1ce554d66a383143094d74f7a4fa6991.ppt
- Количество слайдов: 40
 State HIE Technical Assistance: Preliminary Guidance for Program Participants on Electronic Prescribing August 20 th, 2010
State HIE Technical Assistance: Preliminary Guidance for Program Participants on Electronic Prescribing August 20 th, 2010
 e. Prescribing encompasses many processes… • Checking pharmacy benefit eligibility • Reviewing a patient’s formulary and benefit information, including prior authorization requirements, based upon eligibility status • Applying clinical decision support (CDS) tools such as dosing calculators or rules to avoid errors or identify potential contraindications • Generating a medication prescription or a prescription renewal using a software application that includes computerized provider order entry (CPOE) capabilities • Maintaining active medication and medication allergy lists • Completing prior authorization requirements and receiving prior authorization approval • Obtaining and reviewing medication history and fill status information from multiple sources • Receiving and responding to medication refill requests • Electronically transmitting a prescription to a pharmacy (and related bidirectional information exchange) Our primary focus today 1 Program Information Notice (PIN) available at: http: //www. healthit. hhs. gov/state. HIE 2
e. Prescribing encompasses many processes… • Checking pharmacy benefit eligibility • Reviewing a patient’s formulary and benefit information, including prior authorization requirements, based upon eligibility status • Applying clinical decision support (CDS) tools such as dosing calculators or rules to avoid errors or identify potential contraindications • Generating a medication prescription or a prescription renewal using a software application that includes computerized provider order entry (CPOE) capabilities • Maintaining active medication and medication allergy lists • Completing prior authorization requirements and receiving prior authorization approval • Obtaining and reviewing medication history and fill status information from multiple sources • Receiving and responding to medication refill requests • Electronically transmitting a prescription to a pharmacy (and related bidirectional information exchange) Our primary focus today 1 Program Information Notice (PIN) available at: http: //www. healthit. hhs. gov/state. HIE 2
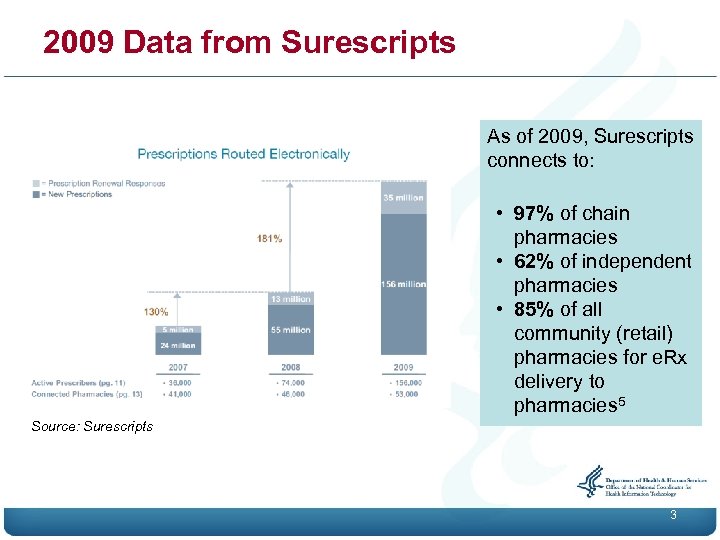 2009 Data from Surescripts As of 2009, Surescripts connects to: • 97% of chain pharmacies • 62% of independent pharmacies • 85% of all community (retail) pharmacies for e. Rx delivery to pharmacies 5 Source: Surescripts 3
2009 Data from Surescripts As of 2009, Surescripts connects to: • 97% of chain pharmacies • 62% of independent pharmacies • 85% of all community (retail) pharmacies for e. Rx delivery to pharmacies 5 Source: Surescripts 3
 Stage 1 Meaningful Use Definitions and Standards Stage 1 Meaningful Use definition 2 A certified EHR (or e. Rx module) must… “generate and transmit permissible prescriptions electronically. . . using certified EHR technology. ” • The concept of “permissible” prescriptions refers to restrictions on the electronic prescribing of controlled substances (EPCS). • The e. Rx requirement does not apply to eligible hospitals or critical access hospitals (CAHs) as part of the 2011 Stage 1 objectives, though it will likely be a component of future stage objectives 4
Stage 1 Meaningful Use Definitions and Standards Stage 1 Meaningful Use definition 2 A certified EHR (or e. Rx module) must… “generate and transmit permissible prescriptions electronically. . . using certified EHR technology. ” • The concept of “permissible” prescriptions refers to restrictions on the electronic prescribing of controlled substances (EPCS). • The e. Rx requirement does not apply to eligible hospitals or critical access hospitals (CAHs) as part of the 2011 Stage 1 objectives, though it will likely be a component of future stage objectives 4
 Stage 1 Meaningful Use Definitions and Standards (cont. ) • The final rule on MU also requires CPOE by EPs, eligible hospitals and CAHs POE C e. Rx – CPOE is the provider's use of computer assistance to directly enter medical orders from a computer or mobile device. • The final rule limits the requirement for CPOE in Stage 1 to medication orders for both EPs and hospitals and distinguishes between CPOE and the electronic transmission of an order (for prescriptions, e. Rx). 5
Stage 1 Meaningful Use Definitions and Standards (cont. ) • The final rule on MU also requires CPOE by EPs, eligible hospitals and CAHs POE C e. Rx – CPOE is the provider's use of computer assistance to directly enter medical orders from a computer or mobile device. • The final rule limits the requirement for CPOE in Stage 1 to medication orders for both EPs and hospitals and distinguishes between CPOE and the electronic transmission of an order (for prescriptions, e. Rx). 5
 State or SDE Responsibilities for e. Prescribing • Initiate a transparent multi-stakeholder process to set goals and conduct gap analysis • Monitor and track meaningful use HIE capabilities in the state, including the percent of pharmacies accepting electronic prescribing and refill requests • Assure trust of information sharing through a privacy and security framework for HIE • Set strategy to meet gaps in HIE capabilities for meaningful use • Ensure consistency with national policies and standards • Align with Medicaid and public health programs Source: 2010 Program Information Notice 6
