MSCs_for_stroke_treatment.pptx
- Количество слайдов: 15

Stem Cells and Tissue Repair Presentation by Anastassia Kostenko
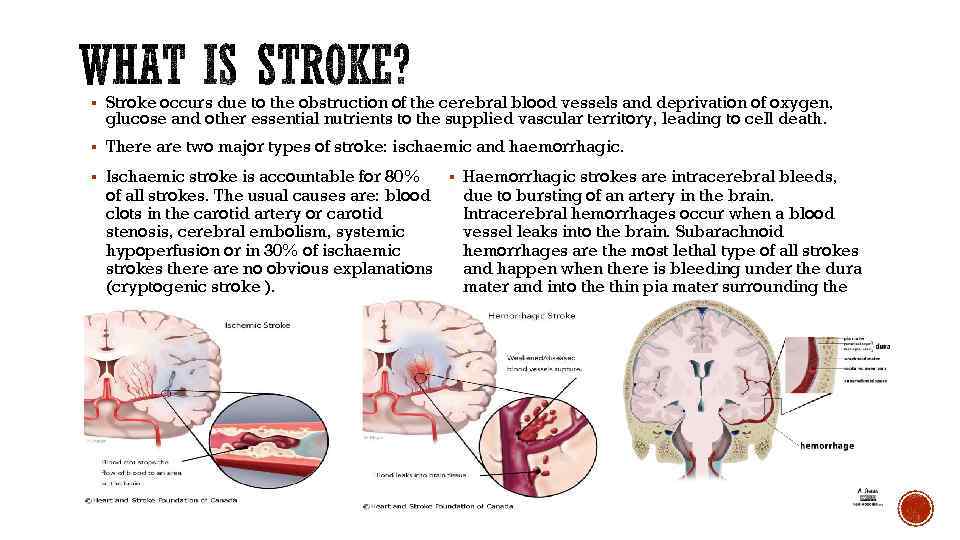
§ Stroke occurs due to the obstruction of the cerebral blood vessels and deprivation of oxygen, glucose and other essential nutrients to the supplied vascular territory, leading to cell death. § There are two major types of stroke: ischaemic and haemorrhagic. § Ischaemic stroke is accountable for 80% of all strokes. The usual causes are: blood clots in the carotid artery or carotid stenosis, cerebral embolism, systemic hypoperfusion or in 30% of ischaemic strokes there are no obvious explanations (cryptogenic stroke ). § Haemorrhagic strokes are intracerebral bleeds, due to bursting of an artery in the brain. Intracerebral hemorrhages occur when a blood vessel leaks into the brain. Subarachnoid hemorrhages are the most lethal type of all strokes and happen when there is bleeding under the dura mater and into the thin pia mater surrounding the brain.
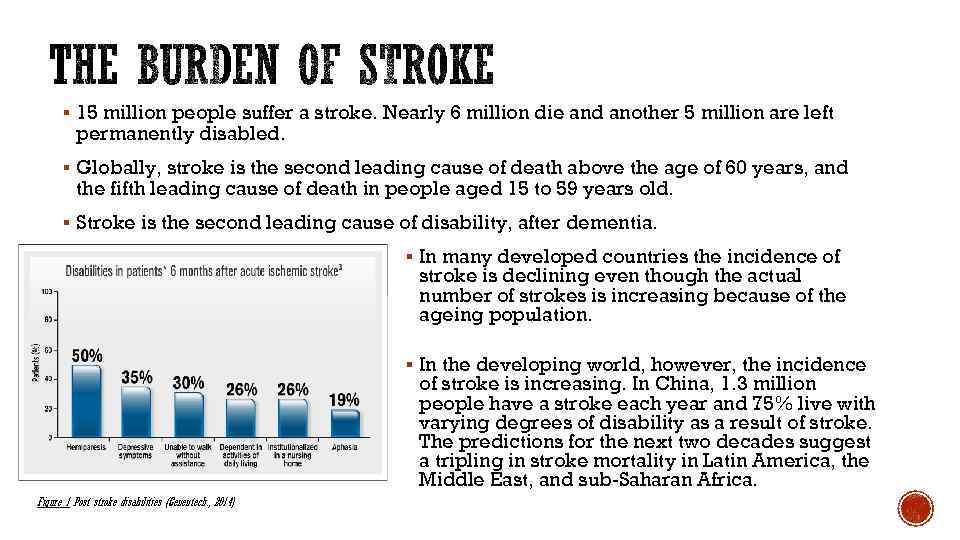
§ 15 million people suffer a stroke. Nearly 6 million die and another 5 million are left permanently disabled. § Globally, stroke is the second leading cause of death above the age of 60 years, and the fifth leading cause of death in people aged 15 to 59 years old. § Stroke is the second leading cause of disability, after dementia. § In many developed countries the incidence of stroke is declining even though the actual number of strokes is increasing because of the ageing population. § In the developing world, however, the incidence of stroke is increasing. In China, 1. 