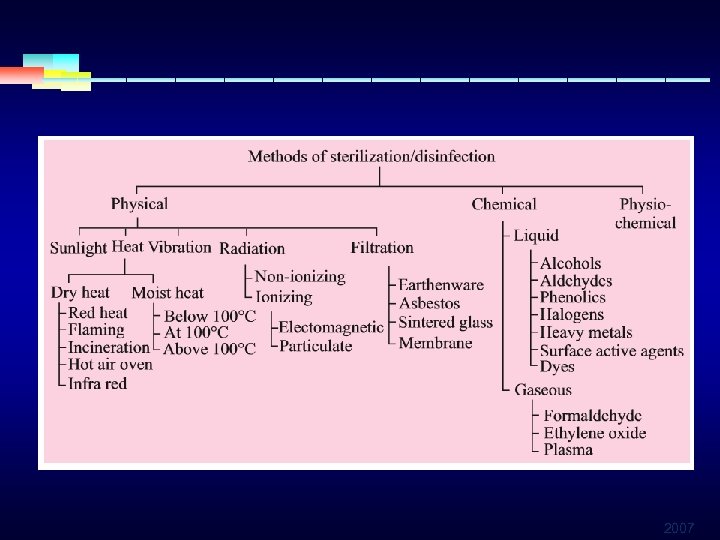 2007
2007
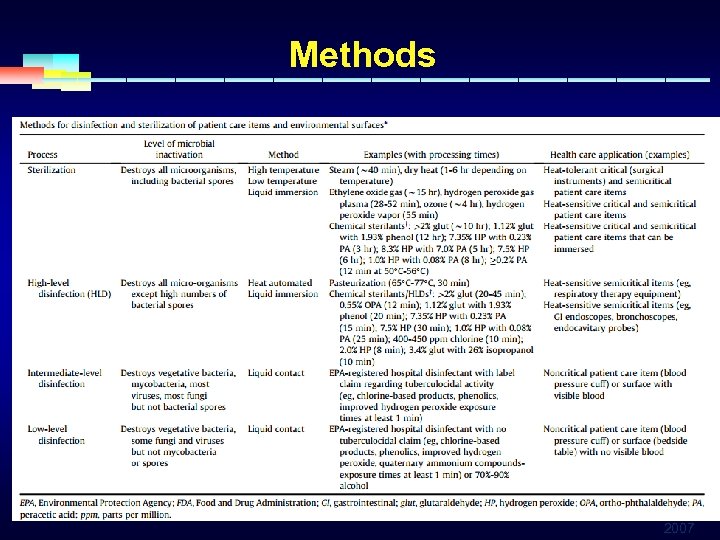 Methods 2007
Methods 2007
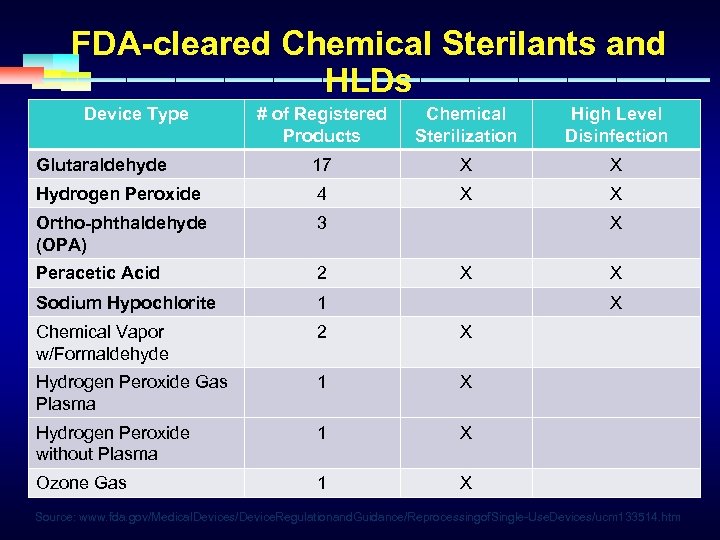 FDA-cleared Chemical Sterilants and HLDs Device Type # of Registered Products Chemical Sterilization High Level Disinfection Glutaraldehyde 17 X X Hydrogen Peroxide 4 X X Ortho-phthaldehyde (OPA) 3 Peracetic Acid 2 Sodium Hypochlorite 1 Chemical Vapor w/Formaldehyde 2 X Hydrogen Peroxide Gas Plasma 1 X Hydrogen Peroxide without Plasma 1 X Ozone Gas 1 X X X Source: www. fda. gov/Medical. Devices/Device. Regulationand. Guidance/Reprocessingof. Single-Use. Devices/ucm 133514. htm
FDA-cleared Chemical Sterilants and HLDs Device Type # of Registered Products Chemical Sterilization High Level Disinfection Glutaraldehyde 17 X X Hydrogen Peroxide 4 X X Ortho-phthaldehyde (OPA) 3 Peracetic Acid 2 Sodium Hypochlorite 1 Chemical Vapor w/Formaldehyde 2 X Hydrogen Peroxide Gas Plasma 1 X Hydrogen Peroxide without Plasma 1 X Ozone Gas 1 X X X Source: www. fda. gov/Medical. Devices/Device. Regulationand. Guidance/Reprocessingof. Single-Use. Devices/ucm 133514. htm
 Instrument Processing Area § Use a designated processing area to control quality and ensure safety § Divide processing area into work areas § Receiving, cleaning, and decontamination § Preparation and packaging § Sterilization § Storage 2007
Instrument Processing Area § Use a designated processing area to control quality and ensure safety § Divide processing area into work areas § Receiving, cleaning, and decontamination § Preparation and packaging § Sterilization § Storage 2007
 Automated Cleaning § § § Ultrasonic cleaner Instrument washer Washer-disinfector 2007
Automated Cleaning § § § Ultrasonic cleaner Instrument washer Washer-disinfector 2007
 Manual Cleaning § § Soak until ready to clean Wear heavy-duty utility gloves, mask, eyewear, and protective clothing 2007
Manual Cleaning § § Soak until ready to clean Wear heavy-duty utility gloves, mask, eyewear, and protective clothing 2007
 Preparation and Packaging § § Critical and semi-critical items that will be stored should be wrapped or placed in containers before heat sterilization Hinged instruments opened and unlocked Place a chemical indicator inside the pack Wear heavy-duty, puncture-resistant utility gloves 2007
