ac2c3bc43b1243496c12044b28436292.ppt
- Количество слайдов: 56
 Start off on the Right Foot: How to Plan and Maintain a Solid Training Program Stephen A. Rydberg BA RLATG Rebecca Serriello BS CVT RLATG Rebecca Mc. Carthy BA RLATG
Start off on the Right Foot: How to Plan and Maintain a Solid Training Program Stephen A. Rydberg BA RLATG Rebecca Serriello BS CVT RLATG Rebecca Mc. Carthy BA RLATG
 Agenda • Stephen Rydberg- “Formulating the Plan” • Rebecca Serriello- “New User Facility Orientation” • Rebecca Mc. Carthy- “On The Job Training Modules and Beyond”
Agenda • Stephen Rydberg- “Formulating the Plan” • Rebecca Serriello- “New User Facility Orientation” • Rebecca Mc. Carthy- “On The Job Training Modules and Beyond”
 Who we are-Genzyme • >11, 000 employees worldwide • Helping patients in 100 countries • 17 manufacturing sites • 9 genetic testing lab sites • 19 major marketed products • 2008 revenue of $4. 6 billion • 85 locations in >40 countries • Henri Termeer: Chairman, CEO
Who we are-Genzyme • >11, 000 employees worldwide • Helping patients in 100 countries • 17 manufacturing sites • 9 genetic testing lab sites • 19 major marketed products • 2008 revenue of $4. 6 billion • 85 locations in >40 countries • Henri Termeer: Chairman, CEO
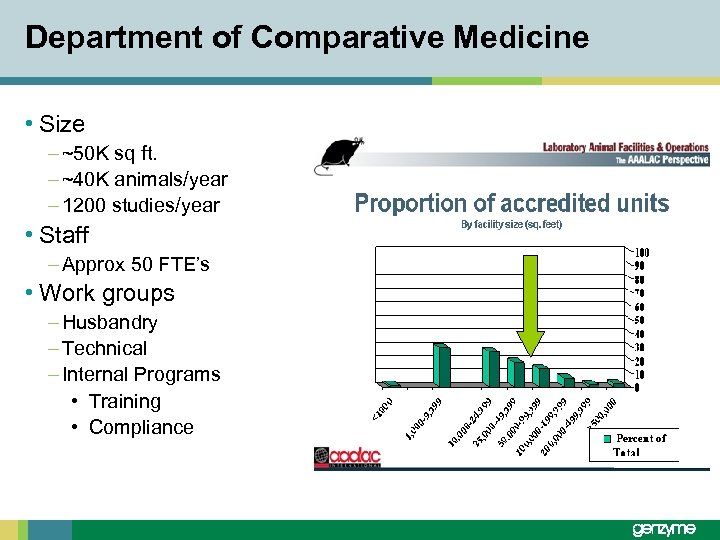 Department of Comparative Medicine • Size – ~50 K sq ft. – ~40 K animals/year – 1200 studies/year • Staff – Approx 50 FTE’s • Work groups – Husbandry – Technical – Internal Programs • Training • Compliance
Department of Comparative Medicine • Size – ~50 K sq ft. – ~40 K animals/year – 1200 studies/year • Staff – Approx 50 FTE’s • Work groups – Husbandry – Technical – Internal Programs • Training • Compliance
 Who we are. . . and where we came from • Then (pre-2003) –Informal –Small staff • Now –Structured –Larger Staff
Who we are. . . and where we came from • Then (pre-2003) –Informal –Small staff • Now –Structured –Larger Staff
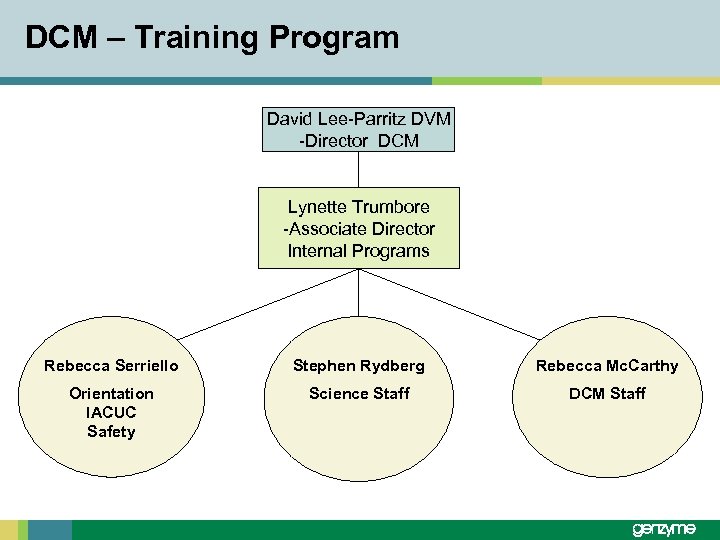 DCM – Training Program David Lee-Parritz DVM -Director DCM Lynette Trumbore -Associate Director Internal Programs Rebecca Serriello Stephen Rydberg Rebecca Mc. Carthy Orientation IACUC Safety Science Staff DCM Staff
DCM – Training Program David Lee-Parritz DVM -Director DCM Lynette Trumbore -Associate Director Internal Programs Rebecca Serriello Stephen Rydberg Rebecca Mc. Carthy Orientation IACUC Safety Science Staff DCM Staff
 Formulating the plan Stephen A. Rydberg BA, RLATG Training Specialist Principal Department of Comparative Medicine Genzyme
