bcd691b43a46f130f39a7d0d2128326e.ppt
- Количество слайдов: 47

State of the Field: Microbicide Science and Trials M 2008 Preconference Workshop Delhi, India Sharon Hillier, Ph. D University of Pittsburgh Microbicide Trials Network February 24, 2008

A microbicide is a product that can be applied to the vaginal or rectal mucosa with the intention of preventing or significantly reducing the transmission of sexually transmitted infections including HIV infection
Topical Microbicides ¢ ¢ ¢ Ideal if option available for use with sex or daily (independent of sex) Local drug levels can be high and systemic exposure low Ø Maximum drug: virus level Ø Lower chance for adverse events related to systemic drug No one prevention option will satisfy all; multiple approaches needed to address acceptability preferences

Types of Microbicide Delivery Platforms Vaginal applicator for gel Vaginal ring Vaginal film ¢ Ideally long acting, safe, effective, low cost and user-friendly ¢ Potential for combinations of drugs to increase effectiveness
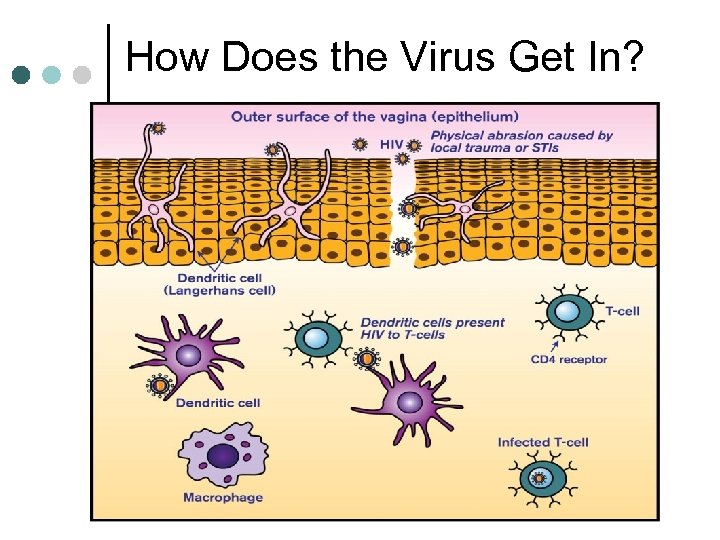
How Does the Virus Get In?
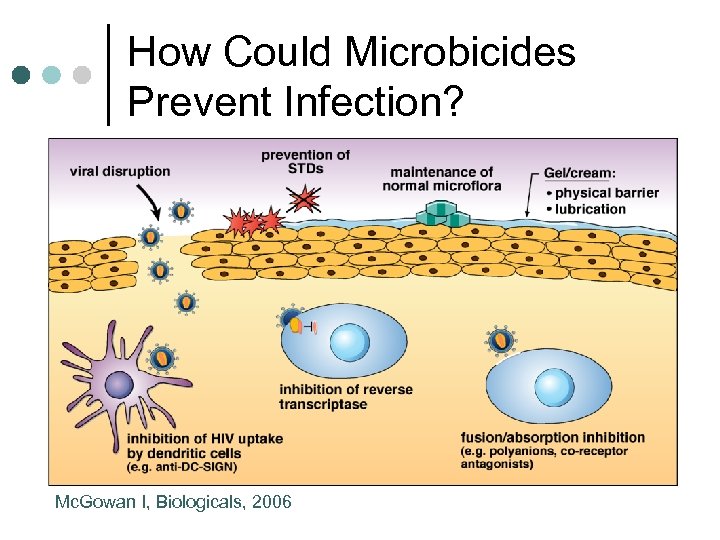
How Could Microbicides Prevent Infection? Mc. Gowan I, Biologicals, 2006

Who is Developing Microbicides?

Microbicide Manufacturers ¢ Gilead Sciences Inc. ¢ Star. Pharma Holdings Ltd ¢ Indevus Pharmaceuticals Inc. ¢ Reprotect LLC ¢ Tibotec Pharmaceuticals Ltd

