fc01dff946849f93123a478229391588.ppt
- Количество слайдов: 69

Spring 2008 Cohort Studies, Relative Risk, and Attributable Risk STAT 6395 Filardo and Ng
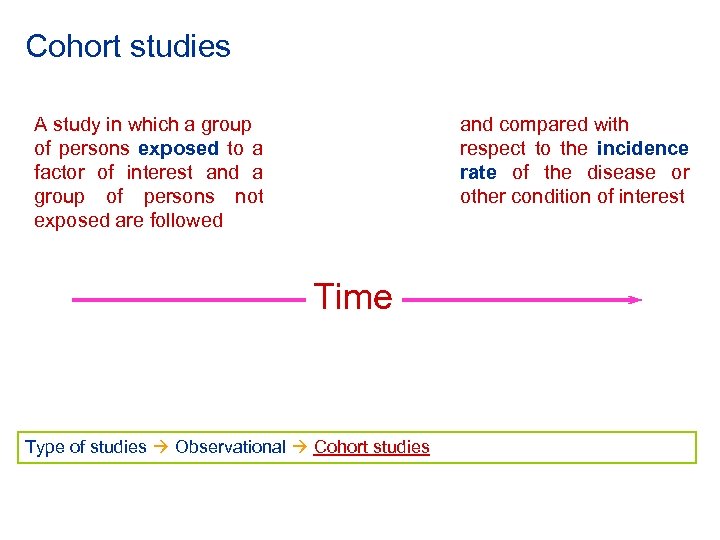
Cohort studies A study in which a group of persons exposed to a factor of interest and a group of persons not exposed are followed and compared with respect to the incidence rate of the disease or other condition of interest Time Type of studies Observational Cohort studies
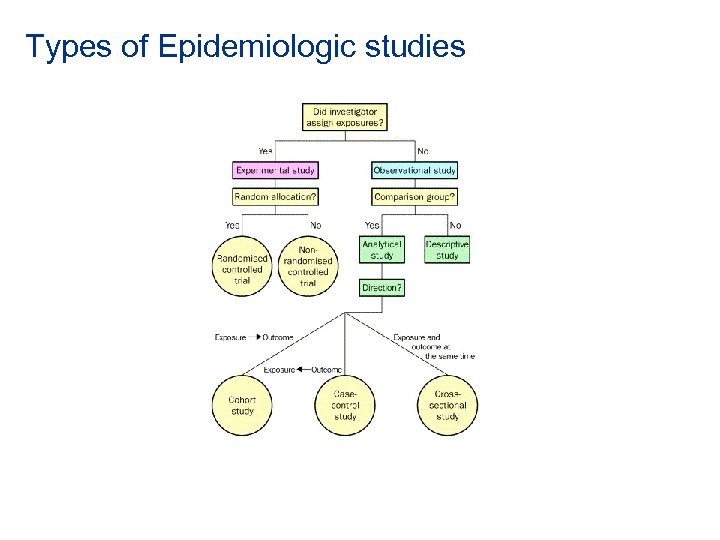
Types of Epidemiologic studies

Cohort A designated group of subjects who are followed (traced) over a period of time Type of studies Observational Cohort studies
Time Type of studies Observational Cohort studies

Cohort studies are also called: • Prospective studies • Retrospective cohort studies • Follow-up studies • Longitudinal studies Type of studies Observational Cohort studies
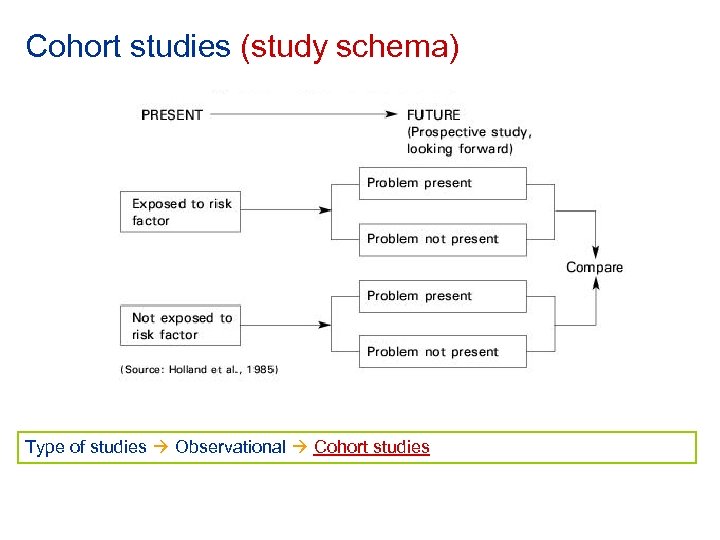
Cohort studies (study schema) Type of studies Observational Cohort studies

Among observational studies, cohort studies are the ‘gold standard’ • Exposure precedes onset of disease (a necessary condition of causality) • No differential recall of exposures by those who develop the disease compared to those who do not (recall bias) • Exposure measurements are taken at baseline, as opposed to querying about past exposures that occurred before the onset of disease or assuming that current levels of biologic markers reflect past exposures (as in case-control studies) Type of studies Observational Cohort studies
Limitations of cohort studies • Large number of study participants • Many years of follow-up • Expensive • Losses to follow-up • Main limitation: observational and not experimental Type of studies Observational Cohort studies

Non exposed comparison group can be internal or external • When the cohort includes both exposed and unexposed individuals, the comparison group is internal, within the cohort • When the entire cohort is exposed, need an external group for purposes of comparison Non exposed group Internal or External

Internal comparison group: Occupational cohort study. Hypothesis: exposure to Chemical X causes one or more types of cancer. • Cohort of workers employed in Factory A • 40% of workers are exposed to Chemical X • 60% of workers are not exposed to Chemical X. • The unexposed workers would serve as in internal comparison group Non exposed group Internal or External

External comparison group: Occupational cohort study • Cohort of workers employed in Factory B • All workers in Factory B are exposed to Chemical X • External comparison group • Workers in Factory C, where the workers have similar demographic • characteristics to the workers in Factory B and not exposed to Chemical X General U. S. population (mortality rates from vital statistics data) Non exposed group Internal or External
Two types of cohorts • Cohort defined by an exposure or group of related exposures • Purpose: test specific hypotheses about the exposure • Study of rare exposures • External comparison group if entire cohort is exposed • Cohort defined by a factor unrelated to any particular exposure • Data collection systems to test multiple hypotheses • Sample of general population of defined geographic area • Convenience sample • Willingness of members to participate • Logistic advantages, such as ease of follow-up Two types of cohorts defined by the exposure or unrelated

Cohort defined by an exposure or group of related exposures • Occupational cohort • Japanese atomic bomb cohort • Cohort of persons treated with radiotherapy for ankylosing spondylitis (inflammatory disease of the spine) • Cohort of persons taking a particular drug • Multicenter AIDS Cohort Study (drug addicts) Two types of cohorts defined by the exposure or unrelated
Cohort defined by a factor unrelated to any particular exposure • Framingham Heart Study • Began in 1948 with 5, 127 participants • Cancer Prevention Study II • Began in 1982 with 1, 184, 657 participants • Nurses Health Study (females) • Began in 1976 with 121, 700 women Two types of cohorts defined by the exposure or unrelated