Formulating the plan Stephen A. Rydberg BA, RLATG Training Specialist Principal Department of Comparative Medicine Genzyme
 Why a training program? • The Guide tells us (pg 13. )…. ”AWRs and PHS Policy require institutions to ensure that people caring for or using animals are qualified to do so. ”
Why a training program? • The Guide tells us (pg 13. )…. ”AWRs and PHS Policy require institutions to ensure that people caring for or using animals are qualified to do so. ”
 What are our needs? • Goals • Self-assessment • Direction • Planning • Integration • Implementation
What are our needs? • Goals • Self-assessment • Direction • Planning • Integration • Implementation
 Goals of a solid training program • Quality science • Animal health and welfare • Safety • Compliance
Goals of a solid training program • Quality science • Animal health and welfare • Safety • Compliance
 Self assessment- Who are we? • Academic – Multi-users with different needs • Post docs, grad students, internal staff • Higher turnover rates • De-centralized
Self assessment- Who are we? • Academic – Multi-users with different needs • Post docs, grad students, internal staff • Higher turnover rates • De-centralized
 Self assessment cont’d • Private Biotech – Similar focus users • Scientists, Internal staff • Lower turnover rates • More centralized • Contract Lab – Similar focus users • Internal staff • Highly centralized
Self assessment cont’d • Private Biotech – Similar focus users • Scientists, Internal staff • Lower turnover rates • More centralized • Contract Lab – Similar focus users • Internal staff • Highly centralized
 Self assessment cont’d • How big are we? – Number of “noses” – Square footage-Multiple buildings – Staffing
Self assessment cont’d • How big are we? – Number of “noses” – Square footage-Multiple buildings – Staffing
 What is our Direction?
What is our Direction?
 Other things to consider • Compliance issues –Animals/people harmed –Are too many mistakes happening? –Is data being rejected by the FDA, journals, etc. ? –Group is getting too large –Are outside resources inadequate? –Does it take too long to get people trained?
Other things to consider • Compliance issues –Animals/people harmed –Are too many mistakes happening? –Is data being rejected by the FDA, journals, etc. ? –Group is getting too large –Are outside resources inadequate? –Does it take too long to get people trained?
 Who will assist in the designs? • How can we involve our staff? –Canvas program members to see what works best? –Work from a “template”? • Previous institution – Familiarity – Comfort –Start from the bottom-up?
