682caa2b6398abcaf02310ebcba009a1.ppt
- Количество слайдов: 71
Stability Q 1(R 2)

Role Of Stability • Safety and efficacy of drug product are established during development via clinical studies • Quality is established for identify, strength, quality and purity • If drug product stability changes beyond established acceptance criteria, established safety and efficacy are no longer applicable. • Change of Drug Stability would risk patient safety – Quality of finished products decrease – Potential sub-potent or over-dose products – Potential toxic unknown impurities • Uncontrolled process → product investigation → product recalls • c. GMP violations → consent decree → criminal prosecution
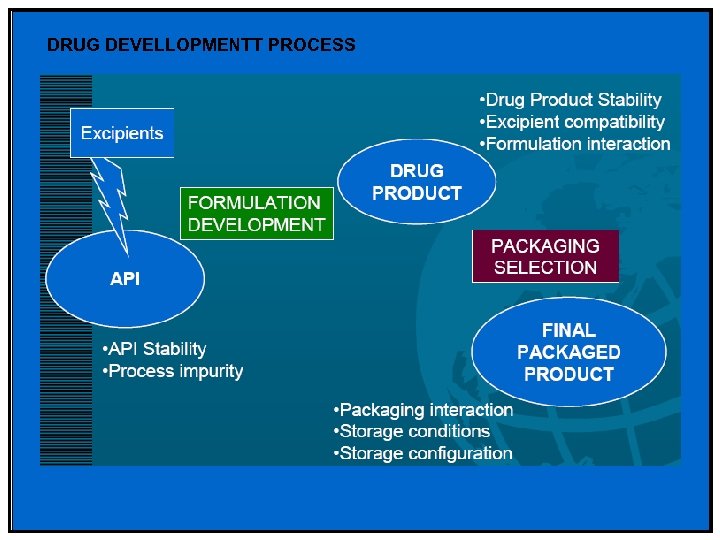
DRUG DEVELLOPMENTT PROCESS

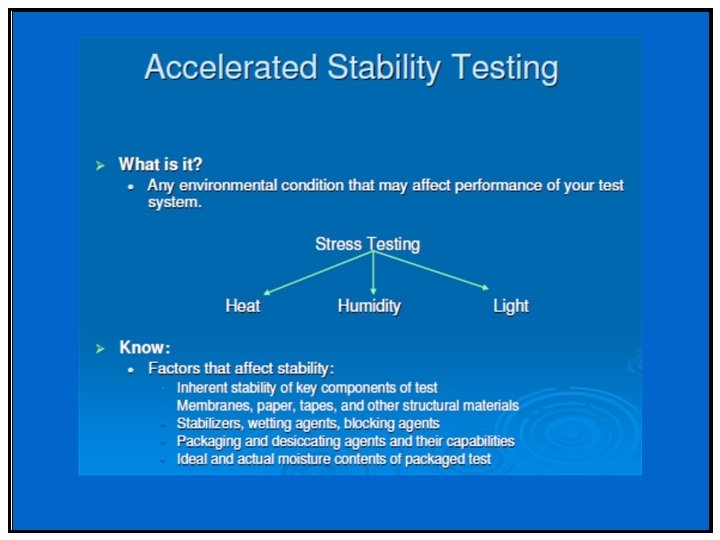
Factors affecting Drug Stability • Stability of the Active Pharmaceutical Ingredient (API) from storage • Interaction between the API and excipient Formulation Development • Selection of dosage form • Manufacturing process of drug product • Selection of container closure packaging system • Effect of storage (temperature, humidity and light) • Selection of marketing image • Handling of the finished products

Purpose of Stability Testing • The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the influence of a variety of environmental factors such as temperature, humidity and light. • Stability testing permits the establishment of recommended storage conditions, retest periods, and shelf-lives.

