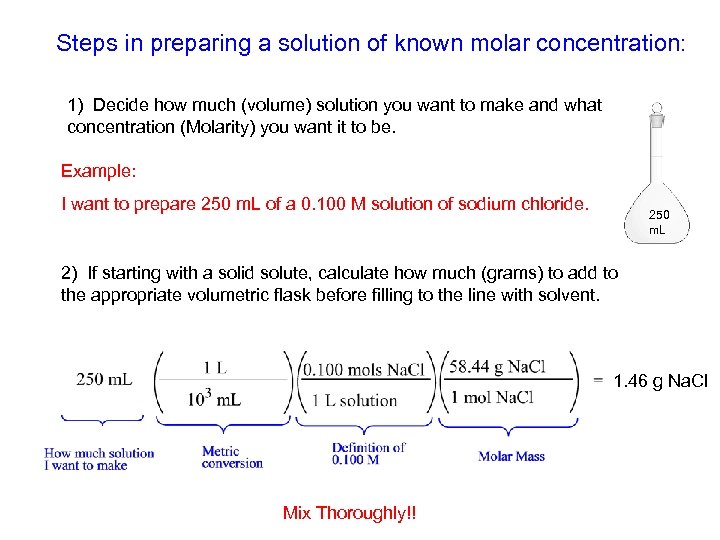
 Steps in preparing a solution of known molar concentration: 1) Decide how much (volume) solution you want to make and what concentration (Molarity) you want it to be. Example: I want to prepare 250 m. L of a 0. 100 M solution of sodium chloride. 250 m. L 2) If starting with a solid solute, calculate how much (grams) to add to the appropriate volumetric flask before filling to the line with solvent. 1. 46 g Na. Cl Mix Thoroughly!!
Steps in preparing a solution of known molar concentration: 1) Decide how much (volume) solution you want to make and what concentration (Molarity) you want it to be. Example: I want to prepare 250 m. L of a 0. 100 M solution of sodium chloride. 250 m. L 2) If starting with a solid solute, calculate how much (grams) to add to the appropriate volumetric flask before filling to the line with solvent. 1. 46 g Na. Cl Mix Thoroughly!!
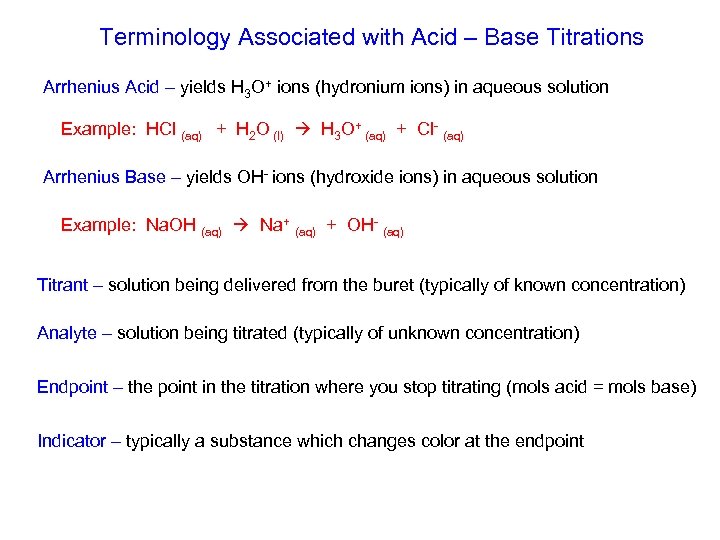 Terminology Associated with Acid – Base Titrations Arrhenius Acid – yields H 3 O+ ions (hydronium ions) in aqueous solution Example: HCl (aq) + H 2 O (l) H 3 O+ (aq) + Cl- (aq) Arrhenius Base – yields OH- ions (hydroxide ions) in aqueous solution Example: Na. OH (aq) Na+ (aq) + OH- (aq) Titrant – solution being delivered from the buret (typically of known concentration) Analyte – solution being titrated (typically of unknown concentration) Endpoint – the point in the titration where you stop titrating (mols acid = mols base) Indicator – typically a substance which changes color at the endpoint
Terminology Associated with Acid – Base Titrations Arrhenius Acid – yields H 3 O+ ions (hydronium ions) in aqueous solution Example: HCl (aq) + H 2 O (l) H 3 O+ (aq) + Cl- (aq) Arrhenius Base – yields OH- ions (hydroxide ions) in aqueous solution Example: Na. OH (aq) Na+ (aq) + OH- (aq) Titrant – solution being delivered from the buret (typically of known concentration) Analyte – solution being titrated (typically of unknown concentration) Endpoint – the point in the titration where you stop titrating (mols acid = mols base) Indicator – typically a substance which changes color at the endpoint
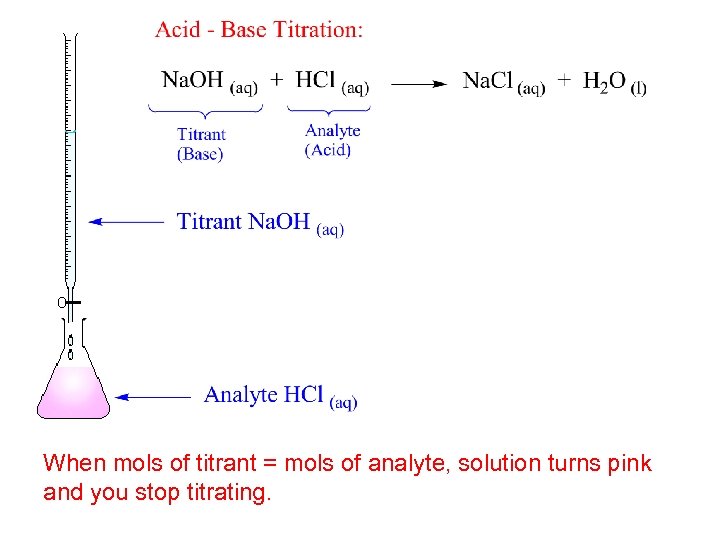 When mols of titrant = mols of analyte, solution turns pink and you stop titrating.