State or SDE Responsibilities for e. Prescribing • Initiate a transparent multi-stakeholder process to set goals and conduct gap analysis • Monitor and track meaningful use HIE capabilities in the state, including the percent of pharmacies accepting electronic prescribing and refill requests • Assure trust of information sharing through a privacy and security framework for HIE • Set strategy to meet gaps in HIE capabilities for meaningful use • Ensure consistency with national policies and standards • Align with Medicaid and public health programs Source: 2010 Program Information Notice 6
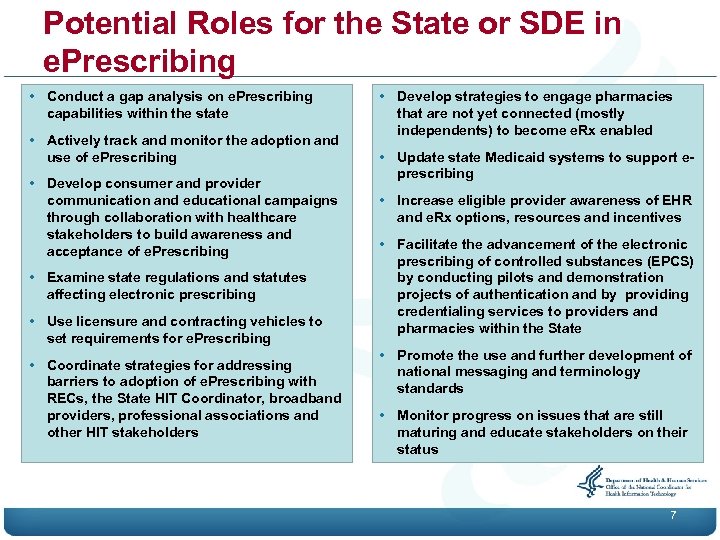 Potential Roles for the State or SDE in e. Prescribing • Conduct a gap analysis on e. Prescribing capabilities within the state • Actively track and monitor the adoption and use of e. Prescribing • Develop consumer and provider communication and educational campaigns through collaboration with healthcare stakeholders to build awareness and acceptance of e. Prescribing • Examine state regulations and statutes affecting electronic prescribing • Use licensure and contracting vehicles to set requirements for e. Prescribing • Coordinate strategies for addressing barriers to adoption of e. Prescribing with RECs, the State HIT Coordinator, broadband providers, professional associations and other HIT stakeholders • Develop strategies to engage pharmacies that are not yet connected (mostly independents) to become e. Rx enabled • Update state Medicaid systems to support eprescribing • Increase eligible provider awareness of EHR and e. Rx options, resources and incentives • Facilitate the advancement of the electronic prescribing of controlled substances (EPCS) by conducting pilots and demonstration projects of authentication and by providing credentialing services to providers and pharmacies within the State • Promote the use and further development of national messaging and terminology standards • Monitor progress on issues that are still maturing and educate stakeholders on their status 7
Potential Roles for the State or SDE in e. Prescribing • Conduct a gap analysis on e. Prescribing capabilities within the state • Actively track and monitor the adoption and use of e. Prescribing • Develop consumer and provider communication and educational campaigns through collaboration with healthcare stakeholders to build awareness and acceptance of e. Prescribing • Examine state regulations and statutes affecting electronic prescribing • Use licensure and contracting vehicles to set requirements for e. Prescribing • Coordinate strategies for addressing barriers to adoption of e. Prescribing with RECs, the State HIT Coordinator, broadband providers, professional associations and other HIT stakeholders • Develop strategies to engage pharmacies that are not yet connected (mostly independents) to become e. Rx enabled • Update state Medicaid systems to support eprescribing • Increase eligible provider awareness of EHR and e. Rx options, resources and incentives • Facilitate the advancement of the electronic prescribing of controlled substances (EPCS) by conducting pilots and demonstration projects of authentication and by providing credentialing services to providers and pharmacies within the State • Promote the use and further development of national messaging and terminology standards • Monitor progress on issues that are still maturing and educate stakeholders on their status 7
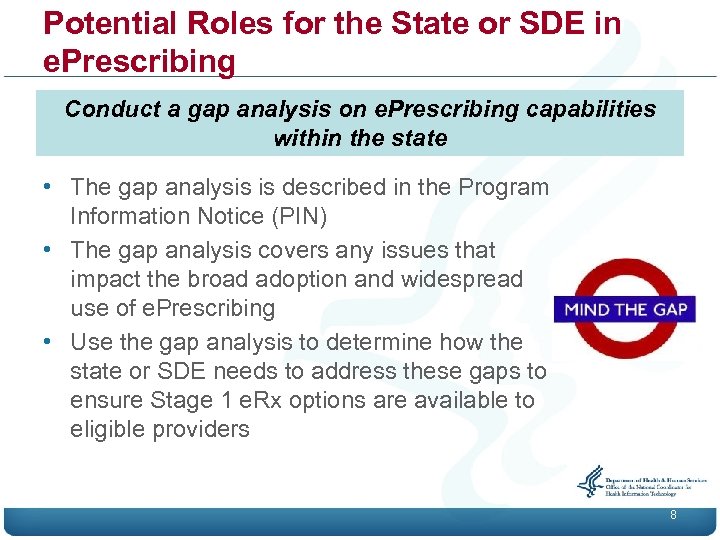 Potential Roles for the State or SDE in e. Prescribing Conduct a gap analysis on e. Prescribing capabilities within the state • The gap analysis is described in the Program Information Notice (PIN) • The gap analysis covers any issues that impact the broad adoption and widespread use of e. Prescribing • Use the gap analysis to determine how the state or SDE needs to address these gaps to ensure Stage 1 e. Rx options are available to eligible providers 8
Potential Roles for the State or SDE in e. Prescribing Conduct a gap analysis on e. Prescribing capabilities within the state • The gap analysis is described in the Program Information Notice (PIN) • The gap analysis covers any issues that impact the broad adoption and widespread use of e. Prescribing • Use the gap analysis to determine how the state or SDE needs to address these gaps to ensure Stage 1 e. Rx options are available to eligible providers 8
 Potential Roles for the State or SDE in e. Prescribing Actively track and monitor the adoption and use of e. Prescribing Gym Membership Adoption • Set public targets for adoption and use of e. Prescribing • Use the many levers available to move your state forward to those targets. Use 9
Potential Roles for the State or SDE in e. Prescribing Actively track and monitor the adoption and use of e. Prescribing Gym Membership Adoption • Set public targets for adoption and use of e. Prescribing • Use the many levers available to move your state forward to those targets. Use 9
