c37bcbe1532b401c3e37a6aacd99148f.ppt
- Количество слайдов: 42
 State-specific surface scattering with laser-prepared molecules Dynamics of Molecular Collisions Asilomar July 2005
State-specific surface scattering with laser-prepared molecules Dynamics of Molecular Collisions Asilomar July 2005
 Outline n INTRODUCTION: Evidence for breakdown of Born. Oppenheimer Approximation for molecules at metal interfaces. n EXPERIMENTAL n Vibrationally promoted ejection of electrons from a surface n Relative importance of vibration and translation on trapping at a surface.
Outline n INTRODUCTION: Evidence for breakdown of Born. Oppenheimer Approximation for molecules at metal interfaces. n EXPERIMENTAL n Vibrationally promoted ejection of electrons from a surface n Relative importance of vibration and translation on trapping at a surface.
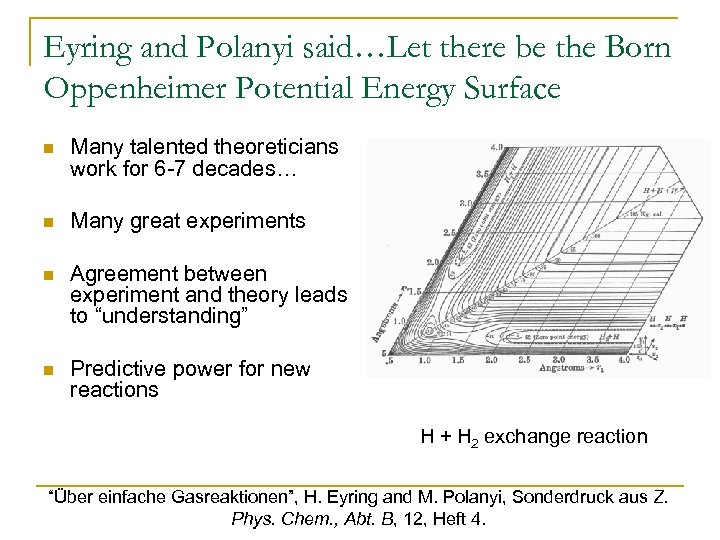 Eyring and Polanyi said…Let there be the Born Oppenheimer Potential Energy Surface n Many talented theoreticians work for 6 -7 decades… n Many great experiments n Agreement between experiment and theory leads to “understanding” n Predictive power for new reactions • H. Eyring, J. Walter, G. E. Kimball, Quantum Chemistry (John Wiley and Sons, New York, 1944). • H. Eyring, H. Gershinowitz, C. E. Sun, J. Chem. Phys. 3, 786 (1935). • J. Hirschfelder, H. Eyring, B. Topely, J. Chem. Phys. 4, 2 170 (1936). H + H exchange reaction “Über einfache Gasreaktionen”, H. Eyring and M. Polanyi, Sonderdruck aus Z. Phys. Chem. , Abt. B, 12, Heft 4.
Eyring and Polanyi said…Let there be the Born Oppenheimer Potential Energy Surface n Many talented theoreticians work for 6 -7 decades… n Many great experiments n Agreement between experiment and theory leads to “understanding” n Predictive power for new reactions • H. Eyring, J. Walter, G. E. Kimball, Quantum Chemistry (John Wiley and Sons, New York, 1944). • H. Eyring, H. Gershinowitz, C. E. Sun, J. Chem. Phys. 3, 786 (1935). • J. Hirschfelder, H. Eyring, B. Topely, J. Chem. Phys. 4, 2 170 (1936). H + H exchange reaction “Über einfache Gasreaktionen”, H. Eyring and M. Polanyi, Sonderdruck aus Z. Phys. Chem. , Abt. B, 12, Heft 4.
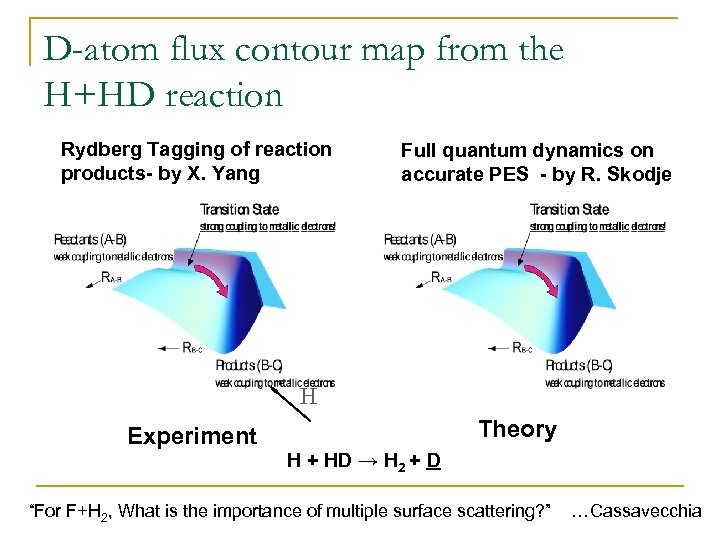 D-atom flux contour map from the H+HD reaction Rydberg Tagging of reaction products- by X. Yang Full quantum dynamics on accurate PES - by R. Skodje H Experiment Theory H + HD → H 2 + D “For F+H 2, What is the importance of multiple surface scattering? ” …Cassavecchia
D-atom flux contour map from the H+HD reaction Rydberg Tagging of reaction products- by X. Yang Full quantum dynamics on accurate PES - by R. Skodje H Experiment Theory H + HD → H 2 + D “For F+H 2, What is the importance of multiple surface scattering? ” …Cassavecchia
 What happens when we apply this strategy to reactions at metal surfaces?
What happens when we apply this strategy to reactions at metal surfaces?
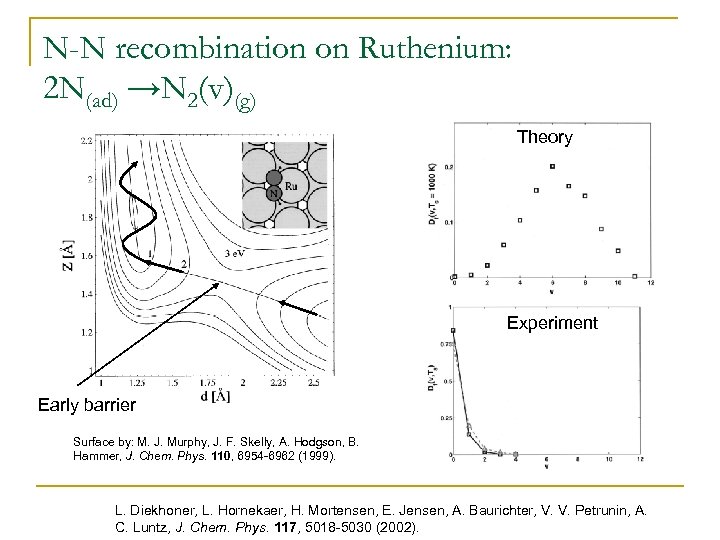 N-N recombination on Ruthenium: 2 N(ad) →N 2(v)(g) Theory Experiment Early barrier Surface by: M. J. Murphy, J. F. Skelly, A. Hodgson, B. Hammer, J. Chem. Phys. 110, 6954 -6962 (1999). L. Diekhoner, L. Hornekaer, H. Mortensen, E. Jensen, A. Baurichter, V. V. Petrunin, A. C. Luntz, J. Chem. Phys. 117, 5018 -5030 (2002).
