78a44eb300f947784ddd4d7b3a50a18c.ppt
- Количество слайдов: 42

SPIRIT IV: Insight Into Subgroups Data Mitchell W. Krucoff, MD, FACC Professor, Medicine/Cardiology Duke University Medical Center Director, Cardiovascular Devices Unit Duke Clinical Research Institute [name of file], 1

DISCLOSURES Mitchell W. Krucoff, MD Consulting Fees – Biosensors International, Terumo Medical Corporation Grants/Contracted Research – Boston Scientific Corporation, Medtronic Cardio. Vascular, Inc. Honoraria – Abbott Vascular, Cordis, a Johnson & Johnson company

Slide Attributions Modified from Gregg Stone Late Breaking Clinical Trials TCT 2009
XIENCE V / PROMUS Everolimus-eluting Stent Everolimus Durable Fluorinated Copolymer ML VISION™ Stent Platform ML VISION™ Stent Delivery System SPIRIT Clinical Trials PROMUS is a private-labeled XIENCE V everolimus-eluting coronary stent system manufactured by Abbott and distributed by Boston Scientific.
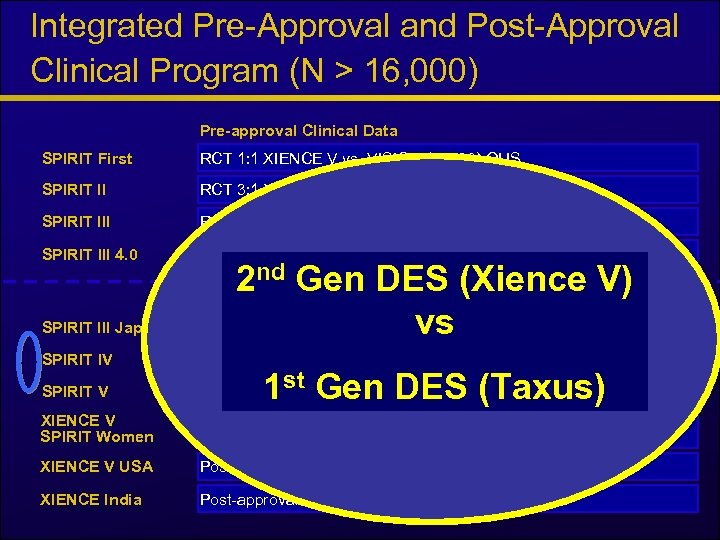
Integrated Pre-Approval and Post-Approval Clinical Program (N > 16, 000) Pre-approval Clinical Data SPIRIT First RCT 1: 1 XIENCE V vs. VISION (n = 60) OUS SPIRIT II RCT 3: 1 XIENCE V vs. TAXUS® (n = 300) OUS SPIRIT III RCT 2: 1 XIENCE V vs. TAXUS (n = 1, 002) US SPIRIT III 4. 