The Microbicide Trials Network ¢ ¢ ¢ NIH funded network PI: Dr. Sharon Hillier Founded in 2006 Based in Pittsburgh Domestic (n=5) and international (n=10) sites funded directly by NIH Conducting Phase 1, 2 and 2 B microbicide studies http: //www. mtnstopshiv. org/ MTN does not do any product development or formulation
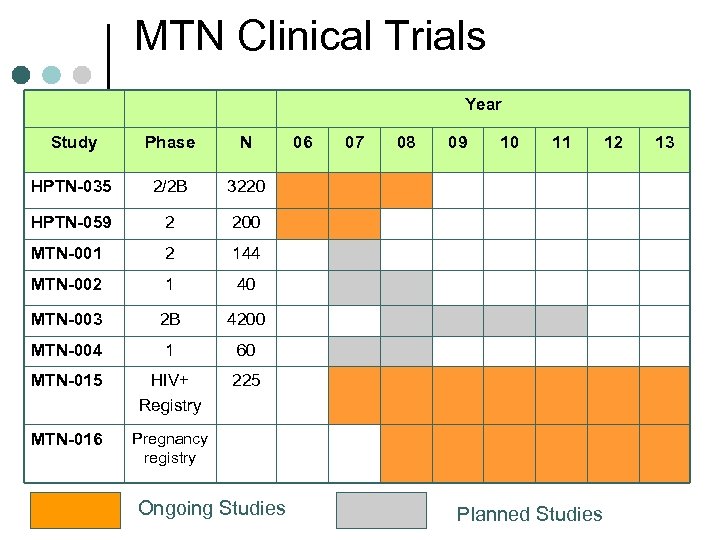
MTN Clinical Trials Year Study Phase N HPTN-035 2/2 B 3220 HPTN-059 2 200 MTN-001 2 144 MTN-002 1 40 MTN-003 2 B 4200 MTN-004 1 60 MTN-015 HIV+ Registry 225 MTN-016 Pregnancy registry Ongoing Studies 06 07 08 09 10 11 Planned Studies 12 13
CONRAD ¢ ¢ Established in 1986 Executive Director: Henry Gabelnick Strategic focus: l l Contraception Prevention of sexual transmission of HIV/AIDS and other infections. http: //www. conrad. org/ Product Portfolio • • • Cellulose sulfate Tenofovir UC-781

International Partnership for Microbicides (IPM) ¢ ¢ IPM is a non-profit product development partnership (PDP) established in 2002 to prevent HIV transmission by accelerating the development and availability of a safe and effective microbicide for use by women in developing countries CEO: Zeda Rosenberg Product Portfolio • • http: //www. ipm-microbicides. org TMC-120 Tenofovir IPM does product formulation and scale-up research

United Kingdom Microbicides Development Program (MDP) ¢ ¢ ¢ Funded through grants from the UK Medical Research Council (MRC) and the UK government Department for International Development (DFID) Conducting the microbicide trial of PRO-2000 Lead investigators- Charles Lacey, Sheena Mc. Cormack http: //www. mdp. mrc. ac. uk/ MRC MDP Trial Sites • • South Africa Tanzania Zambia Uganda

Population Council ¢ Conducts research worldwide to improve policies, programs, and products in three areas l l l ¢ ¢ ¢ HIV and AIDS Poverty, gender, and youth Reproductive health. Just released data from Phase 3 study of Carraguard® Ongoing research program with combination microbicide – PC 815 Director-Naomi Rutenberg Carraguard® Gel http: //www. popcouncil. org/
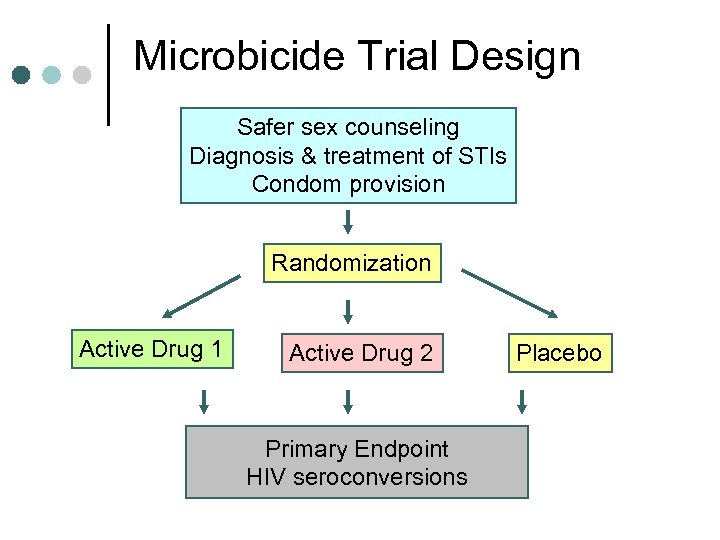
Microbicide Trial Design Safer sex counseling Diagnosis & treatment of STIs Condom provision Randomization Active Drug 1 Active Drug 2 Primary Endpoint HIV seroconversions Placebo
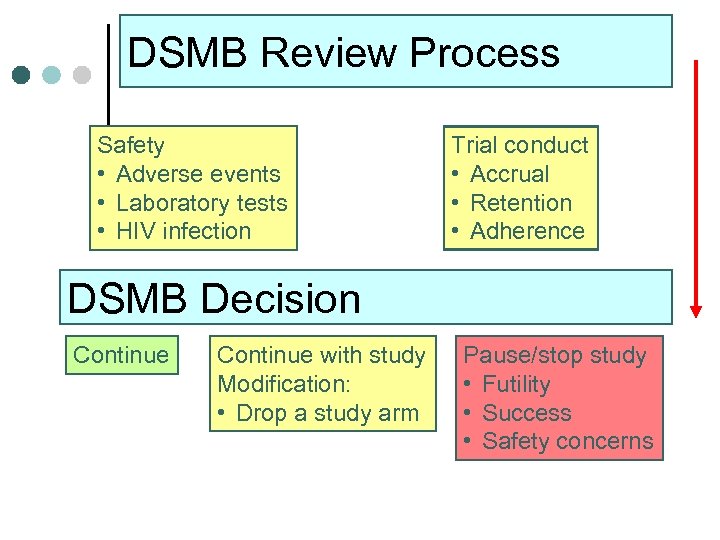
DSMB Review Process Safety • Adverse events • Laboratory tests • HIV infection Trial conduct • Accrual • Retention • Adherence DSMB Decision Continue with study Modification: • Drop a study arm Pause/stop study • Futility • Success • Safety concerns