 Potential Roles for the State or SDE in e. Prescribing Develop consumer and provider communication and educational campaigns through collaboration with healthcare stakeholders to build awareness and acceptance of e. Prescribing • Patients may be confused about how their medication is received at the pharmacy and not understand e. Rx benefits • Most providers will have neither the time nor the detailed knowledge of the patient experience to explain the e. Rx process to patients • Get Connected Campaign 7 is an example e. Rx educational program 10
Potential Roles for the State or SDE in e. Prescribing Develop consumer and provider communication and educational campaigns through collaboration with healthcare stakeholders to build awareness and acceptance of e. Prescribing • Patients may be confused about how their medication is received at the pharmacy and not understand e. Rx benefits • Most providers will have neither the time nor the detailed knowledge of the patient experience to explain the e. Rx process to patients • Get Connected Campaign 7 is an example e. Rx educational program 10
 Potential Roles for the State or SDE in e. Prescribing Examine state regulations and statutes affecting electronic prescribing • States may enact more restrictive rules for the electronic prescribing of controlled substances (EPCS) as the rules for EPCS recently promulgated by the DOJ establish a floor, not a ceiling, for how controlled substances may be electronically prescribed • Each State or SDE must understand any implications of State-specific statutes and educate healthcare stakeholders • Develop consensus on optimal changes to state laws/regulations that address issues and advocate for making those changes 11
Potential Roles for the State or SDE in e. Prescribing Examine state regulations and statutes affecting electronic prescribing • States may enact more restrictive rules for the electronic prescribing of controlled substances (EPCS) as the rules for EPCS recently promulgated by the DOJ establish a floor, not a ceiling, for how controlled substances may be electronically prescribed • Each State or SDE must understand any implications of State-specific statutes and educate healthcare stakeholders • Develop consensus on optimal changes to state laws/regulations that address issues and advocate for making those changes 11
 Potential Roles for the State or SDE in e. Prescribing Use licensure and contracting vehicles to set requirements for e. Prescribing • State boards of pharmacy can play an influential role • At least one state (Ohio) has developed their own certification process for e. Prescribing applications to ensure that they comply with state regulations • A certification process that is limited to reviewing the unique requirements of the State can help to ensure that vendors are in compliance with state-specific rules • Consider collaborating to establish a standardized approach for articulating those unique requirements across states 12
Potential Roles for the State or SDE in e. Prescribing Use licensure and contracting vehicles to set requirements for e. Prescribing • State boards of pharmacy can play an influential role • At least one state (Ohio) has developed their own certification process for e. Prescribing applications to ensure that they comply with state regulations • A certification process that is limited to reviewing the unique requirements of the State can help to ensure that vendors are in compliance with state-specific rules • Consider collaborating to establish a standardized approach for articulating those unique requirements across states 12
 State Example: Minnesota Jennifer Fritz, MPH Minnesota Department of Health Office of Health Information Technology Jennifer. Fritz@state. mn. us 651 -201 -3662 13
State Example: Minnesota Jennifer Fritz, MPH Minnesota Department of Health Office of Health Information Technology Jennifer. Fritz@state. mn. us 651 -201 -3662 13
 Minnesota: Legislation Impacting e. Rx • • 2011 e-Prescribing Mandate [Minnesota Statute 62 J. 497 (2008)] – All providers, group purchasers, prescribers, and dispensers establish and maintain an electronic prescription drug program that complies with applicable standards by January 2011 New Minnesota Law Governing HIE [Chapter 336 (SF 2974) signed May 13, 2010] – Establishes certification requirements and oversight for organizations conducting Health Information Exchange in Minnesota – Allows open, free market for provision of HIE services – Requires State certificate of authority to operate Health Information Organizations (HIOs) or Health Data Intermediaries (HDIs) – Verifies financial sustainability of HIE service providers – Protects consumers – Protects providers
Minnesota: Legislation Impacting e. Rx • • 2011 e-Prescribing Mandate [Minnesota Statute 62 J. 497 (2008)] – All providers, group purchasers, prescribers, and dispensers establish and maintain an electronic prescription drug program that complies with applicable standards by January 2011 New Minnesota Law Governing HIE [Chapter 336 (SF 2974) signed May 13, 2010] – Establishes certification requirements and oversight for organizations conducting Health Information Exchange in Minnesota – Allows open, free market for provision of HIE services – Requires State certificate of authority to operate Health Information Organizations (HIOs) or Health Data Intermediaries (HDIs) – Verifies financial sustainability of HIE service providers – Protects consumers – Protects providers