Drug Product Stability • Stability characteristics of API or Drug Product is a critical quality attribute of pharmaceutical product • Stability Studies are used to: – Establish how product changes over time under critical environmental factors (temperature, heat and light) – Determine appropriate product specifications – Select marketing container closure system – Determine appropriate storage conditions – Justification of expiry of commercial product – Provide necessary medical supplies
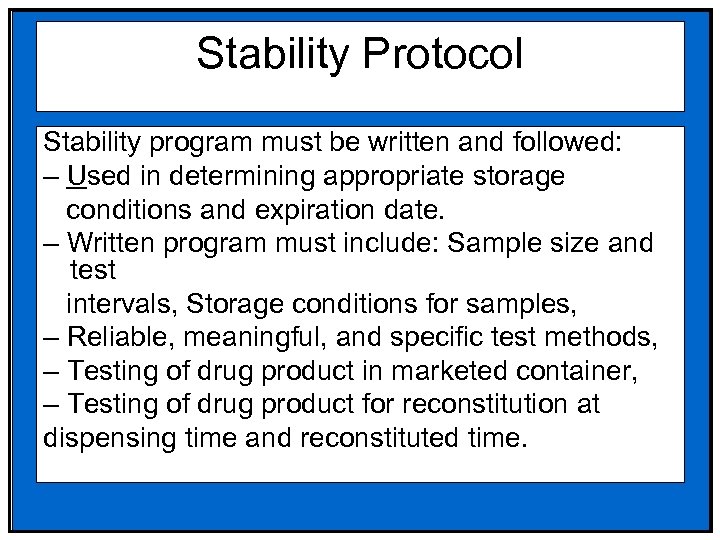
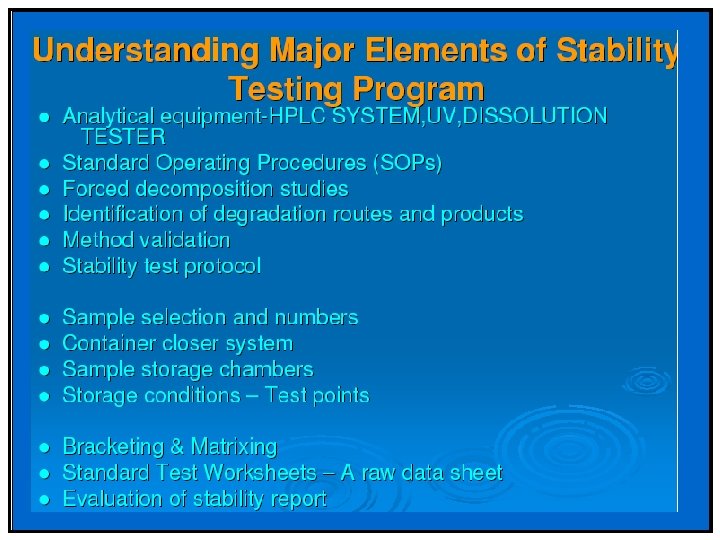
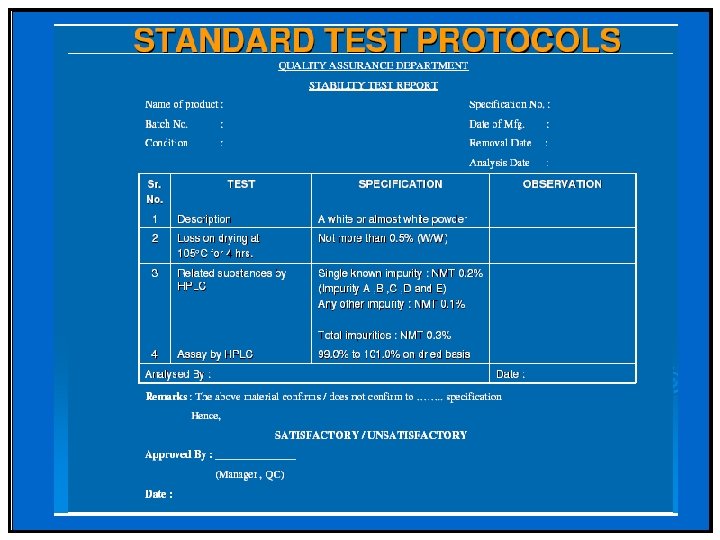
Stability Protocol Stability program must be written and followed: – Used in determining appropriate storage conditions and expiration date. – Written program must include: Sample size and test intervals, Storage conditions for samples, – Reliable, meaningful, and specific test methods, – Testing of drug product in marketed container, – Testing of drug product for reconstitution at dispensing time and reconstituted time.
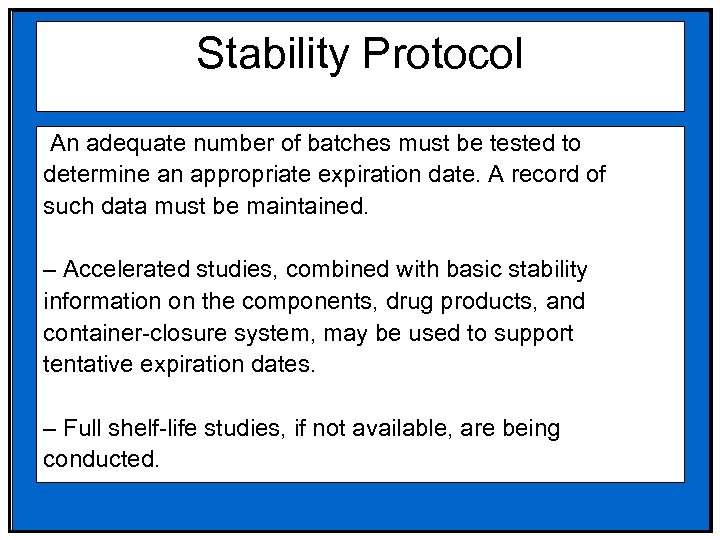
Stability Protocol An adequate number of batches must be tested to determine an appropriate expiration date. A record of such data must be maintained. – Accelerated studies, combined with basic stability information on the components, drug products, and container-closure system, may be used to support tentative expiration dates. – Full shelf-life studies, if not available, are being conducted.
Stability Q 1(R 2) • ICH condition contributing 80% of pharmaceutical market • Outlines minimum stability data package for new drug application. It is not intended for INDs, ANDAs or s. NDAs. • Harmonizes stability requirement for marketing application in EU, Japan and US. Other countries adopt with some modifications. • Must use Validated Stability-Indicating analytical methods • Methods must cover physical, chemical, biological and microbiological attributes.
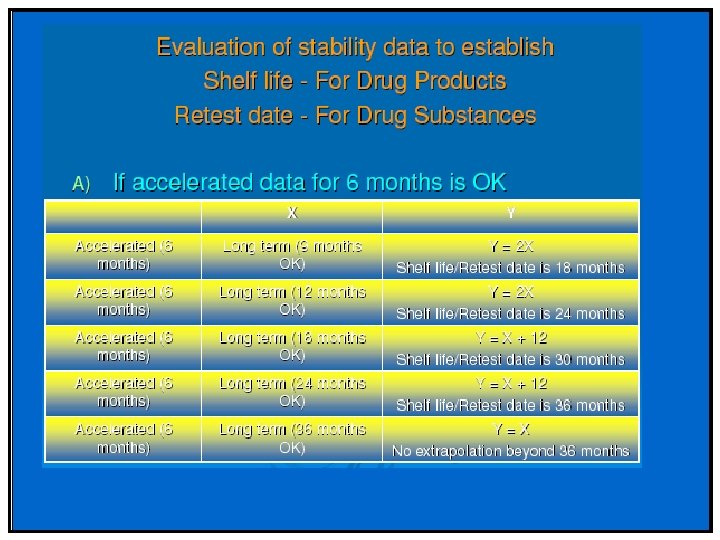
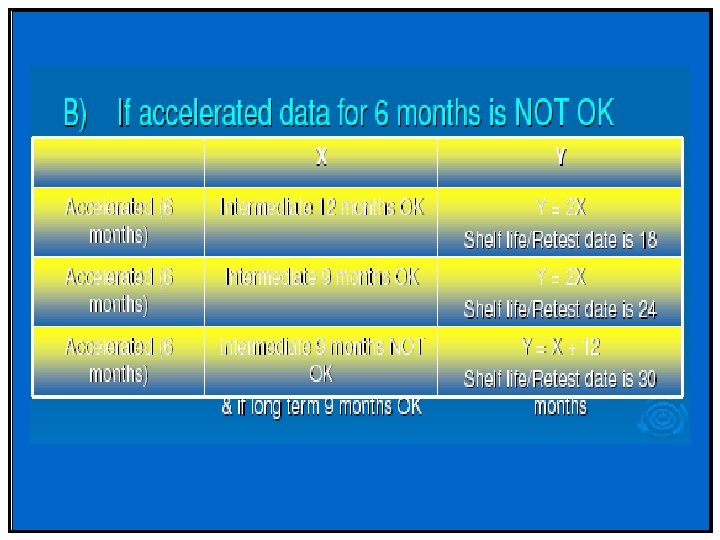
Stability Q 1(R 2) • Studies evaluated under thermal and elevated humidity to cover storage, shipment and subsequent use • Accelerated and intermediate used to evaluate impact of short-term excursions. • Acceptance criteria should include individual and total upper limits for impurities and degradation products • No formal statistical analysis is needed if data show little degradation or variability.