Preparation and Packaging § § Critical and semi-critical items that will be stored should be wrapped or placed in containers before heat sterilization Hinged instruments opened and unlocked Place a chemical indicator inside the pack Wear heavy-duty, puncture-resistant utility gloves 2007
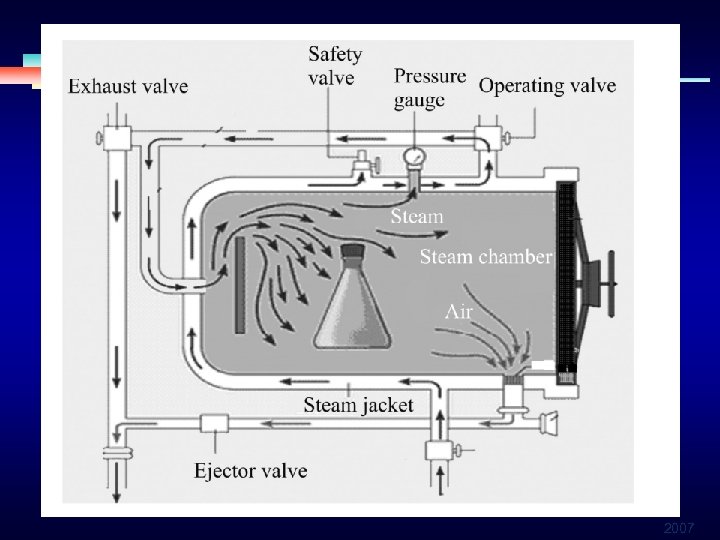
 Heat-Based Sterilization § § § Steam under pressure (autoclaving) ØGravity displacement ØPre-vacuum Dry heat Unsaturated chemical vapor 2007
Heat-Based Sterilization § § § Steam under pressure (autoclaving) ØGravity displacement ØPre-vacuum Dry heat Unsaturated chemical vapor 2007
 2007
2007
 Liquid Chemical Sterilant/Disinfectants § § § Only for heat-sensitive critical and semi-critical devices Powerful, toxic chemicals raise safety concerns Heat tolerant or disposable alternatives are available 2007
Liquid Chemical Sterilant/Disinfectants § § § Only for heat-sensitive critical and semi-critical devices Powerful, toxic chemicals raise safety concerns Heat tolerant or disposable alternatives are available 2007
 Sterilization Monitoring Types of Indicators § Mechanical § Measure time, temperature, pressure § Chemical § Change in color when physical parameter is reached § Biological (spore tests) § Use biological spores to assess the sterilization process directly 2007
Sterilization Monitoring Types of Indicators § Mechanical § Measure time, temperature, pressure § Chemical § Change in color when physical parameter is reached § Biological (spore tests) § Use biological spores to assess the sterilization process directly 2007
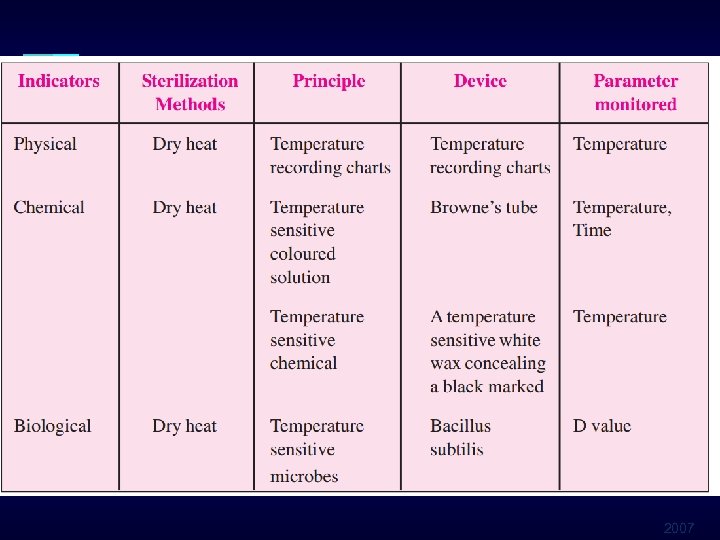 2007
2007
 Storage of Sterile and Clean Items and Supplies § § Use date- or event-related shelf-life practices Examine wrapped items carefully prior to use When packaging of sterile items is damaged, re-clean, re-wrap, and re-sterilize Store clean items in dry, closed, or covered containment 2007
Storage of Sterile and Clean Items and Supplies § § Use date- or event-related shelf-life practices Examine wrapped items carefully prior to use When packaging of sterile items is damaged, re-clean, re-wrap, and re-sterilize Store clean items in dry, closed, or covered containment 2007
 Environmental Infection Control
Environmental Infection Control
 Environmental Surfaces § § § May become contaminated Not directly involved in infectious disease transmission Do not require as stringent decontamination procedures 2007