Cohort defined by a factor unrelated to any particular exposure: advantages • Data collection system to test multiple hypotheses about multiple exposures and disease outcomes • Internal comparison group (unexposed members of the cohort) Two types of cohorts defined by the exposure or unrelated
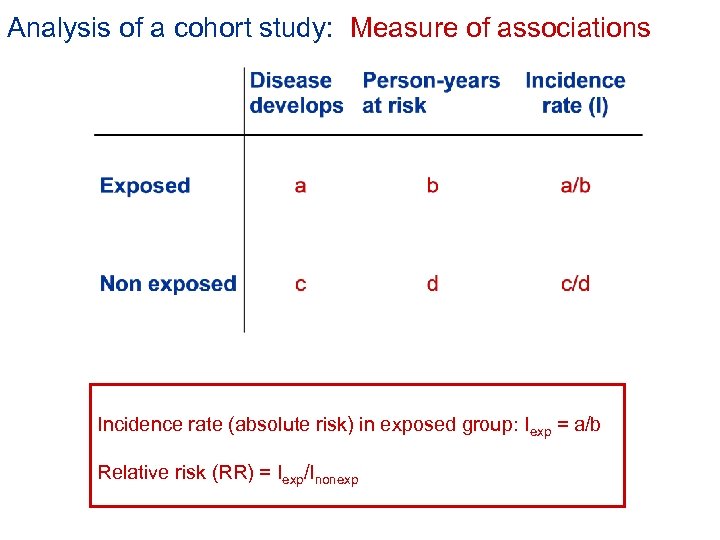
Analysis of a cohort study: Measure of associations Incidence rate (absolute risk) in exposed group: Iexp = a/b Relative risk (RR) = Iexp/Inonexp
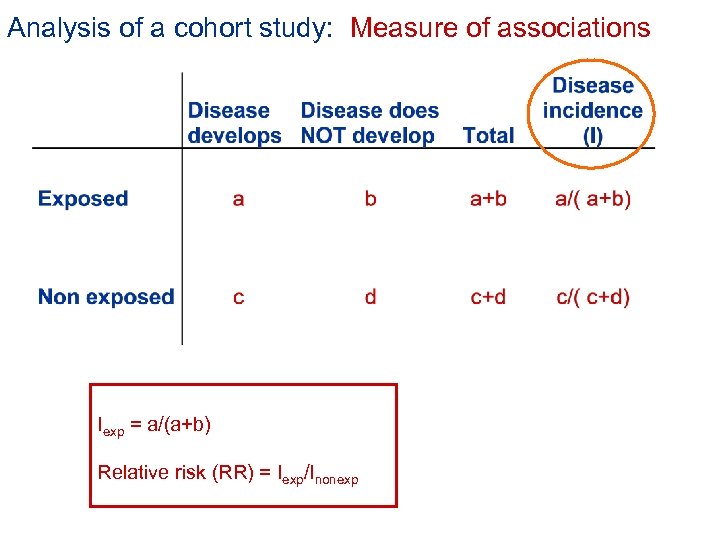
Analysis of a cohort study: Measure of associations Iexp = a/(a+b) Relative risk (RR) = Iexp/Inonexp

Comparison is fundamental to epidemiology: Relative risk (RR) = incidence in exposed /incidence in non exposed • The relative risk is a ratio (dimensionless) • Always make clear which group is exposed and which is non exposed Measure of associations Relative Risk

Interpretation of relative risk (RR) Relative risk is a measure of association between the exposure and the disease • RR = 1 • Risk in exposed = risk in non exposed • No association • RR > 1 • • Risk in exposed > risk in non exposed Positive association The larger the RR, the stronger the association May or may not be causal Measure of associations Relative Risk
Interpretation of relative risk (RR) • RR < 1 • • • Risk in exposed < risk in non exposed Negative association The smaller the RR, the larger the negative association May or may not be causal If causal, indicates a protective effect Measure of associations Relative Risk
Hypothetical cohort study of benzene (exposure) and leukemia (outcome) Hypothesis: benzene exposure increases the risk of leukemia Exposed group to benzene Yes leukemia Non exposed group to benzene No leukemia Follow the two groups for 10 years Time
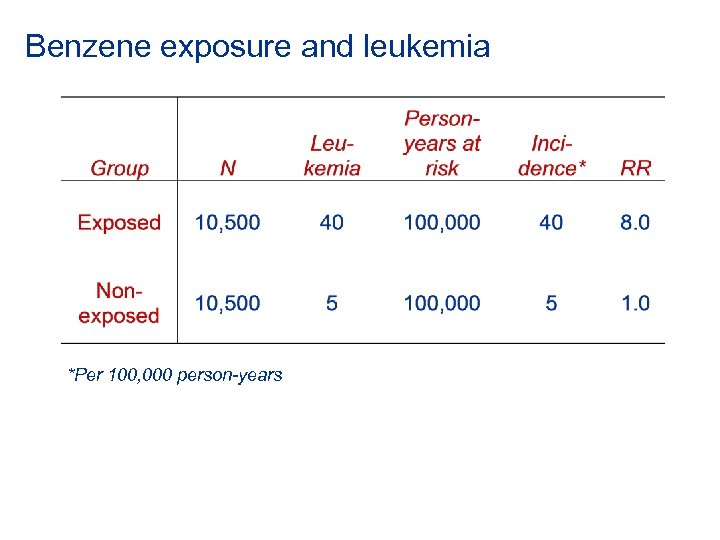
Benzene exposure and leukemia *Per 100, 000 person-years
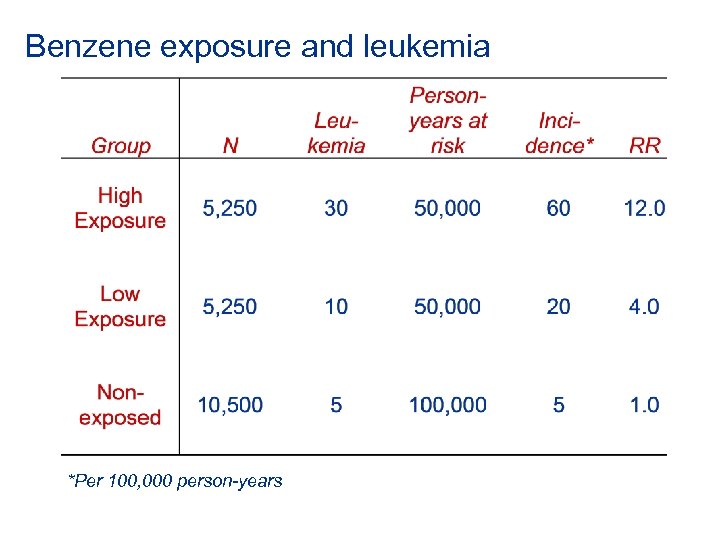
Benzene exposure and leukemia *Per 100, 000 person-years

How much of the disease that occurs can be attributed to a particular exposure? • Relative risk -- is a measure of association between an exposure and a disease • Attributable risk -- the magnitude of disease incidence attributable to a specific exposure • Attributable risk percent -- the percent of disease incidence attributable to a specific exposure The answer to this question tells us how much of the disease we can prevent if we eliminate the exposure Measure of associations Attributable risk (exposure)

Attributable risk for the exposed group • Attributable risk (exposed) = Iexp – Inonexp • Attributable risk percent (exposed) = [(Iexp - Inonexp)/ Iexp] x 100 = [(RR - 1)/RR] x 100 = • Incidence in non exposed group can be considered the background incidence, which would occur regardless of the exposure Measure of associations Attributable risk (exposure)
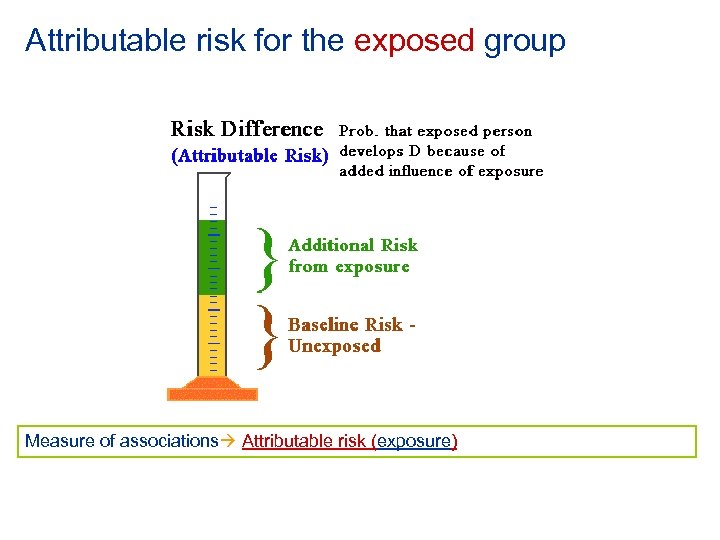
Attributable risk for the exposed group Measure of associations Attributable risk (exposure)

