498075621974bba6d93cfd54a75e01ac.ppt
- Количество слайдов: 48
Spring 2006 Polymer Chemistry Basic Principles and Introduction Prof. Y. M. Lee School of Chemical Engineering, College of Engineering Hanyang University Hanyang Univ.
Spring 2006 We live in a polymer age!! Rubber Elastomers Plastics Fibers Coatings Protein Surfing to the internet Polymers are everywhere !!! Cellulose Adhesives Click the next homepage http: //www. pslc. ws/mactest/leve l 1. htm Hanyang Univ.

Spring 2006 "I just want to say one word to you -- just one word -- 'plastics. '" Advice to Dustin Hoffman's character in The Graduate Hanyang Univ.
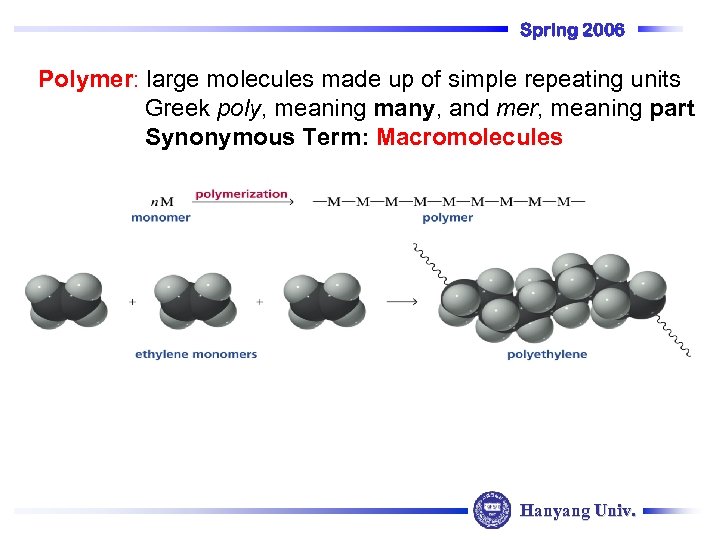
Spring 2006 Polymer: large molecules made up of simple repeating units Greek poly, meaning many, and mer, meaning part Synonymous Term: Macromolecules Hanyang Univ.
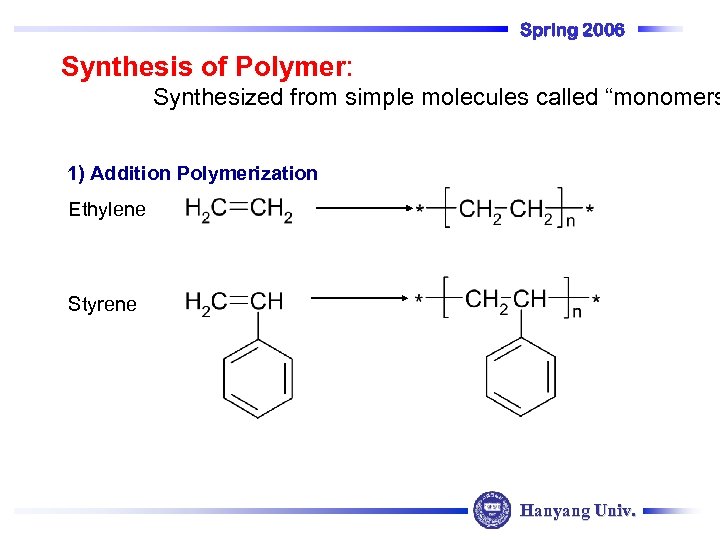
Spring 2006 Synthesis of Polymer: Synthesized from simple molecules called “monomers 1) Addition Polymerization Ethylene Styrene Hanyang Univ.
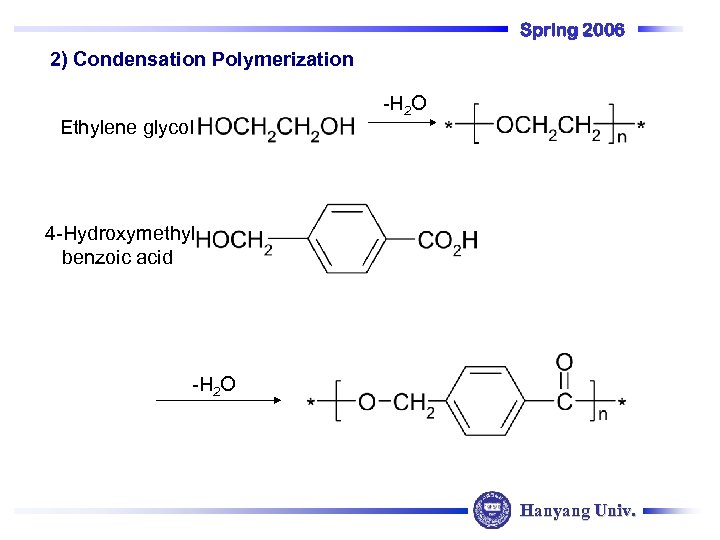
Spring 2006 2) Condensation Polymerization Ethylene glycol -H 2 O 4 -Hydroxymethyl benzoic acid -H 2 O Hanyang Univ.
Spring 2006 Historic Highlights in Polymer Chemistry • 1600 BC - Meso-americans produce Rubber • rubber balls • rubber handles for tools (600 -900 AD) • medicinal chewing gum, rubber boots and clothes (1400 AD) • 1830 AD - Re-invention of Rubber. New vulcanisation with Sulphur - Charles Goodyear • pneumatic tire (Real: 1845 Thomson, but ‘copy’ 1888 Dunlop) • 1846 Gun Cotton by Christian Schönberg • 1866 - Celluloid Wesley Hyatt & Alexander Parkes • billiard balls Hanyang Univ.

Spring 2006 Meso-American Rubber • Latex from Castilla Elastica • Liquid extracted from Ipomoea Alba (morning glory vine) Mixing causes: Latex coagulation and purification Introduction of plasticizers Thermal curing: Crystalline entanglements Chemical crosslinking via sulfonyl chlorides and acids Hanyang Univ.

Spring 2006 • 1907 - Bakelite Leo Baekeland • electrical insulator • light-weight war machinery • 1924 Concept of Macromolecules H. Staudinger (Nobel Prize 1953) • 1929 Concepts of Addition and Condensation polymers, Wallace H. Carothers • neoprene • polyesters • nylons • 1929 Plastisizing PVC by Waldo Semon • 1938 TEFLON by Roy Plunkett • 1943/1949 Silly Putty by James Wright/Peter Hodgson • 1953/1954 Polyethylene/polypropylene Karl Ziegler & Giulio Natta (Nobel Prize 1963) Hanyang Univ.
Spring 2006 • 1974 Paul J. Flory Nobel Prize • Flory temperature • Chain Transfer • Universal constant • 1991 Pierre-Gilles de Gennes Nobel Prize • Reptation model • 2000 Heeger, Macdiarmid, Shirakawa Nobel Prize • conductive polymers • 2002 - John B. Fenn, Koichi Tanaka, Kurt Wüthrich Nobel Prize • Structural determination biomacromolecules Hanyang Univ.

Spring 2006 Important Advances in Polymer Science • High thermal and oxidation-stable polymer: high performance aerospace applications • Engineering plastics – polymers designed to replace metals • High strength aromatic fibers – a variety of applications from tire cord to cables for anchoring oceanic oil-drilling platforms • Non flammable polymers – emit a minimum of smoke or toxic fumes • Degradable polymers – allow controlled release of drugs or agricultural chemicals • Polymer for a broad spectrum of medical applications – from degradable sutures to artificial organs • Conducting polymers – exhibit electrical conductivities comparable to those of metals • Polymer that serve as insoluble support for catalysts or for automated Hanyang Univ. protein or nucleic acid synthesis (Bruce Merrifield, who originated solid-

Spring 2006 Quiz Surfing to the internet University of Southern Mississippi Polymer Science Learning Center -------------------------------General Polymer Knowledge Test Click the next homepage http: //www. pslc. ws/quizzes/poly 0. htm If you take quizzes more than once, you will get different questions, so try them again. Hanyang Univ.