Environmental Surfaces § § § May become contaminated Not directly involved in infectious disease transmission Do not require as stringent decontamination procedures 2007
 Categories of Environmental Surfaces § Clinical contact surfaces ØHigh potential for direct contamination from spray or spatter or by contact with DHCP’s gloved hand § Housekeeping surfaces ØDo not come into contact with patients or devices ØLimited risk of disease transmission 2007
Categories of Environmental Surfaces § Clinical contact surfaces ØHigh potential for direct contamination from spray or spatter or by contact with DHCP’s gloved hand § Housekeeping surfaces ØDo not come into contact with patients or devices ØLimited risk of disease transmission 2007
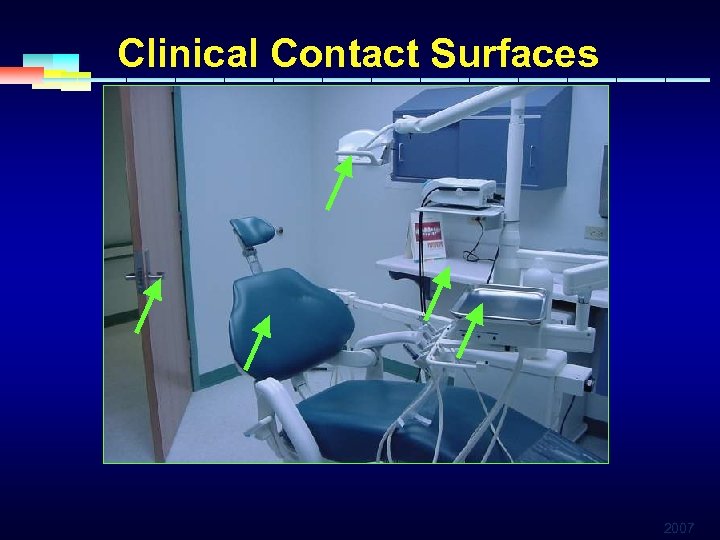 Clinical Contact Surfaces 2007
Clinical Contact Surfaces 2007
 Housekeeping Surfaces 2007
Housekeeping Surfaces 2007
 General Cleaning Recommendations § Use barrier precautions (e. g. , heavy-duty utility gloves, masks, protective eyewear) when cleaning and disinfecting environmental surfaces § Physical removal of microorganisms by cleaning is as important as the disinfection process § Follow manufacturer’s instructions for proper use of EPA-registered hospital disinfectants § Do not use sterilant/high-level disinfectants on environmental surfaces 2007
General Cleaning Recommendations § Use barrier precautions (e. g. , heavy-duty utility gloves, masks, protective eyewear) when cleaning and disinfecting environmental surfaces § Physical removal of microorganisms by cleaning is as important as the disinfection process § Follow manufacturer’s instructions for proper use of EPA-registered hospital disinfectants § Do not use sterilant/high-level disinfectants on environmental surfaces 2007
 Cleaning Clinical Contact Surfaces § Risk of transmitting infections greater than for housekeeping surfaces § Surface barriers can be used and changed between patients OR § Clean then disinfect using an EPA-registered low- (HIV/HBV claim) to intermediate-level (tuberculocidal claim) hospital disinfectant 2007
Cleaning Clinical Contact Surfaces § Risk of transmitting infections greater than for housekeeping surfaces § Surface barriers can be used and changed between patients OR § Clean then disinfect using an EPA-registered low- (HIV/HBV claim) to intermediate-level (tuberculocidal claim) hospital disinfectant 2007