0 Registry 4. 0 mm (n = 80) US SPIRIT III Japan 2 nd Gen Clinical Data(Xience V) DES Ongoing and Planned Registry (n = 88) Japan vs SPIRIT IV RCT XIENCE V vs. TAXUS 2: 1 Continued Access (n = 3, 690) US SPIRIT V Registry (n = 2, 700), RCT Diabetics 2: 1 vs. TAXUS (n = 300) OUS XIENCE V SPIRIT Women Registry (n = 1, 550) RCT 2: 1 vs. CYPHER® (n = 450) OUS XIENCE V USA Post-approval Registry – real world (n ~ 5, 000) US XIENCE India Post-approval Registry – real world (n ~ 1, 000) OUS 1 st Gen DES (Taxus)
XIENCE V Clinical Data: The SPIRIT “Family” of Trials n 1 -3 year follow up n Other key subgroups n Stent thrombosis & DAPT duration
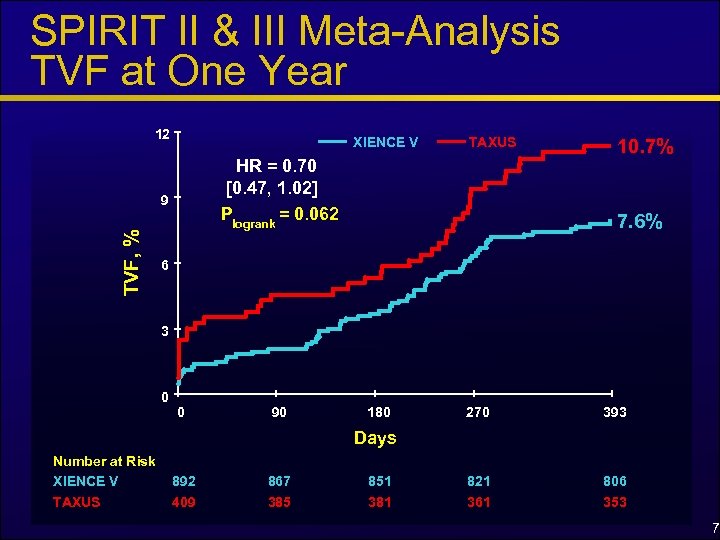
SPIRIT II & III Meta-Analysis TVF at One Year 12 XIENCE V HR = 0. 70 [0. 47, 1. 02] Plogrank = 0. 062 9 TVF, % TAXUS 10. 7% 7. 6% 6 3 0 0 90 180 270 393 Days Number at Risk XIENCE V 892 867 851 821 806 TAXUS 409 385 381 361 353 7
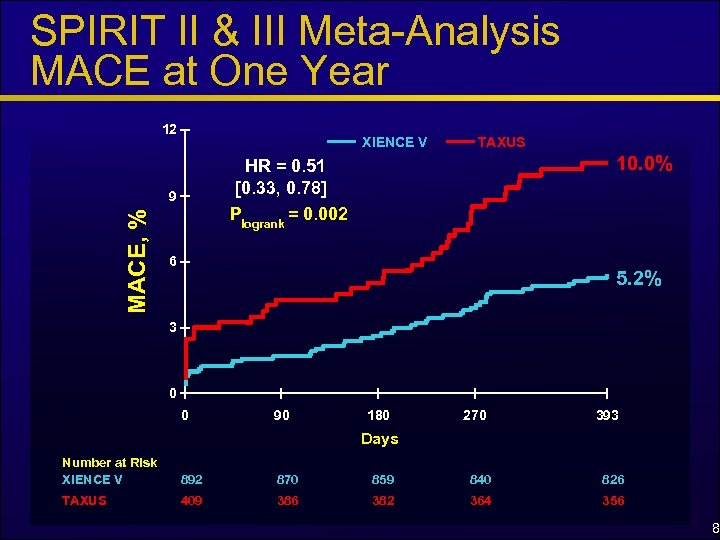
SPIRIT II & III Meta-Analysis MACE at One Year 12 XIENCE V 10. 0% HR = 0. 51 [0. 33, 0. 78] Plogrank = 0. 002 9 MACE, % TAXUS 6 5. 2% 3 0 0 90 180 270 393 Days Number at Risk XIENCE V 892 870 859 840 826 TAXUS 409 386 382 364 356 8