Reasons for Premature Study Discontinuation ¢ ¢ ¢ Overwhelming evidence of efficacy Safety concerns Futility l Inability to achieve primary study endpoint New treatments or prevention strategies emerge that change clinical equipoise Economic considerations Social or political issues

Why Were Some Studies Stopped by the DSMB? ¢ C 31 G (Savvy™) l l ¢ Stopped due to futility Insufficient HIV seroconversions (endpoints) to determine whether product effective Cellulose sulfate (CS) studies l CONRAD study stopped because of safety concerns • Unequal distribution of HIV endpoints • CS group had more endpoints than anticipated • CS might increase risk of HIV transmission l FHI study showed no increased risk of HIV acquisition but also no evidence of efficacy (benefit) http: //www. global-campaign. org/CS-background. htm#interim

Population Council Study of Carraguard ü ü ü USAID & Gates Foundation Carraguard vs placebo S Africa (Durban; Cape Town and Medunsa) 6203 women enrolled First phase 3 trial of microbicides to be completed Safe but not effective Can now be used as a delivery vehicle for next generation product

Effectiveness Studies of Savvy (C 31 G), Cellulose Sulfate, and Carraguard PRODUCT STUDY OUTCOME Savvy (C 31 G) Trial stopped due to futility, trend toward harm in frequent users Cellulose Sulfate CONRAD FHI Carraguard Stopped, evidence of harm No evidence of harm Trial completed, no benefit
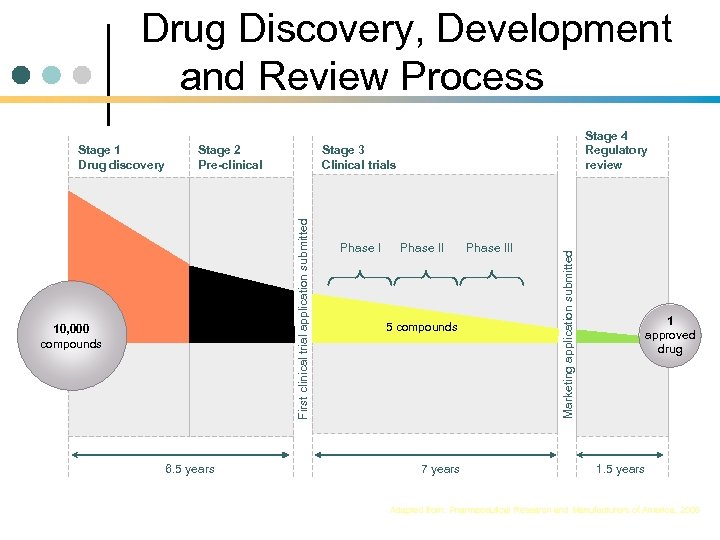
