85403cee706239e27d6d60ddba1c39a8.ppt
- Количество слайдов: 22
 Status Report: Medicaid Preferred Drug List Program Presentation to the: Joint Commission on Health Care Behavioral Health Subcommittee Patrick W. Finnerty, Director Department of Medical Assistance Services August 4, 2004 Richmond, Virginia
Status Report: Medicaid Preferred Drug List Program Presentation to the: Joint Commission on Health Care Behavioral Health Subcommittee Patrick W. Finnerty, Director Department of Medical Assistance Services August 4, 2004 Richmond, Virginia
 Presentation Outline Background of PDL Development Current Status Review of Antidepressants 2
Presentation Outline Background of PDL Development Current Status Review of Antidepressants 2
 2003 Appropriations Act Required DMAS to Implement A PDL n Item 325(ZZ. 1) of the 2003 Appropriations Act directed DMAS to: – Implement PDL program no later than Jan. 1, 2004 – Seek input from physicians, pharmacists, pharmaceutical manufacturers, patient advocates, and others – Form a Pharmacy & Therapeutics (P&T) Committee – Ensure drugs on the PDL are safe and clinically effective before considering cost effectiveness – Include several key provisions: 72 -hour emergency supply; 24 -hour prior authorization process; expedited review of denials; and consumer/provider training and education – Report to General Assembly on main design components 3
2003 Appropriations Act Required DMAS to Implement A PDL n Item 325(ZZ. 1) of the 2003 Appropriations Act directed DMAS to: – Implement PDL program no later than Jan. 1, 2004 – Seek input from physicians, pharmacists, pharmaceutical manufacturers, patient advocates, and others – Form a Pharmacy & Therapeutics (P&T) Committee – Ensure drugs on the PDL are safe and clinically effective before considering cost effectiveness – Include several key provisions: 72 -hour emergency supply; 24 -hour prior authorization process; expedited review of denials; and consumer/provider training and education – Report to General Assembly on main design components 3
 What is a Preferred Drug List (PDL) Program? n PDL is a prior authorization program that divides Medicaid covered prescription drugs into two categories: – (1) Those that are available with no prior authorization, known as “preferred” drugs that are selected based on safety and clinical efficacy first, then on cost-effectiveness. – (2) Those that are available with prior authorization, known as “nonpreferred” drugs. n Virginia Medicaid’s PDL applies only to the fee-for-service program; MCOs have their own PDLs or formularies 4
What is a Preferred Drug List (PDL) Program? n PDL is a prior authorization program that divides Medicaid covered prescription drugs into two categories: – (1) Those that are available with no prior authorization, known as “preferred” drugs that are selected based on safety and clinical efficacy first, then on cost-effectiveness. – (2) Those that are available with prior authorization, known as “nonpreferred” drugs. n Virginia Medicaid’s PDL applies only to the fee-for-service program; MCOs have their own PDLs or formularies 4