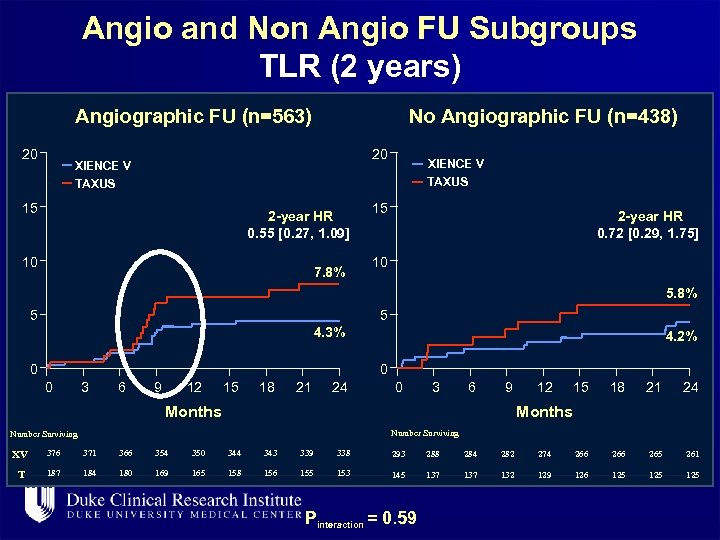
Angio and Non Angio FU Subgroups TLR (2 years) Angiographic FU (n=563) 20 No Angiographic FU (n=438) 20 XIENCE V TAXUS 15 2 -year HR 0. 55 [0. 27, 1. 09] 10 7. 8% XIENCE V TAXUS 15 2 -year HR 0. 72 [0. 29, 1. 75] 10 5. 8% 5 5 4. 3% 0 4. 2% 0 0 3 6 9 12 15 18 21 24 0 3 6 9 Months 12 15 18 21 24 Months Number Surviving XV 376 371 366 354 350 344 343 339 338 293 288 284 282 274 266 265 261 T. 187 184 180 169 165 158 156 155 153 145 137 132 129 126 125 125 Pinteraction = 0. 59
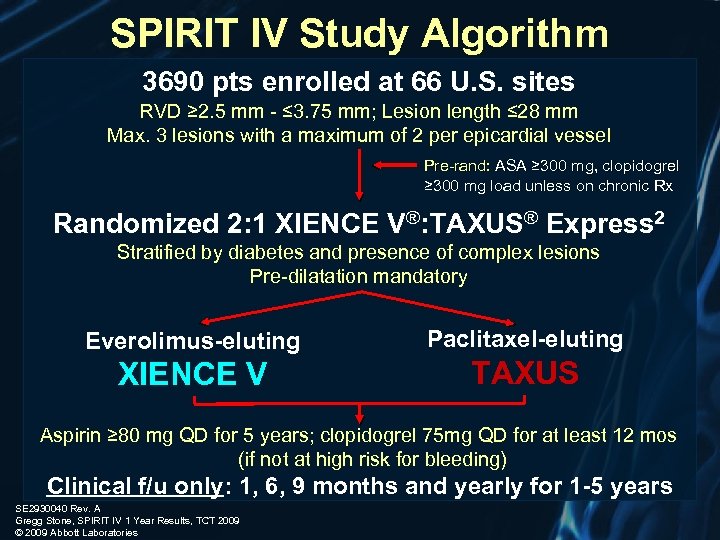
SPIRIT IV Study Algorithm 3690 pts enrolled at 66 U. S. sites RVD ≥ 2. 5 mm - ≤ 3. 75 mm; Lesion length ≤ 28 mm Max. 3 lesions with a maximum of 2 per epicardial vessel Pre-rand: ASA ≥ 300 mg, clopidogrel ≥ 300 mg load unless on chronic Rx Randomized 2: 1 XIENCE V®: TAXUS® Express 2 Stratified by diabetes and presence of complex lesions Pre-dilatation mandatory Everolimus-eluting Paclitaxel-eluting XIENCE V TAXUS Aspirin ≥ 80 mg QD for 5 years; clopidogrel 75 mg QD for at least 12 mos (if not at high risk for bleeding) Clinical f/u only: 1, 6, 9 months and yearly for 1 -5 years SE 2930040 Rev. A Gregg Stone, SPIRIT IV 1 Year Results, TCT 2009 © 2009 Abbott Laboratories