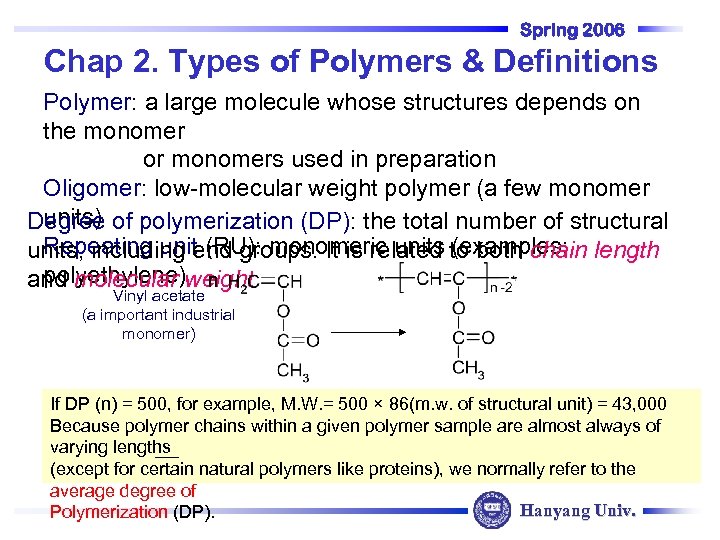
Spring 2006 Chap 2. Types of Polymers & Definitions Polymer: a large molecule whose structures depends on the monomer or monomers used in preparation Oligomer: low-molecular weight polymer (a few monomer units) Degree of polymerization (DP): the total number of structural Repeating unit (RU): monomeric units (examples: units, including end groups. It is related to both chain length polyethylene) n and molecular weight Vinyl acetate (a important industrial monomer) If DP (n) = 500, for example, M. W. = 500 × 86(m. w. of structural unit) = 43, 000 Because polymer chains within a given polymer sample are almost always of varying lengths (except for certain natural polymers like proteins), we normally refer to the average degree of Hanyang Univ. Polymerization (DP).
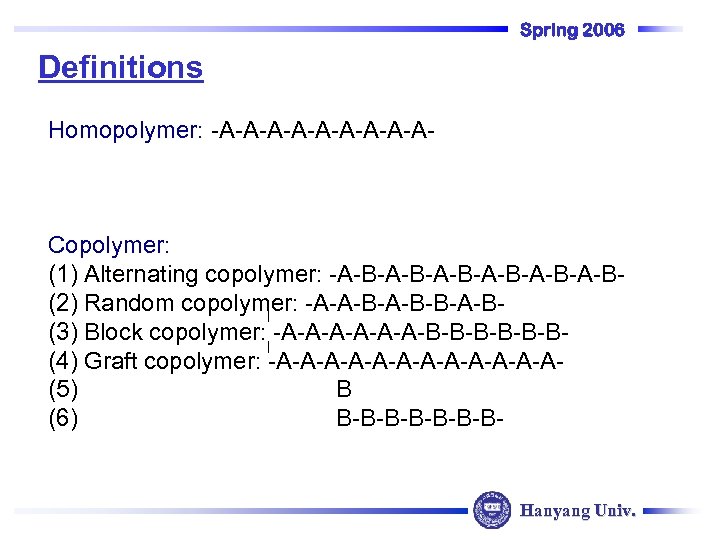
Spring 2006 Definitions Homopolymer: -A-A-A-A-A- Copolymer: (1) Alternating copolymer: -A-B-A-B-A-B(2) Random copolymer: -A-A-B-B-A-B(3) Block copolymer: -A-A-A-B-B-B(4) Graft copolymer: -A-A-A-A-A-A(5) B (6) B-B-B-B- Hanyang Univ.
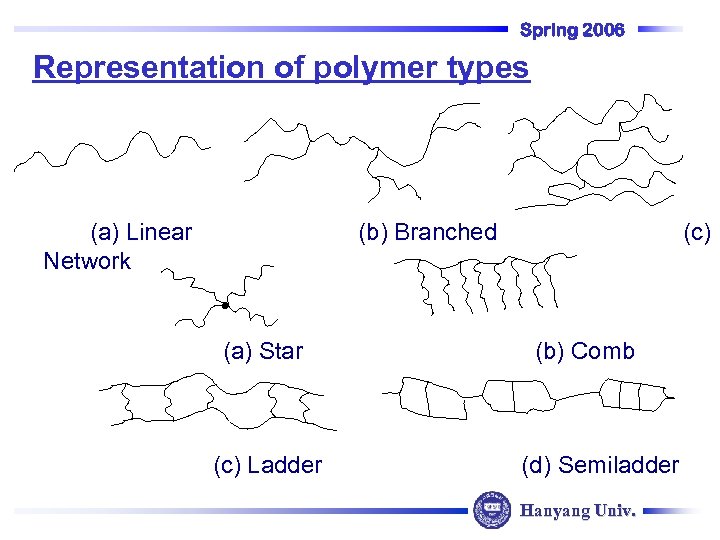
Spring 2006 Representation of polymer types (a) Linear Network (b) Branched (a) Star (c) Ladder (c) (b) Comb (d) Semiladder Hanyang Univ.
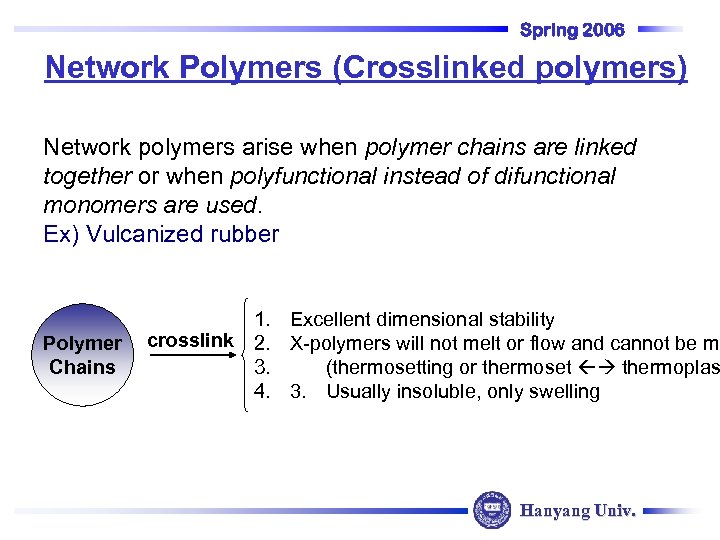
Spring 2006 Network Polymers (Crosslinked polymers) Network polymers arise when polymer chains are linked together or when polyfunctional instead of difunctional monomers are used. Ex) Vulcanized rubber Polymer Chains 1. Excellent dimensional stability crosslink 2. X-polymers will not melt or flow and cannot be mo 3. (thermosetting or thermoset thermoplas 4. 3. Usually insoluble, only swelling Hanyang Univ.