 Pharmacy & Therapeutics Committee Member n n n Randy Axelrod (MD) (Chairman) Roy Beveridge (MD) Avtar Dhillon (MD) James Reinhard (MD) Arthur Garson, Jr (MD) Mariann Johnson (MD) Eleanor (Sue) Cantrell (MD) Christine Tully (MD) Mark Szalwinski (Pharmacist) (Vice Chairman) Gill Abernathy (Pharmacist) Mark Oley (Pharmacist) Renita Warren (Pharmacist) Background Anthem Chief Medical Officer Oncologist/Patient Advocate Psychiatrist (CSB) Psychiatrist (DMHMRSAS) Dean, UVA Med. School Family Practice Local Health District Director Geriatrician, VCU/MCV Sentara Health Care INOVA Health System Westwood Pharmacy Edloe’s Pharmacies 5
Pharmacy & Therapeutics Committee Member n n n Randy Axelrod (MD) (Chairman) Roy Beveridge (MD) Avtar Dhillon (MD) James Reinhard (MD) Arthur Garson, Jr (MD) Mariann Johnson (MD) Eleanor (Sue) Cantrell (MD) Christine Tully (MD) Mark Szalwinski (Pharmacist) (Vice Chairman) Gill Abernathy (Pharmacist) Mark Oley (Pharmacist) Renita Warren (Pharmacist) Background Anthem Chief Medical Officer Oncologist/Patient Advocate Psychiatrist (CSB) Psychiatrist (DMHMRSAS) Dean, UVA Med. School Family Practice Local Health District Director Geriatrician, VCU/MCV Sentara Health Care INOVA Health System Westwood Pharmacy Edloe’s Pharmacies 5
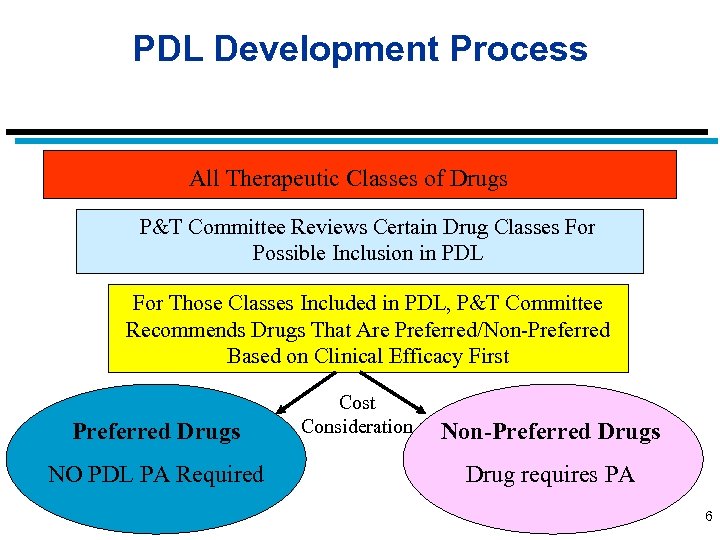 PDL Development Process All Therapeutic Classes of Drugs P&T Committee Reviews Certain Drug Classes For Possible Inclusion in PDL For Those Classes Included in PDL, P&T Committee Recommends Drugs That Are Preferred/Non-Preferred Based on Clinical Efficacy First Preferred Drugs NO PDL PA Required Cost Consideration Non-Preferred Drugs Drug requires PA 6
PDL Development Process All Therapeutic Classes of Drugs P&T Committee Reviews Certain Drug Classes For Possible Inclusion in PDL For Those Classes Included in PDL, P&T Committee Recommends Drugs That Are Preferred/Non-Preferred Based on Clinical Efficacy First Preferred Drugs NO PDL PA Required Cost Consideration Non-Preferred Drugs Drug requires PA 6
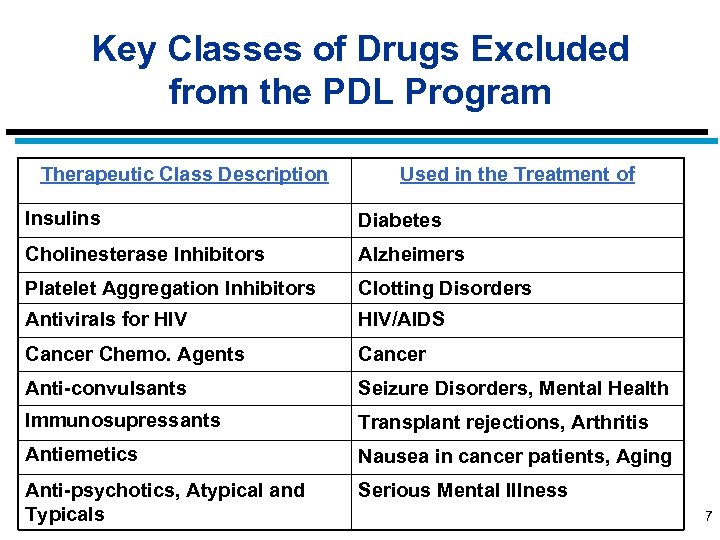 Key Classes of Drugs Excluded from the PDL Program Therapeutic Class Description Used in the Treatment of Insulins Diabetes Cholinesterase Inhibitors Alzheimers Platelet Aggregation Inhibitors Clotting Disorders Antivirals for HIV/AIDS Cancer Chemo. Agents Cancer Anti-convulsants Seizure Disorders, Mental Health Immunosupressants Transplant rejections, Arthritis Antiemetics Nausea in cancer patients, Aging Anti-psychotics, Atypical and Typicals Serious Mental Illness 7
Key Classes of Drugs Excluded from the PDL Program Therapeutic Class Description Used in the Treatment of Insulins Diabetes Cholinesterase Inhibitors Alzheimers Platelet Aggregation Inhibitors Clotting Disorders Antivirals for HIV/AIDS Cancer Chemo. Agents Cancer Anti-convulsants Seizure Disorders, Mental Health Immunosupressants Transplant rejections, Arthritis Antiemetics Nausea in cancer patients, Aging Anti-psychotics, Atypical and Typicals Serious Mental Illness 7