![TLF Through 1 Year HR [95%CI] = 0. 61 [0. 46, 0. 82] p=0. TLF Through 1 Year HR [95%CI] = 0. 61 [0. 46, 0. 82] p=0.](https://present5.com/presentation/78a44eb300f947784ddd4d7b3a50a18c/image-11.jpg)
TLF Through 1 Year HR [95%CI] = 0. 61 [0. 46, 0. 82] p=0. 0008 Target lesion failure (%) XIENCE V TAXUS 6. 6% Δ 2. 7% 3. 9% Months Number at risk XIENCE V 2458 2390 2362 2323 2298 TAXUS 1229 1165 1137 1119 1104 TLF = cardiac death, target vessel MI, or ischemia-driven TLR SE 2930040 Rev. A Gregg Stone, SPIRIT IV 1 Year Results, TCT 2009 © 2009 Abbott Laboratories
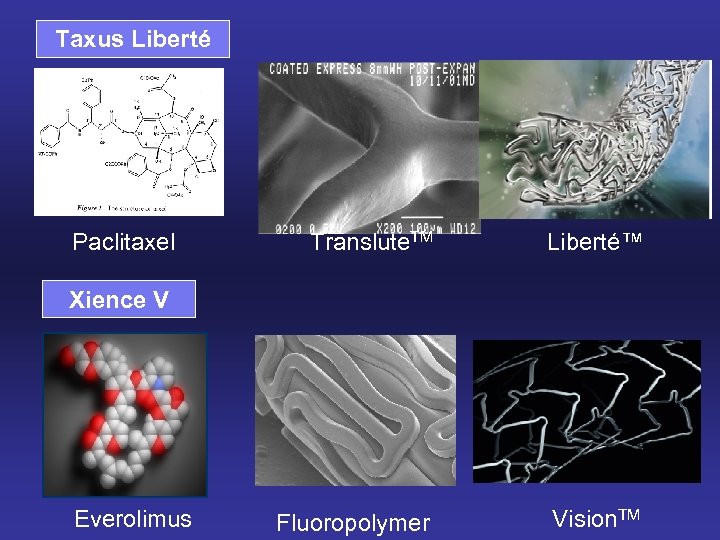
Taxus Liberté Paclitaxel Translute. TM Liberté™ Fluoropolymer Vision. TM Xience V Everolimus

Compare Trial Peter Smits Maasstad Ziekenhuis Rotterdam The Netherlands
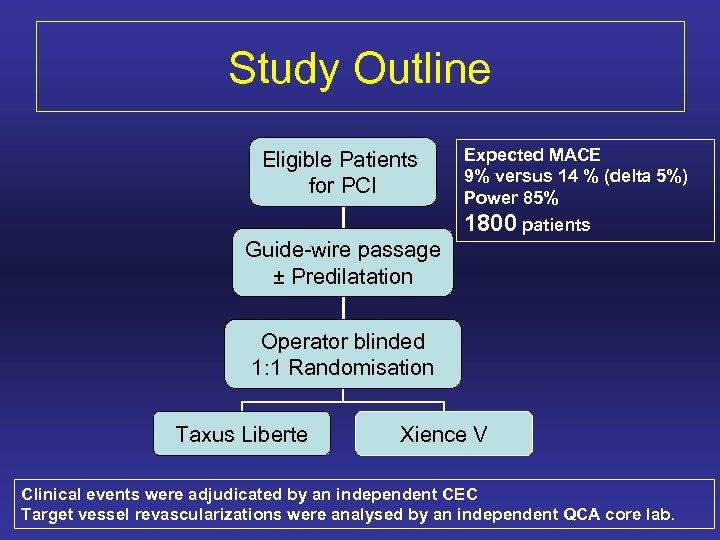
Study Outline Eligible Patients for PCI Expected MACE 9% versus 14 % (delta 5%) Power 85% 1800 patients Guide-wire passage ± Predilatation Operator blinded 1: 1 Randomisation Taxus Liberte Xience V Clinical events were adjudicated by an independent CEC Target vessel revascularizations were analysed by an independent QCA core lab.