Drug Discovery, Development and Review Process 250 compounds 10, 000 compounds 6. 5 years Stage 3 Clinical trials Phase II 5 compounds 7 years Phase III Marketing application submitted Stage 2 Pre-clinical First clinical trial application submitted Stage 1 Drug discovery Stage 4 Regulatory review 1 approved drug 1. 5 years Adapted from: Pharmaceutical Research and Manufacturers of America, 2006
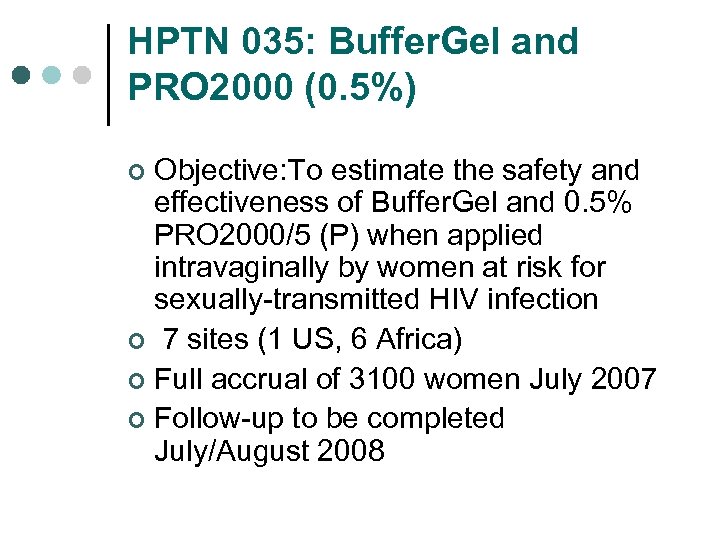
HPTN 035: Buffer. Gel and PRO 2000 (0. 5%) Objective: To estimate the safety and effectiveness of Buffer. Gel and 0. 5% PRO 2000/5 (P) when applied intravaginally by women at risk for sexually-transmitted HIV infection ¢ 7 sites (1 US, 6 Africa) ¢ Full accrual of 3100 women July 2007 ¢ Follow-up to be completed July/August 2008 ¢
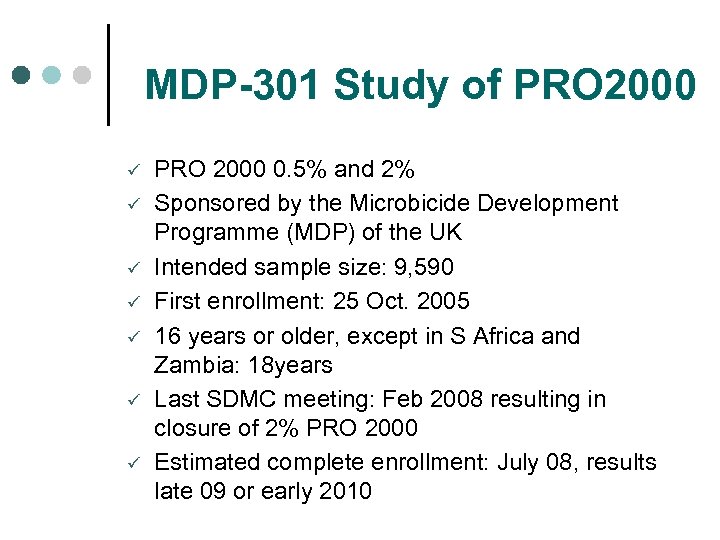
MDP-301 Study of PRO 2000 ü ü ü ü PRO 2000 0. 5% and 2% Sponsored by the Microbicide Development Programme (MDP) of the UK Intended sample size: 9, 590 First enrollment: 25 Oct. 2005 16 years or older, except in S Africa and Zambia: 18 years Last SDMC meeting: Feb 2008 resulting in closure of 2% PRO 2000 Estimated complete enrollment: July 08, results late 09 or early 2010
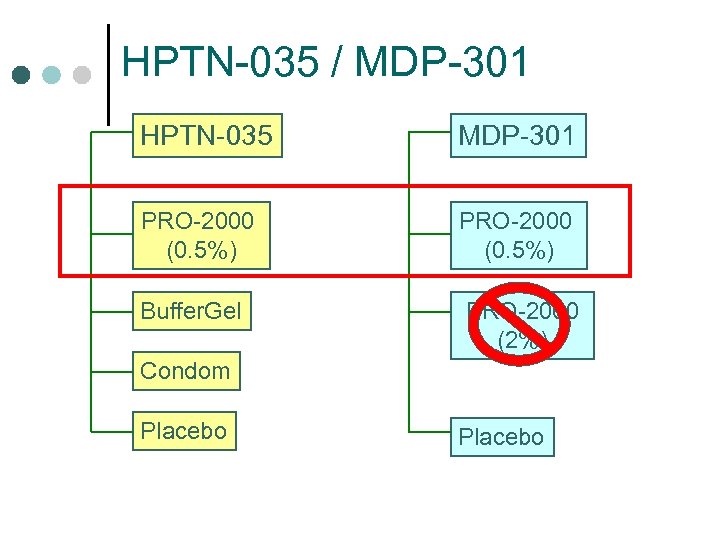
HPTN-035 / MDP-301 HPTN-035 MDP-301 PRO-2000 (0. 5%) Buffer. Gel PRO-2000 (2%) Condom Placebo

The Ideal HIV Prevention Drug ¢ Pr. EP = Pre-Exposure Prophylaxis l l oral, vaginal, rectal Specific antiretroviral microbicide One dose daily – or less ¢ Potent, good tissue penetration at site of infection ¢ Well tolerated, safe (including pregnancy) ¢ Minimal drug interactions ¢ Minimal resistance, preserve options ¢ Affordable: drug cost and monitoring ¢