Who will assist in the designs? • How can we involve our staff? –Canvas program members to see what works best? –Work from a “template”? • Previous institution – Familiarity – Comfort –Start from the bottom-up?
 Planning • Organization –Who will be in charge? –What tasks will we train? –Who will staff this group? • How do we identify a qualified trainer? • Technical skill-AALAS certifications • Experience • Educational challenges – Manual vs. didactic training – Adult learners – ESL
Planning • Organization –Who will be in charge? –What tasks will we train? –Who will staff this group? • How do we identify a qualified trainer? • Technical skill-AALAS certifications • Experience • Educational challenges – Manual vs. didactic training – Adult learners – ESL
 Planning continued • How many staff do we really need? • Can we utilize training “assistance items” such as videos and online training? • Documentation –Paper records –Electronic capture (database)
Planning continued • How many staff do we really need? • Can we utilize training “assistance items” such as videos and online training? • Documentation –Paper records –Electronic capture (database)
 Integration • Compliance married with training – IACUC and QA requirements – If it’s not going to be accepted…why do it? • Tracking training – Assigning “qualifications” • Based upon title • Based upon duties • Make training available – Training plans • Management involvement is key – Ensures staff is available, motivated and accountable • Maximizes trainer time – Ensures trainer is available, prepared and accountable • Training goals are clearly defined and attainable
Integration • Compliance married with training – IACUC and QA requirements – If it’s not going to be accepted…why do it? • Tracking training – Assigning “qualifications” • Based upon title • Based upon duties • Make training available – Training plans • Management involvement is key – Ensures staff is available, motivated and accountable • Maximizes trainer time – Ensures trainer is available, prepared and accountable • Training goals are clearly defined and attainable
 Implementation • Communication and scheduling –Training database-automated reminders –Highly organized trainers –Centralized scheduler of trainers • Paper-trails/E-mail notices –Ease of access to scheduling –“Hard copy” that communication was sent –Ability to forward to another trainer for coverage
Implementation • Communication and scheduling –Training database-automated reminders –Highly organized trainers –Centralized scheduler of trainers • Paper-trails/E-mail notices –Ease of access to scheduling –“Hard copy” that communication was sent –Ability to forward to another trainer for coverage
 Don’t forget about the trainer! • LAWTE involvement • AALAS involvement (local branches as well) • Outside training –Presentation skills –Scientific/technical writing –Computer skills –May not be directly related to animal science • Allowing time to fit into their schedule –Trainer’s lament…”I’m too busy!” –Schedule yourself
Don’t forget about the trainer! • LAWTE involvement • AALAS involvement (local branches as well) • Outside training –Presentation skills –Scientific/technical writing –Computer skills –May not be directly related to animal science • Allowing time to fit into their schedule –Trainer’s lament…”I’m too busy!” –Schedule yourself
 New User Facility Orientation Rebecca Serriello, CVT, RLATG Training Specialist Senior Department of Comparative Medicine Genzyme
New User Facility Orientation Rebecca Serriello, CVT, RLATG Training Specialist Senior Department of Comparative Medicine Genzyme
 Welcome to the facility!
Welcome to the facility!