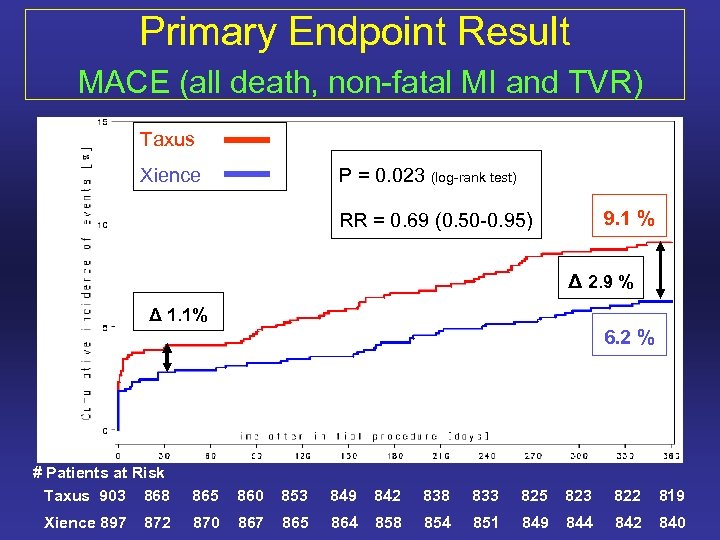
Primary Endpoint Result MACE (all death, non-fatal MI and TVR) Taxus Xience P = 0. 023 (log-rank test) 9. 1 % RR = 0. 69 (0. 50 -0. 95) Δ 2. 9 % Δ 1. 1% 6. 2 % # Patients at Risk Taxus 903 868 Xience 897 872 865 860 853 849 842 838 833 825 823 822 819 870 867 865 864 858 854 851 849 844 842 840
![XIENCE V: 2 year results [name of file], 18 XIENCE V: 2 year results [name of file], 18](https://present5.com/presentation/78a44eb300f947784ddd4d7b3a50a18c/image-16.jpg)
XIENCE V: 2 year results [name of file], 18

Stone G et al, Circulation. 2009; 119: 680 -686
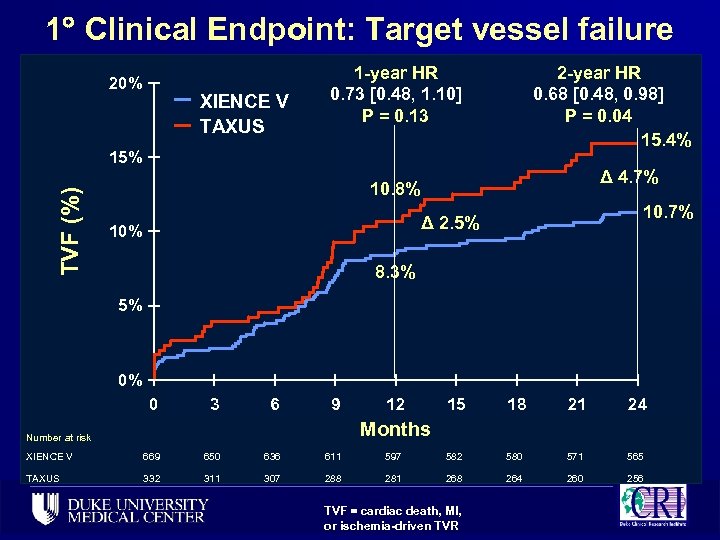
1 Clinical Endpoint: Target vessel failure 20% XIENCE V TAXUS 1 -year HR 0. 73 [0. 48, 1. 10] P = 0. 13 2 -year HR 0. 68 [0. 48, 0. 98] P = 0. 04 15. 4% TVF (%) 15% Δ 4. 7% 10. 8% 10. 7% Δ 2. 5% 10% 8. 3% 5% 0% 0 3 6 9 12 15 18 21 24 Months Number at risk XIENCE V 669 650 636 611 597 582 580 571 565 TAXUS 332 311 307 288 281 268 264 260 256 TVF = cardiac death, MI, or ischemia-driven TVR
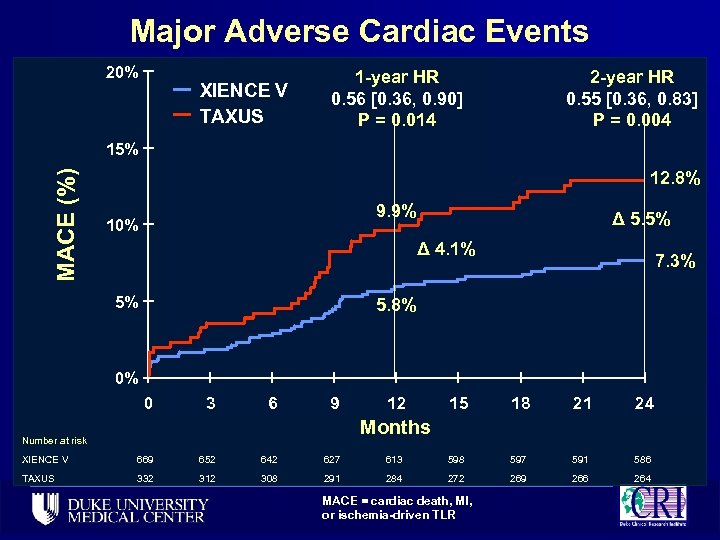