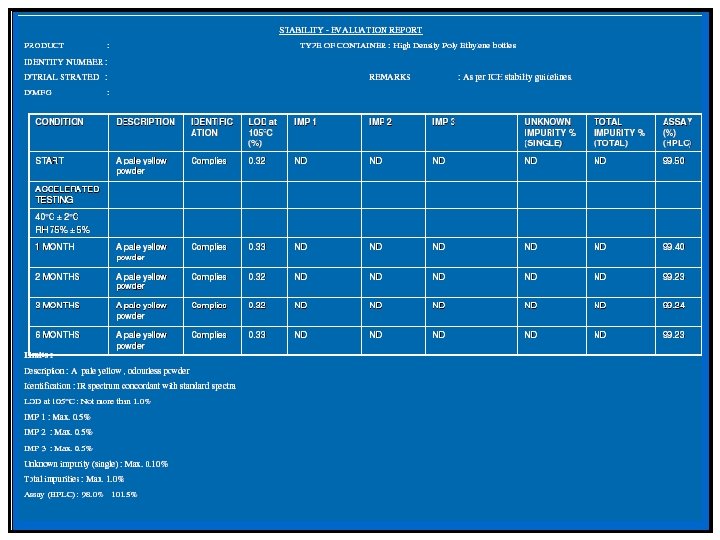
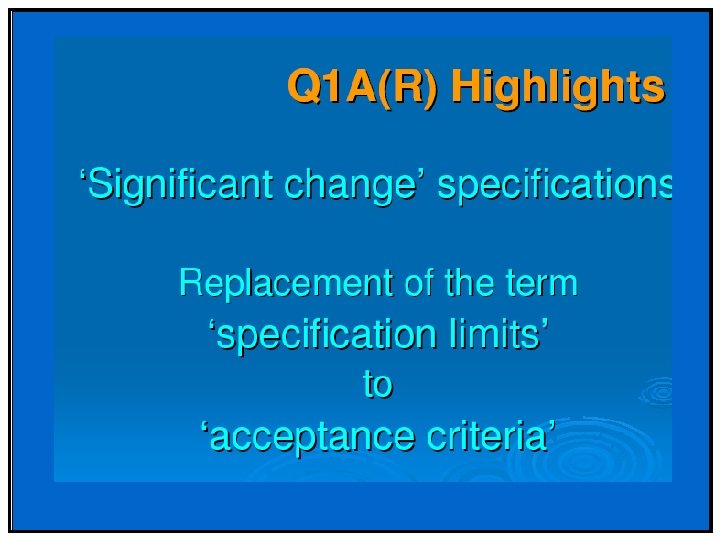
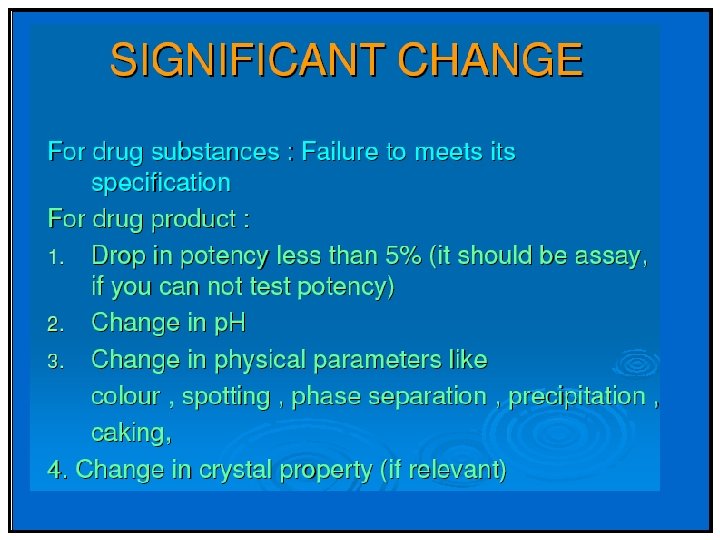
API Significant change is defined as failure to meet the specification. Drug Product - 5 percent potency change from the initial assay value; -Any specified degradant exceeding its acceptance criteria -Failure to meet acceptance criteria for appearance and physical properties (e. g. , color, phase separation, resuspendibility, delivery per actuation, caking, hardness); and as appropriate to the product type; -The p. H exceeding its acceptance criteria; and Dissolution exceeding the acceptance criteria for 12 dosage units.
Photostability: Two types of studies, exposure is cumulative from the light sources • Forced degradation study to generate potential degradation products – 2 X exposure to UV and fluorescent sources • Confirmatory study to confirm product and package performance – NLT 1. 2 million lux hours + 200 watts/hrs per sq. meter Light Sources: – Option 1 • Dual output light sources, such as D 65/ID 65 • Simulates artificial day light fluorescent lamp • Use Xenon or Metal Halide – Option 2 • Tandem exposure to single light source types • Cool white fluorescent lamp + near UV fluorescent lamp (320 -400 nm) • Accumulate exposure under one, then the other

Bracketing: • • • Used for packaging extremes Assume that the intermediates are represented by the extremes For a range of strengths, strengths must be identical or very closely related in composition Can be applied to different container sizes or fills of same packaging system Bracketing design is NOT appropriate if extremes are not demonstrated.