Topical Microbicides Containing Antiretroviral Drugs = Topical Pr. EP ¢ ¢ ¢ Products that can be applied topically for prevention of HIV. Ideal if option available for use with sex or daily (independent of sex) Local drug levels can be high and systemic exposure low Ø Maximum drug: virus level Ø Lower chance for adverse events related to systemic drug Ø May be safer for pregnant or breastfeeding women
ARV-Based Microbicides Tenofovir MIV UC-781 TMC 120 (Dapivirine)

1% Tenofovir Vaginal Gel ¢ Active ingredient is tenofovir, an antiretroviral ¢ Has specific action against HIV and proven safety and activity as a therapeutic agent ¢ Provided in pre-filled applicators ¢ Low levels of drug in the blood ¢ Low frequency of side effects
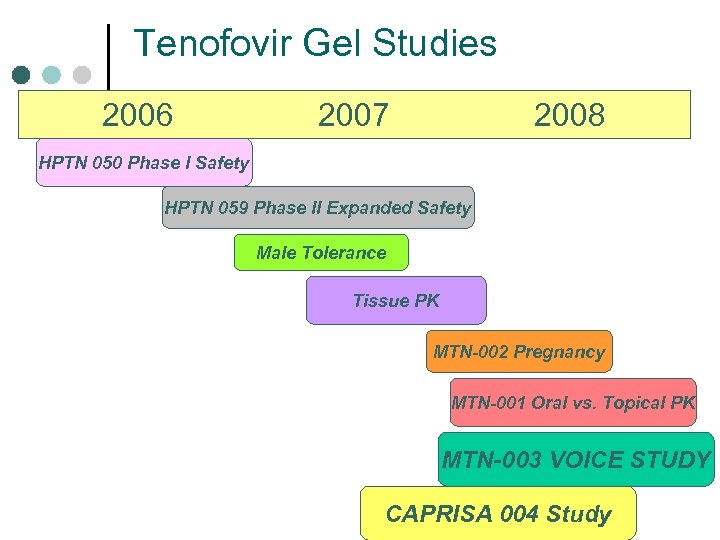
Tenofovir Gel Studies 2006 2007 2008 HPTN 050 Phase I Safety HPTN 059 Phase II Expanded Safety Male Tolerance Tissue PK MTN-002 Pregnancy MTN-001 Oral vs. Topical PK MTN-003 VOICE STUDY CAPRISA 004 Study
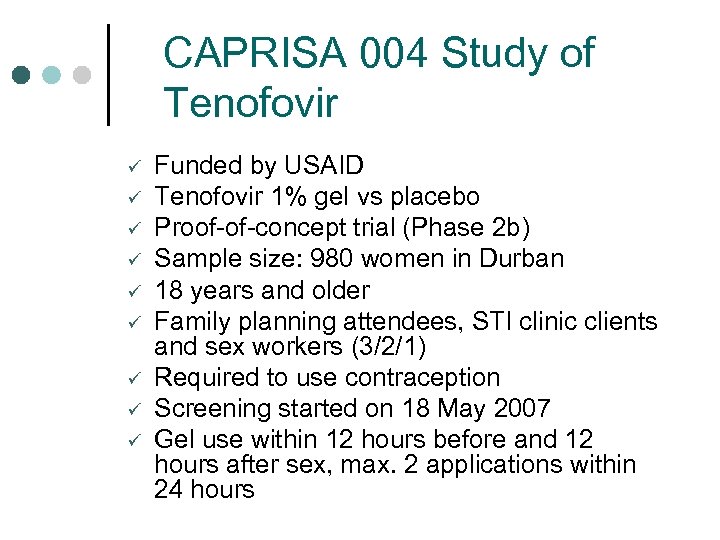
CAPRISA 004 Study of Tenofovir ü ü ü ü ü Funded by USAID Tenofovir 1% gel vs placebo Proof-of-concept trial (Phase 2 b) Sample size: 980 women in Durban 18 years and older Family planning attendees, STI clinic clients and sex workers (3/2/1) Required to use contraception Screening started on 18 May 2007 Gel use within 12 hours before and 12 hours after sex, max. 2 applications within 24 hours

Coital Dosing in CAPRISA 004 Participants advised to use gel which is in single-use, pre-filled applicators, as follows: ¢ ¢ ¢ Coitally dependent use - 2 doses of gel per sex act Participants asked to apply the first dose of the assigned gel within 12 hours prior to coitus and to apply a second dose as soon as possible, within 12 hours, after coitus. Not more than two doses of gel in a 24 -hour period.