3 million people have a stroke each year and 75% live with varying degrees of disability as a result of stroke. The predictions for the next two decades suggest a tripling in stroke mortality in Latin America, the Middle East, and sub-Saharan Africa. Figure 1 Post stroke disabilities (Genentech. , 2014)
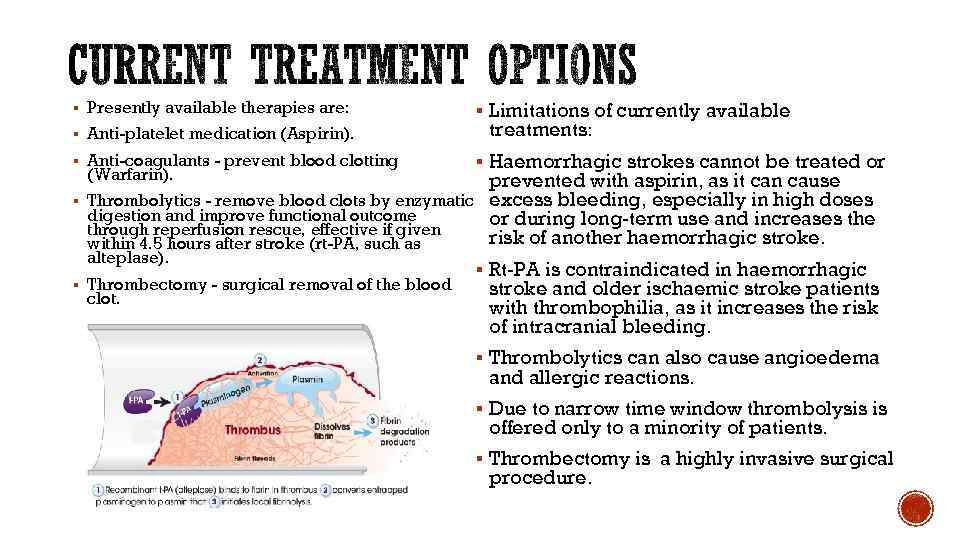
§ Presently available therapies are: § Anti-platelet medication (Aspirin). § Anti-coagulants - prevent blood clotting (Warfarin). § § § Limitations of currently available treatments: § Haemorrhagic strokes cannot be treated or prevented with aspirin, as it can cause Thrombolytics - remove blood clots by enzymatic excess bleeding, especially in high doses digestion and improve functional outcome or during long-term use and increases the through reperfusion rescue, effective if given risk of another haemorrhagic stroke. within 4. 5 hours after stroke (rt-PA, such as alteplase). § Rt-PA is contraindicated in haemorrhagic Thrombectomy - surgical removal of the blood stroke and older ischaemic stroke patients clot. with thrombophilia, as it increases the risk of intracranial bleeding. § Thrombolytics can also cause angioedema and allergic reactions. § Due to narrow time window thrombolysis is offered only to a minority of patients. § Thrombectomy is a highly invasive surgical procedure.