 Minnesota Statewide Implementation Plan and Companion Guides § A Prescription for Meeting Minnesota’s 2015 Interoperable Electronic Health Record Mandate: A Statewide Implementation Plan (2008 Edition) § Guide 1: Addressing Common Barriers to the Adoption of EHRs Released 2008 § Guide 2: Standards Recommended to Achieve Interoperability in MN Released 2008, Updated June 2010 § Guide 3: A Practical Guide to e. Prescribing Released June 2009 § Guide 4: A Practical Guide to Effective Use of EHR Systems Released June 2009
Minnesota Statewide Implementation Plan and Companion Guides § A Prescription for Meeting Minnesota’s 2015 Interoperable Electronic Health Record Mandate: A Statewide Implementation Plan (2008 Edition) § Guide 1: Addressing Common Barriers to the Adoption of EHRs Released 2008 § Guide 2: Standards Recommended to Achieve Interoperability in MN Released 2008, Updated June 2010 § Guide 3: A Practical Guide to e. Prescribing Released June 2009 § Guide 4: A Practical Guide to Effective Use of EHR Systems Released June 2009
 Potential Roles for the State or SDE in e. Prescribing Coordinate strategies for addressing barriers to adoption of e. Prescribing with RECs, the State HIT Coordinator, broadband providers, professional associations and other HIT stakeholders • Coordinate with stakeholders that are involved (or need to become involved) in e. Prescribing • Work closely with RECs to facilitate adoption, implementation and meaningful use of certified EHRs (including e. Rx capabilities) among eligible providers 16
Potential Roles for the State or SDE in e. Prescribing Coordinate strategies for addressing barriers to adoption of e. Prescribing with RECs, the State HIT Coordinator, broadband providers, professional associations and other HIT stakeholders • Coordinate with stakeholders that are involved (or need to become involved) in e. Prescribing • Work closely with RECs to facilitate adoption, implementation and meaningful use of certified EHRs (including e. Rx capabilities) among eligible providers 16
 Potential Roles for the State or SDE in e. Prescribing Develop strategies to engage pharmacies that are not yet connected (mostly independents) to become e. Rx enabled—especially in areas where there are no local pharmacies with the capability • Only 62% of independent pharmacies had connected in 2009 • Use the gap analysis to identify areas where providers and patients do not have reasonable expectation of finding a pharmacy that accepts prescriptions electronically • Focus on areas of greatest need to create a critical mass of e. Rxcapable pharmacies • Consider developing special incentives to support independent pharmacy adoption of e. Prescribing 17
Potential Roles for the State or SDE in e. Prescribing Develop strategies to engage pharmacies that are not yet connected (mostly independents) to become e. Rx enabled—especially in areas where there are no local pharmacies with the capability • Only 62% of independent pharmacies had connected in 2009 • Use the gap analysis to identify areas where providers and patients do not have reasonable expectation of finding a pharmacy that accepts prescriptions electronically • Focus on areas of greatest need to create a critical mass of e. Rxcapable pharmacies • Consider developing special incentives to support independent pharmacy adoption of e. Prescribing 17
 State Example: Rhode Island Jennie Chiller Director, REC Program Management jchiller@riqi. org 401 -276 -9141 ext 278 Rhode Island Quality Institute http: //www. riqi. org RI Regional Extension Center http: //www. Doc. EHRtalk. org 18
State Example: Rhode Island Jennie Chiller Director, REC Program Management jchiller@riqi. org 401 -276 -9141 ext 278 Rhode Island Quality Institute http: //www. riqi. org RI Regional Extension Center http: //www. Doc. EHRtalk. org 18
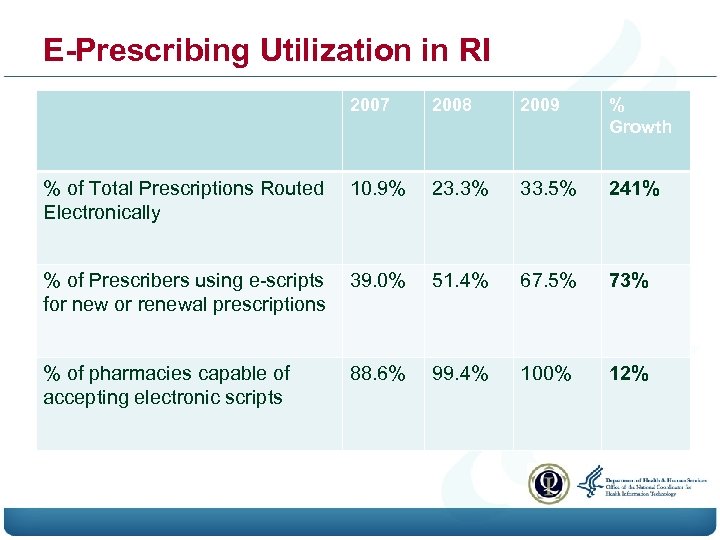 E-Prescribing Utilization in RI 2007 2008 2009 % Growth % of Total Prescriptions Routed Electronically 10. 9% 23. 3% 33. 5% 241% % of Prescribers using e-scripts for new or renewal prescriptions 39. 0% 51. 4% 67. 5% 73% % of pharmacies capable of accepting electronic scripts 88. 6% 99. 4% 100% 12%
E-Prescribing Utilization in RI 2007 2008 2009 % Growth % of Total Prescriptions Routed Electronically 10. 9% 23. 3% 33. 5% 241% % of Prescribers using e-scripts for new or renewal prescriptions 39. 0% 51. 4% 67. 5% 73% % of pharmacies capable of accepting electronic scripts 88. 6% 99. 4% 100% 12%
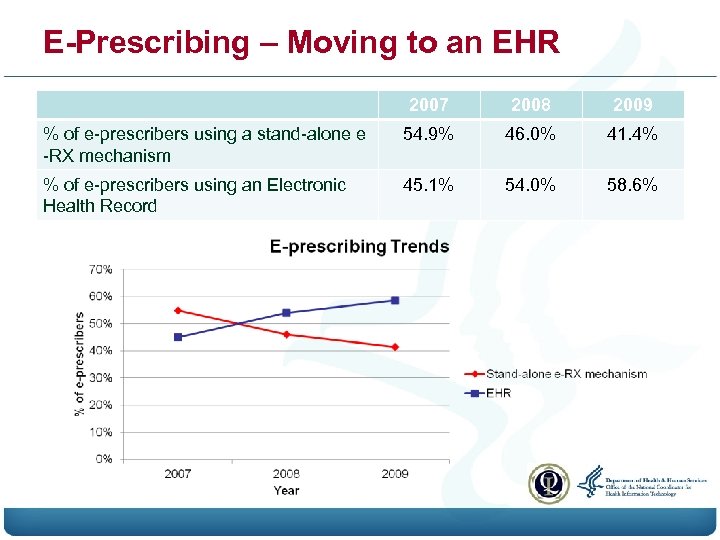 E-Prescribing – Moving to an EHR 2007 2008 2009 % of e-prescribers using a stand-alone e -RX mechanism 54. 9% 46. 0% 41. 4% % of e-prescribers using an Electronic Health Record 45. 1% 54. 0% 58. 6%
E-Prescribing – Moving to an EHR 2007 2008 2009 % of e-prescribers using a stand-alone e -RX mechanism 54. 9% 46. 0% 41. 4% % of e-prescribers using an Electronic Health Record 45. 1% 54. 0% 58. 6%
 E-Prescribing Strategies • High Prescribers Campaign – Target top 500 prescribers and understand their barriers and drivers – Implement strategies based on results in 2011 • Education and Outreach – Spread knowledge around incentives, benefits, and best practices via vendor meetings, office visits, payer -incentive matrix • Leverage Physician Champions – Share local case studies, visit provider offices, utilize Doc. EHRtalk. org • Legislation introduced by RI Department of Health – requires all practitioners to have access to eprescribing in the location(s) where they practice.