N-N recombination on Ruthenium: 2 N(ad) →N 2(v)(g) Theory Experiment Early barrier Surface by: M. J. Murphy, J. F. Skelly, A. Hodgson, B. Hammer, J. Chem. Phys. 110, 6954 -6962 (1999). L. Diekhoner, L. Hornekaer, H. Mortensen, E. Jensen, A. Baurichter, V. V. Petrunin, A. C. Luntz, J. Chem. Phys. 117, 5018 -5030 (2002).
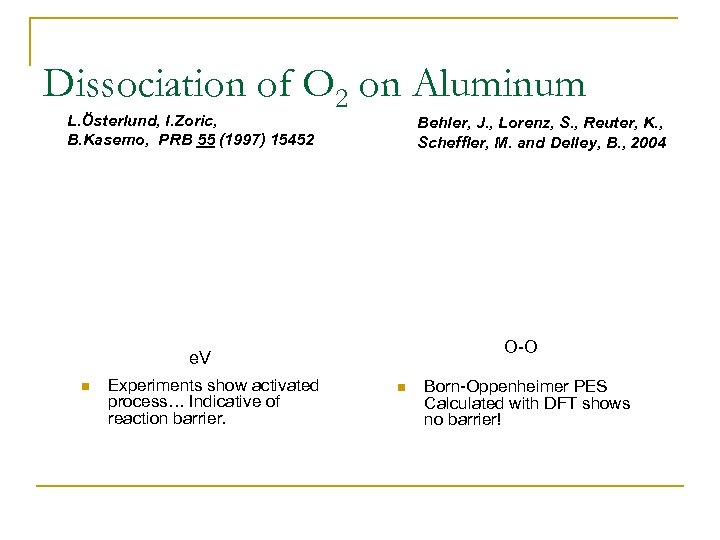 Dissociation of O 2 on Aluminum L. Österlund, I. Zoric, B. Kasemo, PRB 55 (1997) 15452 Behler, J. , Lorenz, S. , Reuter, K. , Scheffler, M. and Delley, B. , 2004 O-O e. V n Experiments show activated process… Indicative of reaction barrier. n Born-Oppenheimer PES Calculated with DFT shows no barrier!
Dissociation of O 2 on Aluminum L. Österlund, I. Zoric, B. Kasemo, PRB 55 (1997) 15452 Behler, J. , Lorenz, S. , Reuter, K. , Scheffler, M. and Delley, B. , 2004 O-O e. V n Experiments show activated process… Indicative of reaction barrier. n Born-Oppenheimer PES Calculated with DFT shows no barrier!
 Both results imply: Important energy transfer between the reactive center and the surface n N 2 reaction on Ruthenium q Many vibrational quanta appear to be lost from the molecule during the 10’s of femto-seconds required for the product to leave the surface n O 2 reaction on Aluminum q Despite no electronically adiabatic barrier on the potential energy surface, translation energy is need to induce the reaction n Possible important energy transfer couplings q To phonons: vibration of the metal substrate ü To electrons: creating excited electron hole pairs (EHP’s)
Both results imply: Important energy transfer between the reactive center and the surface n N 2 reaction on Ruthenium q Many vibrational quanta appear to be lost from the molecule during the 10’s of femto-seconds required for the product to leave the surface n O 2 reaction on Aluminum q Despite no electronically adiabatic barrier on the potential energy surface, translation energy is need to induce the reaction n Possible important energy transfer couplings q To phonons: vibration of the metal substrate ü To electrons: creating excited electron hole pairs (EHP’s)
 Born-Oppenheimer Approximation at metal surfaces is less obviously correct n The stronger attraction of a molecule to a surface, proportional to the inverse third power of distance compared to the inverse sixth power in the gas phase, can lower the energies of more polarizable excited electronic states, bringing them nearer in energy to the ground state. n More dramatically, positively or negatively charged molecules at surfaces are stabilized by an image potential proportional to the first power of the distance, frequently resulting in the crossing or avoided crossing of ionic and neutral potential energy surfaces. n Metals bind electrons more weakly (work functions are generally less than 5 e. V) than gas phase molecules (Ionization potentials are generally more than 8 e. V). n Finally, metal surfaces exhibit a continuum of electronic states, the conduction band, for which there is no energy separation whatever between electronic states. Electron-hole pair (EHP) transitions between electronic levels in the conduction band can provide a mechanism for energy transfer with an adsorbate molecule and perhaps even call into question the applicability of the concept of motion evolving on a PES. Excellent review: J. C. Tully, Ann. Rev. Phys. Chem. 51, 153 (2000).
Born-Oppenheimer Approximation at metal surfaces is less obviously correct n The stronger attraction of a molecule to a surface, proportional to the inverse third power of distance compared to the inverse sixth power in the gas phase, can lower the energies of more polarizable excited electronic states, bringing them nearer in energy to the ground state. n More dramatically, positively or negatively charged molecules at surfaces are stabilized by an image potential proportional to the first power of the distance, frequently resulting in the crossing or avoided crossing of ionic and neutral potential energy surfaces. n Metals bind electrons more weakly (work functions are generally less than 5 e. V) than gas phase molecules (Ionization potentials are generally more than 8 e. V). n Finally, metal surfaces exhibit a continuum of electronic states, the conduction band, for which there is no energy separation whatever between electronic states. Electron-hole pair (EHP) transitions between electronic levels in the conduction band can provide a mechanism for energy transfer with an adsorbate molecule and perhaps even call into question the applicability of the concept of motion evolving on a PES. Excellent review: J. C. Tully, Ann. Rev. Phys. Chem. 51, 153 (2000).
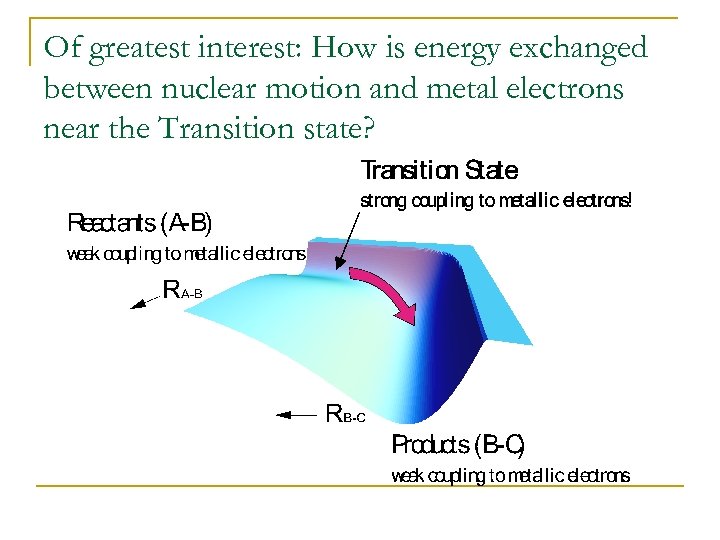 Of greatest interest: How is energy exchanged between nuclear motion and metal electrons near the Transition state?