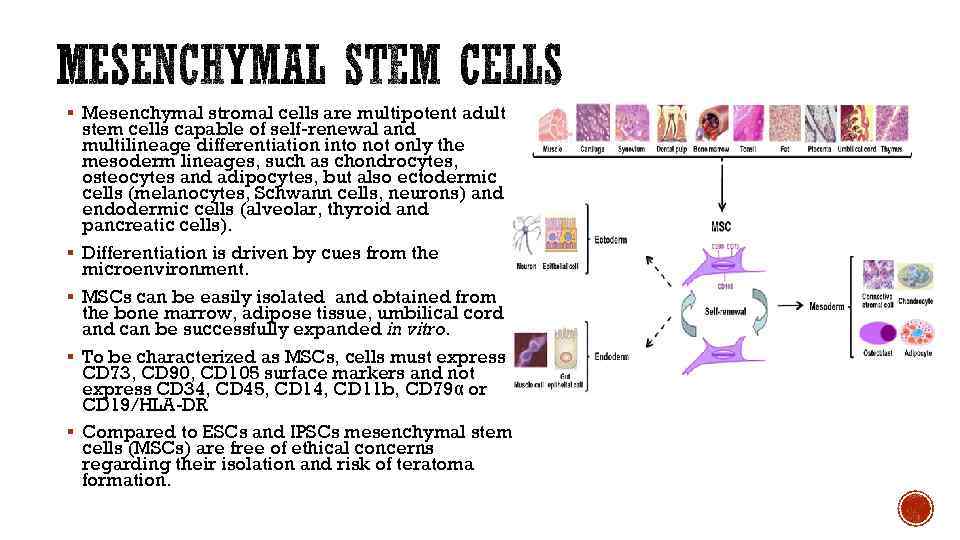
§ Mesenchymal stromal cells are multipotent adult § § stem cells capable of self-renewal and multilineage differentiation into not only the mesoderm lineages, such as chondrocytes, osteocytes and adipocytes, but also ectodermic cells (melanocytes, Schwann cells, neurons) and endodermic cells (alveolar, thyroid and pancreatic cells). Differentiation is driven by cues from the microenvironment. MSCs can be easily isolated and obtained from the bone marrow, adipose tissue, umbilical cord and can be successfully expanded in vitro. To be characterized as MSCs, cells must express CD 73, CD 90, CD 105 surface markers and not express CD 34, CD 45, CD 14, CD 11 b, CD 79α or CD 19/HLA-DR Compared to ESCs and IPSCs mesenchymal stem cells (MSCs) are free of ethical concerns regarding their isolation and risk of teratoma formation.

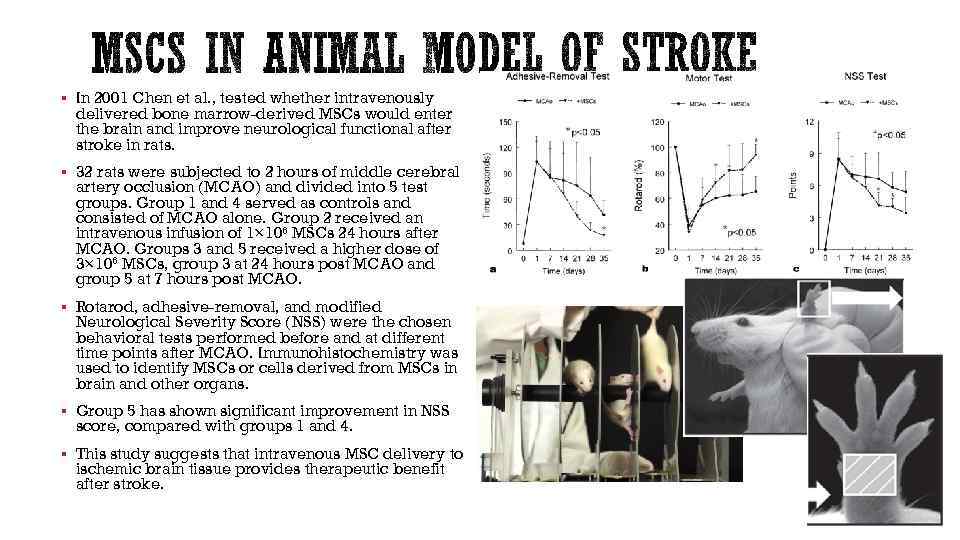
§ In 2001 Chen et al. , tested whether intravenously delivered bone marrow-derived MSCs would enter the brain and improve neurological functional after stroke in rats. § 32 rats were subjected