The VOICE Study: Vaginal and Oral Interventions to Control the Epidemic ¢ Phase IIb trial with five study groups testing two different HIV prevention approaches in women: - A once-a-day antiretroviral tablet (Pr. EP) - A once-a-day application of a vaginal gel ¢ 4, 200 women to be enrolled at 10 centers in Africa ¢ Target start date Fall 2008
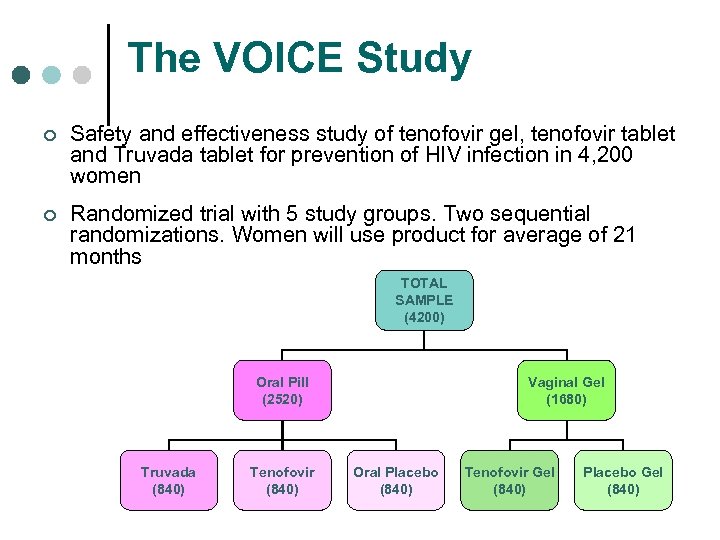
The VOICE Study ¢ Safety and effectiveness study of tenofovir gel, tenofovir tablet and Truvada tablet for prevention of HIV infection in 4, 200 women ¢ Randomized trial with 5 study groups. Two sequential randomizations. Women will use product for average of 21 months TOTAL SAMPLE (4200) Oral Pill (2520) Truvada (840) Tenofovir (840) Vaginal Gel (1680) Oral Placebo (840) Tenofovir Gel (840) Placebo Gel (840)
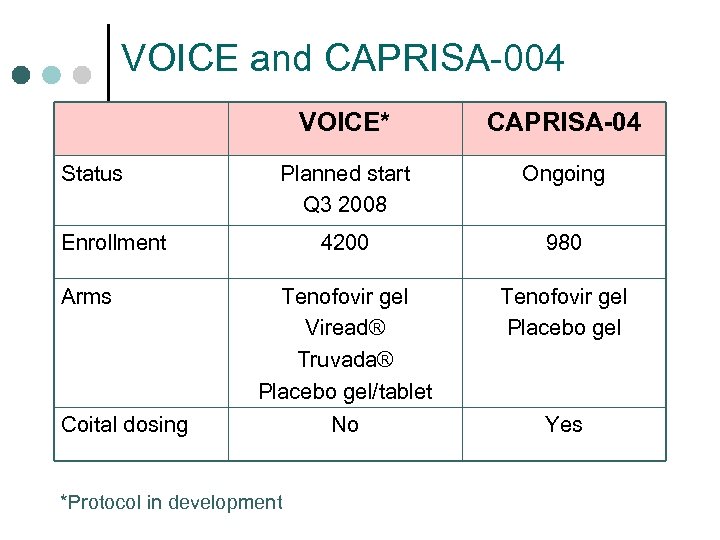
VOICE and CAPRISA-004 VOICE* Status CAPRISA-04 Planned start Q 3 2008 Ongoing 4200 980 Tenofovir gel Viread® Truvada® Placebo gel/tablet Tenofovir gel Placebo gel No Yes Enrollment Arms Coital dosing *Protocol in development
Why VOICE? Tenofovir Gel Truvada Which is safer? Which is effective? Which will women use?