Of greatest interest: How is energy exchanged between nuclear motion and metal electrons near the Transition state?
 Experimental Approach
Experimental Approach
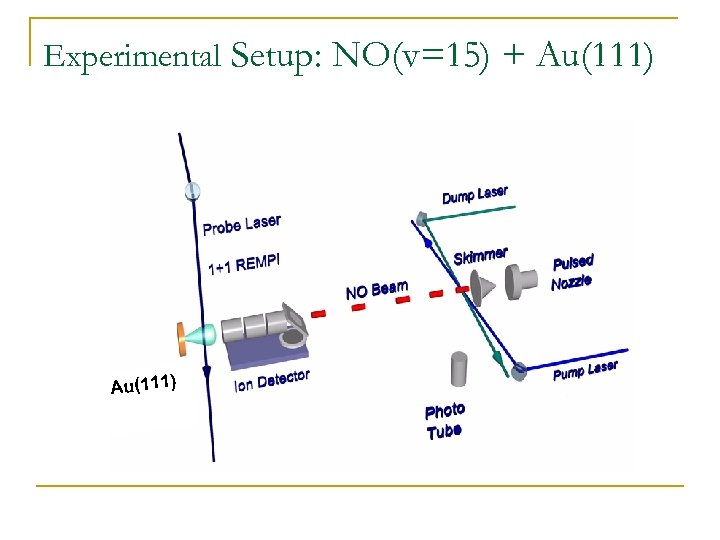 Experimental Setup: NO(v=15) + Au(111)
Experimental Setup: NO(v=15) + Au(111)
 Optical Pumping on Nitric Oxide n Capabilities q q q Franck-Condon Pumping of low vibrational states (v=0 -10) Stimulated Emission Pumping of high vibrational states (v<27, 5. 1 e. V) Laser induced fluorescence detection Resonance enhanced multiphoton Ionization detection A few percent of sample may be excited.
Optical Pumping on Nitric Oxide n Capabilities q q q Franck-Condon Pumping of low vibrational states (v=0 -10) Stimulated Emission Pumping of high vibrational states (v<27, 5. 1 e. V) Laser induced fluorescence detection Resonance enhanced multiphoton Ionization detection A few percent of sample may be excited.
 Large amplitude vibration a mimic of the kind of motion near the transition state! Numerical Solution to the Vibrational Schrödinger Equation for NO
Large amplitude vibration a mimic of the kind of motion near the transition state! Numerical Solution to the Vibrational Schrödinger Equation for NO
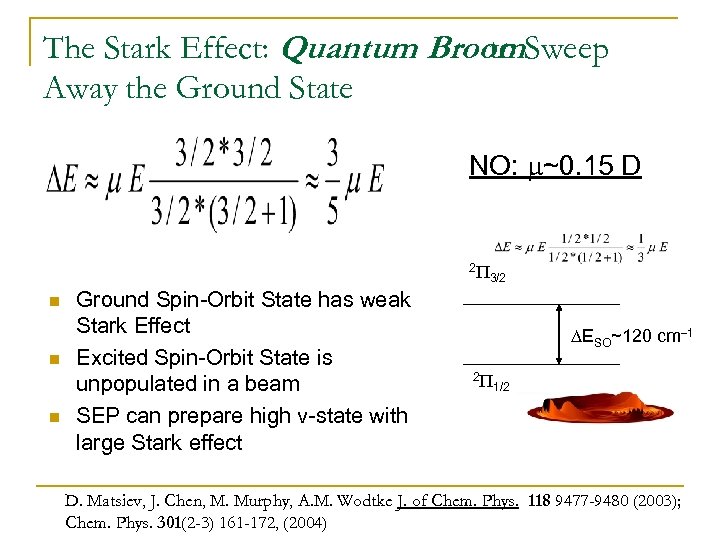 The Stark Effect: Quantum Broom. Sweep to Away the Ground State NO: m~0. 15 D 2 P n n n Ground Spin-Orbit State has weak Stark Effect Excited Spin-Orbit State is unpopulated in a beam SEP can prepare high v-state with large Stark effect 3/2 DESO~120 cm-1 2 P 1/2 D. Matsiev, J. Chen, M. Murphy, A. M. Wodtke J. of Chem. Phys. 118 9477 -9480 (2003); Chem. Phys. 301(2 -3) 161 -172, (2004)
The Stark Effect: Quantum Broom. Sweep to Away the Ground State NO: m~0. 15 D 2 P n n n Ground Spin-Orbit State has weak Stark Effect Excited Spin-Orbit State is unpopulated in a beam SEP can prepare high v-state with large Stark effect 3/2 DESO~120 cm-1 2 P 1/2 D. Matsiev, J. Chen, M. Murphy, A. M. Wodtke J. of Chem. Phys. 118 9477 -9480 (2003); Chem. Phys. 301(2 -3) 161 -172, (2004)
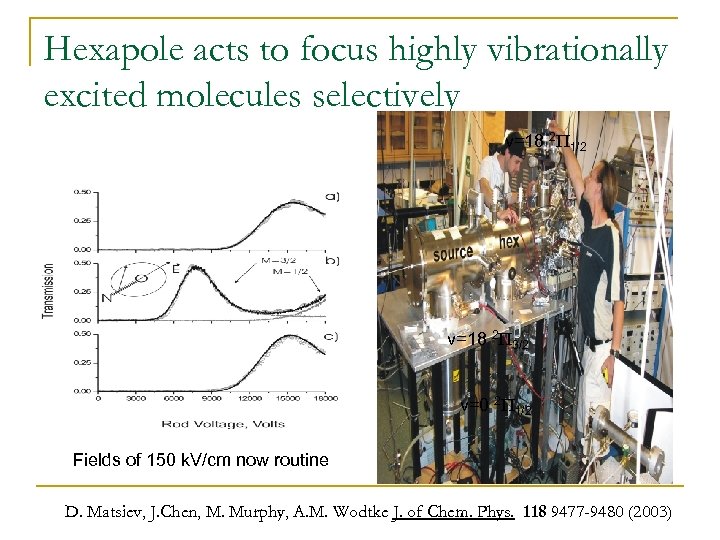 Hexapole acts to focus highly vibrationally excited molecules selectively v=18 2 P 1/2 v=18 2 P 3/2 v=0 2 P 1/2 Fields of 150 k. V/cm now routine D. Matsiev, J. Chen, M. Murphy, A. M. Wodtke J. of Chem. Phys. 118 9477 -9480 (2003)
Hexapole acts to focus highly vibrationally excited molecules selectively v=18 2 P 1/2 v=18 2 P 3/2 v=0 2 P 1/2 Fields of 150 k. V/cm now routine D. Matsiev, J. Chen, M. Murphy, A. M. Wodtke J. of Chem. Phys. 118 9477 -9480 (2003)
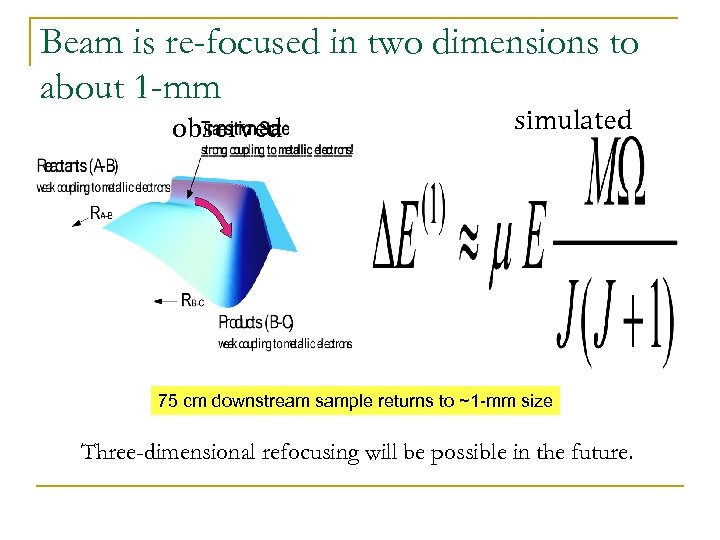 Beam is re-focused in two dimensions to about 1 -mm observed simulated 75 cm downstream sample returns to ~1 -mm size Three-dimensional refocusing will be possible in the future.