to 2 hours of middle cerebral artery occlusion (MCAO) and divided into 5 test groups. Group 1 and 4 served as controls and consisted of MCAO alone. Group 2 received an intravenous infusion of 1× 106 MSCs 24 hours after MCAO. Groups 3 and 5 received a higher dose of 3× 106 MSCs, group 3 at 24 hours post MCAO and group 5 at 7 hours post MCAO. § Rotarod, adhesive-removal, and modified Neurological Severity Score (NSS) were the chosen behavioral tests performed before and at different time points after MCAO. Immunohistochemistry was used to identify MSCs or cells derived from MSCs in brain and other organs. § Group 5 has shown significant improvement in NSS score, compared with groups 1 and 4. § This study suggests that intravenous MSC delivery to ischemic brain tissue provides therapeutic benefit after stroke.

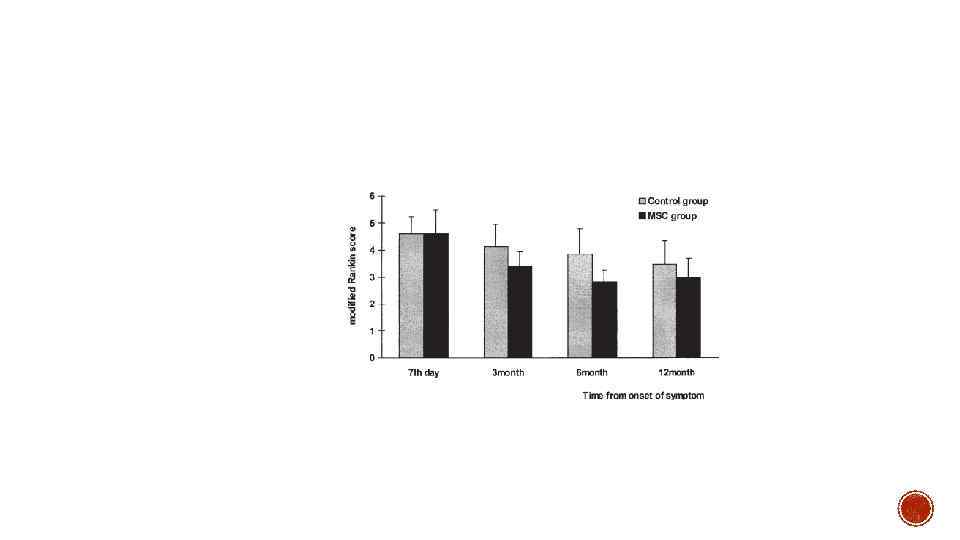
§ Bang et al. , (2005) have conducted one of the very first randomized controlled phase I/II clinical trials in order to assess the safety, feasibility and efficacy of intravenous delivery of culture-expanded MSCs in post-stroke patients.