 Cleaning Housekeeping Surfaces § § § Routinely clean with soap and water or an EPAregistered detergent/hospital disinfectant routinely Clean mops and cloths and allow to dry thoroughly before re-using Prepare fresh cleaning and disinfecting solutions daily and per manufacturer recommendations 2007
Cleaning Housekeeping Surfaces § § § Routinely clean with soap and water or an EPAregistered detergent/hospital disinfectant routinely Clean mops and cloths and allow to dry thoroughly before re-using Prepare fresh cleaning and disinfecting solutions daily and per manufacturer recommendations 2007
 Medical Waste § Medical Waste: Not considered infectious, thus can be discarded in regular trash § Regulated Medical Waste: Poses a potential risk of infection during handling and disposal 2007
Medical Waste § Medical Waste: Not considered infectious, thus can be discarded in regular trash § Regulated Medical Waste: Poses a potential risk of infection during handling and disposal 2007
 Regulated Medical Waste Management § § § Properly labeled containment to prevent injuries and leakage Medical wastes are “treated” in accordance with state and local EPA regulations Processes for regulated waste include autoclaving and incineration 2007
Regulated Medical Waste Management § § § Properly labeled containment to prevent injuries and leakage Medical wastes are “treated” in accordance with state and local EPA regulations Processes for regulated waste include autoclaving and incineration 2007
 8 -TIBBİ ATIK YÖNETİMİ PROSEDÜRÜ § 1. AMAÇ: Atıkların; Tıbbi Atıkların Kontrolü Yönetmeliğine uygun olarak toplanması, taşınması, geçici depolanması ve ilgili birimlere tesliminin sağlanmasıdır. Tıbbi atık yönetiminin uygulama amacı; tıbbi atıkların hastanemiz sağlık personeline ve çevreye zarar vermeden bertaraf edilmelerinin sağlanmasıdır. § § § 2. KAPSAM: Hastanenin tüm birimlerini kapsar. 3. SORUMLULAR: Başhemşire, başhemşire yardımcıları, birim sorumluları, sorumlu hemşireler, temizlik şirketi sorumlusu, temizlik personeli. § Tıbbi atıklar: Kırmızı § Evsel nitelikteki atıklar: Siyah § Geri kazanılabilen atıklar: Mavi
8 -TIBBİ ATIK YÖNETİMİ PROSEDÜRÜ § 1. AMAÇ: Atıkların; Tıbbi Atıkların Kontrolü Yönetmeliğine uygun olarak toplanması, taşınması, geçici depolanması ve ilgili birimlere tesliminin sağlanmasıdır. Tıbbi atık yönetiminin uygulama amacı; tıbbi atıkların hastanemiz sağlık personeline ve çevreye zarar vermeden bertaraf edilmelerinin sağlanmasıdır. § § § 2. KAPSAM: Hastanenin tüm birimlerini kapsar. 3. SORUMLULAR: Başhemşire, başhemşire yardımcıları, birim sorumluları, sorumlu hemşireler, temizlik şirketi sorumlusu, temizlik personeli. § Tıbbi atıklar: Kırmızı § Evsel nitelikteki atıklar: Siyah § Geri kazanılabilen atıklar: Mavi