E-Prescribing Strategies • High Prescribers Campaign – Target top 500 prescribers and understand their barriers and drivers – Implement strategies based on results in 2011 • Education and Outreach – Spread knowledge around incentives, benefits, and best practices via vendor meetings, office visits, payer -incentive matrix • Leverage Physician Champions – Share local case studies, visit provider offices, utilize Doc. EHRtalk. org • Legislation introduced by RI Department of Health – requires all practitioners to have access to eprescribing in the location(s) where they practice.
 Potential Roles for the State or SDE in e. Prescribing Update state Medicaid systems to support eprescribing • Define strategies and timetables for making medication history and formulary and benefit information available to providers • Accurate and timely access to medication history and formulary data has been demonstrated to reduce duplicative and fraudulent prescriptions and help providers identify potential drug-drug interactions 8 22
Potential Roles for the State or SDE in e. Prescribing Update state Medicaid systems to support eprescribing • Define strategies and timetables for making medication history and formulary and benefit information available to providers • Accurate and timely access to medication history and formulary data has been demonstrated to reduce duplicative and fraudulent prescriptions and help providers identify potential drug-drug interactions 8 22
 Potential Roles for the State or SDE in e. Prescribing Increase eligible provider awareness of EHR and e. Rx options, resources and incentives • Enable pathways to MU by offering or building awareness of certified products that meet the requirements for an e. Rx module – Markets where there is an opportunity for a certified e. Rx module to be added to a certified EHR, since performance of MU requirements in the absence of a certified EHR is not sufficient to achieve MU • States or SDEs could provide additional motivation to EPs by negotiating discounts or other incentives for full-featured certified EHRs that include e. Rx functionality on their behalf 23
Potential Roles for the State or SDE in e. Prescribing Increase eligible provider awareness of EHR and e. Rx options, resources and incentives • Enable pathways to MU by offering or building awareness of certified products that meet the requirements for an e. Rx module – Markets where there is an opportunity for a certified e. Rx module to be added to a certified EHR, since performance of MU requirements in the absence of a certified EHR is not sufficient to achieve MU • States or SDEs could provide additional motivation to EPs by negotiating discounts or other incentives for full-featured certified EHRs that include e. Rx functionality on their behalf 23
 State Example: Florida Walt Culbertson Program Director The Center for the Advancement of Health IT A Regional Extension Center serving Northern and Rural Florida Office: 904 -230 -1336 Cell: 904 -651 -1805 walt. culbertson@advancehealthit. org http: //www. advancehealth. IT. org http: //www. chcalliance. org 24
State Example: Florida Walt Culbertson Program Director The Center for the Advancement of Health IT A Regional Extension Center serving Northern and Rural Florida Office: 904 -230 -1336 Cell: 904 -651 -1805 walt. culbertson@advancehealthit. org http: //www. advancehealth. IT. org http: //www. chcalliance. org 24
 e. Prescribe Florida • e. Prescribe Florida worked to increase patient safety and meet the needs of the Florida public by establishing a collaborative framework that helps achieve an understanding of the benefits of electronic prescribing, while fostering education and implementation efforts to accelerate physician adoption and cooperation among prescribing constituents. e. Prescribe Florida Members Steering Committee and Advisory Council Medical and Pharmacy Associations • Florida Academy of Family Physicians • Florida Hospital Association • Florida Chapter of the American College of Cardiology • Florida Chapter, American Society of Consultant Pharmacists • Florida Medical Association • Pharmacy Provider Services Corporation • Florida Pharmacy Association • Florida Osteopathic Medical Association Health Plans • Aetna • Av. Med • Blue Cross and Blue Shield of Florida • CIGNA Health. Care • Humana • Health First Healthplans • United. Healthcare Pharmacies and Pharmaceuticals • Albertsons • Astra. Zeneca • CVS • Novartis • Publix • Walgreens • Wal-Mart • Winn-Dixie Electronic Networks • • Collaborative Planning December 2006 Began Operations February 2007 – – – 1, 274 active e-prescribers (4%) in 2005 1, 210 active e-prescribers (4%) in 2006 2, 331 active e-prescribers (7%) in 2007 4, 497 active e-prescribers (14%) in 2008 7, 238 active e-prescribers (23%) in 2009 • e. Rx. Network • Surescripts • Script. Save State Agencies and Programs • Agency for Health Care Administration (AHCA) • Florida Medicaid • Florida Drug Control Office of the Governor • FMQAI - Quality Assurance Organization Other Stakeholders • University of South Florida (USF) • Florida Chapters of HIMSS • Rural Health Partnership • Well. Florida Steering Committee Members Source: Surescripts, June 2010 25
e. Prescribe Florida • e. Prescribe Florida worked to increase patient safety and meet the needs of the Florida public by establishing a collaborative framework that helps achieve an understanding of the benefits of electronic prescribing, while fostering education and implementation efforts to accelerate physician adoption and cooperation among prescribing constituents. e. Prescribe Florida Members Steering Committee and Advisory Council Medical and Pharmacy Associations • Florida Academy of Family Physicians • Florida Hospital Association • Florida Chapter of the American College of Cardiology • Florida Chapter, American Society of Consultant Pharmacists • Florida Medical Association • Pharmacy Provider Services Corporation • Florida Pharmacy Association • Florida Osteopathic Medical Association Health Plans • Aetna • Av. Med • Blue Cross and Blue Shield of Florida • CIGNA Health. Care • Humana • Health First Healthplans • United. Healthcare Pharmacies and Pharmaceuticals • Albertsons • Astra. Zeneca • CVS • Novartis • Publix • Walgreens • Wal-Mart • Winn-Dixie Electronic Networks • • Collaborative Planning December 2006 Began Operations February 2007 – – – 1, 274 active e-prescribers (4%) in 2005 1, 210 active e-prescribers (4%) in 2006 2, 331 active e-prescribers (7%) in 2007 4, 497 active e-prescribers (14%) in 2008 7, 238 active e-prescribers (23%) in 2009 • e. Rx. Network • Surescripts • Script. Save State Agencies and Programs • Agency for Health Care Administration (AHCA) • Florida Medicaid • Florida Drug Control Office of the Governor • FMQAI - Quality Assurance Organization Other Stakeholders • University of South Florida (USF) • Florida Chapters of HIMSS • Rural Health Partnership • Well. Florida Steering Committee Members Source: Surescripts, June 2010 25