Why a head to head trial or oral vs vaginal regimens? ¢ Each approach carries specific theoretical and operational advantages l l ¢ ¢ vaginal use may confer less systemic toxicity oral use is less closely linked to sexual practices, and can be administered by the woman without knowledge of her partner Theoretical reasons to favor either approach for efficacy and/or selection of resistance Only a head-to-head trial of these two approaches will answer this question
TMC 120 (Dapivirine) § § § § NNRTI developed by Tibotec/J&J, licensed to IPM (2004) Developed originally as therapeutic, 11 clinical studies conducted via oral administration Highly potent ARV Low cytotoxicity, non-mutagenic, non-teratogenic Easily manufactured, cheap Stable drug substance H C CH CN N IP clarity N N N CH H H Multiple dosage forms 3 3 3
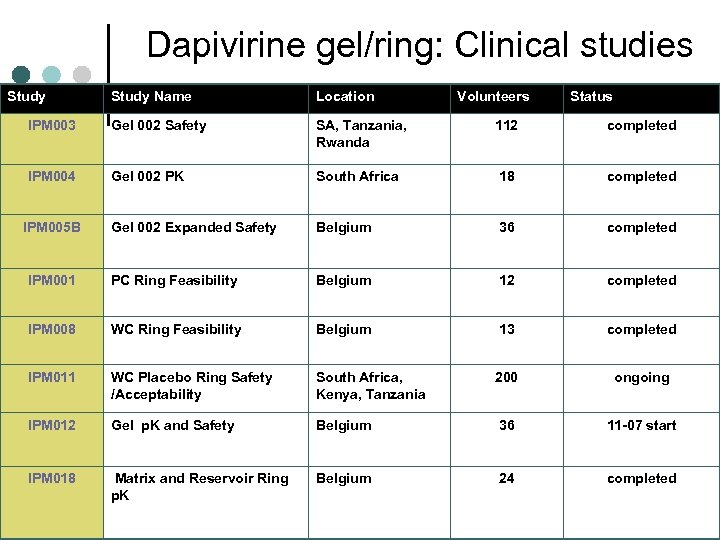
Dapivirine gel/ring: Clinical studies Study Name Location IPM 003 Gel 002 Safety SA, Tanzania, Rwanda 112 completed IPM 004 Gel 002 PK South Africa 18 completed Gel 002 Expanded Safety Belgium 36 completed IPM 001 PC Ring Feasibility Belgium 12 completed IPM 008 WC Ring Feasibility Belgium 13 completed IPM 011 WC Placebo Ring Safety /Acceptability South Africa, Kenya, Tanzania 200 ongoing IPM 012 Gel p. K and Safety Belgium 36 11 -07 start IPM 018 Matrix and Reservoir Ring p. K Belgium 24 completed IPM 005 B Volunteers Status

Planned trial: International Partnership for Microbicides ü ü TMC 120 – no final decision on formulation yet: gel or ring or both Directly monitored treatment No sample size calculations yet Planned start at the end of 2008
UC-781 ¢ ¢ ¢ Carboxanilide type of NNRTI Potent anti-HIV-1 activity (n. M range) Ø Tight binding to HIV-1 RT Ø Active against cell-free and cellassociated virus Little to no cytotoxicity (> M) Active against RT inhibitor resistant strains Ø Reduced likelihood for resistance selection Exhibits so-called “Memory Effect”
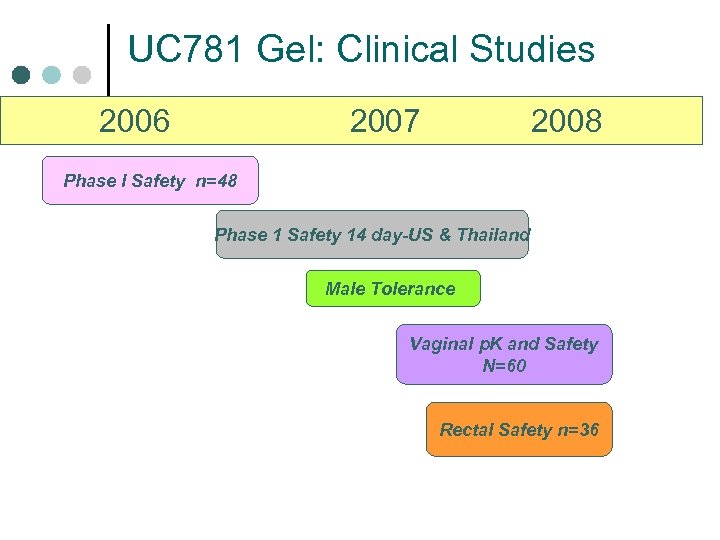
UC 781 Gel: Clinical Studies 2006 2007 2008 Phase I Safety n=48 Phase 1 Safety 14 day-US & Thailand Male Tolerance Vaginal p. K and Safety N=60 Rectal Safety n=36
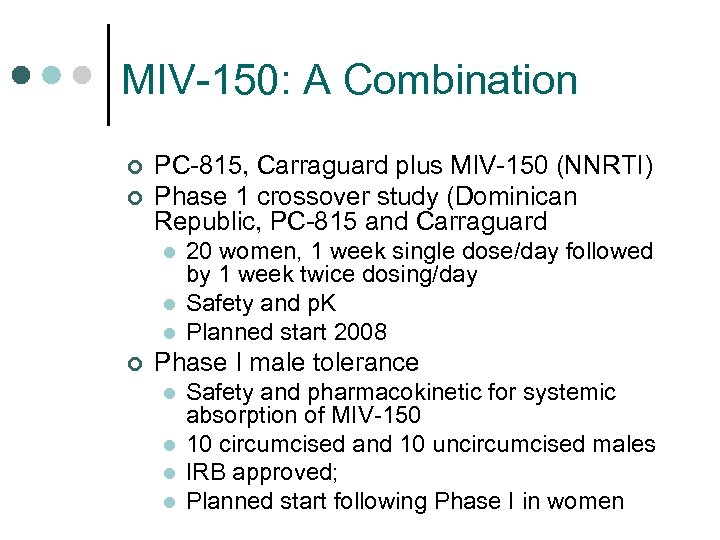
MIV-150: A Combination ¢ ¢ PC-815, Carraguard plus MIV-150 (NNRTI) Phase 1 crossover study (Dominican Republic, PC-815 and Carraguard l l l ¢ 20 women, 1 week single dose/day followed by 1 week twice dosing/day Safety and p. K Planned start 2008 Phase I male tolerance l l Safety and pharmacokinetic for systemic absorption of MIV-150 10 circumcised and 10 uncircumcised males IRB approved; Planned start following Phase I in women