 13 Drug Classes Were Included in the PDL Program for January 2004 Therapeutic Class Description Used in the Treatment of: n Proton Pump Inhibitors (PPIs) n Gastrointestinal Disorders n H 2 Antagonists n Gastrointestinal Disorders n Nasal Steroids n Allergies, Asthma, Other Respiratory Illness n Second Generation Antihistamines n Allergic Conditions n Selective Cox-2 Inhibitors n Inflammatory Conditions n HMG Co. A Reductase Inhibitors (Statins) n High Cholesterol and Dyslipidemia n Sedative Hypnotics n Insomnia n Beta Adrenergics n Asthma and Other Respiratory Illness n Inhaled Corticosteroids n Asthma and Other Respiratory Illness n ACE Inhibitors n Hypertension/Other Cardiovascular Illness n Angiotensin II Receptor Blockers (ARBs) n Hypertension/Other Cardiovascular Illness n Calcium Channel Blockers (CCBs) n Hypertension/Other Cardiovascular Illness n Beta Blockers n Hypertension/Other Cardiovascular Illness 8
13 Drug Classes Were Included in the PDL Program for January 2004 Therapeutic Class Description Used in the Treatment of: n Proton Pump Inhibitors (PPIs) n Gastrointestinal Disorders n H 2 Antagonists n Gastrointestinal Disorders n Nasal Steroids n Allergies, Asthma, Other Respiratory Illness n Second Generation Antihistamines n Allergic Conditions n Selective Cox-2 Inhibitors n Inflammatory Conditions n HMG Co. A Reductase Inhibitors (Statins) n High Cholesterol and Dyslipidemia n Sedative Hypnotics n Insomnia n Beta Adrenergics n Asthma and Other Respiratory Illness n Inhaled Corticosteroids n Asthma and Other Respiratory Illness n ACE Inhibitors n Hypertension/Other Cardiovascular Illness n Angiotensin II Receptor Blockers (ARBs) n Hypertension/Other Cardiovascular Illness n Calcium Channel Blockers (CCBs) n Hypertension/Other Cardiovascular Illness n Beta Blockers n Hypertension/Other Cardiovascular Illness 8
 Drug Classes Added to PDL Program in April 2004 Therapeutic Class Description Used in The Treatment of: n Oral Hypoglycemics n Diabetes n Leukotriene Modifiers n Allergic Conditions/Asthma n Bisphonates n Osteoporosis n Traditional NSAIDs n Inflammatory Conditions n Serotonin Receptor Agonists n Migraine Headache n Oral Antifungals n Nail Fungal Infections 9
Drug Classes Added to PDL Program in April 2004 Therapeutic Class Description Used in The Treatment of: n Oral Hypoglycemics n Diabetes n Leukotriene Modifiers n Allergic Conditions/Asthma n Bisphonates n Osteoporosis n Traditional NSAIDs n Inflammatory Conditions n Serotonin Receptor Agonists n Migraine Headache n Oral Antifungals n Nail Fungal Infections 9
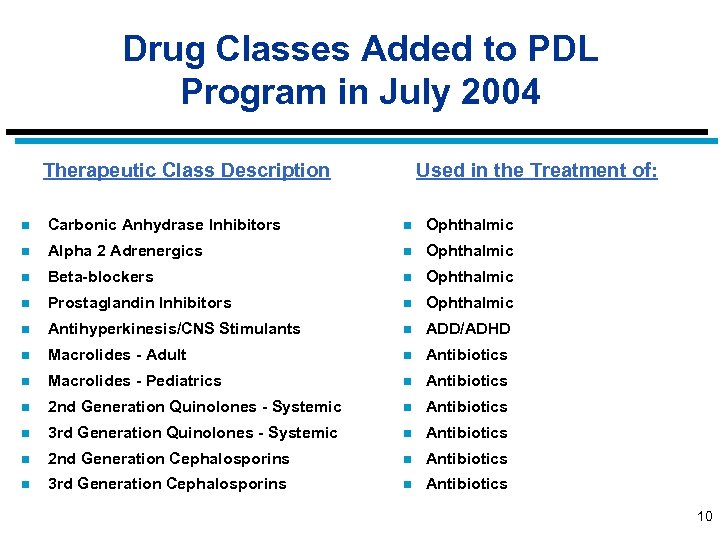 Drug Classes Added to PDL Program in July 2004 Therapeutic Class Description Used in the Treatment of: n Carbonic Anhydrase Inhibitors n Ophthalmic n Alpha 2 Adrenergics n Ophthalmic n Beta-blockers n Ophthalmic n Prostaglandin Inhibitors n Ophthalmic n Antihyperkinesis/CNS Stimulants n ADD/ADHD n Macrolides - Adult n Antibiotics n Macrolides - Pediatrics n Antibiotics n 2 nd Generation Quinolones - Systemic n Antibiotics n 3 rd Generation Quinolones - Systemic n Antibiotics n 2 nd Generation Cephalosporins n Antibiotics n 3 rd Generation Cephalosporins n Antibiotics 10
Drug Classes Added to PDL Program in July 2004 Therapeutic Class Description Used in the Treatment of: n Carbonic Anhydrase Inhibitors n Ophthalmic n Alpha 2 Adrenergics n Ophthalmic n Beta-blockers n Ophthalmic n Prostaglandin Inhibitors n Ophthalmic n Antihyperkinesis/CNS Stimulants n ADD/ADHD n Macrolides - Adult n Antibiotics n Macrolides - Pediatrics n Antibiotics n 2 nd Generation Quinolones - Systemic n Antibiotics n 3 rd Generation Quinolones - Systemic n Antibiotics n 2 nd Generation Cephalosporins n Antibiotics n 3 rd Generation Cephalosporins n Antibiotics 10