 e. Prescribe Florida Approach 1. Provide Education – Prescribers – Stakeholders (Payers, PBMs, Pharmacies, Patients) – Others (Media, Government, Law Enforcement, Academic Medical, etc. ) 2. Promote Consistency – Payer and PBM information delivery • E&B • Med History • Formulary – Pharmacy receipt and processing – Vendors • Applications Functionality • Training and Implementation Support – Prescribers • How to select an application • How to implement and make successful • How to seek funding and financing alternatives 3. Promote Incentives and Funding 26
e. Prescribe Florida Approach 1. Provide Education – Prescribers – Stakeholders (Payers, PBMs, Pharmacies, Patients) – Others (Media, Government, Law Enforcement, Academic Medical, etc. ) 2. Promote Consistency – Payer and PBM information delivery • E&B • Med History • Formulary – Pharmacy receipt and processing – Vendors • Applications Functionality • Training and Implementation Support – Prescribers • How to select an application • How to implement and make successful • How to seek funding and financing alternatives 3. Promote Incentives and Funding 26
 Potential Roles for the State or SDE in e. Prescribing Facilitate the advancement of the electronic prescribing of controlled substances (EPCS) by conducting pilots and demonstration projects of authentication and by providing credentialing services to providers and pharmacies within the State • States or SDEs could choose to play a role in facilitating or offering services related to: – Identity proofing, – Issuance of authentication credentials such as digital certificates, – Certifying e. Rx applications and pharmacy information systems, – Conducting audits as required under the Do. J rule 27
Potential Roles for the State or SDE in e. Prescribing Facilitate the advancement of the electronic prescribing of controlled substances (EPCS) by conducting pilots and demonstration projects of authentication and by providing credentialing services to providers and pharmacies within the State • States or SDEs could choose to play a role in facilitating or offering services related to: – Identity proofing, – Issuance of authentication credentials such as digital certificates, – Certifying e. Rx applications and pharmacy information systems, – Conducting audits as required under the Do. J rule 27
 Potential Roles for the State or SDE in e. Prescribing Promote the use and further development of national messaging and terminology standards e. Rx Messaging e. Rx Terminology • MU final rule identified the Medicare Part D adopted standards as the required transaction standard for e. Rx 12 • For terminology, ONC has adopted a standard that requires certified applications to use “any source vocabulary that is included in Rx. Norm” 14 • Application providers use various code sets within their systems, while still promoting Rx. Norm as a crosswalk between the disparate code sets • NCPDP SCRIPT 8. 1 or NCPDP SCRIPT 10. 6 • On July 1 st, 2010, CMS issued an IFR that identifies NCPDP SCRIPT 10. 6 as a backward compatible update of the previously adopted NCPDP SCRIPT 8. 1 for Medicare Part D electronic prescribing. 13 28
Potential Roles for the State or SDE in e. Prescribing Promote the use and further development of national messaging and terminology standards e. Rx Messaging e. Rx Terminology • MU final rule identified the Medicare Part D adopted standards as the required transaction standard for e. Rx 12 • For terminology, ONC has adopted a standard that requires certified applications to use “any source vocabulary that is included in Rx. Norm” 14 • Application providers use various code sets within their systems, while still promoting Rx. Norm as a crosswalk between the disparate code sets • NCPDP SCRIPT 8. 1 or NCPDP SCRIPT 10. 6 • On July 1 st, 2010, CMS issued an IFR that identifies NCPDP SCRIPT 10. 6 as a backward compatible update of the previously adopted NCPDP SCRIPT 8. 1 for Medicare Part D electronic prescribing. 13 28
 Potential Roles for the State or SDE in e. Prescribing Monitor progress on issues that are still maturing and educate stakeholders on their status • Future work is needed on such issues as: – Creating a standardized, structured and codified sig (the ordering information that is included as part of the prescription —e. g. , “take two pills by mouth at bedtime”); – Embedding prior authorization into the electronic prescribing process; and – Ensuring that hospital-based CPOE systems (that typically use HL 7 messaging to send prescriptions to inpatient pharmacy) are able to transmit prescriptions to community or mail order pharmacies during emergency department or outpatient clinic visits or following a patient’s discharge. • Though the issues presented above are not required for meaningful use in 2011, they are needed to address current limitations in e. Prescribing and, once addressed, will impact the quality and efficiency of care 29
Potential Roles for the State or SDE in e. Prescribing Monitor progress on issues that are still maturing and educate stakeholders on their status • Future work is needed on such issues as: – Creating a standardized, structured and codified sig (the ordering information that is included as part of the prescription —e. g. , “take two pills by mouth at bedtime”); – Embedding prior authorization into the electronic prescribing process; and – Ensuring that hospital-based CPOE systems (that typically use HL 7 messaging to send prescriptions to inpatient pharmacy) are able to transmit prescriptions to community or mail order pharmacies during emergency department or outpatient clinic visits or following a patient’s discharge. • Though the issues presented above are not required for meaningful use in 2011, they are needed to address current limitations in e. Prescribing and, once addressed, will impact the quality and efficiency of care 29
 State Example: Tennessee Will Rice Executive Director Office of e-Health Initiatives State of Tennessee 310 Great Circle Road 4 th Floor, East Wing Nashville, TN 37243 Cell: (615) 584 -2212 Email: Will. Rice@tn. gov 30
State Example: Tennessee Will Rice Executive Director Office of e-Health Initiatives State of Tennessee 310 Great Circle Road 4 th Floor, East Wing Nashville, TN 37243 Cell: (615) 584 -2212 Email: Will. Rice@tn. gov 30
 Impetus for Medication Management RFI • Opportunity to improve care and lower costs through leveraging multi-state purchasing power/demand • Driver for service: meaningful use criteria – Drug formulary checks – E-Prescribing requirements – Medication history and allergy lists
Impetus for Medication Management RFI • Opportunity to improve care and lower costs through leveraging multi-state purchasing power/demand • Driver for service: meaningful use criteria – Drug formulary checks – E-Prescribing requirements – Medication history and allergy lists
 Goals for the RFI • Needed to map out current technology and data connectivity landscape: – Other potentially comprehensive data sources besides Surescripts? – Vendors that can gather and reconcile disparate data sources to integrate into EHRs? – What technology and data can be provided at the point of care? – Recognizing all states are different, what are the types of services states can provide that will most dramatically lower costs and improve quality?