 Monitoring Options § § § Water testing laboratory In-office testing with self-contained kits Follow recommendations provided by the manufacturer of the dental unit or waterline treatment product for monitoring water quality 2007
Monitoring Options § § § Water testing laboratory In-office testing with self-contained kits Follow recommendations provided by the manufacturer of the dental unit or waterline treatment product for monitoring water quality 2007
 Parenteral Medications § Definition: Medications that are injected into the body § Cases of disease transmission have been reported § Handle safely to prevent transmission of infections 2007
Parenteral Medications § Definition: Medications that are injected into the body § Cases of disease transmission have been reported § Handle safely to prevent transmission of infections 2007
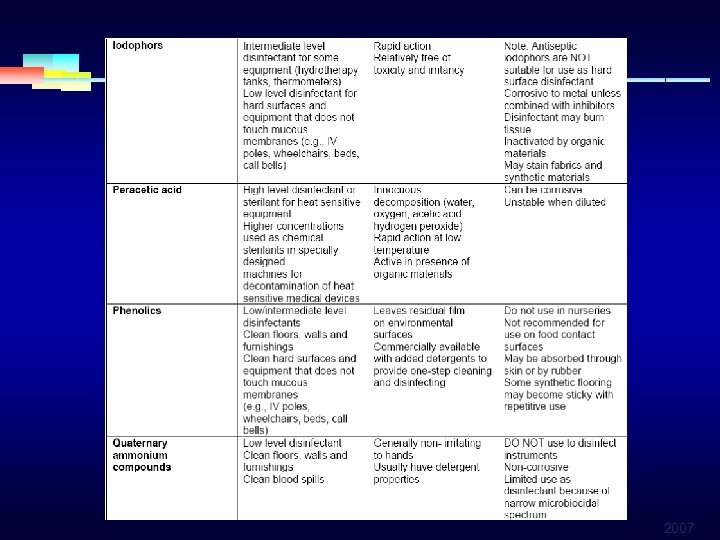
 LOW LEVEL DISINFECTANTS § PHENOLIC DISINFECTANTS: § Effective against bacteria (gram+) and enveloped § § viruses Not effective against nonenveloped viruses and spores Active in the presence of organic material Used for decontamination of hospital environment Not recommended for semicritical items 2007
LOW LEVEL DISINFECTANTS § PHENOLIC DISINFECTANTS: § Effective against bacteria (gram+) and enveloped § § viruses Not effective against nonenveloped viruses and spores Active in the presence of organic material Used for decontamination of hospital environment Not recommended for semicritical items 2007
 LOW LEVEL DISINFECTANTS § QUARTERNARY AMMONIUM COMPOUNDS: § Widely used as disinfectants § Contraindicated as antiseptics § Not effective against nonenveloped viruses, fungi and bacterial spores § Commonly used in ordinary environment sanitation of noncritical surfaces 2007
LOW LEVEL DISINFECTANTS § QUARTERNARY AMMONIUM COMPOUNDS: § Widely used as disinfectants § Contraindicated as antiseptics § Not effective against nonenveloped viruses, fungi and bacterial spores § Commonly used in ordinary environment sanitation of noncritical surfaces 2007
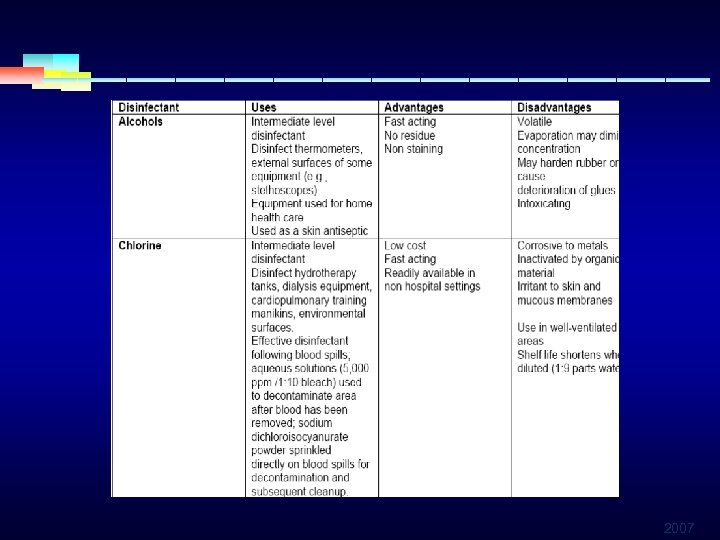