Beam is re-focused in two dimensions to about 1 -mm observed simulated 75 cm downstream sample returns to ~1 -mm size Three-dimensional refocusing will be possible in the future.
 When it is all put together, a machine that can… n …transport optically prepared molecules to UHV surface science chamber and refocus them. n …enrich beam in concentration of high-v molecules. n …select M-states for orientation studies. n …Provide vibrational-state specific dipole moments.
When it is all put together, a machine that can… n …transport optically prepared molecules to UHV surface science chamber and refocus them. n …enrich beam in concentration of high-v molecules. n …select M-states for orientation studies. n …Provide vibrational-state specific dipole moments.
 Scattering from a simple inert metal surface NO(v=15) from Au(111) and comparison to Li. F insulator
Scattering from a simple inert metal surface NO(v=15) from Au(111) and comparison to Li. F insulator
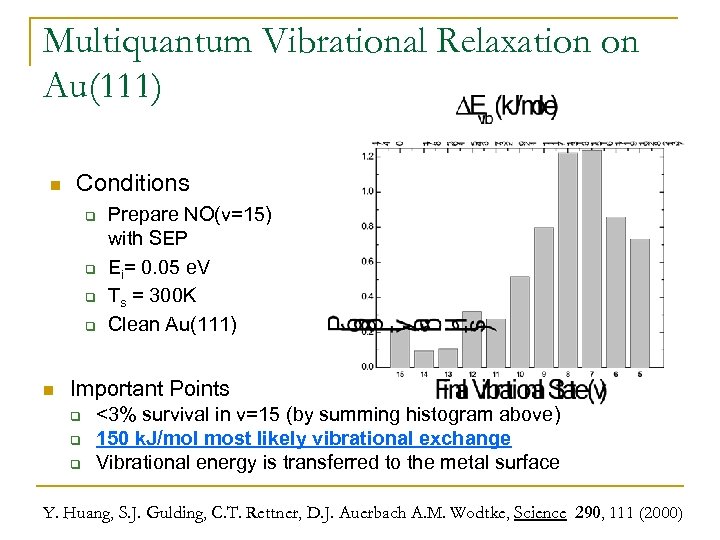 Multiquantum Vibrational Relaxation on Au(111) n Conditions q q n Prepare NO(v=15) with SEP Ei= 0. 05 e. V Ts = 300 K Clean Au(111) Important Points q q q <3% survival in v=15 (by summing histogram above) 150 k. J/mol most likely vibrational exchange Vibrational energy is transferred to the metal surface Y. Huang, S. J. Gulding, C. T. Rettner, D. J. Auerbach A. M. Wodtke, Science 290, 111 (2000)
Multiquantum Vibrational Relaxation on Au(111) n Conditions q q n Prepare NO(v=15) with SEP Ei= 0. 05 e. V Ts = 300 K Clean Au(111) Important Points q q q <3% survival in v=15 (by summing histogram above) 150 k. J/mol most likely vibrational exchange Vibrational energy is transferred to the metal surface Y. Huang, S. J. Gulding, C. T. Rettner, D. J. Auerbach A. M. Wodtke, Science 290, 111 (2000)
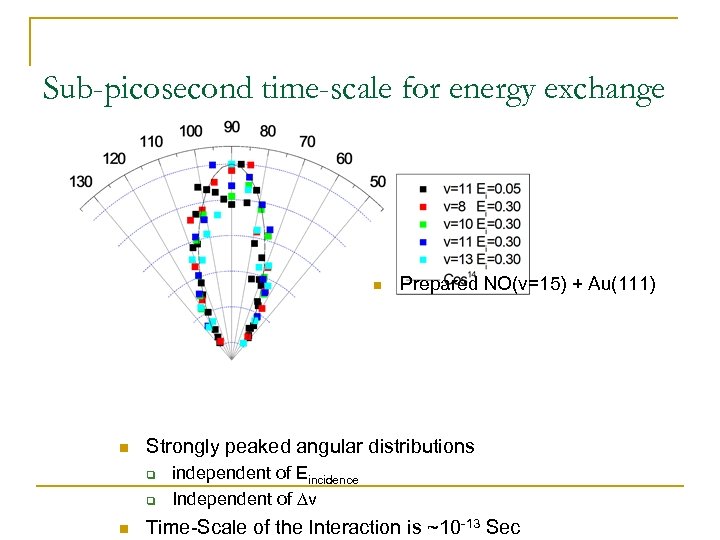 Sub-picosecond time-scale for energy exchange n n Strongly peaked angular distributions q q n Prepared NO(v=15) + Au(111) independent of Eincidence Independent of Dv Time-Scale of the Interaction is ~10 -13 Sec
Sub-picosecond time-scale for energy exchange n n Strongly peaked angular distributions q q n Prepared NO(v=15) + Au(111) independent of Eincidence Independent of Dv Time-Scale of the Interaction is ~10 -13 Sec
 Quite different dynamics observed on insulators.
Quite different dynamics observed on insulators.