Goals for the RFI • Needed to map out current technology and data connectivity landscape: – Other potentially comprehensive data sources besides Surescripts? – Vendors that can gather and reconcile disparate data sources to integrate into EHRs? – What technology and data can be provided at the point of care? – Recognizing all states are different, what are the types of services states can provide that will most dramatically lower costs and improve quality?
 RFI Process • Tennessee engaged other states through a variety of channels • Participating states include: Alabama, California, Colorado, Georgia, Hawaii, Iowa, Maine, Missouri, New York, North Carolina, South Carolina, Tennessee, Utah • 21 responses received, demos now complete, and preparing to discuss next steps
RFI Process • Tennessee engaged other states through a variety of channels • Participating states include: Alabama, California, Colorado, Georgia, Hawaii, Iowa, Maine, Missouri, New York, North Carolina, South Carolina, Tennessee, Utah • 21 responses received, demos now complete, and preparing to discuss next steps
 Initial Market Findings • All vendors at least partly rely on Surescripts • Some vendors have already begun assembling additional data networks • Med reconciliation technology is available and capable – connecting data sources will be the main challenge • Impressive decision support capabilities at point of care (including research-based alerts and patient cost-sharing) • No one vendor can “do it all”
Initial Market Findings • All vendors at least partly rely on Surescripts • Some vendors have already begun assembling additional data networks • Med reconciliation technology is available and capable – connecting data sources will be the main challenge • Impressive decision support capabilities at point of care (including research-based alerts and patient cost-sharing) • No one vendor can “do it all”
 Legislative or Regulatory Action • States have enacted legislative powers to encourage or mandate the adoption of e. Prescribing technology. • • • Arizona – A 2008 executive order was aimed at significantly increasing patient safety through the use of e-prescribing in Arizona. California – A 2006 executive order established a e-prescribing requirement by all providers by 2010. New Hampshire – A 2006 executive order mandated healthcare providers to implement e-prescribing by October 2008. Tennessee – The e-Prescribe Tennessee program to advise and support the state as it implements a strategy for e-prescribing adoption. Rhode Island – The “Anywhere, Anytime Health Information” platform set a goal of 75 percent of all prescriptions be completed electronically. Rhode Islands medical professional associations developed policy supporting e. Prescribing adoption. Minnesota – A 2007 bill provided $14 M in assistance for rural providers to meet e. Prescribing and other HIT mandates. State statute requires all hospitals and health care providers implement e-prescribing by January 1, 2011, and interoperable EHRs by January 1, 2015. 35
Legislative or Regulatory Action • States have enacted legislative powers to encourage or mandate the adoption of e. Prescribing technology. • • • Arizona – A 2008 executive order was aimed at significantly increasing patient safety through the use of e-prescribing in Arizona. California – A 2006 executive order established a e-prescribing requirement by all providers by 2010. New Hampshire – A 2006 executive order mandated healthcare providers to implement e-prescribing by October 2008. Tennessee – The e-Prescribe Tennessee program to advise and support the state as it implements a strategy for e-prescribing adoption. Rhode Island – The “Anywhere, Anytime Health Information” platform set a goal of 75 percent of all prescriptions be completed electronically. Rhode Islands medical professional associations developed policy supporting e. Prescribing adoption. Minnesota – A 2007 bill provided $14 M in assistance for rural providers to meet e. Prescribing and other HIT mandates. State statute requires all hospitals and health care providers implement e-prescribing by January 1, 2011, and interoperable EHRs by January 1, 2015. 35
 About the State HIE TA Program • Who We Are: Our team is dedicated to providing upto-date technical assistance to states and SDEs. – We are comprised of a team of subject matter experts (SMEs) with deep expertise in several areas, including legal and policy, technical architecture, e-prescribing, etc. Our SMEs work directly with states in many capacities, including one-on-one consults. • Services we provide: Funded by ONC and thus offered at no cost to your program, including: – – – – One-on-one and group consultations Specific guidance and recommended practices Inter-state collaboration and mentoring opportunities Informational webinars Market analysis reports Toolkit modules Speaking engagements 36
About the State HIE TA Program • Who We Are: Our team is dedicated to providing upto-date technical assistance to states and SDEs. – We are comprised of a team of subject matter experts (SMEs) with deep expertise in several areas, including legal and policy, technical architecture, e-prescribing, etc. Our SMEs work directly with states in many capacities, including one-on-one consults. • Services we provide: Funded by ONC and thus offered at no cost to your program, including: – – – – One-on-one and group consultations Specific guidance and recommended practices Inter-state collaboration and mentoring opportunities Informational webinars Market analysis reports Toolkit modules Speaking engagements 36