 INTERMEDIATE LEVEL DISINFECTANTS § ALCOHOLS § Ethyl alcohol § Isopropyl alcohol § Rapidly bactericidal against vegetative forms of bacteria § Effective against M. tuberculosis, fungi, enveloped viruses 2007
INTERMEDIATE LEVEL DISINFECTANTS § ALCOHOLS § Ethyl alcohol § Isopropyl alcohol § Rapidly bactericidal against vegetative forms of bacteria § Effective against M. tuberculosis, fungi, enveloped viruses 2007
 INTERMEDIATE LEVEL DISINFECTANTS § ALCOHOLS § Optimum bactericidal concentration is 60 -90 % in § § water Commonly used topical antiseptics (hand) Also used to disinfect the surface of medical equipment May not penetrate organic material Flammable, irriates tissue, expensive for general use 2007
INTERMEDIATE LEVEL DISINFECTANTS § ALCOHOLS § Optimum bactericidal concentration is 60 -90 % in § § water Commonly used topical antiseptics (hand) Also used to disinfect the surface of medical equipment May not penetrate organic material Flammable, irriates tissue, expensive for general use 2007
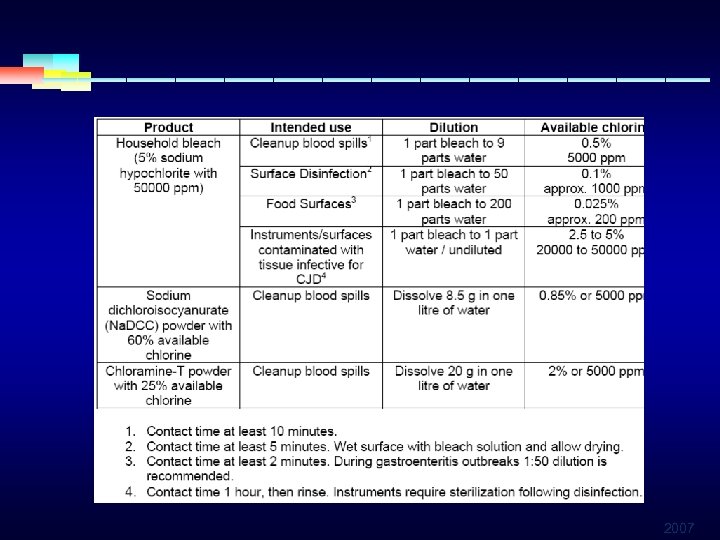
 INTERMEDIATE LEVEL DISINFECTANTS § HYPOCHLORITES § Most widely used of the chlorine disinfectants § Most common product is 4 -6 % sodium hypochlorite § Inexpensive and fast acting § May produce skin and ocular irritation or gis burns § Corrosive to metals in high concent, inactivated by organic material § Release toxic chlorine gas when mixed with ammonia or acid 2007
INTERMEDIATE LEVEL DISINFECTANTS § HYPOCHLORITES § Most widely used of the chlorine disinfectants § Most common product is 4 -6 % sodium hypochlorite § Inexpensive and fast acting § May produce skin and ocular irritation or gis burns § Corrosive to metals in high concent, inactivated by organic material § Release toxic chlorine gas when mixed with ammonia or acid 2007
 INTERMEDIATE LEVEL DISINFECTANTS § HYPOCHLORITES § Eliminate enveloped and non envelopedviruses § Effective against fungi, bacteria, algae, but not spores § Broad spectrum of antimicrobial activity § Most recommended for decontamination of hepatitis and HIV viruses § Used as solution in water (20. 000 ppm) 2007
INTERMEDIATE LEVEL DISINFECTANTS § HYPOCHLORITES § Eliminate enveloped and non envelopedviruses § Effective against fungi, bacteria, algae, but not spores § Broad spectrum of antimicrobial activity § Most recommended for decontamination of hepatitis and HIV viruses § Used as solution in water (20. 000 ppm) 2007