Major Adverse Cardiac Events 20% XIENCE V TAXUS 1 -year HR 0. 56 [0. 36, 0. 90] P = 0. 014 2 -year HR 0. 55 [0. 36, 0. 83] P = 0. 004 MACE (%) 15% 12. 8% 9. 9% 10% Δ 5. 5% Δ 4. 1% 5% 7. 3% 5. 8% 0% 0 3 6 9 12 15 18 21 24 Months Number at risk XIENCE V 669 652 642 627 613 598 597 591 586 TAXUS 332 312 308 291 284 272 269 266 264 MACE = cardiac death, MI, or ischemia-driven TLR
![XIENCE V: 3 year results [name of file], 22 XIENCE V: 3 year results [name of file], 22](https://present5.com/presentation/78a44eb300f947784ddd4d7b3a50a18c/image-20.jpg)
XIENCE V: 3 year results [name of file], 22
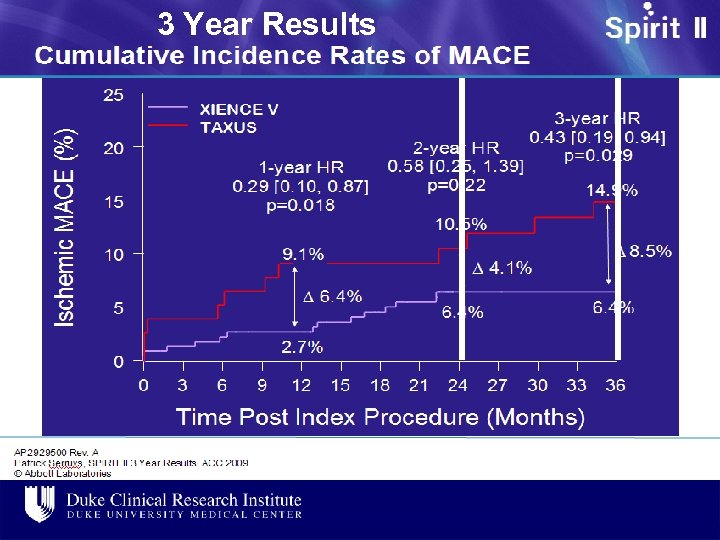
3 Year Results
![XIENCE V: Key Clinical Subgroups [name of file], 24 XIENCE V: Key Clinical Subgroups [name of file], 24](https://present5.com/presentation/78a44eb300f947784ddd4d7b3a50a18c/image-22.jpg)
XIENCE V: Key Clinical Subgroups [name of file], 24
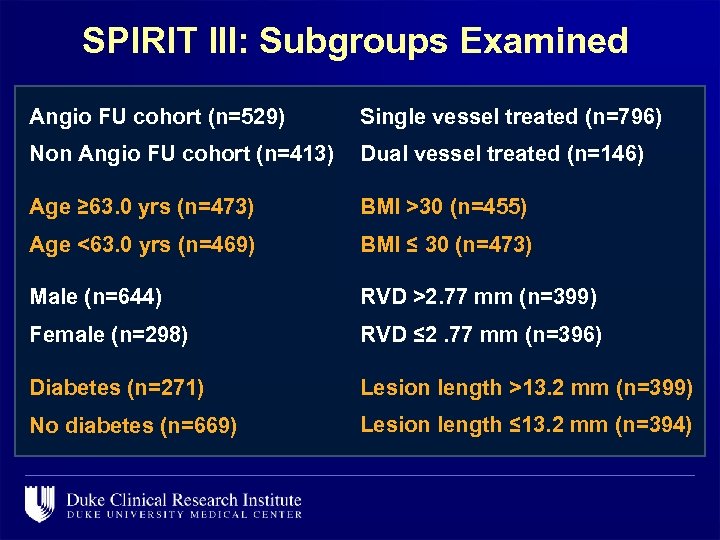
SPIRIT III: Subgroups Examined Angio FU cohort (n=529) Single vessel treated (n=796) Non Angio FU cohort (n=413) Dual vessel treated (n=146) Age ≥ 63. 0 yrs (n=473) BMI >30 (n=455) Age <63. 0 yrs (n=469) BMI ≤ 30 (n=473) Male (n=644) RVD >2. 77 mm (n=399) Female (n=298) RVD ≤ 2. 77 mm (n=396) Diabetes (n=271) Lesion length >13. 2 mm (n=399) No diabetes (n=669) Lesion length ≤ 13. 2 mm (n=394)