Attributable risk (exposed) tells us the most we can hope to accomplish in reducing the risk of disease among the exposed if we totally eliminated the exposure Measure of associations Attributable risk (exposure)
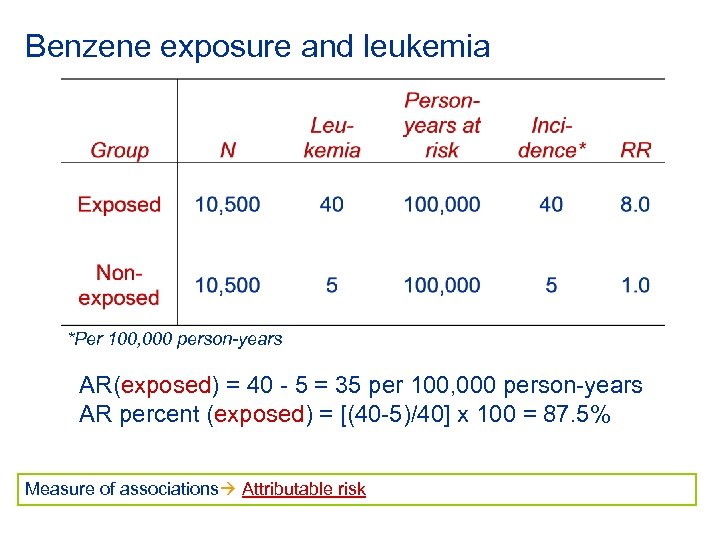
Benzene exposure and leukemia *Per 100, 000 person-years AR(exposed) = 40 - 5 = 35 per 100, 000 person-years AR percent (exposed) = [(40 -5)/40] x 100 = 87. 5% Measure of associations Attributable risk
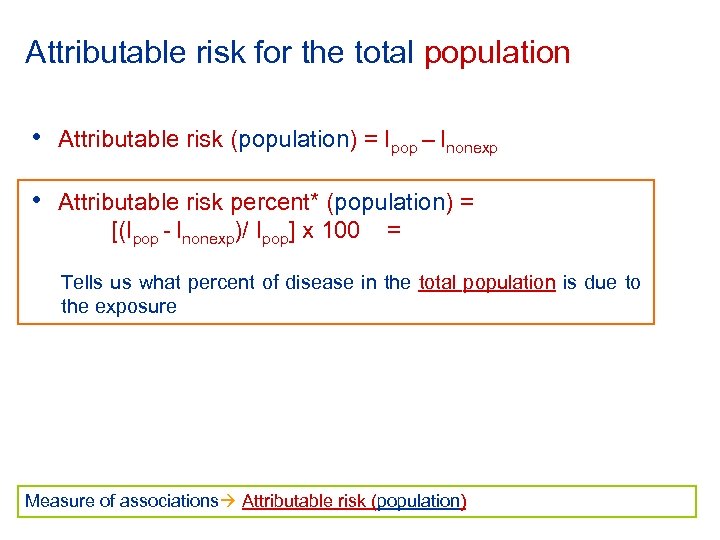
Attributable risk for the total population • Attributable risk (population) = Ipop – Inonexp • Attributable risk percent* (population) = [(Ipop - Inonexp)/ Ipop] x 100 = Tells us what percent of disease in the total population is due to the exposure Measure of associations Attributable risk (population)