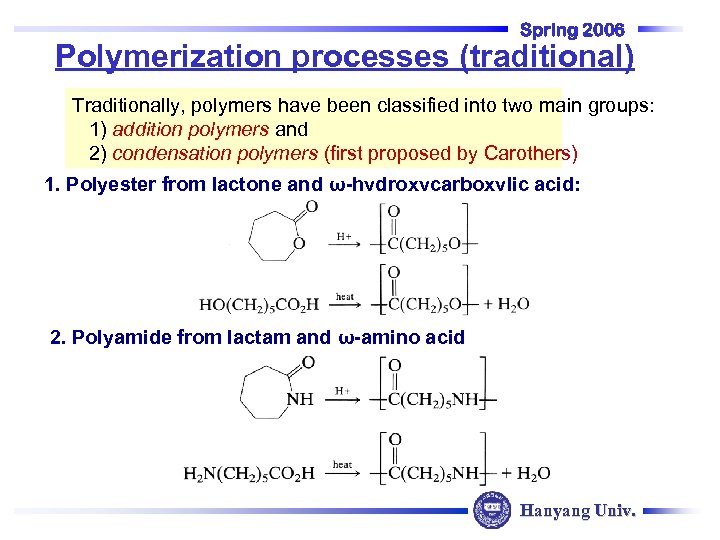
Spring 2006 Polymerization processes (traditional) Traditionally, polymers have been classified into two main groups: 1) addition polymers and 2) condensation polymers (first proposed by Carothers) 1. Polyester from lactone and ω-hydroxycarboxylic acid: 2. Polyamide from lactam and ω-amino acid Hanyang Univ.
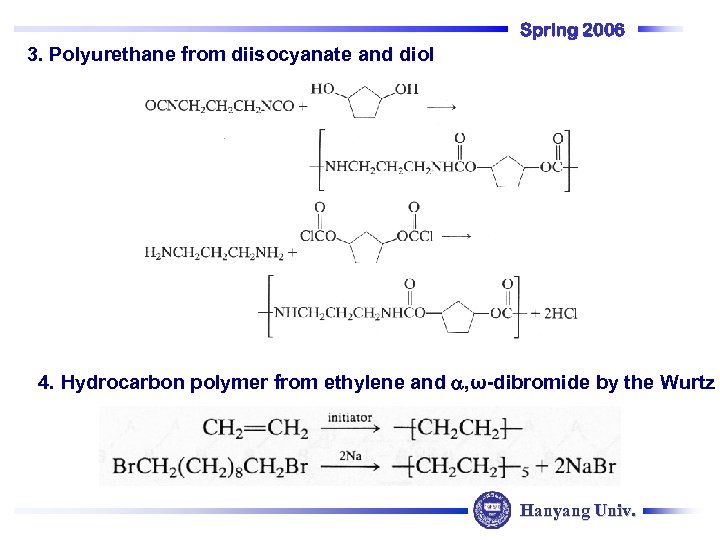
Spring 2006 3. Polyurethane from diisocyanate and diol 4. Hydrocarbon polymer from ethylene and , ω-dibromide by the Wurtz Hanyang Univ.
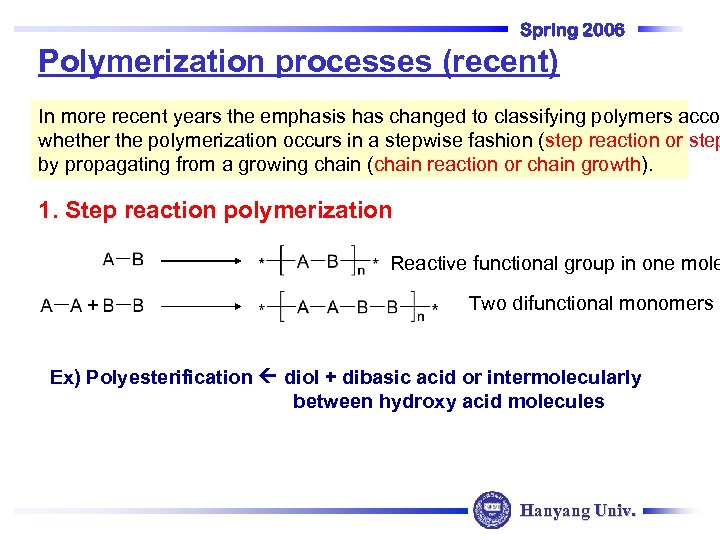
Spring 2006 Polymerization processes (recent) In more recent years the emphasis has changed to classifying polymers acco whether the polymerization occurs in a stepwise fashion (step reaction or step by propagating from a growing chain (chain reaction or chain growth). 1. Step reaction polymerization Reactive functional group in one mole + Two difunctional monomers Ex) Polyesterification diol + dibasic acid or intermolecularly between hydroxy acid molecules Hanyang Univ.
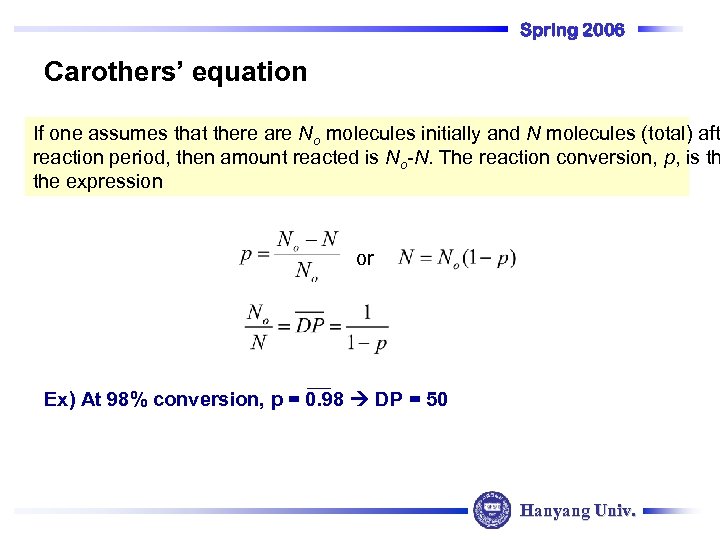
Spring 2006 Carothers’ equation If one assumes that there are No molecules initially and N molecules (total) aft reaction period, then amount reacted is No-N. The reaction conversion, p, is th the expression or Ex) At 98% conversion, p = 0. 98 DP = 50 Hanyang Univ.
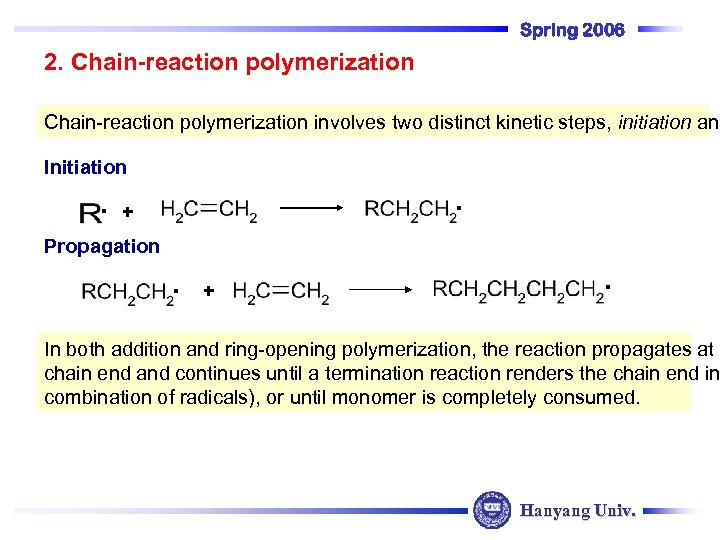
Spring 2006 2. Chain-reaction polymerization involves two distinct kinetic steps, initiation and Initiation . . + Propagation . + . In both addition and ring-opening polymerization, the reaction propagates at a chain end and continues until a termination reaction renders the chain end in combination of radicals), or until monomer is completely consumed. Hanyang Univ.