 Orientation • Department of Comparative Medicine (DCM) • Science staff –Scientists –Research Associate/Assistant –Intern • Facilities Department & Contractors
Orientation • Department of Comparative Medicine (DCM) • Science staff –Scientists –Research Associate/Assistant –Intern • Facilities Department & Contractors
 Orientation Goals- Animal Welfare • Don’t hurt the animals • Maintain Biosecurity
Orientation Goals- Animal Welfare • Don’t hurt the animals • Maintain Biosecurity
 Orientation Goals- Safety • Don’t hurt yourself • Protect the public
Orientation Goals- Safety • Don’t hurt yourself • Protect the public
 Orientation Goals- Good Science • Maintain equipment • Write it down when it happens
Orientation Goals- Good Science • Maintain equipment • Write it down when it happens
 Orientation Goals- Company’s Resources • Protect your company’s interests • Protect your job
Orientation Goals- Company’s Resources • Protect your company’s interests • Protect your job
 Why Orientation? • General principles of animal care, safety and science • Site specific features – How to get around – Where do I find things? – How to get help • Compliance- IACUC, QA, documentation • Unique needs of the individual
Why Orientation? • General principles of animal care, safety and science • Site specific features – How to get around – Where do I find things? – How to get help • Compliance- IACUC, QA, documentation • Unique needs of the individual
 Animal Facility Orientation at Genzyme • Introduction • Reading materials – SOPs – Safety modules and documents – Facility manual • Facility Tour • Wrap up
Animal Facility Orientation at Genzyme • Introduction • Reading materials – SOPs – Safety modules and documents – Facility manual • Facility Tour • Wrap up
 Orientation DCM & Science Staff • SOPs – Large amount of information • Required – Access procedures & PPE – Labeling of Chemicals – Storage of test materials – IACUC – Veterinary care – Good documentation & use of data forms
Orientation DCM & Science Staff • SOPs – Large amount of information • Required – Access procedures & PPE – Labeling of Chemicals – Storage of test materials – IACUC – Veterinary care – Good documentation & use of data forms
 Orientation for DCM & Science Staff • Facility Manual • Facility Usage Agreement – IACUC, HR & legal approved document
Orientation for DCM & Science Staff • Facility Manual • Facility Usage Agreement – IACUC, HR & legal approved document
 Facility Tour • Biosecurity • Chemical waste satellite area • PPE • Fire evacuation policy, muster area • Animal related risks
Facility Tour • Biosecurity • Chemical waste satellite area • PPE • Fire evacuation policy, muster area • Animal related risks
 Contractors/Facilities Orientation • Facility access SOP • Document training • Facility tour • Importance of biosecurity • Educate
Contractors/Facilities Orientation • Facility access SOP • Document training • Facility tour • Importance of biosecurity • Educate
 Next steps… • Document training • Identify further training • Communication – Security/Manager – Portal links – SOPs – Point of contact information – IACUC personnel amendment
Next steps… • Document training • Identify further training • Communication – Security/Manager – Portal links – SOPs – Point of contact information – IACUC personnel amendment
 Orientation today… surgeon tomorrow
Orientation today… surgeon tomorrow
 On The Job Training Modules and Beyond Rebecca Mc. Carthy Training Specialist Senior Genzyme LAWTE 2009
On The Job Training Modules and Beyond Rebecca Mc. Carthy Training Specialist Senior Genzyme LAWTE 2009