Attributable risk (population) tells us the most we can hope to accomplish in reducing the incidence of disease in the total population if we totally eliminated the exposure Measure of associations Attributable risk (population)
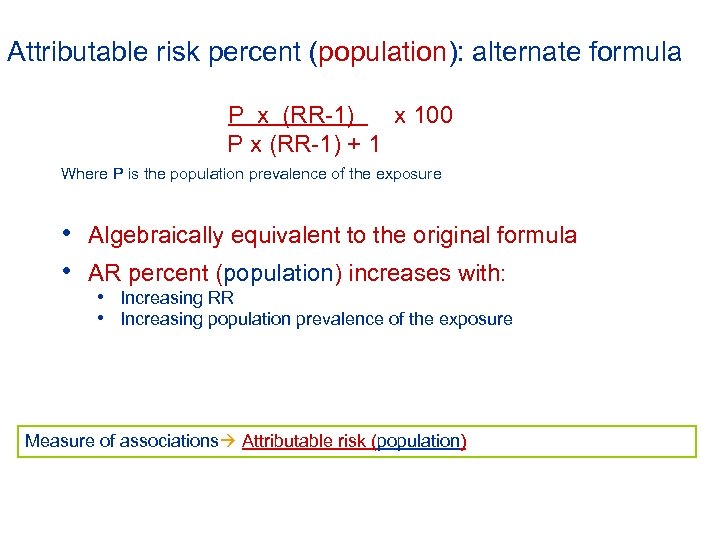
Attributable risk percent (population): alternate formula P x (RR-1) x 100 P x (RR-1) + 1 Where P is the population prevalence of the exposure • Algebraically equivalent to the original formula • AR percent (population) increases with: • Increasing RR • Increasing population prevalence of the exposure Measure of associations Attributable risk (population)
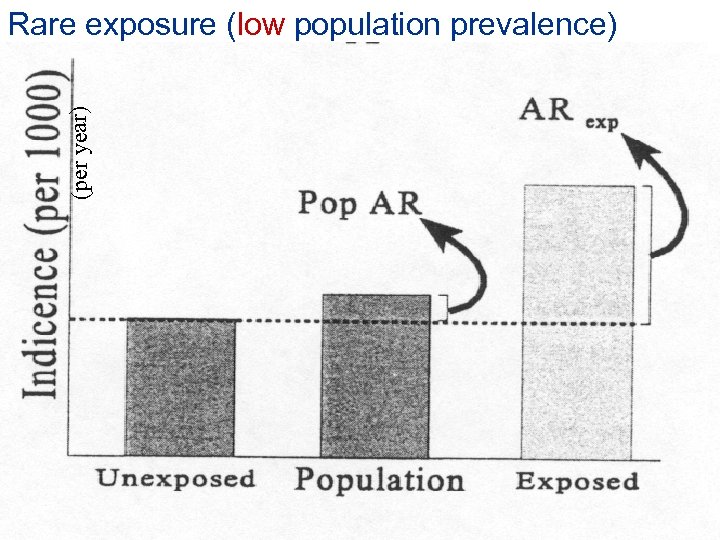
Rare exposure (low population prevalence) (per year) RR = 2
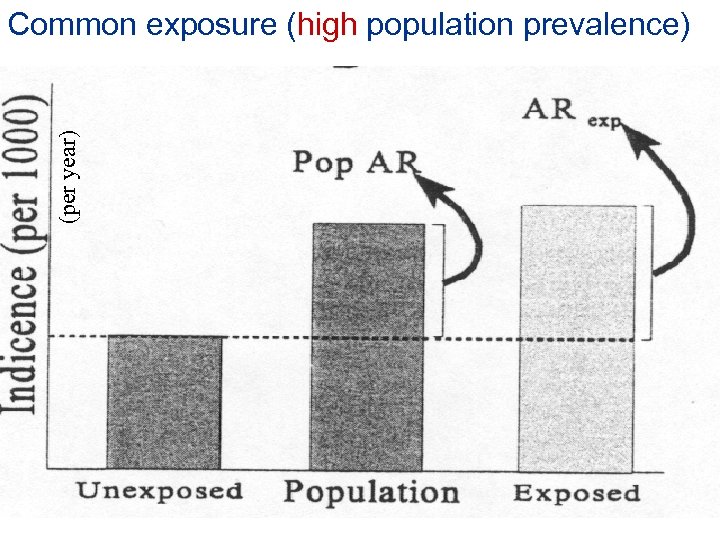
(per year) Common exposure (high population prevalence)
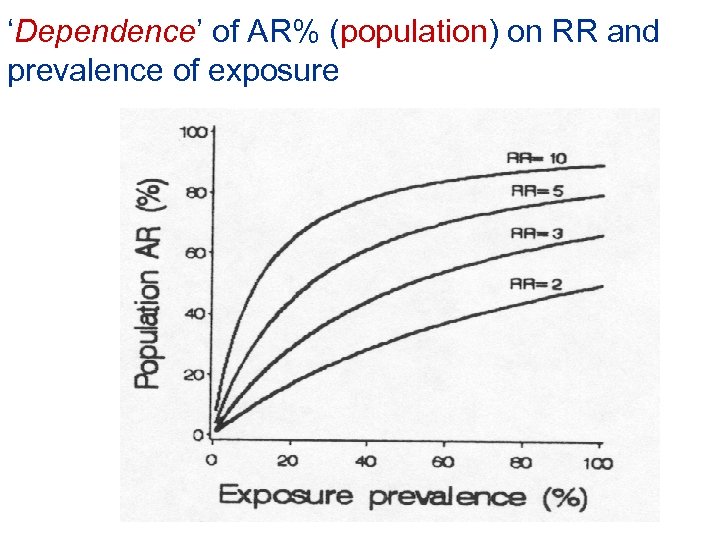
‘Dependence’ of AR% (population) on RR and prevalence of exposure
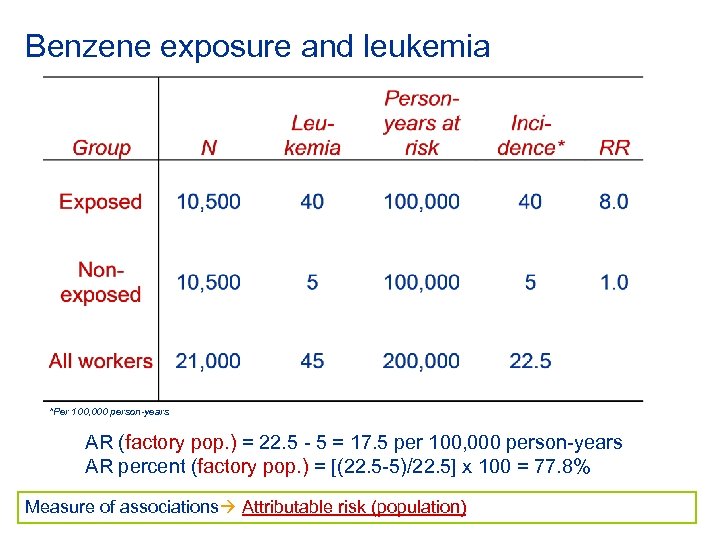
Benzene exposure and leukemia *Per 100, 000 person-years AR (factory pop. ) = 22. 5 - 5 = 17. 5 per 100, 000 person-years AR percent (factory pop. ) = [(22. 5 -5)/22. 5] x 100 = 77. 8% Measure of associations Attributable risk (population)
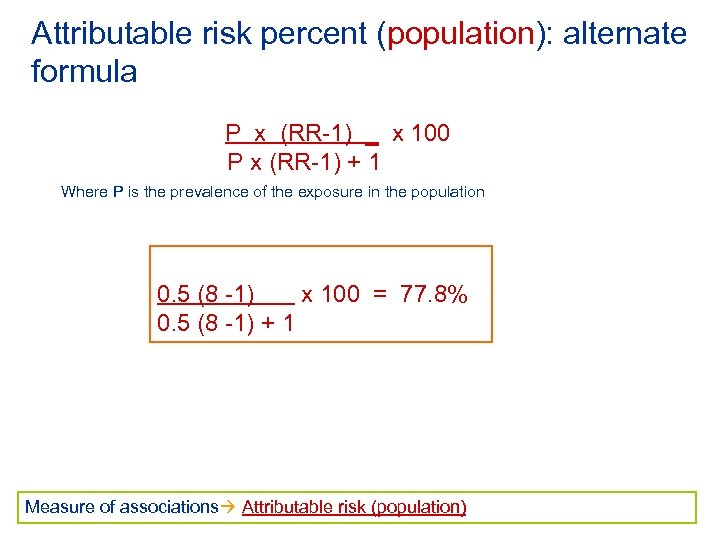
Attributable risk percent (population): alternate formula P x (RR-1) _ x 100 P x (RR-1) + 1 Where P is the prevalence of the exposure in the population 0. 5 (8 -1) x 100 = 77. 8% 0. 5 (8 -1) + 1 Measure of associations Attributable risk (population)
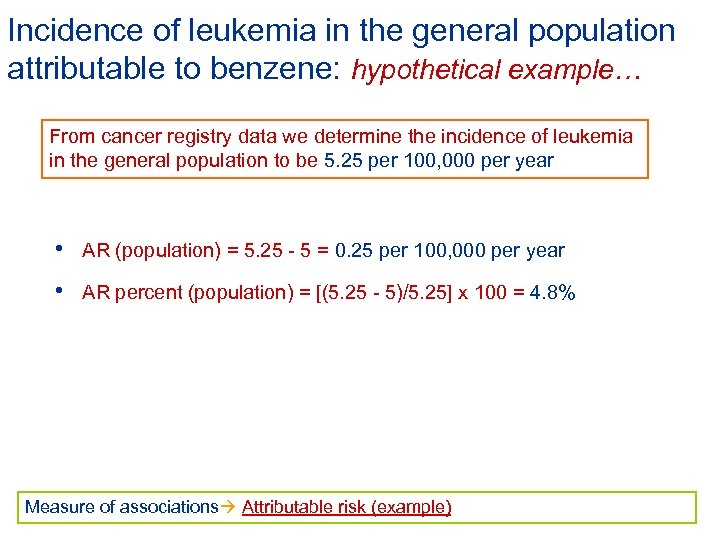
Incidence of leukemia in the general population attributable to benzene: hypothetical example… From cancer registry data we determine the incidence of leukemia in the general population to be 5. 25 per 100, 000 per year • AR (population) = 5. 25 - 5 = 0. 25 per 100, 000 per year • AR percent (population) = [(5. 25 - 5)/5. 25] x 100 = 4. 8% Measure of associations Attributable risk (example)
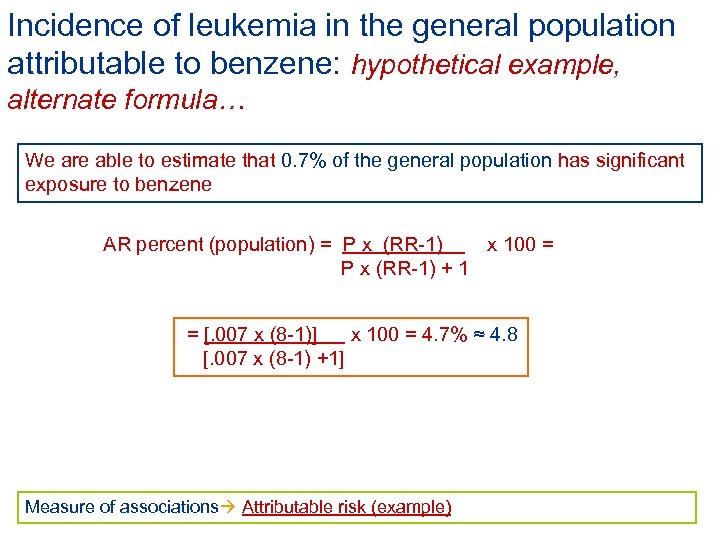
Incidence of leukemia in the general population attributable to benzene: hypothetical example, alternate formula… We are able to estimate that 0. 7% of the general population has significant exposure to benzene AR percent (population) = P x (RR-1) x 100 = P x (RR-1) + 1 = [. 007 x (8 -1)] x 100 = 4. 7% ≈ 4. 8 [. 007 x (8 -1) +1] Measure of associations Attributable risk (example)