Rectal Microbicides
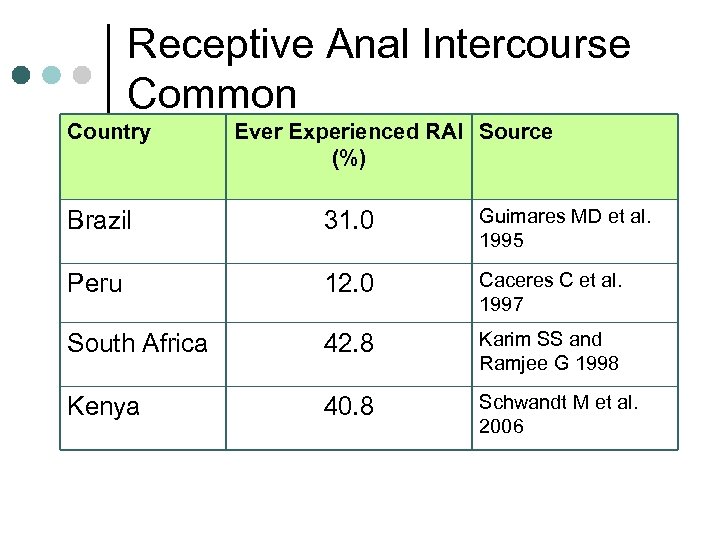
Receptive Anal Intercourse Common Country Ever Experienced RAI Source (%) Brazil 31. 0 Guimares MD et al. 1995 Peru 12. 0 Caceres C et al. 1997 South Africa 42. 8 Karim SS and Ramjee G 1998 Kenya 40. 8 Schwandt M et al. 2006
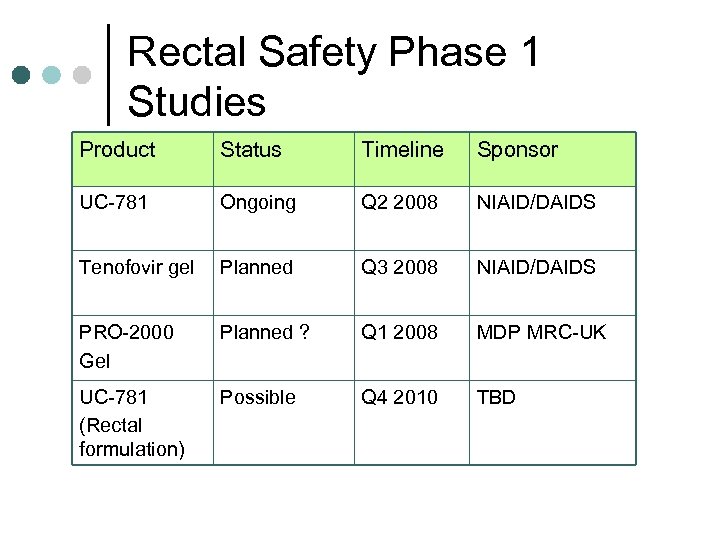
Rectal Safety Phase 1 Studies Product Status Timeline Sponsor UC-781 Ongoing Q 2 2008 NIAID/DAIDS Tenofovir gel Planned Q 3 2008 NIAID/DAIDS PRO-2000 Gel Planned ? Q 1 2008 MDP MRC-UK UC-781 (Rectal formulation) Possible Q 4 2010 TBD

State of the Field and Science First generation products have been evaluated in efficacy studies with no level of effectiveness detected to date ¢ Potent antiretroviral microbicides (topical Pr. EP) now in efficacy studies ¢ Oral versus topical microbicide use is a key scientific question ¢ Rectal safety studies have started ¢ Community engagement in research agenda is more critical than ever ¢

Ten years ago, 1 percent of women in South Africa had contracted HIV; today the number is 25 percent. These women are living a nightmare, but we in rich countries are the ones who have to wake up. We need to develop prevention tools that can give women a chance to defend themselves. We need to deliver them as soon as they’re available, and we need to deploy now the prevention tools we already have.
bcd691b43a46f130f39a7d0d2128326e.ppt