§ MSC therapies are delivered via intravenous, inta-arterial, or intracerebral injections. Intra-arterial infusion is very effective as it attains maximum cell concentration in the damaged area supplied by that artery. However, at least minor surgery is needed for access, which may cause blockage of local capillaries with transplanted cells (Borlongan et al. , 2011). Intracerebral injections are invasive and require anaesthesia. MSCs are surgically transplanted directly into the ischaemically damaged brain area, which can interrupt the blood brain barrier (BBB). For clinical use, it is best that an open BBB and access to penubra are available; therefore controlling the timing of therapy is necessary. Despite the limitations, intracerebral MSC delivery showed effective engraftment, especially in inflammatory conditions (Borlongan et al. , 2011). Intra-venous stem cell administration is considered safer, than site directed transplantation. Prolonged injection at certain intervals of even high doses of stem cells is possible, with no contraindications. Nevertheless, only a small number of IV injected cells transmigrate and reach the site of injury (Chen et al. , 2006
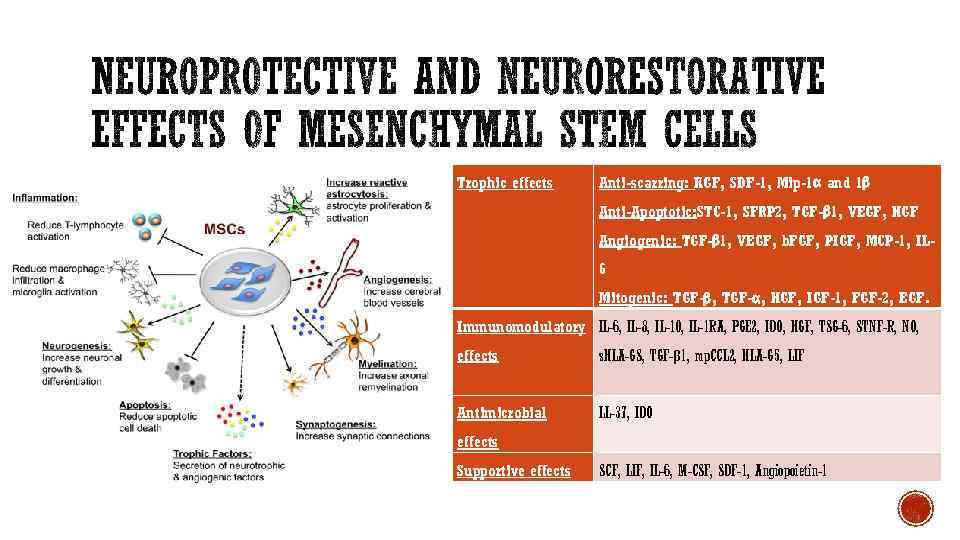
Trophic effects Anti-scarring: KGF, SDF-1, Mip-1α and 1β Anti-Apoptotic: STC-1, SFRP 2, TGF-β 1, VEGF, HGF Angiogenic: TGF-β 1, VEGF, b. FGF, PIGF, MCP-1, IL 6 Mitogenic: TGF-β, TGF-α, HGF, IGF-1, FGF-2, EGF. Immunomodulatory IL-6, IL-8, IL-10, IL-1 RA, PGE 2, IDO, HGF, TSG-6, STNF-R, NO, effects s. HLA-GS, TGF-β 1, mp. CCL 2, HLA-G 5, LIF Antimicrobial LL-37, IDO effects Supportive effects SCF, LIF, IL-6, M-CSF, SDF-1, Angiopoietin-1
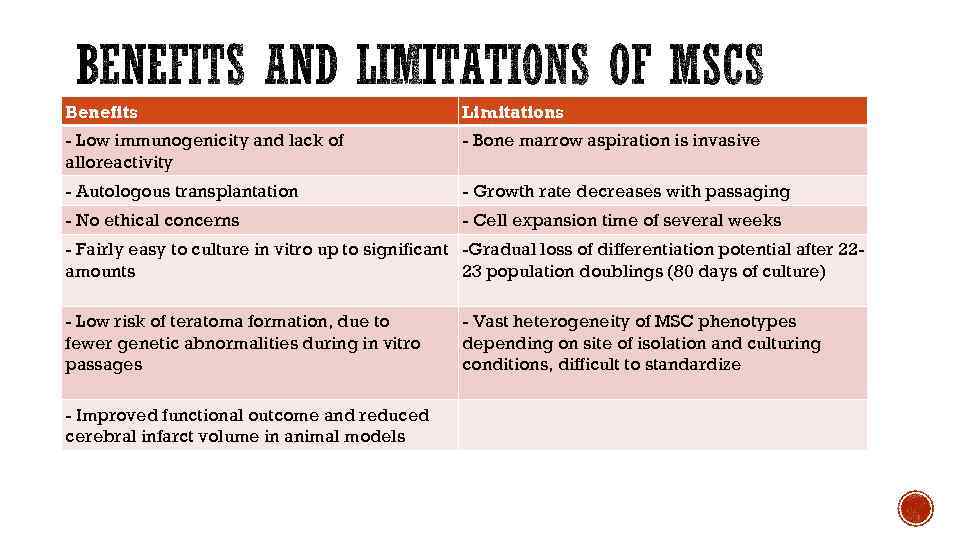
Benefits Limitations - Low immunogenicity and lack of alloreactivity - Bone marrow aspiration is invasive - Autologous transplantation - Growth rate decreases with passaging - No ethical concerns - Cell expansion time of several weeks - Fairly easy to culture in vitro up to significant -Gradual loss of differentiation potential after 22 amounts 23 population doublings (80 days of culture) - Low risk of teratoma formation, due to fewer genetic abnormalities during in vitro passages - Improved functional outcome and reduced cerebral infarct volume in animal models - Vast heterogeneity of MSC phenotypes depending on site of isolation and culturing conditions, difficult to standardize