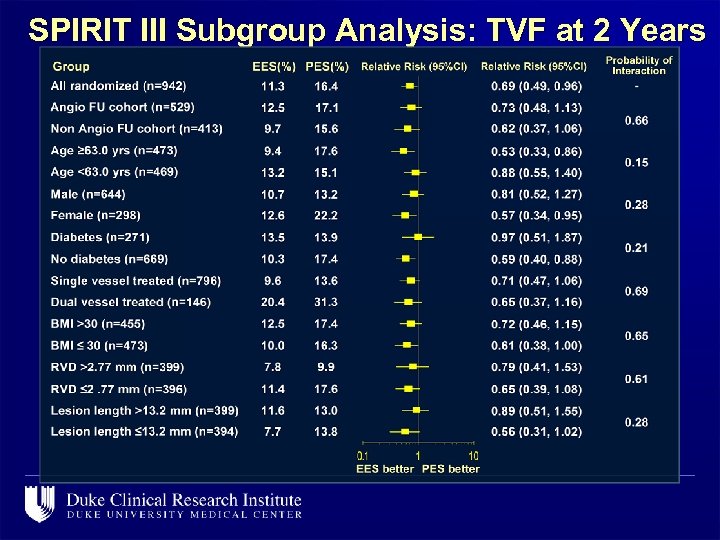
SPIRIT III Subgroup Analysis: TVF at 2 Years
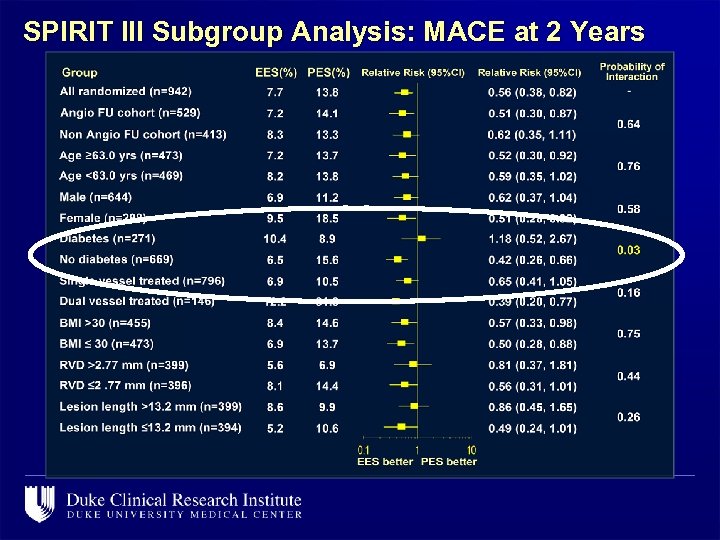
SPIRIT III Subgroup Analysis: MACE at 2 Years

SPIRIT IV: 11 Subgroups Examined Age < 65 (n=1993) Age ≥ 65 (n=1618) Male (n=2450) Female (n=1161) Lesion number = 1 (n=2710) Lesion number = 2 or 3 (n=901) - Lesion number = 2 (n=784) - Lesion number = 3 (n=117) Hypertension* (n=2778) No hypertension* (n=828) Lesion length ≤ median (13. 3 mm; n=1349) Lesion length > median (13. 3 mm; n=1346) Hypercholesterolemia* (n=2701) No hypercholesterolemia* (n=852) RVD ≤ median (2. 75 mm; n=1352) RVD > median (2. 75 mm; n=1351) BMI ≥ 30 (n=1758) BMI < 30 (n=1853) No diabetes (n=2467) Diabetes (n=1140) - Diabetics not requiring insulin (n=826) - Diabetics requiring insulin (n=314) Stable angina (n=2085) No stable angina (n=1458) Bailout stent required (n=221) No bailout stent required (n=3390) RVD and lesion length from single lesion treated subgroup. * Requiring medication RVD range (min, max; mm): X = (1. 