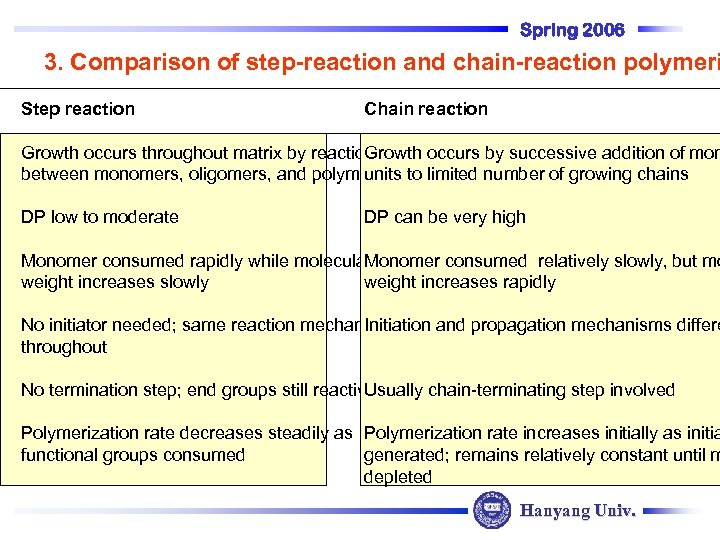
Spring 2006 3. Comparison of step-reaction and chain-reaction polymeri Step reaction Chain reaction Growth occurs throughout matrix by reaction Growth occurs by successive addition of mon between monomers, oligomers, and polymers to limited number of growing chains units DP low to moderate DP can be very high Monomer consumed rapidly while molecular Monomer consumed relatively slowly, but mo weight increases slowly weight increases rapidly No initiator needed; same reaction mechanism Initiation and propagation mechanisms differe throughout No termination step; end groups still reactive Usually chain-terminating step involved Polymerization rate decreases steadily as Polymerization rate increases initially as initia functional groups consumed generated; remains relatively constant until m depleted Hanyang Univ.
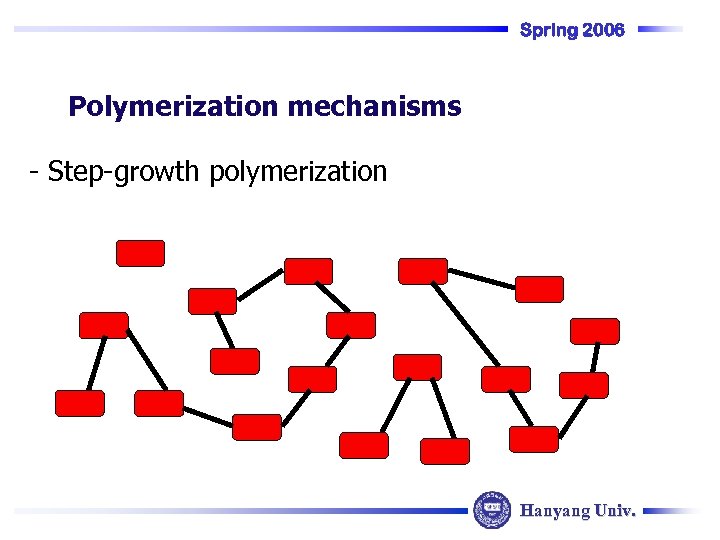
Spring 2006 Polymerization mechanisms - Step-growth polymerization Hanyang Univ.
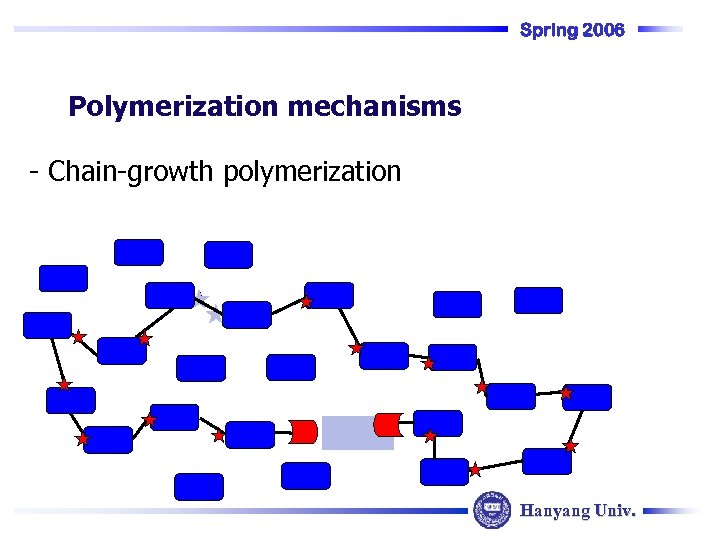
Spring 2006 Polymerization mechanisms - Chain-growth polymerization Hanyang Univ.
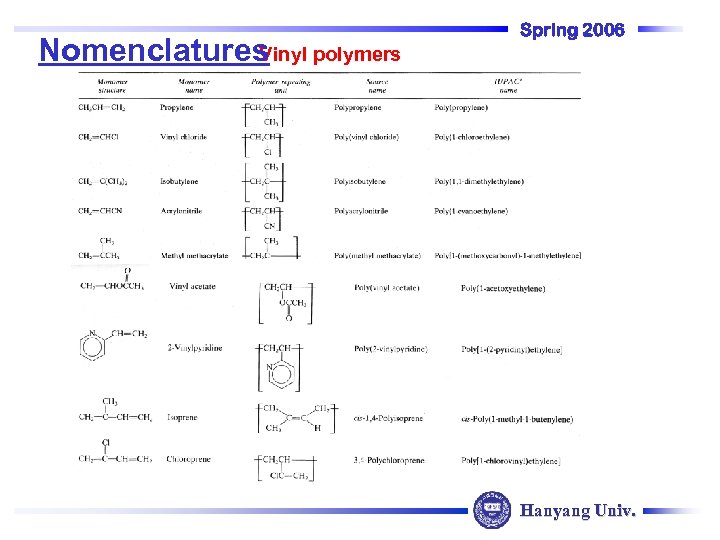
Vinyl polymers Nomenclatures Spring 2006 Hanyang Univ.
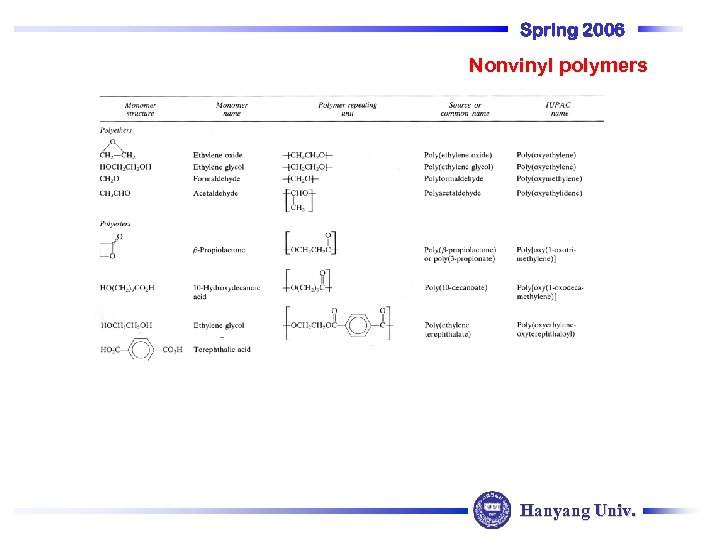
Spring 2006 Nonvinyl polymers Hanyang Univ.