 INTERMEDIATE LEVEL DISINFECTANTS § IODINE AND IODOPHOR § Formulations in soaps (surgical scrubs) § Bactericidal, sporicidal, virucidal, fungicidal § Require a prolonged contact time § Is neutralized in the presence of organic material § Frequent application needed § Irrigate tissues, corrosive § Used for disinfection of some medical equipment (not silicone catheters) 2007
INTERMEDIATE LEVEL DISINFECTANTS § IODINE AND IODOPHOR § Formulations in soaps (surgical scrubs) § Bactericidal, sporicidal, virucidal, fungicidal § Require a prolonged contact time § Is neutralized in the presence of organic material § Frequent application needed § Irrigate tissues, corrosive § Used for disinfection of some medical equipment (not silicone catheters) 2007
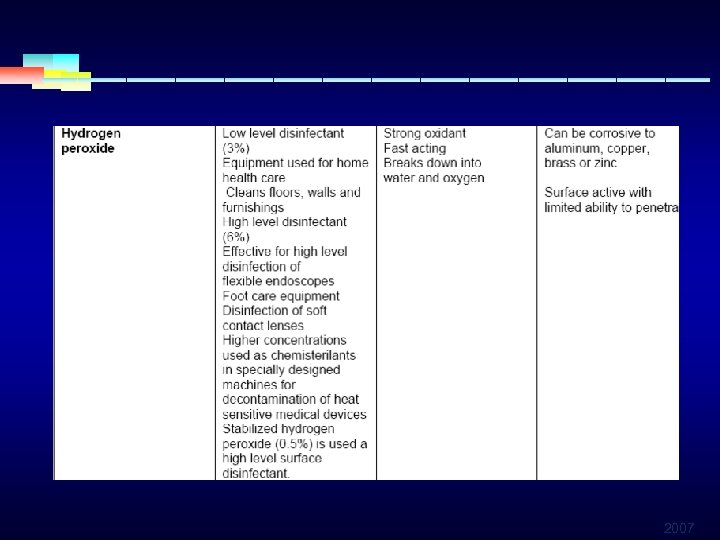
 HIGH LEVEL DISINFECTANTS § HYDROGEN PEROXIDE § § § Often used as antiseptic to clean wounds Greatest effect against anaerobic bacteria Effective against a broad range of pathogens Damage tissues in high concentrations Provides high levelof disinfection in 5 min May be blended with iodophors, quarterner ammonia, paracetic acid 2007
HIGH LEVEL DISINFECTANTS § HYDROGEN PEROXIDE § § § Often used as antiseptic to clean wounds Greatest effect against anaerobic bacteria Effective against a broad range of pathogens Damage tissues in high concentrations Provides high levelof disinfection in 5 min May be blended with iodophors, quarterner ammonia, paracetic acid 2007
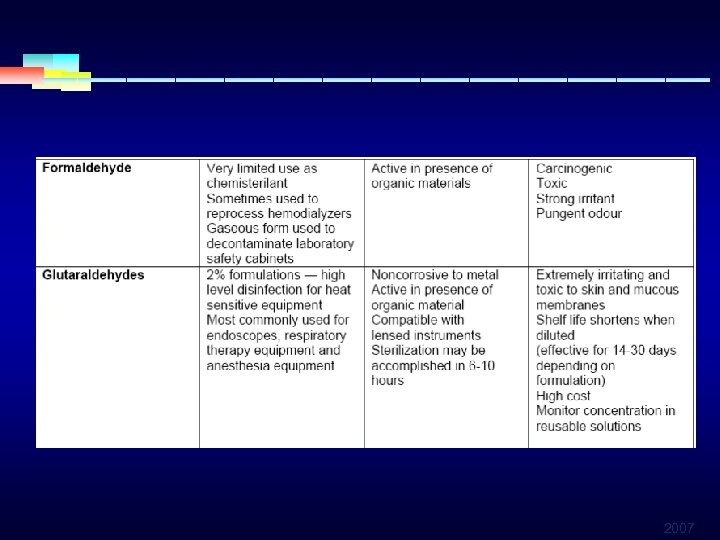
 HIGH LEVEL DISINFECTANTS § GLUTERALDEHYDE § Has a wide germicidal spectrum § Used as disinfectant or sterilant § Highly toxic § Only used in a well ventilated setting 2007
HIGH LEVEL DISINFECTANTS § GLUTERALDEHYDE § Has a wide germicidal spectrum § Used as disinfectant or sterilant § Highly toxic § Only used in a well ventilated setting 2007
 HIGH LEVEL DISINFECTANTS § FORMALDEHYDE § Used as disinfectant or sterilant § Sold and used as a water-based solution called formalin § Has a wide range spectrum of effect § Potential carcinogen, require limited direct contact § Limited use for irritating fumes and the pungent odor 2007
HIGH LEVEL DISINFECTANTS § FORMALDEHYDE § Used as disinfectant or sterilant § Sold and used as a water-based solution called formalin § Has a wide range spectrum of effect § Potential carcinogen, require limited direct contact § Limited use for irritating fumes and the pungent odor 2007