 Terminology • Qualifications=Curriculum • Components=Skills • OJT=Tool for trainers to train a skill –Skills –Didactic>SOP, video, AALAS manual, lectures • Module=Reference/Take home material • Proficiency
Terminology • Qualifications=Curriculum • Components=Skills • OJT=Tool for trainers to train a skill –Skills –Didactic>SOP, video, AALAS manual, lectures • Module=Reference/Take home material • Proficiency
 Curriculum • Group of SOPs and skills required to execute the requirements of a functional job description • Example: Rodent Technician I must be able to perform basic technical procedures on study – Curriculum=Rodent Substance Administration, Rodent Basic Blood Collection, Study Outline/Protocol Review, Rodent Handling and Restraint, Rodent Health Monitoring, Rodent Tissue Harvesting, Rodent Basic Urine Collection – 6 Skills=Drug Calculations, Rodent Basic Injectable Administration, Small Animal Scale Balancing – 7 SOPs=Research Facility Operations-Rodents, Procedure for Reporting Lab Animal Adverse Events, Administration of Substances within the Animal Research Facility
Curriculum • Group of SOPs and skills required to execute the requirements of a functional job description • Example: Rodent Technician I must be able to perform basic technical procedures on study – Curriculum=Rodent Substance Administration, Rodent Basic Blood Collection, Study Outline/Protocol Review, Rodent Handling and Restraint, Rodent Health Monitoring, Rodent Tissue Harvesting, Rodent Basic Urine Collection – 6 Skills=Drug Calculations, Rodent Basic Injectable Administration, Small Animal Scale Balancing – 7 SOPs=Research Facility Operations-Rodents, Procedure for Reporting Lab Animal Adverse Events, Administration of Substances within the Animal Research Facility
 Skills/Techniques (Rodent Substance Administration) 1. Basic Injectable Administration (IP, SQ, IM) 2. Rodent Oral Dosing 3. Drug Calculations 4. Small Animal Scale Balancing/Leveling and Calibration
Skills/Techniques (Rodent Substance Administration) 1. Basic Injectable Administration (IP, SQ, IM) 2. Rodent Oral Dosing 3. Drug Calculations 4. Small Animal Scale Balancing/Leveling and Calibration
 OJT • Didactic-SOPs, AALAS manual, video • Materials/Equipment to perform skill-Needles • Critical Points • Proficiency Criteria • Progress
OJT • Didactic-SOPs, AALAS manual, video • Materials/Equipment to perform skill-Needles • Critical Points • Proficiency Criteria • Progress
 OJT Rodent Basic Injectable Administration IP Injections-Mouse
OJT Rodent Basic Injectable Administration IP Injections-Mouse
 OJT Rodent Basic Injectable Administration IP Injections-Mouse
OJT Rodent Basic Injectable Administration IP Injections-Mouse
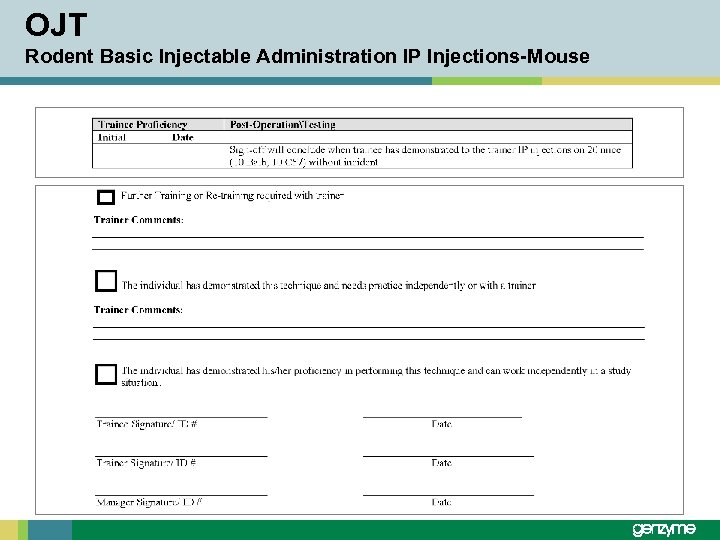 OJT Rodent Basic Injectable Administration IP Injections-Mouse
OJT Rodent Basic Injectable Administration IP Injections-Mouse
 Step-by-Step Guide • Accompanies OJT • Contains same information as OJT • Step-by-step guide on how to perform the skill/prerequisite skills • Remains with the trainee as reference guide
Step-by-Step Guide • Accompanies OJT • Contains same information as OJT • Step-by-step guide on how to perform the skill/prerequisite skills • Remains with the trainee as reference guide
 Proficiency • Demonstrates trainee can perform the skill –Reliably –Accurately –Efficiently • Criteria established by Trainers / Veterinarians / Scientist • One day to several weeks depending on task and previous experience