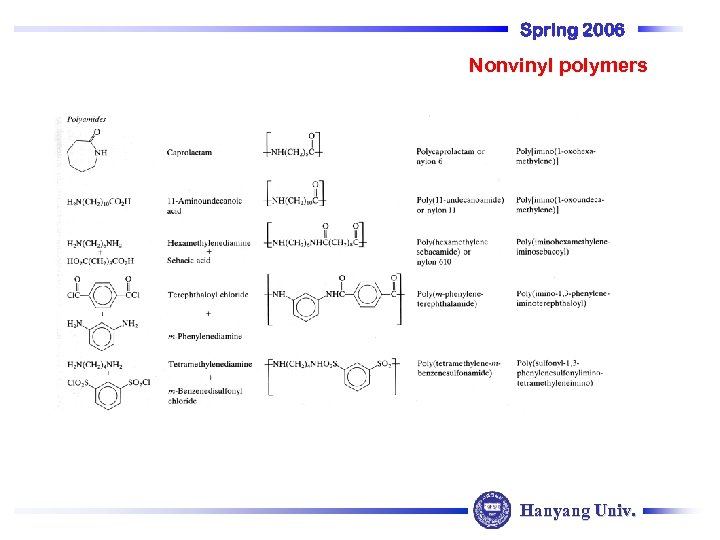
Spring 2006 Nonvinyl polymers Hanyang Univ.

Spring 2006 Quiz 2 Surfing to the internet University of Southern Mississippi Polymer Science Learning Center -------------------------------Naming of polymers: What works and doesn’t Click the next homepage http: //www. pslc. ws/quizzes/assess/NAMING/NA MING. HTM If you take quizzes more than once, you will get different questions, so try them again. Hanyang Univ.
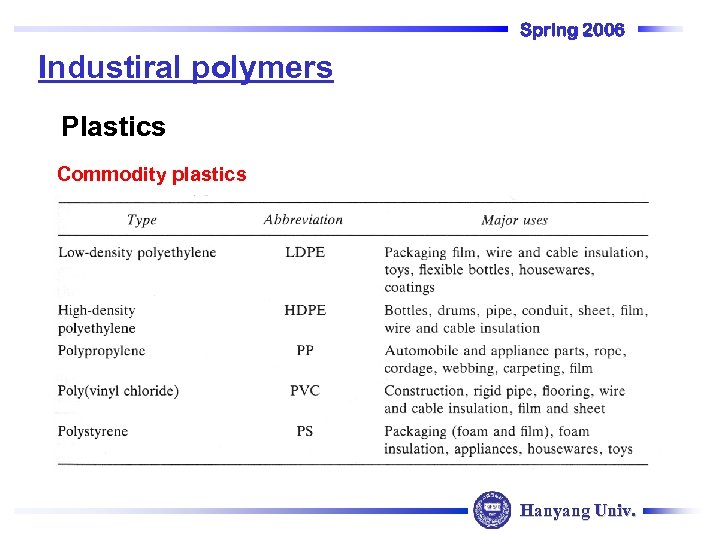
Spring 2006 Industiral polymers Plastics Commodity plastics Hanyang Univ.
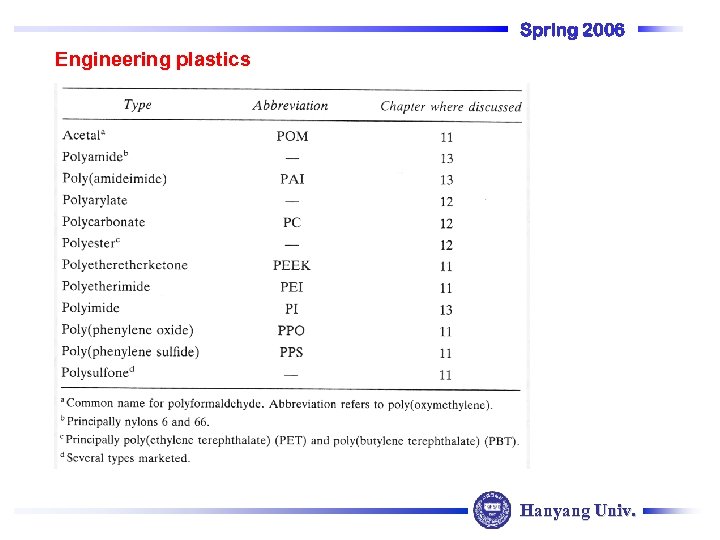
Spring 2006 Engineering plastics Hanyang Univ.
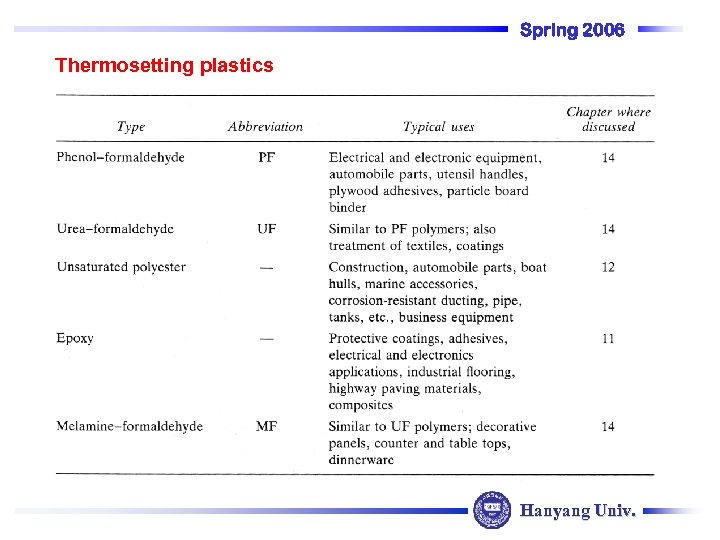
Spring 2006 Thermosetting plastics Hanyang Univ.
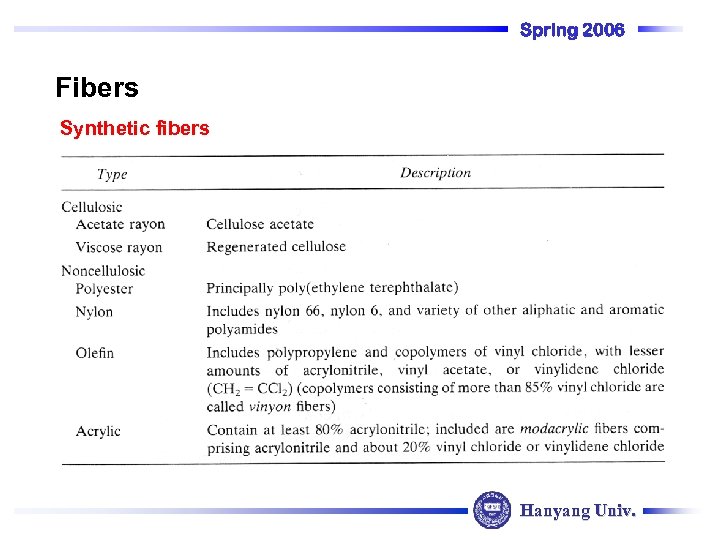
Spring 2006 Fibers Synthetic fibers Hanyang Univ.