 Appendix A: Resources and Tools • • Program Information Notice (PIN) http: //www. statehieresources. org/wp-content/uploads/2010/07/Program. Information-Notice-to-States-for-HTML_7 -6_1028 AM. htm Surescripts 2009 National Progress Report on E-Prescribing http: //www. surescripts. com/ downloads/npr/national-progress-report. pdf MU Final Rule http: //edocket. access. gpo. gov/2010/pdf/E 9 -31217. pdf MU Standards and Certification Criteria Final Rule http: //edocket. access. gpo. gov/2010/pdf/2010 -17210. pdf ONC Overview of EHR Certification Process http: //www. healthit. hhs. gov/certification MU Notice of Proposed Rule Making (NPRM) http: //edocket. access. gpo. gov/2010/pdf/E 9 -31217. pdf MU Interim Final Rule (IFR) http: //edocket. access. gpo. gov/2010/pdf/E 9 -31216. pdf 37
Appendix A: Resources and Tools • • Program Information Notice (PIN) http: //www. statehieresources. org/wp-content/uploads/2010/07/Program. Information-Notice-to-States-for-HTML_7 -6_1028 AM. htm Surescripts 2009 National Progress Report on E-Prescribing http: //www. surescripts. com/ downloads/npr/national-progress-report. pdf MU Final Rule http: //edocket. access. gpo. gov/2010/pdf/E 9 -31217. pdf MU Standards and Certification Criteria Final Rule http: //edocket. access. gpo. gov/2010/pdf/2010 -17210. pdf ONC Overview of EHR Certification Process http: //www. healthit. hhs. gov/certification MU Notice of Proposed Rule Making (NPRM) http: //edocket. access. gpo. gov/2010/pdf/E 9 -31217. pdf MU Interim Final Rule (IFR) http: //edocket. access. gpo. gov/2010/pdf/E 9 -31216. pdf 37
 Appendix A: Resources and Tools (cont. ) • • • HIT Policy Workgroup Web Site http: //www. healthit. hhs. gov/portal/server. pt? Community. ID=1474&space. ID=14& parentname=&control=Set. Community&parentid=&in_hi_userid=11673&Page. ID= 0&space=Community. Page DOJ Electronic Prescriptions for Controlled Substance Web Site http: //www. deadiversion. usdoj. gov/ecomm/e_rx/index. html National Council for Prescription Drug Programs Web Site http: //www. ncpdp. org. / National Library of Medicine Rx. Norm Release Documentation File for 11/02/2009 Full Release http: //www. nlm. nih. gov/research/umls/rxnorm/docs/2009/rxnorm_doco_full 1102 2009. html Electronic Prescribing Readiness Assessment – Sponsored by medical specialty organizations, practice associations and The Center for Improving Medication Management. Site includes a tool for querying by ZIP Code a database of pharmacies participating in e. Prescribing. www. getrxconnected. com 38
Appendix A: Resources and Tools (cont. ) • • • HIT Policy Workgroup Web Site http: //www. healthit. hhs. gov/portal/server. pt? Community. ID=1474&space. ID=14& parentname=&control=Set. Community&parentid=&in_hi_userid=11673&Page. ID= 0&space=Community. Page DOJ Electronic Prescriptions for Controlled Substance Web Site http: //www. deadiversion. usdoj. gov/ecomm/e_rx/index. html National Council for Prescription Drug Programs Web Site http: //www. ncpdp. org. / National Library of Medicine Rx. Norm Release Documentation File for 11/02/2009 Full Release http: //www. nlm. nih. gov/research/umls/rxnorm/docs/2009/rxnorm_doco_full 1102 2009. html Electronic Prescribing Readiness Assessment – Sponsored by medical specialty organizations, practice associations and The Center for Improving Medication Management. Site includes a tool for querying by ZIP Code a database of pharmacies participating in e. Prescribing. www. getrxconnected. com 38
 Appendix B: Acronyms • • • • CAHs: Critical Access Hospitals CDS: Clinical Decision Support CMS: Center for Medicare and Medicaid Services CPOE: Computerized Provider Order Entry DOJ: Department of Justice EHR: Electronic Health Record EP: Eligible Provider EPCS: Electronic Prescribing of Controlled Substances e. RX: Electronic Prescribing HDI: Health Data Intermediaries HIE: Health Information Exchange HIT: Health Information Technology HIO: Health Information Organization HITECH: Health Information Technology for Economic and Clinical Health Act of 2009 39
Appendix B: Acronyms • • • • CAHs: Critical Access Hospitals CDS: Clinical Decision Support CMS: Center for Medicare and Medicaid Services CPOE: Computerized Provider Order Entry DOJ: Department of Justice EHR: Electronic Health Record EP: Eligible Provider EPCS: Electronic Prescribing of Controlled Substances e. RX: Electronic Prescribing HDI: Health Data Intermediaries HIE: Health Information Exchange HIT: Health Information Technology HIO: Health Information Organization HITECH: Health Information Technology for Economic and Clinical Health Act of 2009 39
 Appendix B: Acronyms (cont. ) • • • • IFR: Interim Final Rule ONC: Office of the National Coordinator for Health Information Technology MIPPA: Medicare Improvements for Patients and Providers Act MMA: Medicare Modernization Act of 2003 MU: Meaningful Use NACDS: National Association of Chain Drug Stores REC: Regional Extension Center NPRM: Notice of Proposed Rule Making ONC: Office of the National Coordinator for Health Information Technology PBM: Pharmacy Benefit Manager PIN: Program Information Notice REC: Regional Extension Center RFI: Request For Information SDE: State Designated Entity 40
Appendix B: Acronyms (cont. ) • • • • IFR: Interim Final Rule ONC: Office of the National Coordinator for Health Information Technology MIPPA: Medicare Improvements for Patients and Providers Act MMA: Medicare Modernization Act of 2003 MU: Meaningful Use NACDS: National Association of Chain Drug Stores REC: Regional Extension Center NPRM: Notice of Proposed Rule Making ONC: Office of the National Coordinator for Health Information Technology PBM: Pharmacy Benefit Manager PIN: Program Information Notice REC: Regional Extension Center RFI: Request For Information SDE: State Designated Entity 40