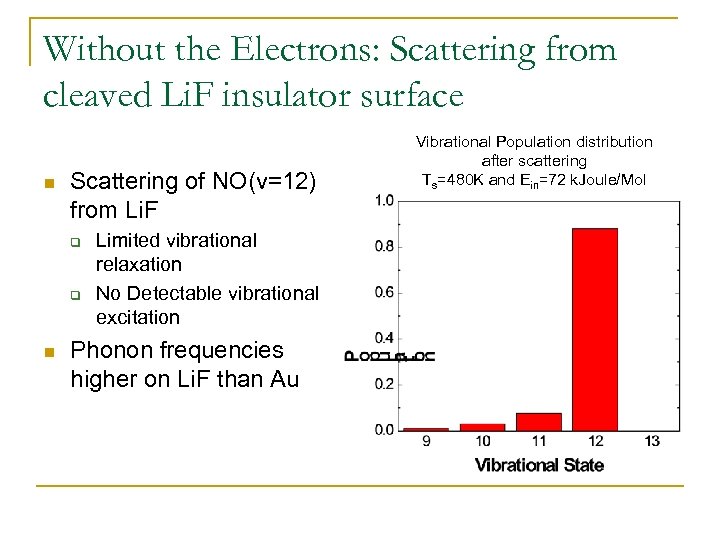 Without the Electrons: Scattering from cleaved Li. F insulator surface n Scattering of NO(v=12) from Li. F q q n Limited vibrational relaxation No Detectable vibrational excitation Phonon frequencies higher on Li. F than Au Vibrational Population distribution after scattering Ts=480 K and Ein=72 k. Joule/Mol
Without the Electrons: Scattering from cleaved Li. F insulator surface n Scattering of NO(v=12) from Li. F q q n Limited vibrational relaxation No Detectable vibrational excitation Phonon frequencies higher on Li. F than Au Vibrational Population distribution after scattering Ts=480 K and Ein=72 k. Joule/Mol
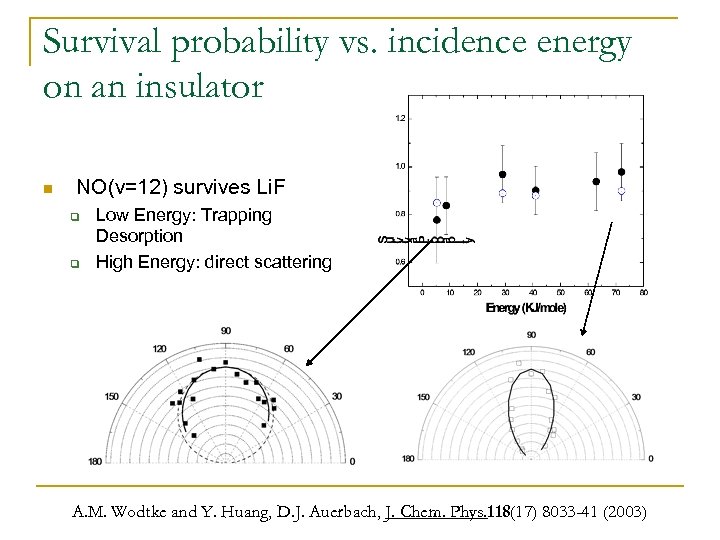 Survival probability vs. incidence energy on an insulator n NO(v=12) survives Li. F q q Low Energy: Trapping Desorption High Energy: direct scattering A. M. Wodtke and Y. Huang, D. J. Auerbach, J. Chem. Phys. 118(17) 8033 -41 (2003)
Survival probability vs. incidence energy on an insulator n NO(v=12) survives Li. F q q Low Energy: Trapping Desorption High Energy: direct scattering A. M. Wodtke and Y. Huang, D. J. Auerbach, J. Chem. Phys. 118(17) 8033 -41 (2003)
 Some ideas about mechanisms Electron transfer appears to play an important role.
Some ideas about mechanisms Electron transfer appears to play an important role.
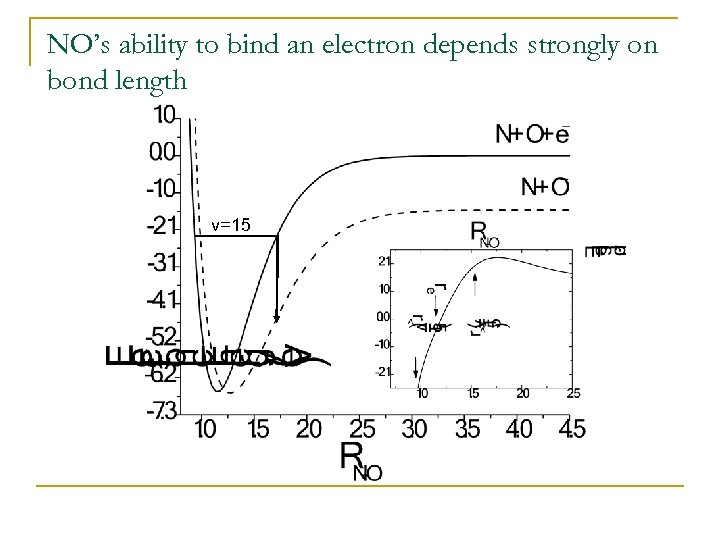 NO’s ability to bind an electron depends strongly on bond length v=15
NO’s ability to bind an electron depends strongly on bond length v=15
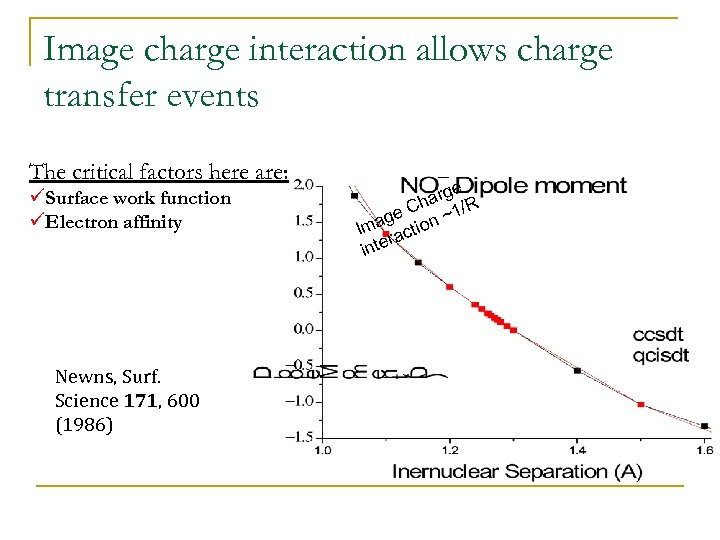 Image charge interaction allows charge transfer events The critical factors here are: üSurface work function üElectron affinity Newns, Surf. Science 171, 600 (1986) e arg R Ch / ge on ~1 a Im acti r inte
Image charge interaction allows charge transfer events The critical factors here are: üSurface work function üElectron affinity Newns, Surf. Science 171, 600 (1986) e arg R Ch / ge on ~1 a Im acti r inte
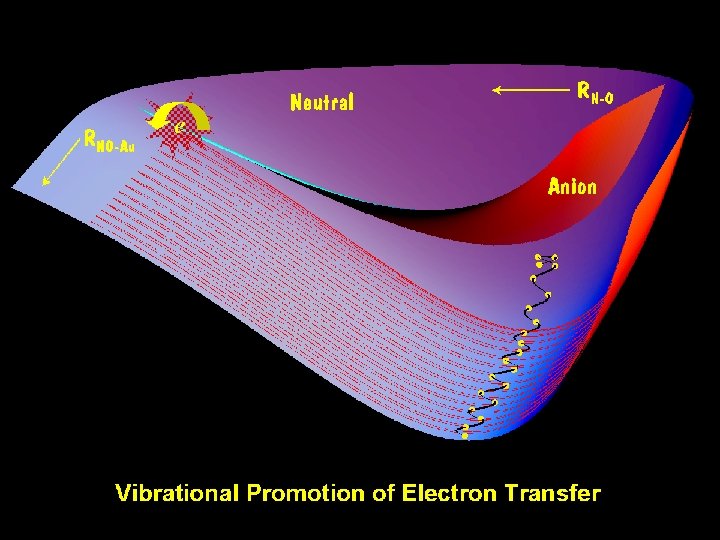 A picture is worth a thousand words
A picture is worth a thousand words
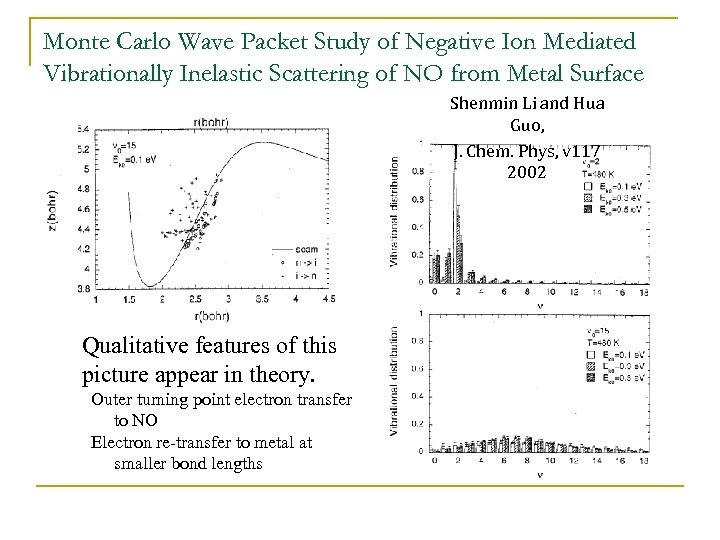 Monte Carlo Wave Packet Study of Negative Ion Mediated Vibrationally Inelastic Scattering of NO from Metal Surface Shenmin Li and Hua Guo, J. Chem. Phys, v 117 2002 • Qualitative features of this picture appear in theory. Outer turning point electron transfer to NO Electron re-transfer to metal at smaller bond lengths