Matrixing: • Define full design and reduced design (with examples) • Assume that the stability of each subset of samples tested represent the stability of all samples at a given time point • Define what is needed for justification • Covering different batches, different strengths, different sizes of the same container and closure, and, possibly, in some cases, different container/closure systems…. ”

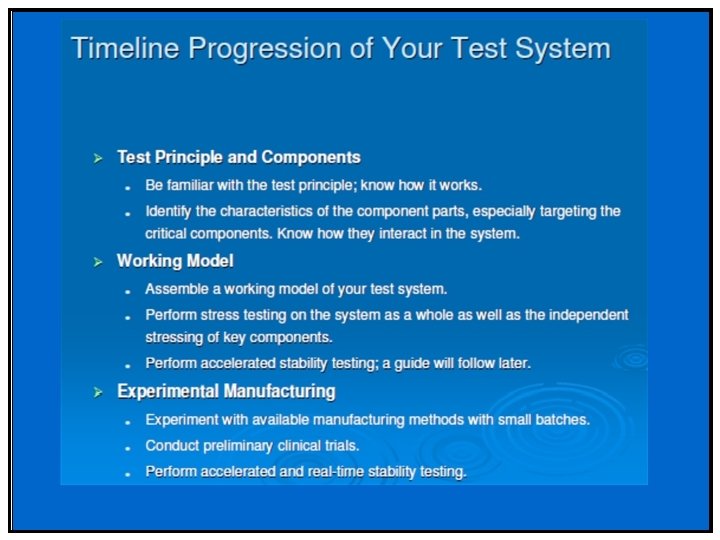
Design Program by Phase • Stability Program varies depending on the phase and clinical study it supports • Stability Study Goals. . . – Identify problems early – Know your product – Minimize repeat study – Identify critical control attributes • i. e. , Particle size • Develop a stable commercial product • Maintain database--stability informatics • Establish systems to cycle back learning • Develop stability strategies to expedite product development
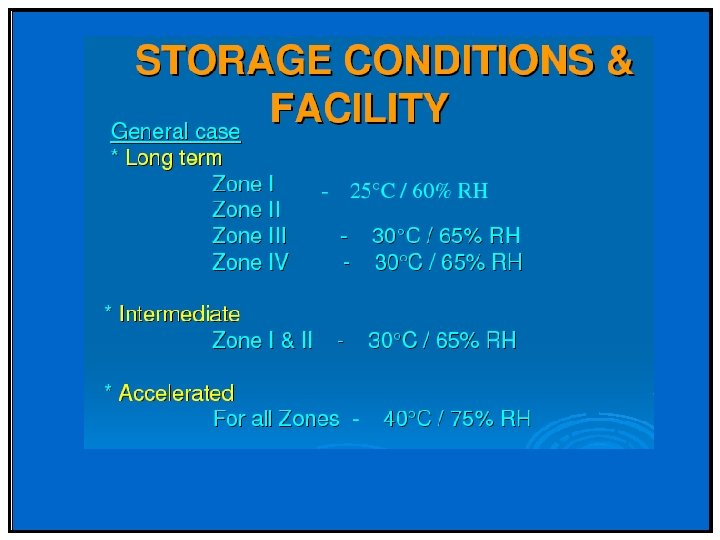
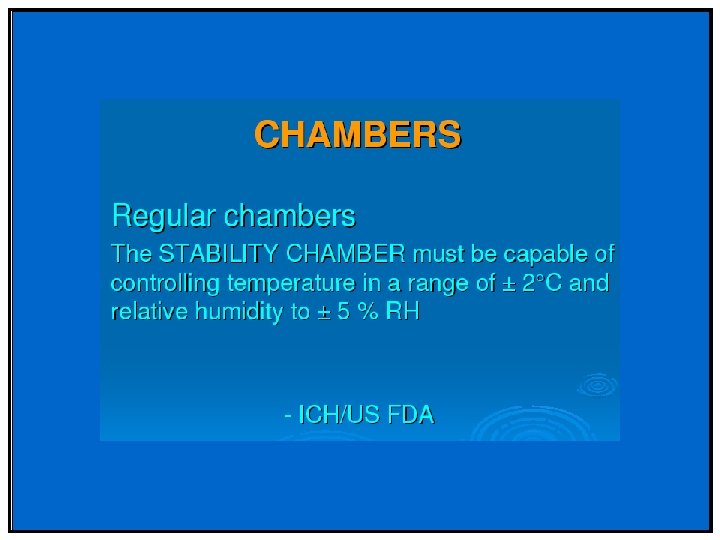
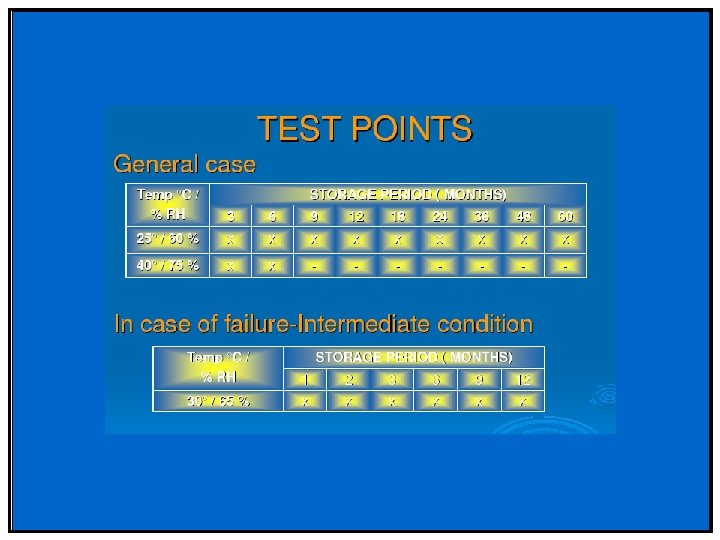
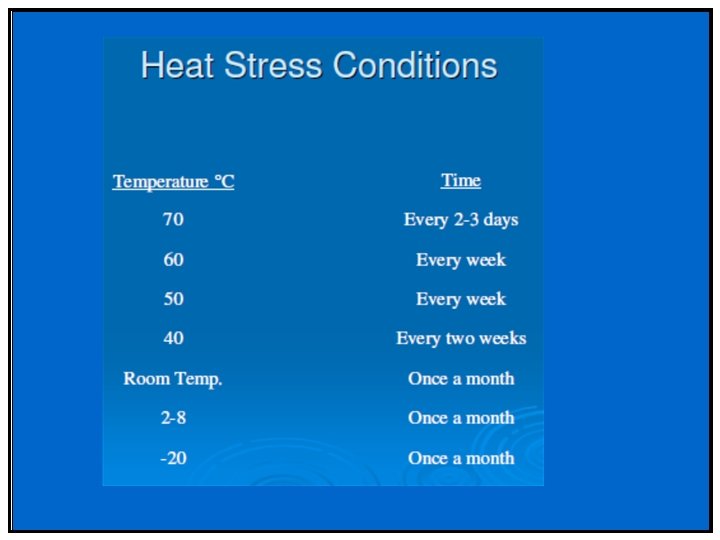
Phase II & III • Stability profile of clinical materials must be monitored • Test stations – 25 °C/60%RH – 30 °C/75%RH (if trial conducted in zone III and IV) – 40 °C/75%RH (Open Dish 1 M) – 40 °C/75%RH – ICH Photostability • What packaging will be required – Is your product moisture, light or heat sensitive – Desiccant needed/filler