 Critical Steps Taken in Implementation Process n Met with more than 40 interested parties to solicit input into design of PDL program. n Formed PDL Implementation Advisory Group n Provided broad access to all PDL information through dedicated web site (www. dmas. virginia. gov) and e-mail (pdlinput@dmas. virginia. gov) n Conducted extensive beta-site testing with independent, chain and long-term care pharmacies. n Phased-in drug classes – “soft edits” for a period, then “hard edits” 11
Critical Steps Taken in Implementation Process n Met with more than 40 interested parties to solicit input into design of PDL program. n Formed PDL Implementation Advisory Group n Provided broad access to all PDL information through dedicated web site (www. dmas. virginia. gov) and e-mail (pdlinput@dmas. virginia. gov) n Conducted extensive beta-site testing with independent, chain and long-term care pharmacies. n Phased-in drug classes – “soft edits” for a period, then “hard edits” 11
 Critical Steps Taken in Implementation Process n Developed an extensive education program – Memorandum and reminder postcard sent to providers – Information (English & Spanish) sent to all recipients – Regional and targeted training programs for pharmacists, health systems, and provider associations – Personal contact made with high volume Medicaid prescribers and pharmacists n Effective September 1, providers can download the PDL to their handheld personal assistants through e. Procates 12
Critical Steps Taken in Implementation Process n Developed an extensive education program – Memorandum and reminder postcard sent to providers – Information (English & Spanish) sent to all recipients – Regional and targeted training programs for pharmacists, health systems, and provider associations – Personal contact made with high volume Medicaid prescribers and pharmacists n Effective September 1, providers can download the PDL to their handheld personal assistants through e. Procates 12
 Presentation Outline Background of PDL Development Current Status Review of Antidepressants 13
Presentation Outline Background of PDL Development Current Status Review of Antidepressants 13
 Implementation Has Gone Very Smoothly n All clinical decisions regarding the PDL and prior authorization process are made by DMAS’ Pharmacy and Therapeutics (P&T) Committee. n PDL compliance rate is high and most changes to “preferred” drugs are being made voluntarily n Patients are getting the drugs they need – There have been 26 “technical” denials but in these cases, the patients still received their drugs – There have been no appeals – 7 of every 10 requests for a PA are approved; in other cases, provider agrees to switch to the preferred drug 14
Implementation Has Gone Very Smoothly n All clinical decisions regarding the PDL and prior authorization process are made by DMAS’ Pharmacy and Therapeutics (P&T) Committee. n PDL compliance rate is high and most changes to “preferred” drugs are being made voluntarily n Patients are getting the drugs they need – There have been 26 “technical” denials but in these cases, the patients still received their drugs – There have been no appeals – 7 of every 10 requests for a PA are approved; in other cases, provider agrees to switch to the preferred drug 14