Relative risk vs. attributable risk • RR is a measure of the strength of an association between an exposure and a disease, and is the measure used in etiologic studies • AR is a measure of how much of the disease incidence is attributable to the exposure, and is useful in assessing the exposure’s public health importance • AR (population) will vary among populations, depending upon the prevalence of the exposure Measure of associations Relative risk vs. attributable risk
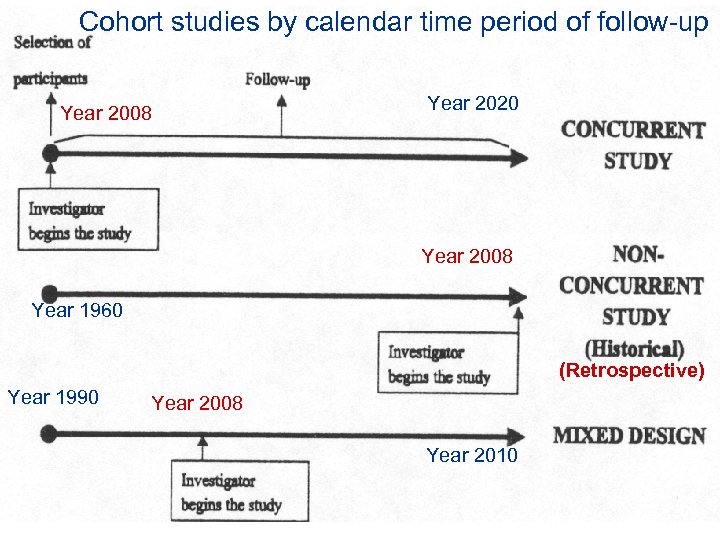
Cohort studies by calendar time period of follow-up Year 2008 Year 2020 Year 2008 Year 1960 (Retrospective) Year 1990 Year 2008 Year 2010
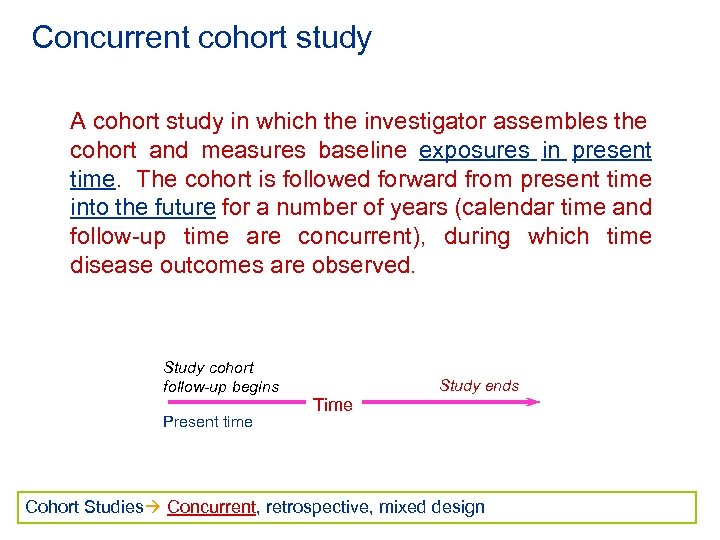
Concurrent cohort study A cohort study in which the investigator assembles the cohort and measures baseline exposures in present time. The cohort is followed forward from present time into the future for a number of years (calendar time and follow-up time are concurrent), during which time disease outcomes are observed. Study cohort follow-up begins Present time Study ends Time Cohort Studies Concurrent, retrospective, mixed design
Concurrent cohort study (cont. ) Advantage: • baseline exposure assessment and methods of follow-up for disease outcome are planned and implemented for purposes of the study Disadvantages • Study takes many years to conduct • High cost Cohort Studies Concurrent, retrospective, mixed design
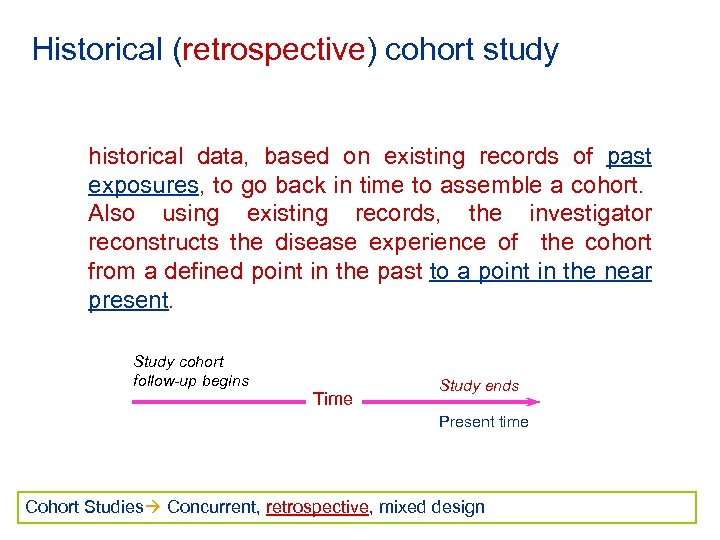
Historical (retrospective) cohort study historical data, based on existing records of past exposures, to go back in time to assemble a cohort. Also using existing records, the investigator reconstructs the disease experience of the cohort from a defined point in the past to a point in the near present. Study cohort follow-up begins Time Study ends Present time Cohort Studies Concurrent, retrospective, mixed design
Historical (retrospective) cohort study (cont. ) Advantage: • less expensive than concurrent studies • less time to conduct than concurrent studies Disadvantages • the quality of exposure or disease outcome data is often inferior to the quality obtained in concurrent studies (due to the reliance on records that usually were collected for a purpose other than conducting an epidemiologic study) Cohort Studies Concurrent, retrospective, mixed design
Mixed design cohort study historical data, based on existing records of past exposures, to go back in time to assemble a cohort. Also using existing records, the investigator reconstructs the disease experience of the cohort from a defined point in the past to a point in the future. Data for the cohort includes data from the past and data from present time into the future. Study cohort follow-up begins Study ends Time Present time Cohort Studies Concurrent, retrospective, mixed design
Concurrent cohort study: Hepatocelluar carcinoma and hepatitis B virus -- a prospective study of 22, 707 men in Taiwan (Beasley et al. ). Hypothesis: hepatitis B virus infection causes hepatocellular carcinoma (HCC) Cohort: male Taiwanese government civil servants Why civil servants? • Life and health insurance system provided almost total ascertainment • of death Retained insurance after retirement Cohort Studies Concurrent (example), retrospective, mixed design
Concurrent cohort study: Hepatocelluar carcinoma and hepatitis B virus -- a prospective study of 22, 707 men in Taiwan (Beasley et al. ). First cohort study of hepatitis B virus infection and Hepatocelluar carcinoma Cohort Studies Concurrent (example), retrospective, mixed design
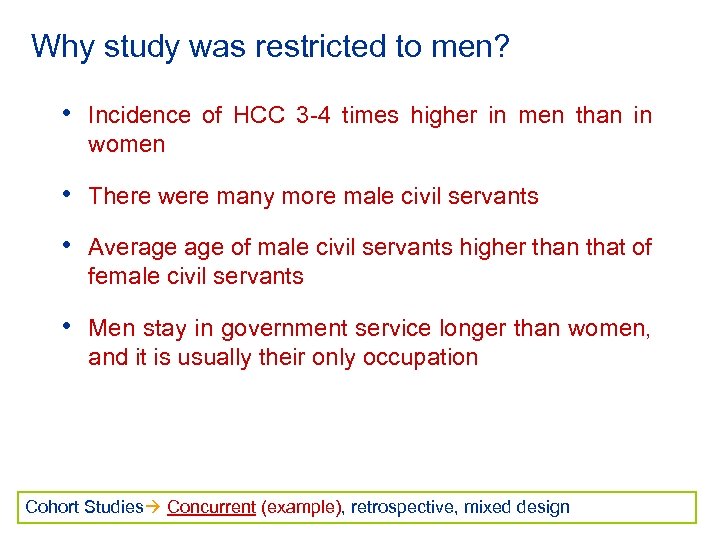
Why study was restricted to men? • Incidence of HCC 3 -4 times higher in men than in women • There were many more male civil servants • Average of male civil servants higher than that of female civil servants • Men stay in government service longer than women, and it is usually their only occupation Cohort Studies Concurrent (example), retrospective, mixed design
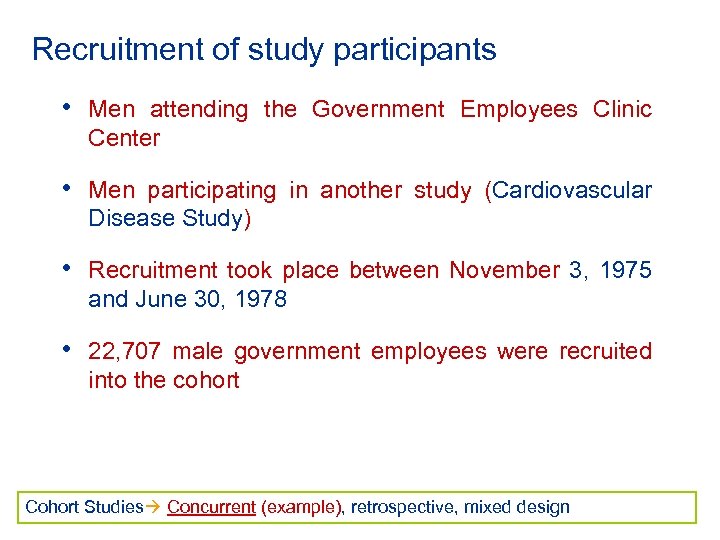
Recruitment of study participants • Men attending the Government Employees Clinic Center • Men participating in another study (Cardiovascular Disease Study) • Recruitment took place between November 3, 1975 and June 30, 1978 • 22, 707 male government employees were recruited into the cohort Cohort Studies Concurrent (example), retrospective, mixed design
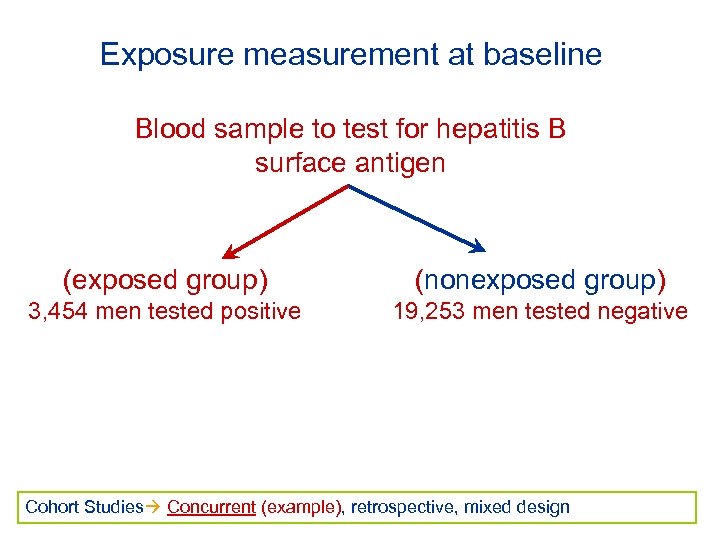
Exposure measurement at baseline Blood sample to test for hepatitis B surface antigen (exposed group) (nonexposed group) 3, 454 men tested positive 19, 253 men tested negative Cohort Studies Concurrent (example), retrospective, mixed design