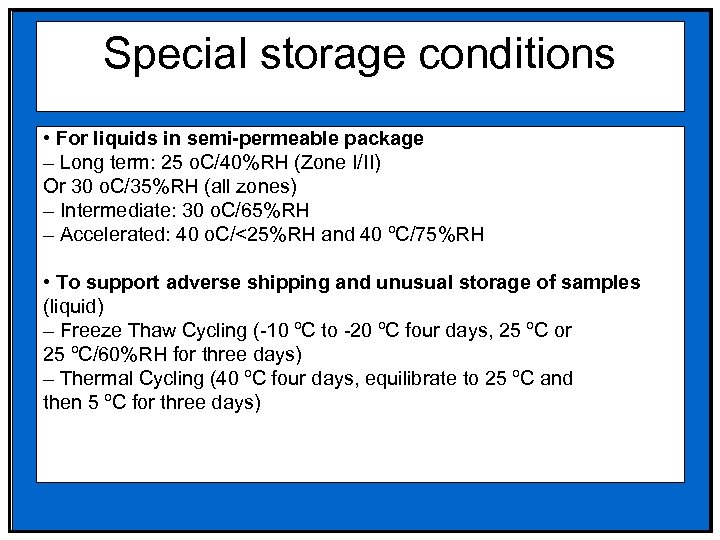
Special storage conditions • For liquids in semi-permeable package – Long term: 25 o. C/40%RH (Zone I/II) Or 30 o. C/35%RH (all zones) – Intermediate: 30 o. C/65%RH – Accelerated: 40 o. C/<25%RH and 40 ºC/75%RH • To support adverse shipping and unusual storage of samples (liquid) – Freeze Thaw Cycling (-10 ºC to -20 ºC four days, 25 ºC or 25 ºC/60%RH for three days) – Thermal Cycling (40 ºC four days, equilibrate to 25 ºC and then 5 ºC for three days)
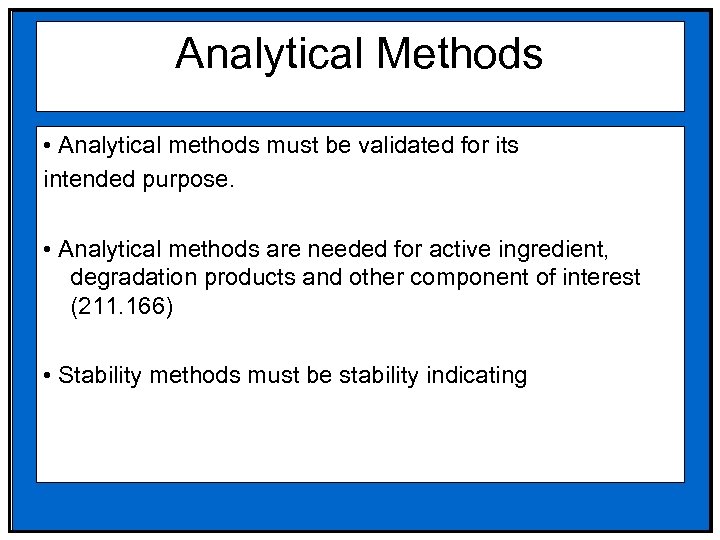
Analytical Methods • Analytical methods must be validated for its intended purpose. • Analytical methods are needed for active ingredient, degradation products and other component of interest (211. 166) • Stability methods must be stability indicating

ICH Guidelines Q 1 AR 2…”The testing should cover, as appropriate, the physical, chemical, biological and microbiological attributes, preservative content, and functionality tests (e. g for a dose delivery system). Analytical procedures should be fully validated and stability indicating. ” Q 2 A… “Validated analytical procedures should be used, irrespective of whether they are for in-process, release, acceptance, or stability testing”.