 Implementation Has Gone Very Smoothly n Call Center is operating extremely well n Very few complaints from providers or clients n In terms of savings, actual Medicaid expenditures are significantly below DMAS’ official forecast. Preliminary savings analysis indicates DMAS is on pace to meet its required savings 15
Implementation Has Gone Very Smoothly n Call Center is operating extremely well n Very few complaints from providers or clients n In terms of savings, actual Medicaid expenditures are significantly below DMAS’ official forecast. Preliminary savings analysis indicates DMAS is on pace to meet its required savings 15
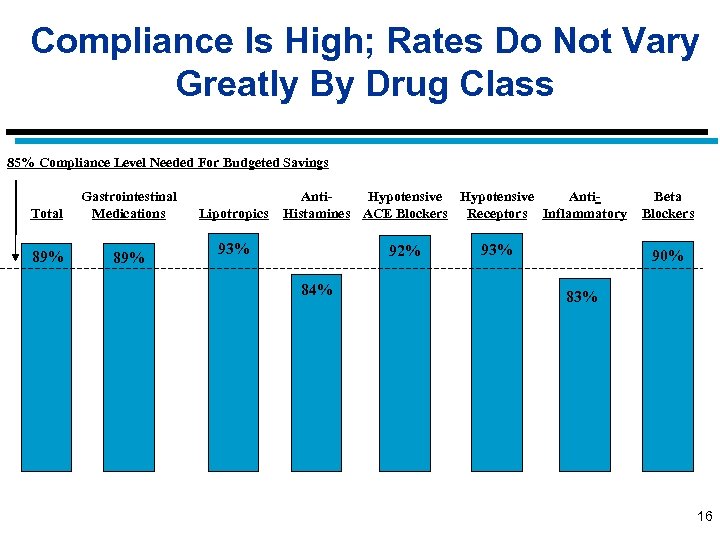 Compliance Is High; Rates Do Not Vary Greatly By Drug Class 85% Compliance Level Needed For Budgeted Savings Total Gastrointestinal Medications 89% Lipotropics Anti. Hypotensive Anti. Histamines ACE Blockers Receptors Inflammatory 93% 92% 84% 93% Beta Blockers 90% 83% 16
Compliance Is High; Rates Do Not Vary Greatly By Drug Class 85% Compliance Level Needed For Budgeted Savings Total Gastrointestinal Medications 89% Lipotropics Anti. Hypotensive Anti. Histamines ACE Blockers Receptors Inflammatory 93% 92% 84% 93% Beta Blockers 90% 83% 16
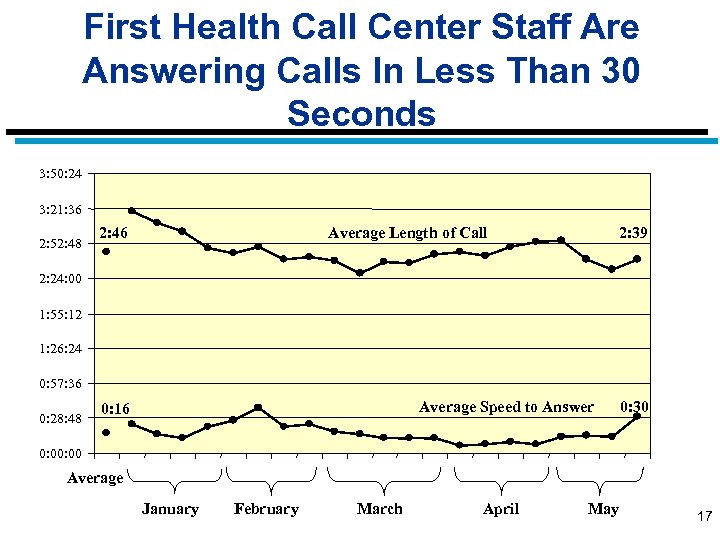 First Health Call Center Staff Are Answering Calls In Less Than 30 Seconds 3: 50: 24 3: 21: 36 2: 52: 48 2: 46 Average Length of Call 2: 39 2: 24: 00 1: 55: 12 1: 26: 24 0: 57: 36 0: 28: 48 Average Speed to Answer 0: 16 0: 30 0: 00 Average January February March April May 17
First Health Call Center Staff Are Answering Calls In Less Than 30 Seconds 3: 50: 24 3: 21: 36 2: 52: 48 2: 46 Average Length of Call 2: 39 2: 24: 00 1: 55: 12 1: 26: 24 0: 57: 36 0: 28: 48 Average Speed to Answer 0: 16 0: 30 0: 00 Average January February March April May 17
 DMAS Will Conduct A Comprehensive Evaluation of PDL Program n Evaluation will address the following key issues: – Has the PDL program been implemented in a way to ensure a high rate of compliance without adversely affecting patient access/care? – What impact has the PDL program had on Medicaid pharmaceutical spending? – Has the PDL program impacted patient health outcomes for Medicaid clients? 18
DMAS Will Conduct A Comprehensive Evaluation of PDL Program n Evaluation will address the following key issues: – Has the PDL program been implemented in a way to ensure a high rate of compliance without adversely affecting patient access/care? – What impact has the PDL program had on Medicaid pharmaceutical spending? – Has the PDL program impacted patient health outcomes for Medicaid clients? 18
 Presentation Outline Background of PDL Development Current Status Review of Antidepressants 19
Presentation Outline Background of PDL Development Current Status Review of Antidepressants 19
 Antidepressants (SSRIs) & Antianxiety Drugs n Medicaid spent approximately $29. 5 million in total funds (net of rebates) on SSRIs ($15. 8), anti-anxiety drugs ($6. 9), and new generation antidepressants ($6. 8) in FY 2003 n The SSRI drug class is the third highest in expenditures n Excluding the SSRIs, anti-anxiety drugs and new generation antidepressants from the PDL would cost approximately $5 million (total funds) annually; a “grandfather” provision would cost roughly half of this amount 20