Proficiency • Demonstrates trainee can perform the skill –Reliably –Accurately –Efficiently • Criteria established by Trainers / Veterinarians / Scientist • One day to several weeks depending on task and previous experience
 Proficiency (Sham) Tests • Skill specific • Demonstrates to scientist that test material is administered properly and produces a physiologic effect on the animal • Administration of a marker to animals by testers and control injector • Serum analyzed for a % level of marker in the animals blood
Proficiency (Sham) Tests • Skill specific • Demonstrates to scientist that test material is administered properly and produces a physiologic effect on the animal • Administration of a marker to animals by testers and control injector • Serum analyzed for a % level of marker in the animals blood
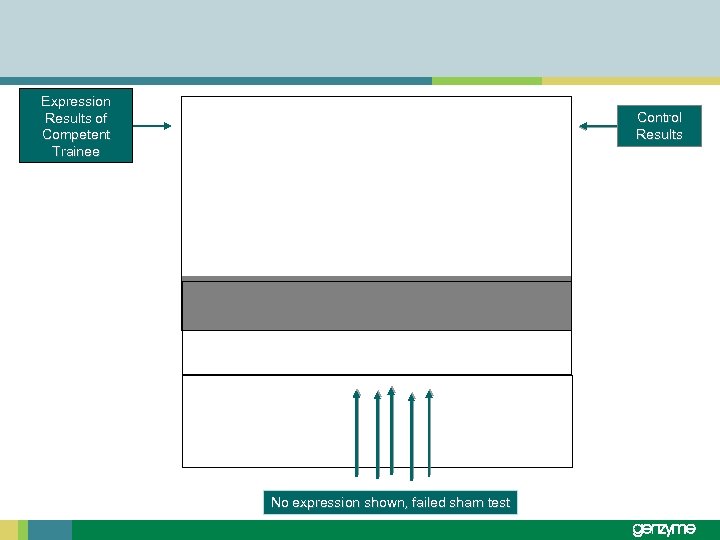 Expression Results of Competent Trainee Control Results No expression shown, failed sham test
Expression Results of Competent Trainee Control Results No expression shown, failed sham test
 Refresher Training • Incident/Accident • Failure to pass sham test • Skill not performed recently • Compliance Issue • SOP requires retraining
Refresher Training • Incident/Accident • Failure to pass sham test • Skill not performed recently • Compliance Issue • SOP requires retraining
 Mentors • Senior technicians • Role models –study management –technical ability –professionalism • Development opportunity • “Train the trainer” –presentation skills –use of training documentation
Mentors • Senior technicians • Role models –study management –technical ability –professionalism • Development opportunity • “Train the trainer” –presentation skills –use of training documentation
 Continuing Education • Group training • Special topics – journal articles – outside consultant (rabbit epidurals) – anesthesia monitoring – pulse oximetry • Address issues – incidents/accidents – Compliance – Safety – animal welfare
Continuing Education • Group training • Special topics – journal articles – outside consultant (rabbit epidurals) – anesthesia monitoring – pulse oximetry • Address issues – incidents/accidents – Compliance – Safety – animal welfare
 The 3 R’s (Russell & Burch) • Refinement > train better, accommodate individuals different modes of learning • Replacement > video, e-learning • Reduction > use of fewer animals with the use of videos, e-learning, use the trainer more efficiently
The 3 R’s (Russell & Burch) • Refinement > train better, accommodate individuals different modes of learning • Replacement > video, e-learning • Reduction > use of fewer animals with the use of videos, e-learning, use the trainer more efficiently
 WRAP-UP • How we plan • Examples/Tools • Follow-through
WRAP-UP • How we plan • Examples/Tools • Follow-through
 Follow-through • Move forward with a purpose…. . while allowing some flexibility –Consistency must be maintained • Buy-in –All in the same boat –Best results • Science • Animals
Follow-through • Move forward with a purpose…. . while allowing some flexibility –Consistency must be maintained • Buy-in –All in the same boat –Best results • Science • Animals
 Follow-through cont’d • Eye on the prize! • Avoid pitfalls to the plan – Don’t play “favorites” • Credibility issues • Sending mixed messages –Confusion –Errors • Continued self evaluation – Internal reviews and audits – Include other groups – Ask for feedback
Follow-through cont’d • Eye on the prize! • Avoid pitfalls to the plan – Don’t play “favorites” • Credibility issues • Sending mixed messages –Confusion –Errors • Continued self evaluation – Internal reviews and audits – Include other groups – Ask for feedback
 Questions?
Questions?