Follow-up methods Cohort followed forward in time from the present (calendar time and follow-up time concurrent) to assess outcome (death from HCC) Cohort Studies Concurrent (example), retrospective, mixed design
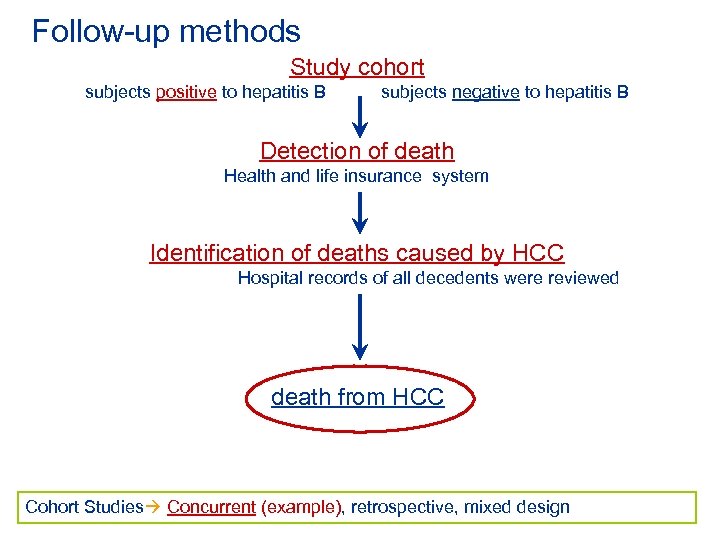
Follow-up methods Study cohort subjects positive to hepatitis B subjects negative to hepatitis B Detection of death Health and life insurance system Identification of deaths caused by HCC Hospital records of all decedents were reviewed death from HCC Cohort Studies Concurrent (example), retrospective, mixed design