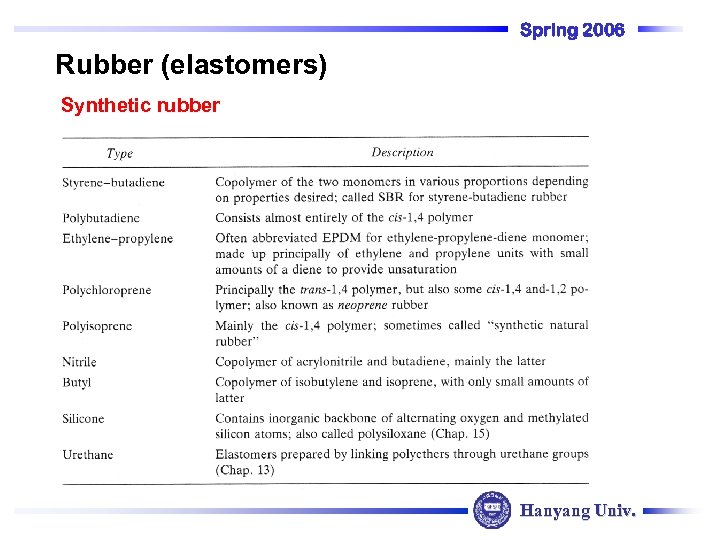
Spring 2006 Rubber (elastomers) Synthetic rubber Hanyang Univ.
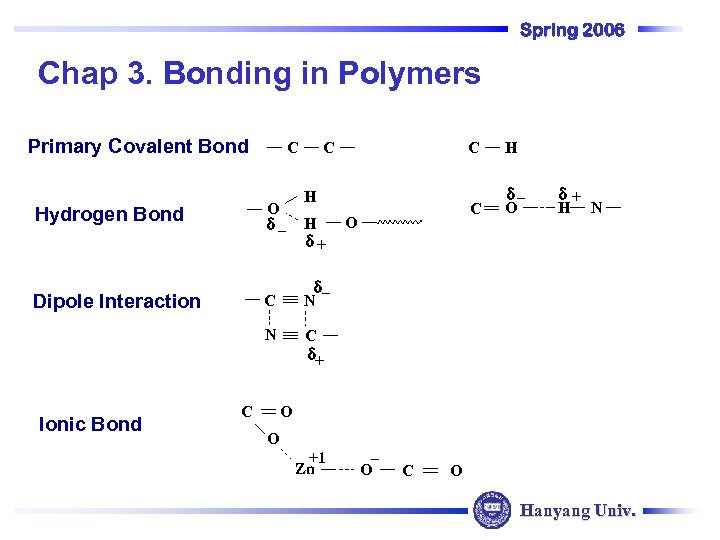
Spring 2006 Chap 3. Bonding in Polymers Primary Covalent Bond C O d_ Hydrogen Bond Dipole Interaction C C H H d+ O H d_ O d+ H N d_ N N Ionic Bond C C d+ O O +1 Zn _ O C O Hanyang Univ.
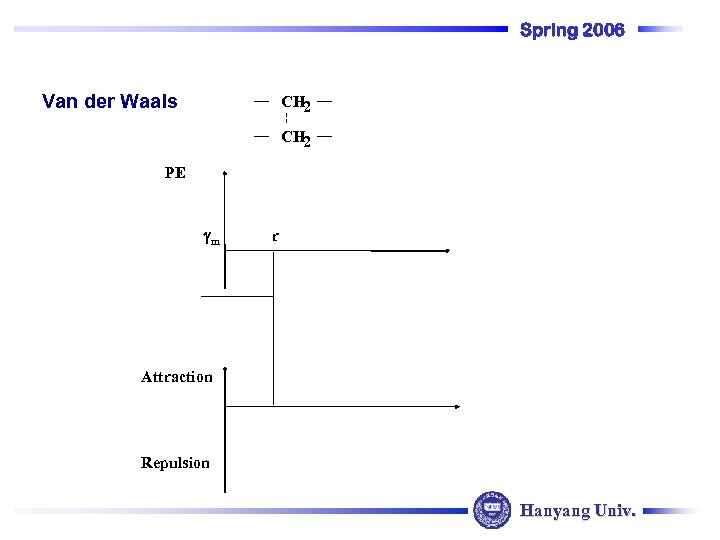
Spring 2006 Van der Waals CH 2 PE m r Attraction Repulsion Hanyang Univ.
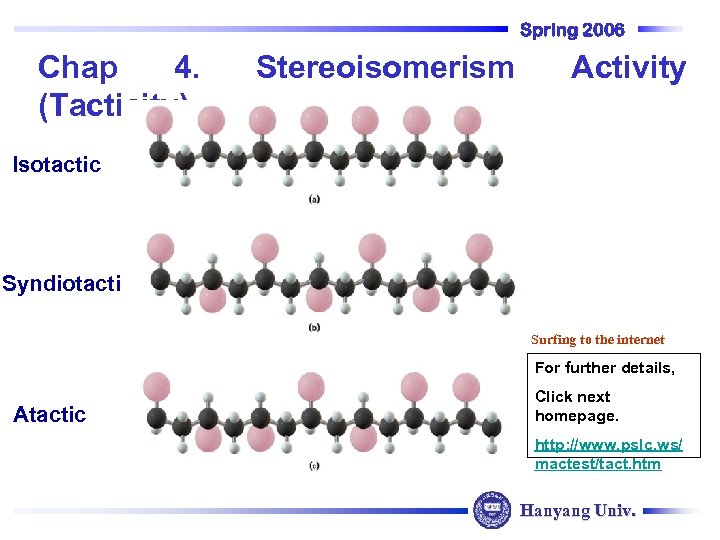
Spring 2006 Chap 4. (Tacticity) Isotactic C Stereoisomerism C CH 3 Syndiotactic C CH 3 C C C C CH 3 C C CH 3 C Activity Surfing to the internet CH 3 For further details, Atactic CH 3 C C Click next homepage. C C C http: //www. pslc. ws/ mactest/tact. htm Hanyang Univ.
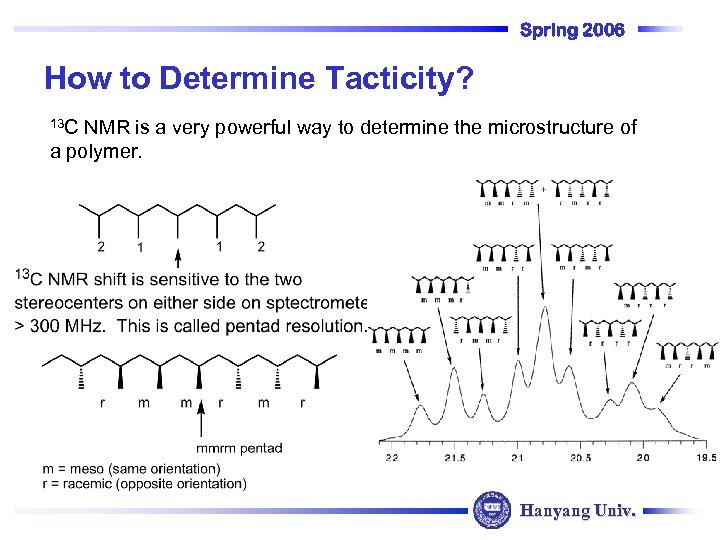
Spring 2006 How to Determine Tacticity? 13 C NMR is a very powerful way to determine the microstructure of a polymer. Hanyang Univ.
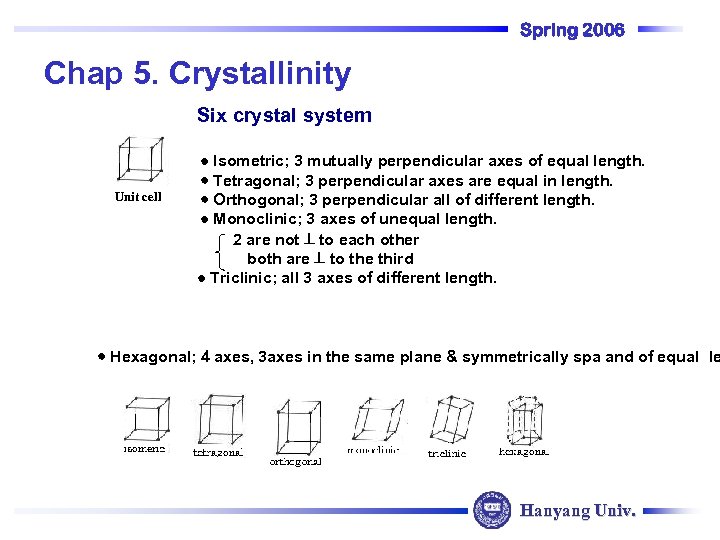
Spring 2006 Chap 5. Crystallinity Six crystal system Unit cell Isometric; 3 mutually perpendicular axes of equal length. Tetragonal; 3 perpendicular axes are equal in length. Orthogonal; 3 perpendicular all of different length. Monoclinic; 3 axes of unequal length. 2 are not to each other both are to the third Triclinic; all 3 axes of different length. Hexagonal; 4 axes, 3 axes in the same plane & symmetrically spa and of equal le Hanyang Univ.