When mols of titrant = mols of analyte, solution turns pink and you stop titrating.
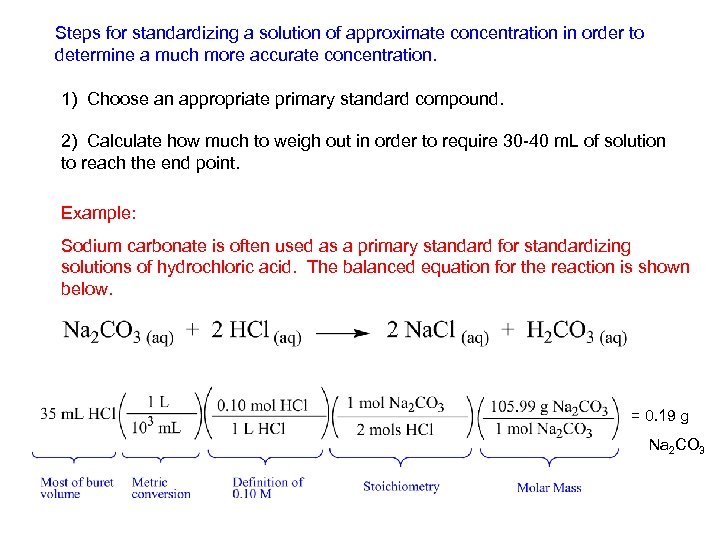 Steps for standardizing a solution of approximate concentration in order to determine a much more accurate concentration. 1) Choose an appropriate primary standard compound. 2) Calculate how much to weigh out in order to require 30 -40 m. L of solution to reach the end point. Example: Sodium carbonate is often used as a primary standard for standardizing solutions of hydrochloric acid. The balanced equation for the reaction is shown below. = 0. 19 g Na 2 CO 3
Steps for standardizing a solution of approximate concentration in order to determine a much more accurate concentration. 1) Choose an appropriate primary standard compound. 2) Calculate how much to weigh out in order to require 30 -40 m. L of solution to reach the end point. Example: Sodium carbonate is often used as a primary standard for standardizing solutions of hydrochloric acid. The balanced equation for the reaction is shown below. = 0. 19 g Na 2 CO 3
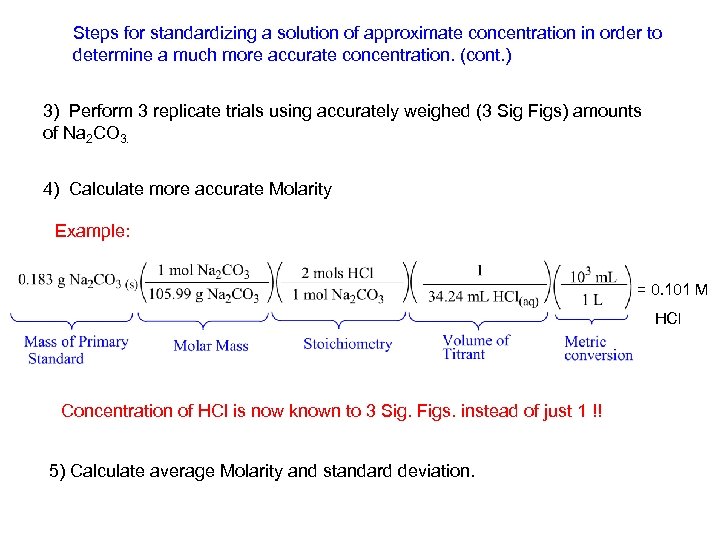 Steps for standardizing a solution of approximate concentration in order to determine a much more accurate concentration. (cont. ) 3) Perform 3 replicate trials using accurately weighed (3 Sig Figs) amounts of Na 2 CO 3. 4) Calculate more accurate Molarity Example: = 0. 101 M HCl Concentration of HCl is now known to 3 Sig. Figs. instead of just 1 !! 5) Calculate average Molarity and standard deviation.
Steps for standardizing a solution of approximate concentration in order to determine a much more accurate concentration. (cont. ) 3) Perform 3 replicate trials using accurately weighed (3 Sig Figs) amounts of Na 2 CO 3. 4) Calculate more accurate Molarity Example: = 0. 101 M HCl Concentration of HCl is now known to 3 Sig. Figs. instead of just 1 !! 5) Calculate average Molarity and standard deviation.