ICH Guidelines • Q 3 B: “Analytical methods should be validated to demonstrate that impurities unique to the new drug substance do not interfere with or are separated from specified and unspecified degradation products in the drug product. ”
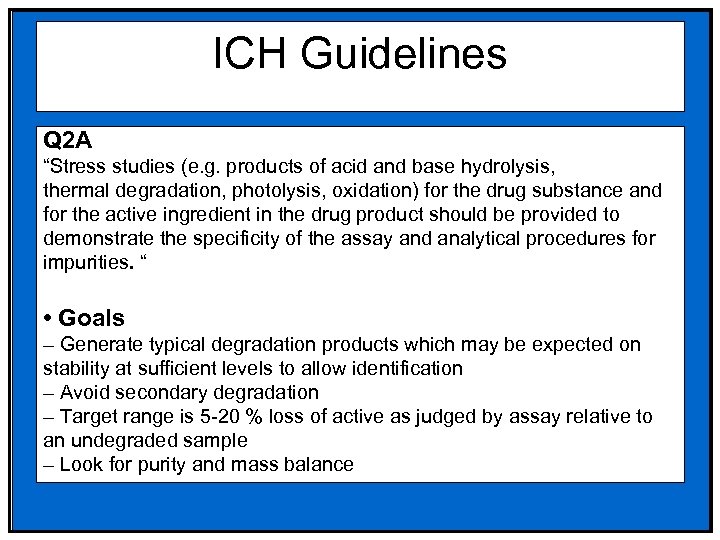
ICH Guidelines Q 2 A “Stress studies (e. g. products of acid and base hydrolysis, thermal degradation, photolysis, oxidation) for the drug substance and for the active ingredient in the drug product should be provided to demonstrate the specificity of the assay and analytical procedures for impurities. “ • Goals – Generate typical degradation products which may be expected on stability at sufficient levels to allow identification – Avoid secondary degradation – Target range is 5 -20 % loss of active as judged by assay relative to an undegraded sample – Look for purity and mass balance
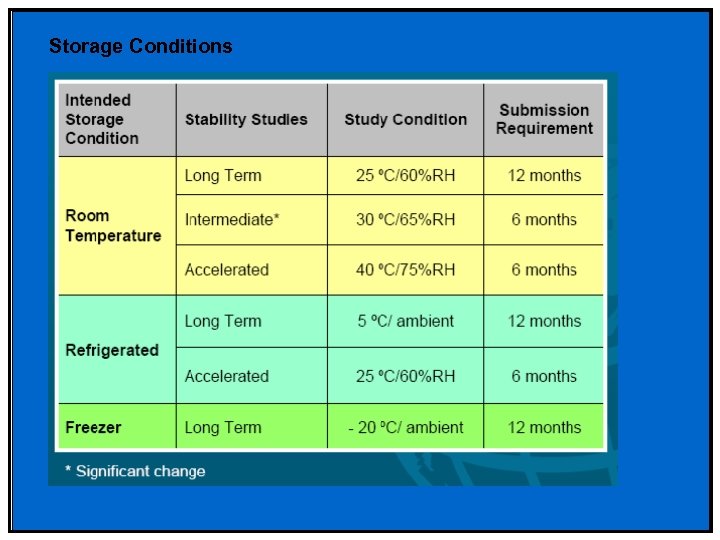
Storage Conditions
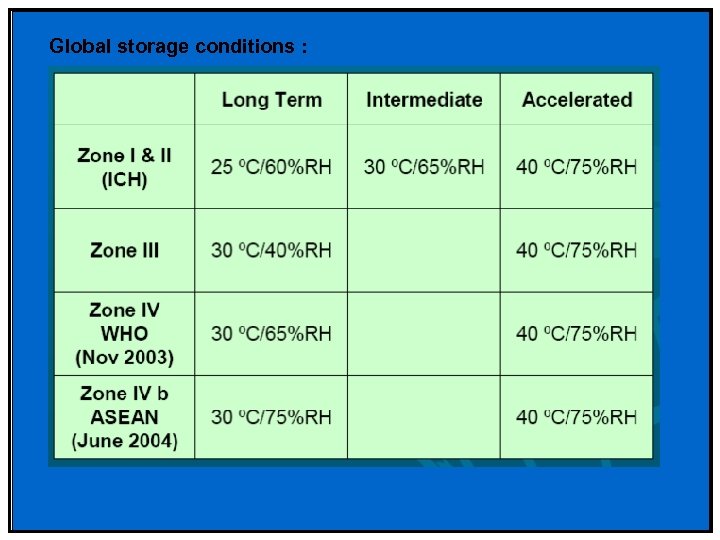
Global storage conditions :
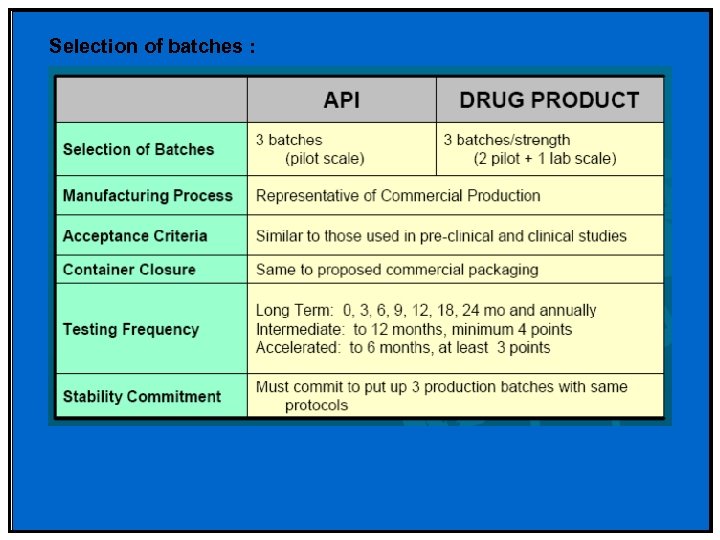
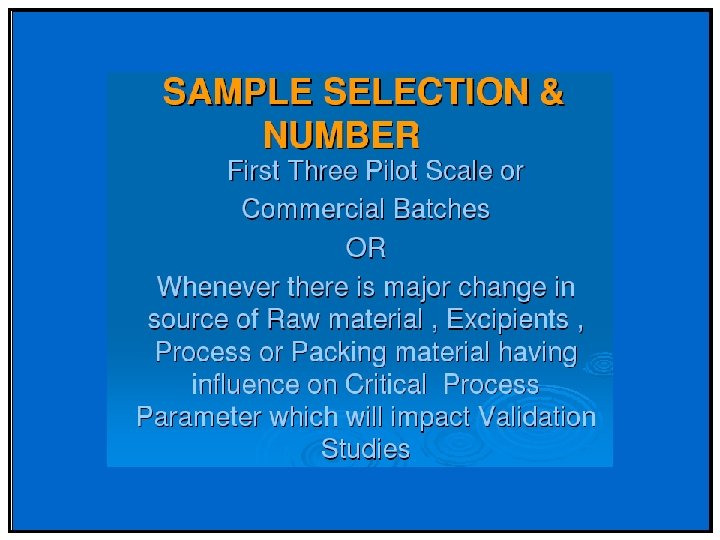
Selection of batches :
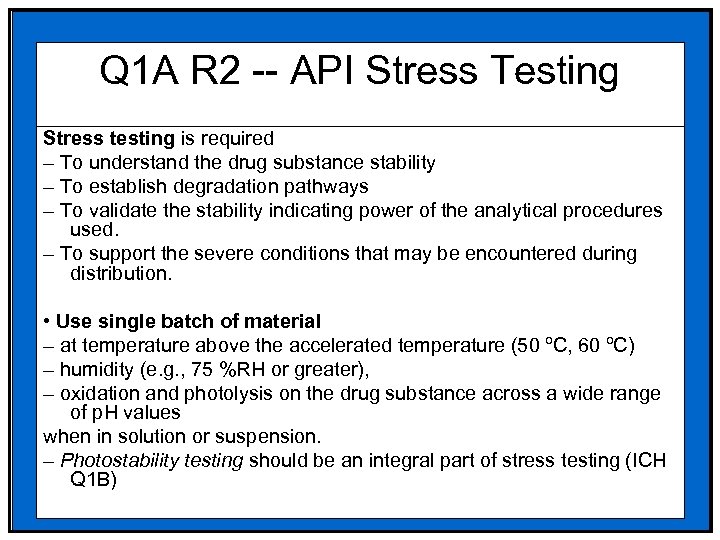
Q 1 A R 2 -- API Stress Testing Stress testing is required – To understand the drug substance stability – To establish degradation pathways – To validate the stability indicating power of the analytical procedures used. – To support the severe conditions that may be encountered during distribution. • Use single batch of material – at temperature above the accelerated temperature (50 ºC, 60 ºC) – humidity (e. g. , 75 %RH or greater), – oxidation and photolysis on the drug substance across a wide range of p. H values when in solution or suspension. – Photostability testing should be an integral part of stress testing (ICH Q 1 B)