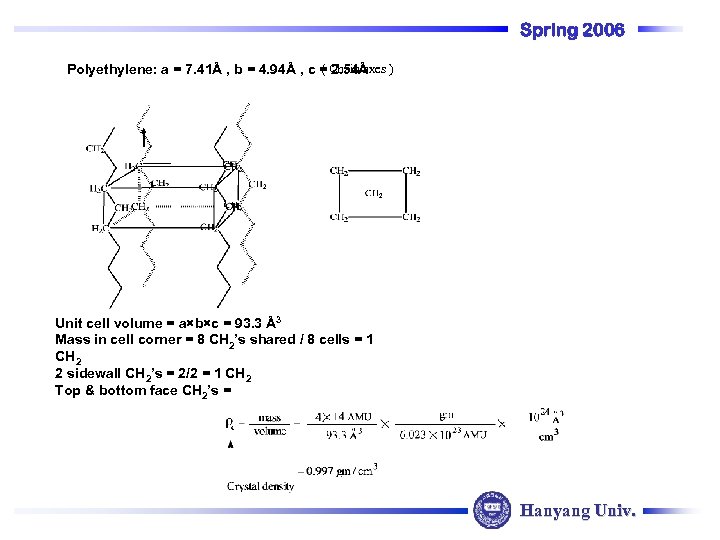
Spring 2006 ( Chain axes Polyethylene: a = 7. 41Å , b = 4. 94Å , c = 2. 54Å ) Unit cell volume = a×b×c = 93. 3 Å3 Mass in cell corner = 8 CH 2’s shared / 8 cells = 1 CH 2 2 sidewall CH 2’s = 2/2 = 1 CH 2 Top & bottom face CH 2’s = Hanyang Univ.
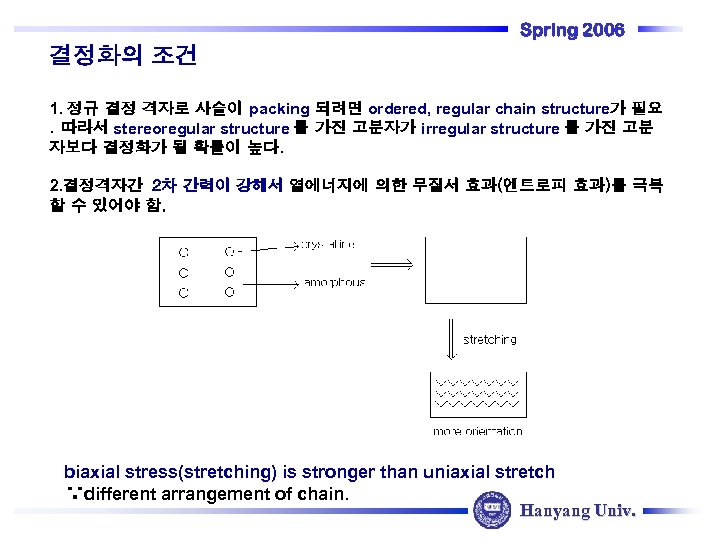
Spring 2006 결정화의 조건 1. 정규 결정 격자로 사슬이 packing 되려면 ordered, regular chain structure가 필요. 따라서 stereoregular structure 를 가진 고분자가 irregular structure 를 가진 고분 자보다 결정화가 될 확률이 높다. 2. 결정격자간 2차 간력이 강해서 열에너지에 의한 무질서 효과(엔트로피 효과)를 극복 할 수 있어야 함. biaxial stress(stretching) is stronger than uniaxial stretch ∵different arrangement of chain. Hanyang Univ.

Spring 2006 Crystallizability 고분자의 화학구조에 의한 고유의 성질 구조의 규칙성 강한 친화력 Crystallinity 가공 history 에 직접 의존 Temperature/time Stress/time Surfing to the internet For further details, Click next homepage. http: //www. pslc. ws/ macrogcss/crystal. h tml Hanyang Univ.
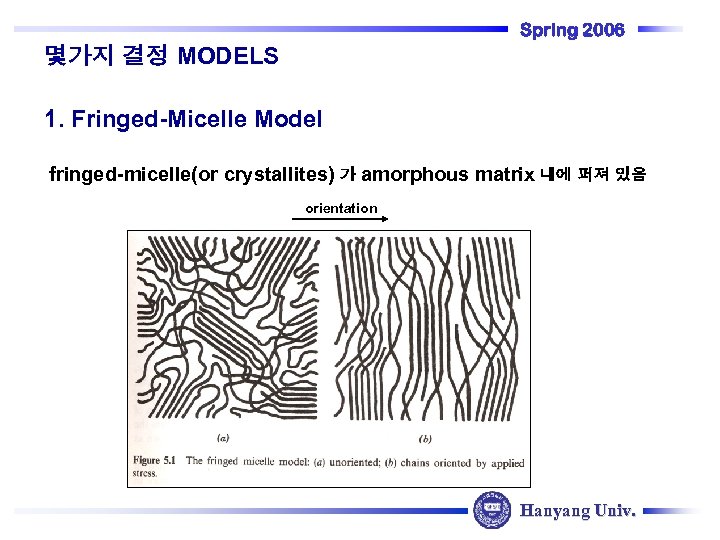
Spring 2006 몇가지 결정 MODELS 1. Fringed-Micelle Model fringed-micelle(or crystallites) 가 amorphous matrix 내에 퍼져 있음 orientation Hanyang Univ.
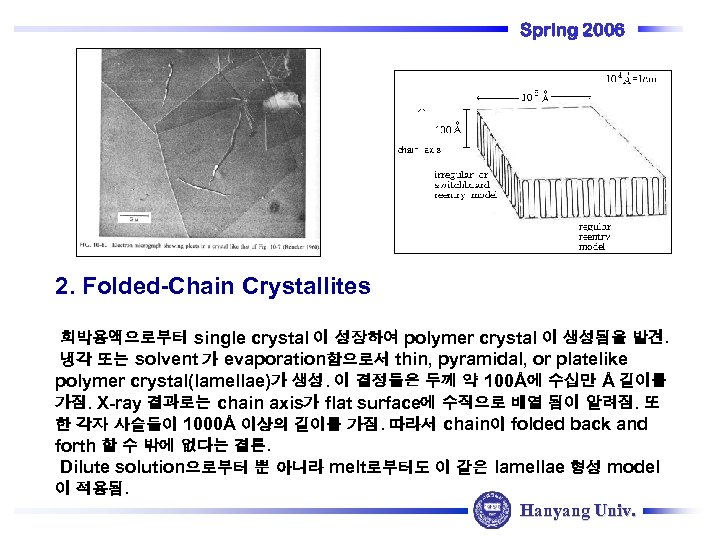
Spring 2006 2. Folded-Chain Crystallites 희박용액으로부터 single crystal 이 성장하여 polymer crystal 이 생성됨을 발견. 냉각 또는 solvent 가 evaporation함으로서 thin, pyramidal, or platelike polymer crystal(lamellae)가 생성. 이 결정들은 두께 약 100Å에 수십만 Å 길이를 가짐. X-ray 결과로는 chain axis가 flat surface에 수직으로 배열 됨이 알려짐. 또 한 각자 사슬들이 1000Å 이상의 길이를 가짐. 따라서 chain이 folded back and forth 할 수 밖에 없다는 결론. Dilute solution으로부터 뿐 아니라 melt로부터도 이 같은 lamellae 형성 model 이 적용됨. Hanyang Univ.