Monte Carlo Wave Packet Study of Negative Ion Mediated Vibrationally Inelastic Scattering of NO from Metal Surface Shenmin Li and Hua Guo, J. Chem. Phys, v 117 2002 • Qualitative features of this picture appear in theory. Outer turning point electron transfer to NO Electron re-transfer to metal at smaller bond lengths
 Does this mean multiquantum relaxation on Au excites a single electron? n If, for example, transient NO- is formed q Dipole derivative for NOduring large amplitude vibration is quite large n q ~8 Debye/Å Dipole function is quite linear. n Might couple one vibration at a time to many electrons as the anion equilibrates to the surface. Can we observe vibrationally induced electron emission from a low work function surface?
Does this mean multiquantum relaxation on Au excites a single electron? n If, for example, transient NO- is formed q Dipole derivative for NOduring large amplitude vibration is quite large n q ~8 Debye/Å Dipole function is quite linear. n Might couple one vibration at a time to many electrons as the anion equilibrates to the surface. Can we observe vibrationally induced electron emission from a low work function surface?
 If you can convert such large amounts of vibrational energy to electronic excitation… …Can you induce electron emission form a low work-function surface?
If you can convert such large amounts of vibrational energy to electronic excitation… …Can you induce electron emission form a low work-function surface?
 Details of the Apparatus View looking down from above View from the molecular beam Photoemission probe of Cs-dose Beam exposure to low work-function surface
Details of the Apparatus View looking down from above View from the molecular beam Photoemission probe of Cs-dose Beam exposure to low work-function surface
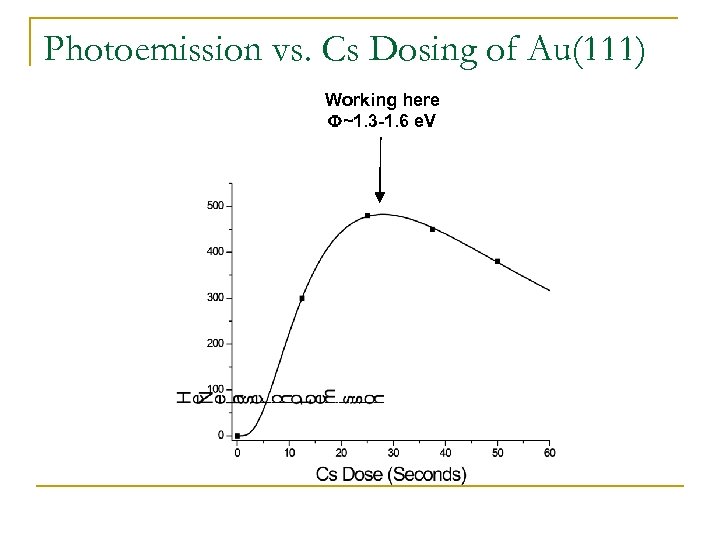 Photoemission vs. Cs Dosing of Au(111) Working here F~1. 3 -1. 6 e. V
Photoemission vs. Cs Dosing of Au(111) Working here F~1. 3 -1. 6 e. V