§ Important considerations to improve future MSC applications: § standardization of practices § full understanding of the potential uses in regenerative medicine is essential. § However, there are no standard isolation methods for MSCs and the harvested populations, which are themselves quite heterogeneous, vary depending on the donor (Phinney and Prockop, 2007). Despite the apparent need for standardization, MSCs are becoming increasingly important in the field of regenerative medicine, having great potential for tissue repair. Specifically, MSCs have properties that inhibit inflammation and immune responses, making them ideal for this field (Phinney and Prockop, 2007). As set protocols are put into place and the large family of MSCs continues to be better understood, MSCs hold the promise of being major players in the future world of regenerative medicine.


§ 1) Doeppner R. , Hermann D. , (2010) Mesenchymal stem cells in the treatment of ischemic stroke: progress and possibilities. Stem Cells Cloning, 3, p. 157 -163 § 2) Murphy M. , Moncivais K. , Caplan A. , (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Experimental & Molecular Medicine, 45 (54), p. 1 -16. § 3) Tsai L. , Wang Z. , Munasinghe J. , Leng Y. , Leeds P. , Chuang D. , (2011) Mesenchymal Stem Cells Primed With Valproate and Lithium Robustly Migrate to Infarcted Regions and Facilitate Recovery in a Stroke Model. Stroke. 42, p. 2932 -2939 § 4) Castillo-Melendez M. , Yawno T. , Jenkin G. , Miller SL. , (2013) Stem cell therapy to protect and repair the developing brain: a review of mechanisms of action of cord blood and amnion epithelial derived cells. Frontiers in neuroscience. 7, (194). Available at: http: //journal. frontiersin. org/Journal/10. 3389/fnins. 2013. 00194/full § Last accessed: 23/02/2015 § 5) Borlogan C. , Glover LE. , Tajiri N. , Kaneko Y. , Freeman TB. , (2011) The Great Migration of Bone Marrow- Derived Stem Cells Toward the Ischemic Brain: Therapeutic Implications for Stroke and Other Neurological Disorders. Progress in Neurobiology. 95 (2), p. 213– 228. § 6) Le Blanc K. , Tammika C. , Rosendahlc K. , Zetterbergc E. , Ringdéna O. , (2003) HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Experimental haematology. 31 (10), p. 890 -896. § 7) Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF (2013) Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin 34: 747– 754 § 8)
MSCs_for_stroke_treatment.pptx