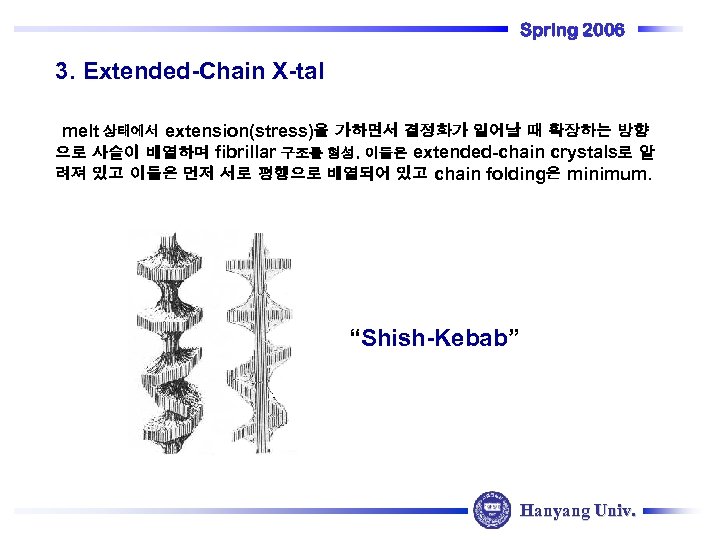
Spring 2006 3. Extended-Chain X-tal melt 상태에서 extension(stress)을 가하면서 결정화가 일어날 때 확장하는 방향 으로 사슬이 배열하며 fibrillar 구조를 형성. 이들은 extended-chain crystals로 알 려져 있고 이들은 먼저 서로 평행으로 배열되어 있고 chain folding은 minimum. “Shish-Kebab” Hanyang Univ.
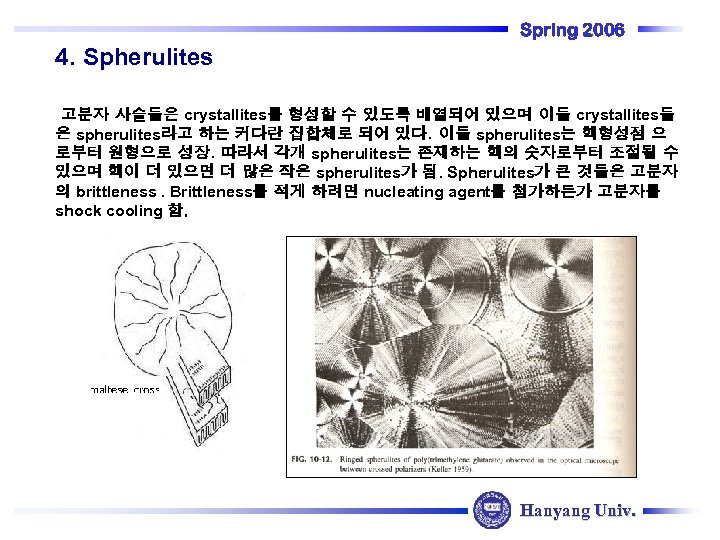
4. Spherulites Spring 2006 고분자 사슬들은 crystallites를 형성할 수 있도록 배열되어 있으며 이들 crystallites들 은 spherulites라고 하는 커다란 집합체로 되어 있다. 이들 spherulites는 핵형성점 으 로부터 원형으로 성장. 따라서 각개 spherulites는 존재하는 핵의 숫자로부터 조절될 수 있으며 핵이 더 있으면 더 많은 작은 spherulites가 됨. Spherulites가 큰 것들은 고분자 의 brittleness. Brittleness를 적게 하려면 nucleating agent를 첨가하든가 고분자를 shock cooling 함. Hanyang Univ.
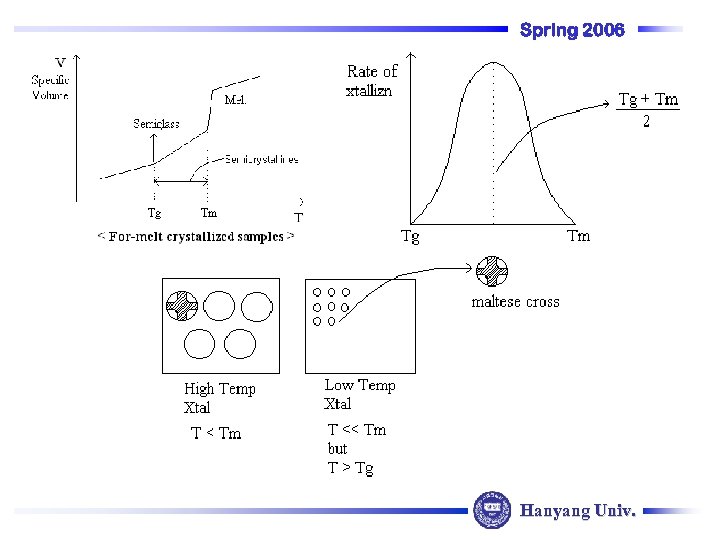
Spring 2006 Hanyang Univ.
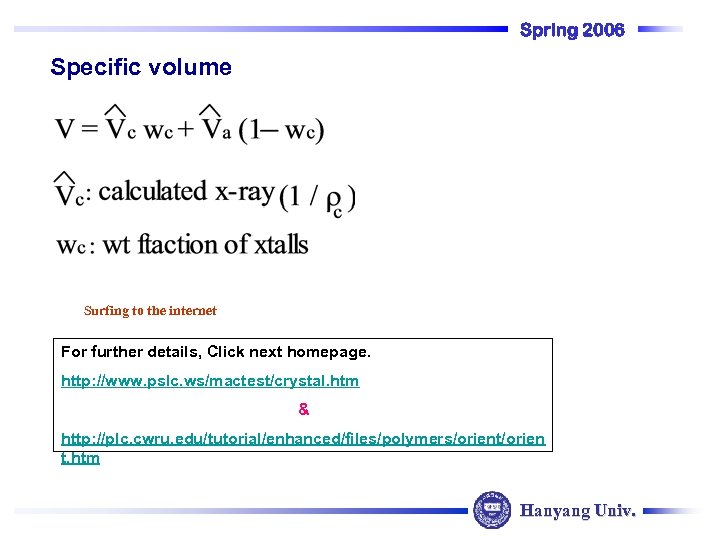
Spring 2006 Specific volume Surfing to the internet For further details, Click next homepage. http: //www. pslc. ws/mactest/crystal. htm & http: //plc. cwru. edu/tutorial/enhanced/files/polymers/orient/orien t. htm Hanyang Univ.
Spring 2006 Polymer Conformation Virtual Experiment Case Western Reserve Univ. Polymer and Liquid Crystals Conformation Lattice Simulation Click the next homepage, experiment part http: //plc. cwru. edu/tutorial/enhanc ed/lab/lattice. htm If you have the trouble viewing this site, See this page http: //plc. cwru. edu/tutorial/enhanc ed/software. html Hanyang Univ.
498075621974bba6d93cfd54a75e01ac.ppt