 HIGH LEVEL DISINFECTANTS § FORMALDEHYDE § Used as disinfectant or sterilant § Sold and used as a water-based solution called formalin § Has a wide range spectrum of effect § Potential carcinogen, require limited direct contact § Limited use for irritating fumes and the pungent odor 2007
HIGH LEVEL DISINFECTANTS § FORMALDEHYDE § Used as disinfectant or sterilant § Sold and used as a water-based solution called formalin § Has a wide range spectrum of effect § Potential carcinogen, require limited direct contact § Limited use for irritating fumes and the pungent odor 2007
 HIGH LEVEL DISINFECTANTS § ORTHO-PHYTHALDEHYDE (OPA) § § § § Similar to gluteraldehyde Has potential advantage compare to glu Has excellent stability Not irritant to eyes, not smell Excellent material compatibility Stains proteins gray (skin) Must be handled with caution 2007
HIGH LEVEL DISINFECTANTS § ORTHO-PHYTHALDEHYDE (OPA) § § § § Similar to gluteraldehyde Has potential advantage compare to glu Has excellent stability Not irritant to eyes, not smell Excellent material compatibility Stains proteins gray (skin) Must be handled with caution 2007
 HIGH LEVEL DISINFECTANTS § PERACETIC ACID § Rapid acts against all organisms § No harmful, leaves no residue § Remains effective in the presence of organic matter § Can corrode some metals § Used in automated machines 2007
HIGH LEVEL DISINFECTANTS § PERACETIC ACID § Rapid acts against all organisms § No harmful, leaves no residue § Remains effective in the presence of organic matter § Can corrode some metals § Used in automated machines 2007
 2007
2007
 2007
2007
 2007
2007
 2007
2007
 2007
2007
 2007
2007
 TUS 2013 § Aşağıdaki kimyasal maddelerden hangisi, sterilizasyon amacıyla kullanılmaz? § A) Glutaraldehit B) Benzalkonyum klorid C) Etilen oksit D) Formaldehit E) Hidrojen peroksit 2007
TUS 2013 § Aşağıdaki kimyasal maddelerden hangisi, sterilizasyon amacıyla kullanılmaz? § A) Glutaraldehit B) Benzalkonyum klorid C) Etilen oksit D) Formaldehit E) Hidrojen peroksit 2007
 TUS 2013 § Aşağıdaki kimyasal maddelerden hangisi, sterilizasyon amacıyla kullanılmaz? § A) Glutaraldehit B) Benzalkonyum klorid C) Etilen oksit D) Formaldehit E) Hidrojen peroksit 2007
TUS 2013 § Aşağıdaki kimyasal maddelerden hangisi, sterilizasyon amacıyla kullanılmaz? § A) Glutaraldehit B) Benzalkonyum klorid C) Etilen oksit D) Formaldehit E) Hidrojen peroksit 2007