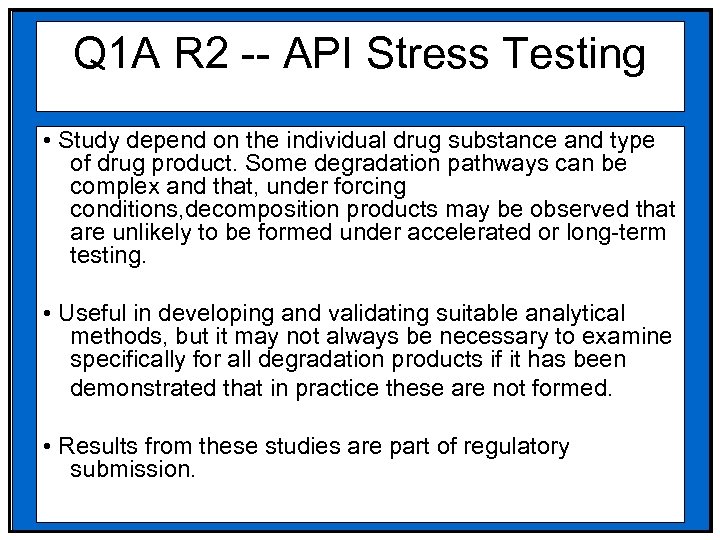
Q 1 A R 2 -- API Stress Testing • Study depend on the individual drug substance and type of drug product. Some degradation pathways can be complex and that, under forcing conditions, decomposition products may be observed that are unlikely to be formed under accelerated or long-term testing. • Useful in developing and validating suitable analytical methods, but it may not always be necessary to examine specifically for all degradation products if it has been demonstrated that in practice these are not formed. • Results from these studies are part of regulatory submission.
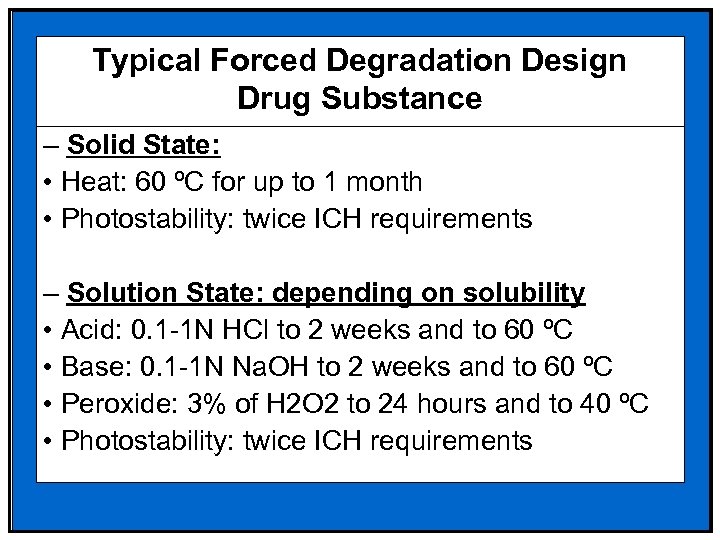
Typical Forced Degradation Design Drug Substance – Solid State: • Heat: 60 ºC for up to 1 month • Photostability: twice ICH requirements – Solution State: depending on solubility • Acid: 0. 1 -1 N HCl to 2 weeks and to 60 ºC • Base: 0. 1 -1 N Na. OH to 2 weeks and to 60 ºC • Peroxide: 3% of H 2 O 2 to 24 hours and to 40 ºC • Photostability: twice ICH requirements
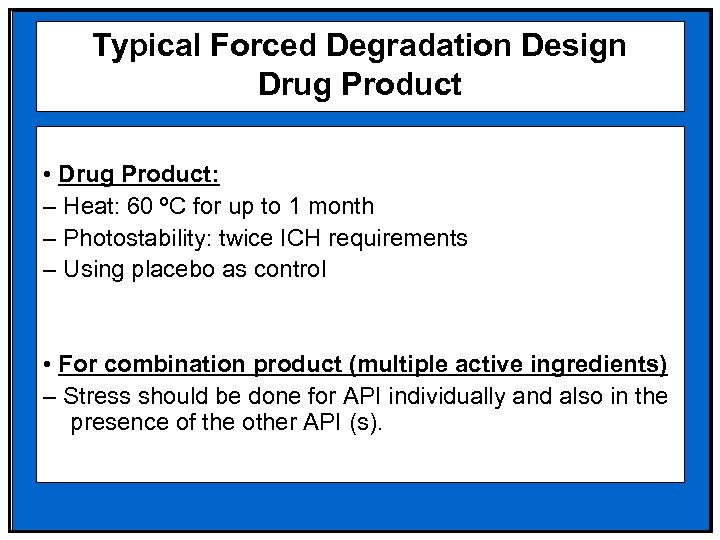
Typical Forced Degradation Design Drug Product • Drug Product: – Heat: 60 ºC for up to 1 month – Photostability: twice ICH requirements – Using placebo as control • For combination product (multiple active ingredients) – Stress should be done for API individually and also in the presence of the other API (s).
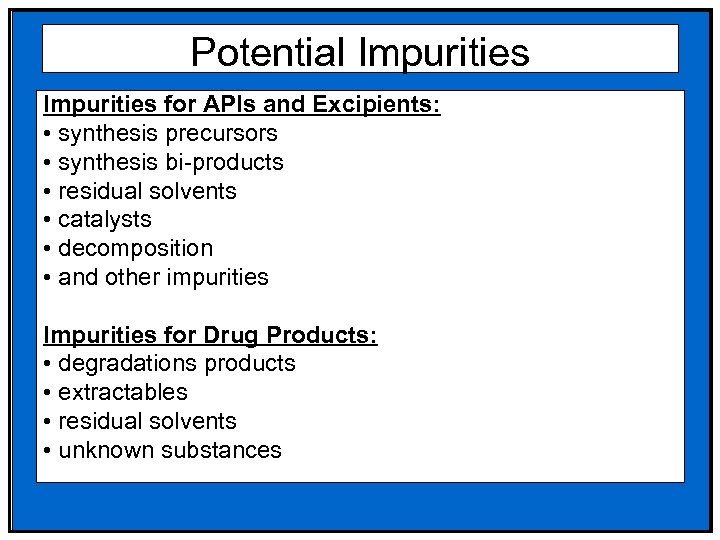
Potential Impurities for APIs and Excipients: • synthesis precursors • synthesis bi-products • residual solvents • catalysts • decomposition • and other impurities Impurities for Drug Products: • degradations products • extractables • residual solvents • unknown substances