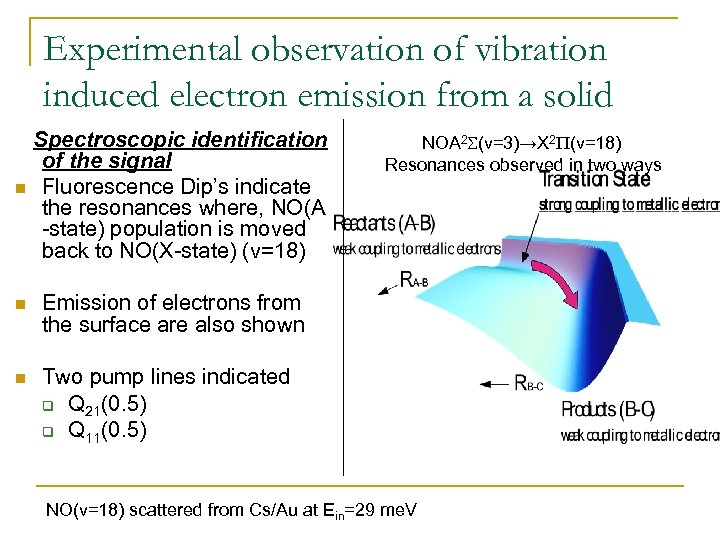 Experimental observation of vibration induced electron emission from a solid n Spectroscopic identification of the signal Fluorescence Dip’s indicate the resonances where, NO(A -state) population is moved back to NO(X-state) (v=18) n Emission of electrons from the surface are also shown n NOA 2 S(v=3)→X 2 P(v=18) Resonances observed in two ways Two pump lines indicated q Q 21(0. 5) q Q 11(0. 5) NO(v=18) scattered from Cs/Au at Ein=29 me. V
Experimental observation of vibration induced electron emission from a solid n Spectroscopic identification of the signal Fluorescence Dip’s indicate the resonances where, NO(A -state) population is moved back to NO(X-state) (v=18) n Emission of electrons from the surface are also shown n NOA 2 S(v=3)→X 2 P(v=18) Resonances observed in two ways Two pump lines indicated q Q 21(0. 5) q Q 11(0. 5) NO(v=18) scattered from Cs/Au at Ein=29 me. V
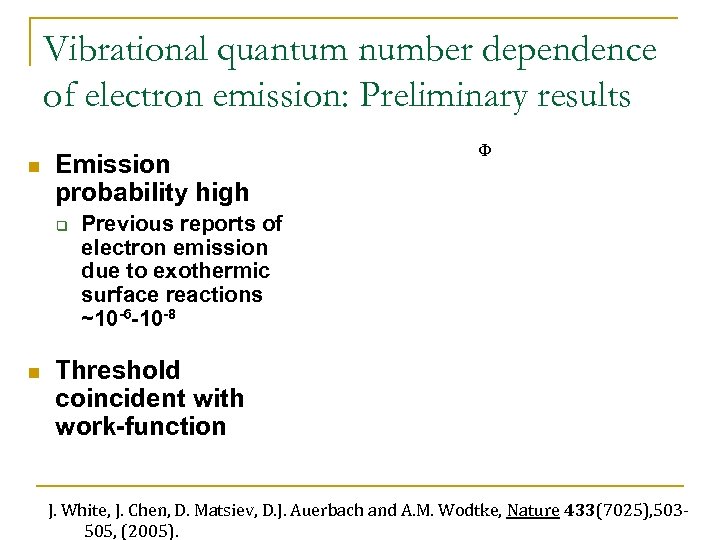 Vibrational quantum number dependence of electron emission: Preliminary results n Emission probability high q n F Previous reports of electron emission due to exothermic surface reactions ~10 -6 -10 -8 Threshold coincident with work-function J. White, J. Chen, D. Matsiev, D. J. Auerbach and A. M. Wodtke, Nature 433(7025), 503505, (2005).
Vibrational quantum number dependence of electron emission: Preliminary results n Emission probability high q n F Previous reports of electron emission due to exothermic surface reactions ~10 -6 -10 -8 Threshold coincident with work-function J. White, J. Chen, D. Matsiev, D. J. Auerbach and A. M. Wodtke, Nature 433(7025), 503505, (2005).
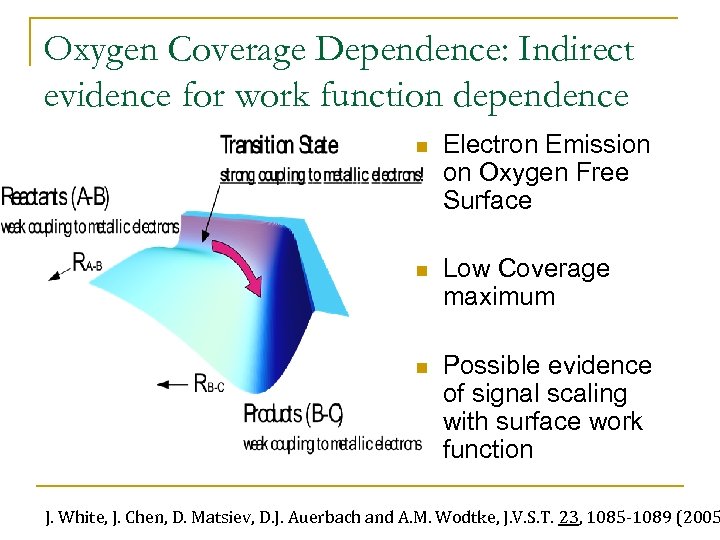 Oxygen Coverage Dependence: Indirect evidence for work function dependence n Electron Emission on Oxygen Free Surface n Low Coverage maximum n Possible evidence of signal scaling with surface work function J. White, J. Chen, D. Matsiev, D. J. Auerbach and A. M. Wodtke, J. V. S. T. 23, 1085 -1089 (2005
Oxygen Coverage Dependence: Indirect evidence for work function dependence n Electron Emission on Oxygen Free Surface n Low Coverage maximum n Possible evidence of signal scaling with surface work function J. White, J. Chen, D. Matsiev, D. J. Auerbach and A. M. Wodtke, J. V. S. T. 23, 1085 -1089 (2005
 Vibrational and Translational Influence on Trapping Few experimental measurements
Vibrational and Translational Influence on Trapping Few experimental measurements