Antidepressants (SSRIs) & Antianxiety Drugs n Medicaid spent approximately $29. 5 million in total funds (net of rebates) on SSRIs ($15. 8), anti-anxiety drugs ($6. 9), and new generation antidepressants ($6. 8) in FY 2003 n The SSRI drug class is the third highest in expenditures n Excluding the SSRIs, anti-anxiety drugs and new generation antidepressants from the PDL would cost approximately $5 million (total funds) annually; a “grandfather” provision would cost roughly half of this amount 20
 2004 Appropriations Act Provides Direction on Review of Antidepressants n Item 326 BB 7 – If DMAS does not exempt antidepressants and antianxiety medications used for the treatment of mental illness from the PDL, it should defer inclusion from PDL until July 1, 2005 – Prior to including these drug classes in the PDL, DMAS shall provide a plan that stipulates mechanisms to: minimize adverse impacts on consumers; ensure appropriate provider education; and ensure inclusion is evidence-based, clinically efficacious and cost-effective – DMAS shall report such plan to the Governor, and Chairman of the House Appropriations and Senate Finance Committees and the Joint Commission on Health Care by January 1, 2005 21
2004 Appropriations Act Provides Direction on Review of Antidepressants n Item 326 BB 7 – If DMAS does not exempt antidepressants and antianxiety medications used for the treatment of mental illness from the PDL, it should defer inclusion from PDL until July 1, 2005 – Prior to including these drug classes in the PDL, DMAS shall provide a plan that stipulates mechanisms to: minimize adverse impacts on consumers; ensure appropriate provider education; and ensure inclusion is evidence-based, clinically efficacious and cost-effective – DMAS shall report such plan to the Governor, and Chairman of the House Appropriations and Senate Finance Committees and the Joint Commission on Health Care by January 1, 2005 21
 DMAS/P&T Committee Approach n Antidepressants and antianxiety drug classes will be reviewed by the P&T Committee on October 6, 2004 n In addition to receiving testimony on scientific evidence from manufacturers, clinicians and others, the P&T Committee also will receive public comments from other interested parties n If the P&T Committee recommends including these drug classes in the PDL, a report will be prepared and submitted as required by the 2004 Appropriations Act. – If recommended for inclusion in the PDL, the effective date would be no earlier than July 1, 2005 22
DMAS/P&T Committee Approach n Antidepressants and antianxiety drug classes will be reviewed by the P&T Committee on October 6, 2004 n In addition to receiving testimony on scientific evidence from manufacturers, clinicians and others, the P&T Committee also will receive public comments from other interested parties n If the P&T Committee recommends including these drug classes in the PDL, a report will be prepared and submitted as required by the 2004 Appropriations Act. – If recommended for inclusion in the PDL, the effective date would be no earlier than July 1, 2005 22