Results of follow-up • Follow-up through December 31, 1980 • 307 members of cohort died • 74 members of cohort had retired and canceled their insurance and could not be traced • About 75, 000 person-years of follow-up; average of 3. 3 years per man Cohort Studies Concurrent (example), retrospective, mixed design
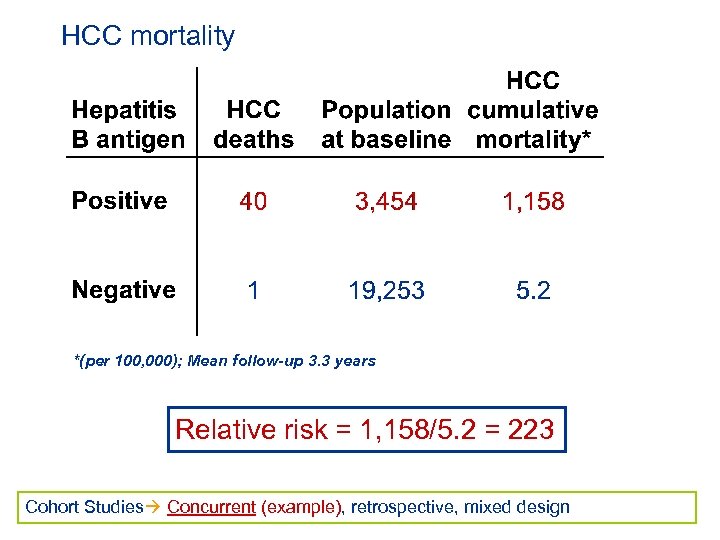
HCC mortality *(per 100, 000); Mean follow-up 3. 3 years Relative risk = 1, 158/5. 2 = 223 Cohort Studies Concurrent (example), retrospective, mixed design
Conclusions • hepatitis B virus infection preceded (causes) the development of Hepatocelluar carcinoma • The estimated relative risk (223) provides strong evidence that hepatitis B virus infection causes Hepatocelluar carcinoma Cohort Studies Concurrent (example), retrospective, mixed design
Comparing incidence rates in different populations Indirect age-adjustment (often used in retrospective cohort studies) uses standard age-specific mortality or incidence rates from an external comparison group (usually the general population) • Calculate the number of deaths or cases of disease expected in the cohort if it had the same age-specific mortality or incidence rates as the external comparison group (usually general population) • Compare the expected number with the actual observed number of deaths or disease in the cohort • Usually also adjust for calendar-year time of death or disease incidence (Selikoff et al. )

Standardized mortality ratio (SMR): observed number of deaths x 100 expected number of deaths Where expected = population of the study cohort X mortality rate of external population Standardized incidence ratio (SIR): observed number of cases x 100 expected number of cases Where expected = population of the study cohort X incidence rate of external population SMR and SIR can be interpreted as relative risks
…a key factor for the interpretation of standardized mortality ratios and incidence ratios is the construction of reliable 95% confidence intervals Cohort Studies Concurrent, retrospective, mixed design
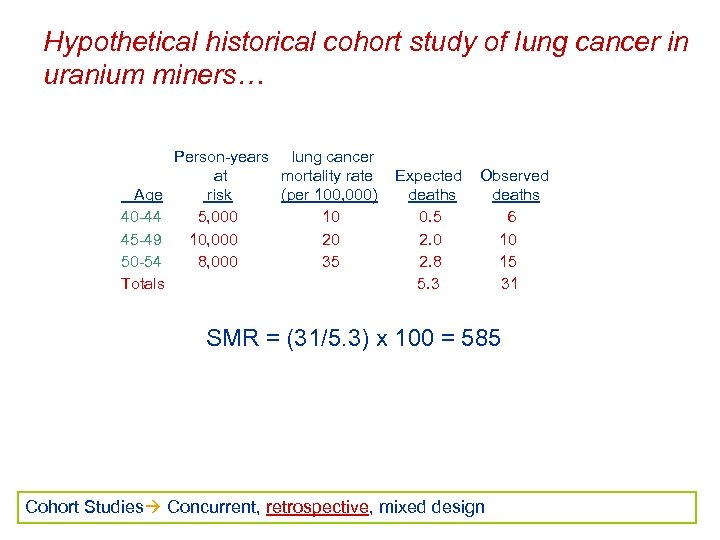
Hypothetical historical cohort study of lung cancer in uranium miners… Age 40 -44 45 -49 50 -54 Totals Person-years lung cancer at mortality rate risk (per 100, 000) 5, 000 10 10, 000 20 8, 000 35 Expected deaths 0. 5 2. 0 2. 8 5. 3 Observed deaths 6 10 15 31 SMR = (31/5. 3) x 100 = 585 Cohort Studies Concurrent, retrospective, mixed design
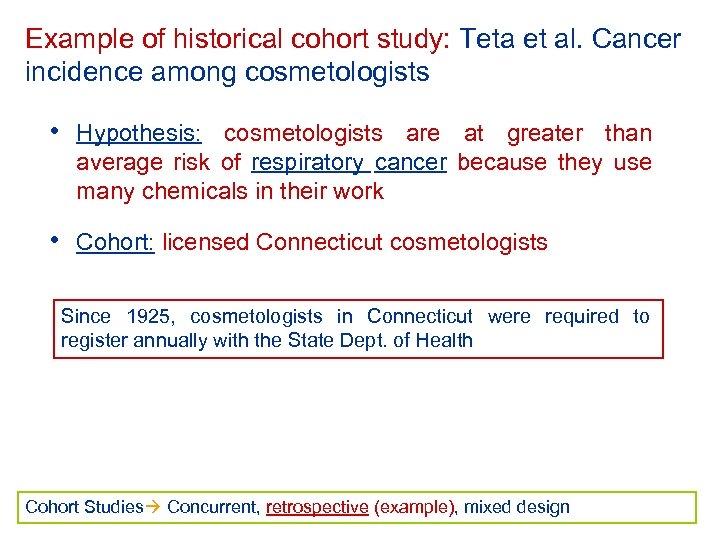
Example of historical cohort study: Teta et al. Cancer incidence among cosmetologists • Hypothesis: cosmetologists are at greater than average risk of respiratory cancer because they use many chemicals in their work • Cohort: licensed Connecticut cosmetologists Since 1925, cosmetologists in Connecticut were required to register annually with the State Dept. of Health Cohort Studies Concurrent, retrospective (example), mixed design
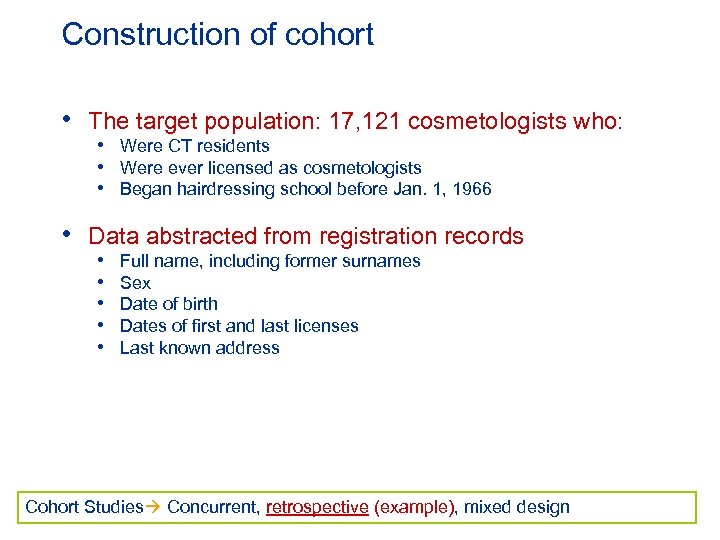
Construction of cohort • The target population: 17, 121 cosmetologists who: • Were CT residents • Were ever licensed as cosmetologists • Began hairdressing school before Jan. 1, 1966 • Data abstracted from registration records • • • Full name, including former surnames Sex Date of birth Dates of first and last licenses Last known address Cohort Studies Concurrent, retrospective (example), mixed design
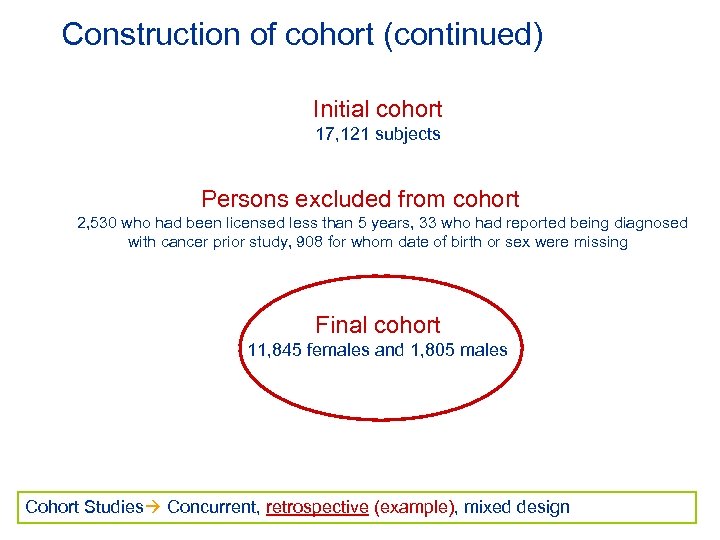
Construction of cohort (continued) Initial cohort 17, 121 subjects Persons excluded from cohort 2, 530 who had been licensed less than 5 years, 33 who had reported being diagnosed with cancer prior study, 908 for whom date of birth or sex were missing Final cohort 11, 845 females and 1, 805 males Cohort Studies Concurrent, retrospective (example), mixed design
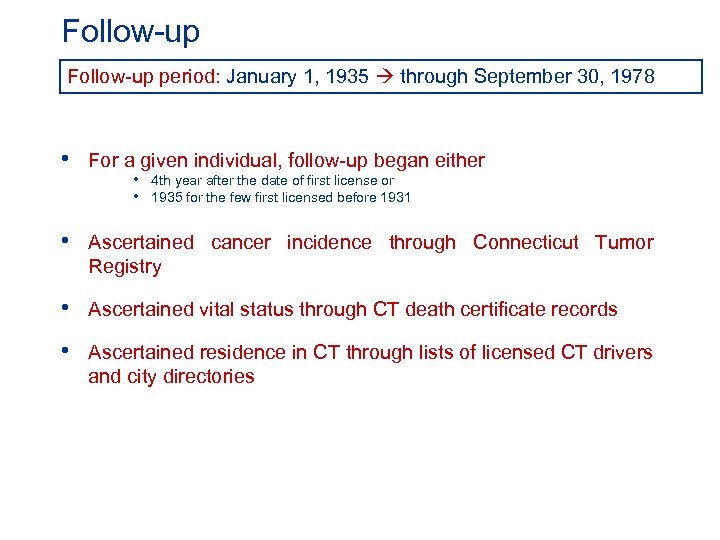
Follow-up period: January 1, 1935 through September 30, 1978 • For a given individual, follow-up began either • 4 th year after the date of first license or • 1935 for the few first licensed before 1931 • Ascertained cancer incidence through Connecticut Tumor Registry • Ascertained vital status through CT death certificate records • Ascertained residence in CT through lists of licensed CT drivers and city directories