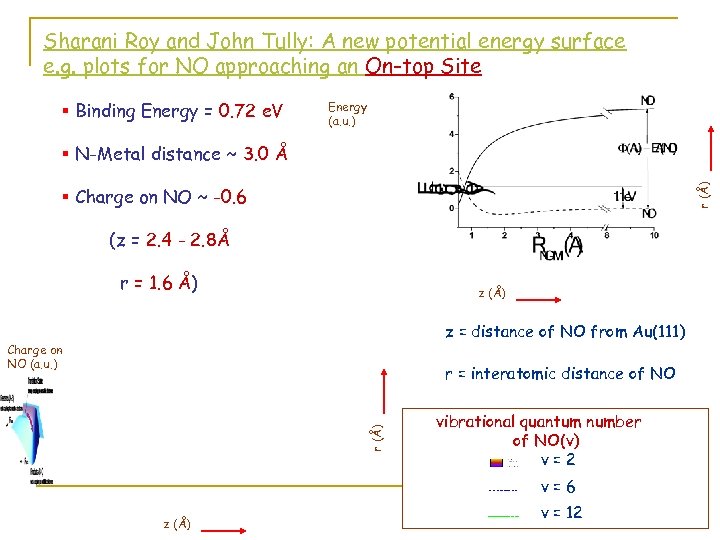 Sharani Roy and John Tully: A new potential energy surface e. g. plots for NO approaching an On-top Site § Binding Energy = 0. 72 e. V Energy (a. u. ) r (Å) § N-Metal distance ~ 3. 0 Å § Charge on NO ~ -0. 6 (z = 2. 4 - 2. 8Å r = 1. 6 Å) z (Å) z = distance of NO from Au(111) Charge on NO (a. u. ) r (Å) r = interatomic distance of NO vibrational quantum number of NO(v) v=2 v=6 z (Å) v = 12
Sharani Roy and John Tully: A new potential energy surface e. g. plots for NO approaching an On-top Site § Binding Energy = 0. 72 e. V Energy (a. u. ) r (Å) § N-Metal distance ~ 3. 0 Å § Charge on NO ~ -0. 6 (z = 2. 4 - 2. 8Å r = 1. 6 Å) z (Å) z = distance of NO from Au(111) Charge on NO (a. u. ) r (Å) r = interatomic distance of NO vibrational quantum number of NO(v) v=2 v=6 z (Å) v = 12
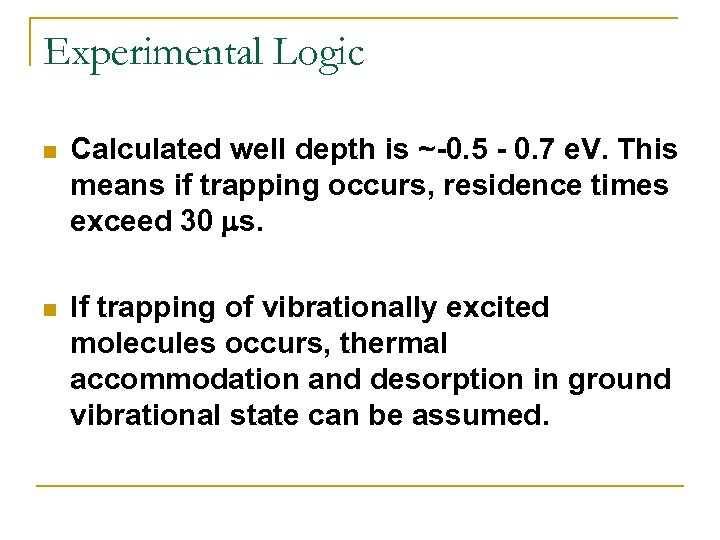 Experimental Logic n Calculated well depth is ~-0. 5 - 0. 7 e. V. This means if trapping occurs, residence times exceed 30 ms. n If trapping of vibrationally excited molecules occurs, thermal accommodation and desorption in ground vibrational state can be assumed.
Experimental Logic n Calculated well depth is ~-0. 5 - 0. 7 e. V. This means if trapping occurs, residence times exceed 30 ms. n If trapping of vibrationally excited molecules occurs, thermal accommodation and desorption in ground vibrational state can be assumed.
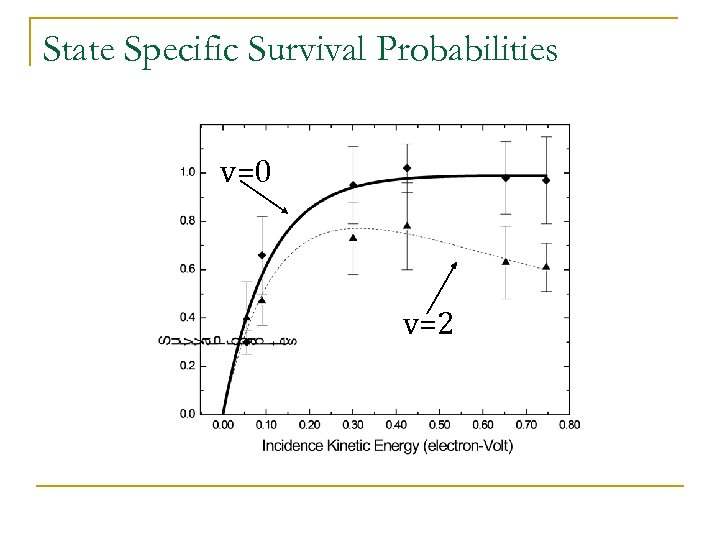 State Specific Survival Probabilities v=0 v=2
State Specific Survival Probabilities v=0 v=2
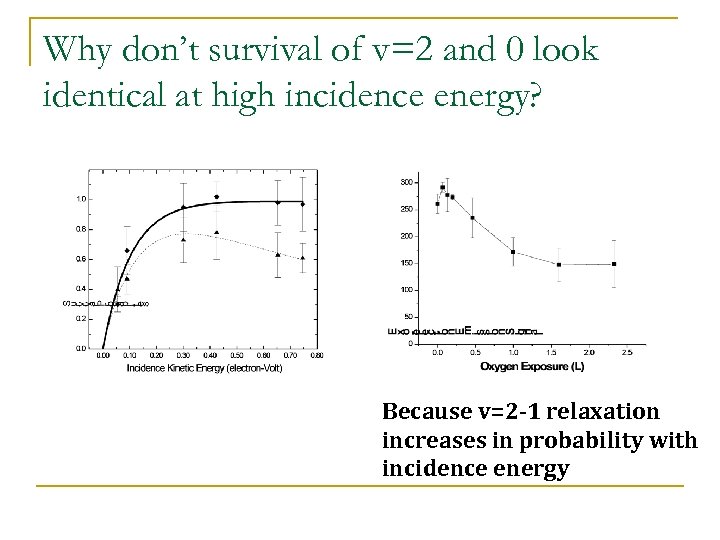 Why don’t survival of v=2 and 0 look identical at high incidence energy? Because v=2 -1 relaxation increases in probability with incidence energy
Why don’t survival of v=2 and 0 look identical at high incidence energy? Because v=2 -1 relaxation increases in probability with incidence energy
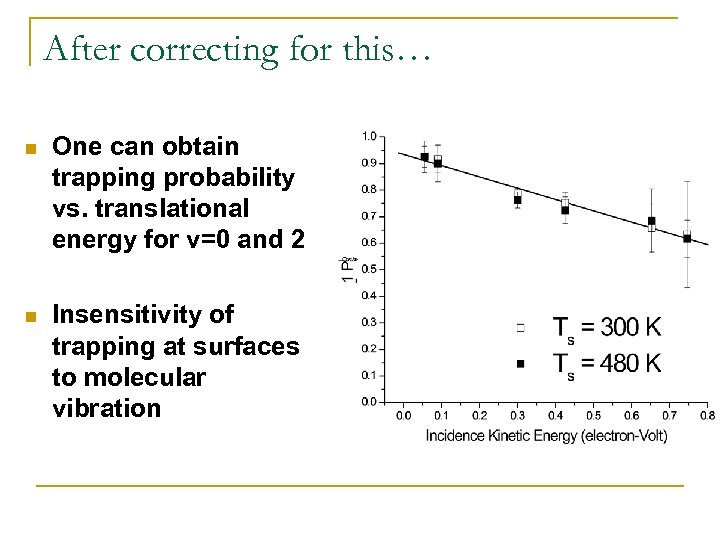 After correcting for this… n One can obtain trapping probability vs. translational energy for v=0 and 2 n Insensitivity of trapping at surfaces to molecular vibration
After correcting for this… n One can obtain trapping probability vs. translational energy for v=0 and 2 n Insensitivity of trapping at surfaces to molecular vibration