Conclusion • Stability is a critical quality attribute of the API and the Drug Product • Stability profile needs to be established for drug product to assure safety, efficacy and quality. • Understand key concepts to develop stability indicating methods. • Understand the Regulatory Requirements versus Scientific Knowledge. • Understand regional versus global concerns to develop stability program • Design strategy for stability study based on data of development batches • Review c. GMPs violations and regulatory observations • Develop stability program and maximize efficiency

DEFINITION STABILITY “The capacity off a drug product/substance to remain within specifications established to ensure its system, identity, strength, purity & quality” The purpose off stability testing is to provide evidence on how the quality off a drug substance or product varies with time under the influence off a variety off environment factor. – Temperature, humidity, light and to set up retest period for the drug substance or a shelf-life for the drug product and recommended storage conditions.

ICH Drug Stability Test Requirements • Scientific in approach • Provide clear mandate to users • Call for good infrastructure and investment in stability testing

HOW TO GO ABOUT STABILITY STUDY ICH International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human use.

ICH - A TRIPARTITE AGREEMENT 17 countries in three regions The world biggest pool for production and consumption of pharmaceuticals

Legal status of guidelines Most of the guidelines have become part of the local regulations in US , Europe and Japan
THE ZONE CONCEPT Distribution of world into Four different zones
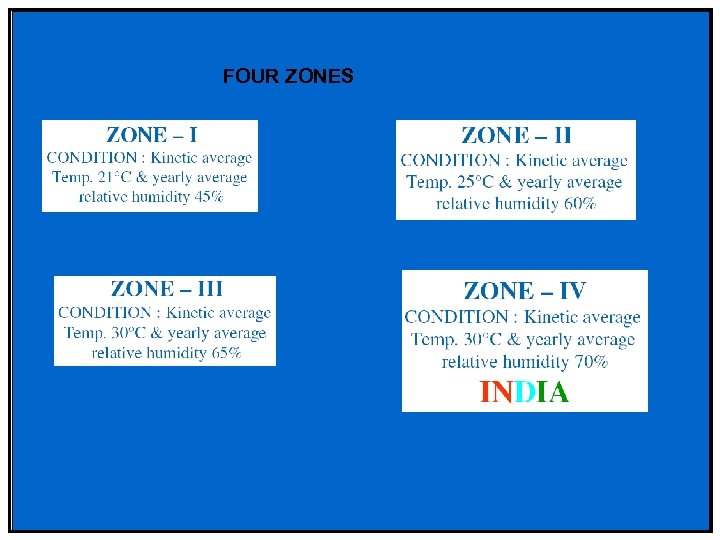
FOUR ZONES




















682caa2b6398abcaf02310ebcba009a1.ppt