Follow-up (continued) • For each cohort member, person-years at risk were counted until one of the following, whichever came first: » Last known year at a CT address » Date of death » Cancer diagnosis » September 30, 1978 • 241, 580 person-years of follow-up
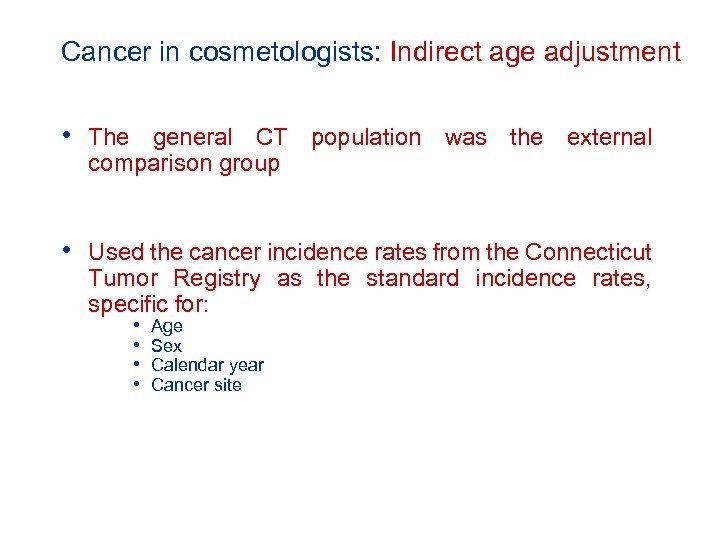
Cancer in cosmetologists: Indirect age adjustment • The general CT population was the external comparison group • Used the cancer incidence rates from the Connecticut Tumor Registry as the standard incidence rates, specific for: • • Age Sex Calendar year Cancer site
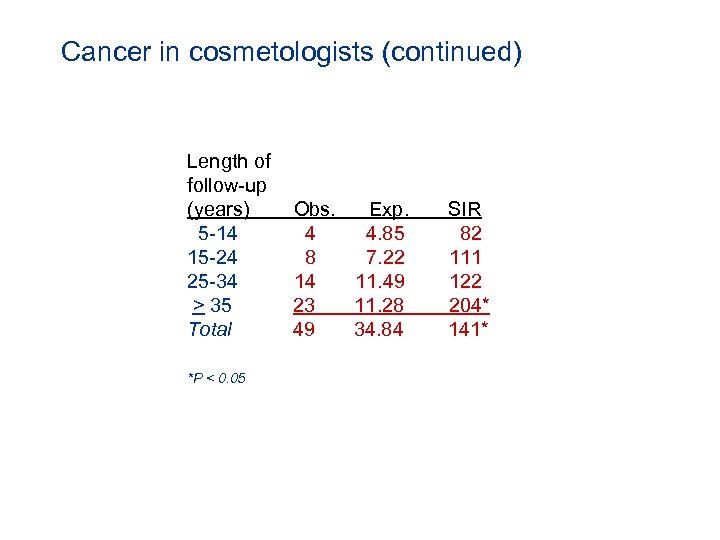
Cancer in cosmetologists (continued) Length of follow-up (years) 5 -14 15 -24 25 -34 > 35 Total *P < 0. 05 Obs. Exp. 4 4. 85 8 7. 22 14 11. 49 23 11. 28 49 34. 84 SIR 82 111 122 204* 141*

Cancer in cosmetologists: limitations • History of specific exposures unavailable • Smoking histories unavailable • Authors present anecdotal evidence that cosmetologists had higher smoking rates than general female population • ‘Adjustment’ for important confounders not possible
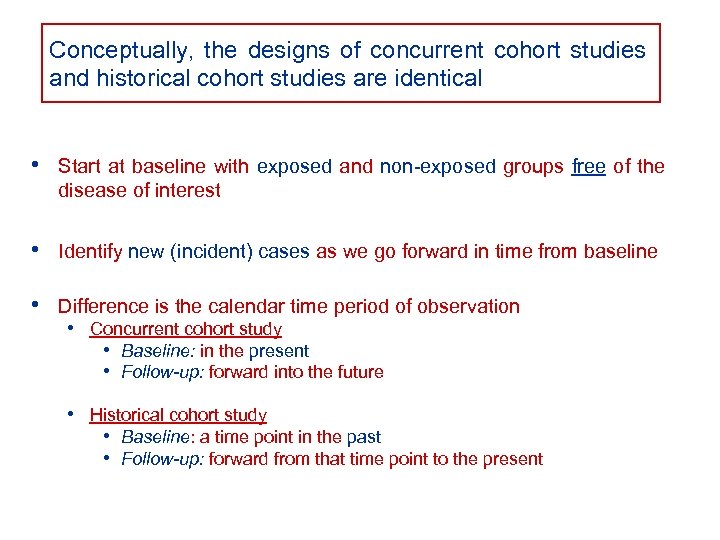
Conceptually, the designs of concurrent cohort studies and historical cohort studies are identical • Start at baseline with exposed and non-exposed groups free of the disease of interest • Identify new (incident) cases as we go forward in time from baseline • Difference is the calendar time period of observation • Concurrent cohort study • Baseline: in the present • Follow-up: forward into the future • Historical cohort study • Baseline: a time point in the past • Follow-up: forward from that time point to the present
fc01dff946849f93123a478229391588.ppt