39, 4. 71), T = (1. 36, 4. 70) Lesion length range (min, max; mm): X = (1. 99, 54. 80), T = (1. 72, 47. 10) SE 2930040 Rev. A Gregg Stone, SPIRIT IV 1 Year Results, TCT 2009 © 2009 Abbott Laboratories
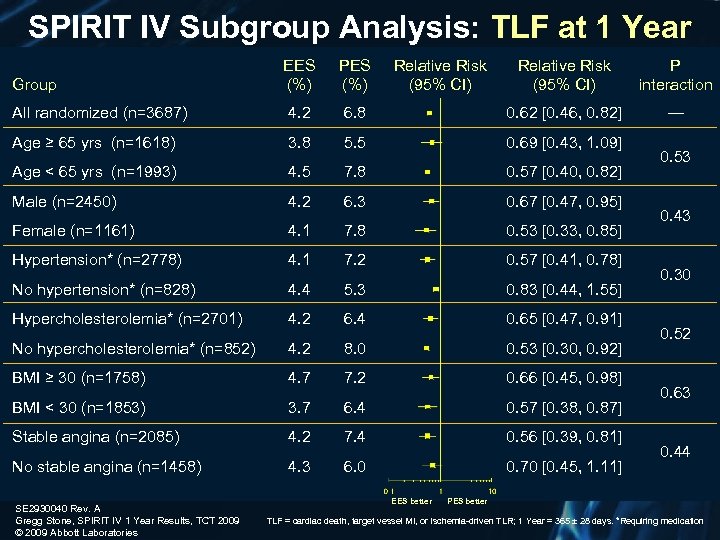
SPIRIT IV Subgroup Analysis: TLF at 1 Year EES (%) PES (%) Relative Risk (95% CI) P interaction All randomized (n=3687) 4. 2 6. 8 0. 62 [0. 46, 0. 82] — Age ≥ 65 yrs (n=1618) 3. 8 5. 5 0. 69 [0. 43, 1. 09] Age < 65 yrs (n=1993) 4. 5 7. 8 0. 57 [0. 40, 0. 82] Male (n=2450) 4. 2 6. 3 0. 67 [0. 47, 0. 95] Female (n=1161) 4. 1 7. 8 0. 53 [0. 33, 0. 85] Hypertension* (n=2778) 4. 1 7. 2 0. 57 [0. 41, 0. 78] No hypertension* (n=828) 4. 4 5. 3 0. 83 [0. 44, 1. 55] Hypercholesterolemia* (n=2701) 4. 2 6. 4 0. 65 [0. 47, 0. 91] No hypercholesterolemia* (n=852) 4. 2 8. 0 0. 53 [0. 30, 0. 92] BMI ≥ 30 (n=1758) 4. 7 7. 2 0. 66 [0. 45, 0. 98] BMI < 30 (n=1853) 3. 7 6. 4 0. 57 [0. 38, 0. 87] Stable angina (n=2085) 4. 2 7. 4 0. 56 [0. 39, 0. 81] No stable angina (n=1458) 4. 3 6. 0 0. 70 [0. 45, 1. 11] Group SE 2930040 Rev. A Gregg Stone, SPIRIT IV 1 Year Results, TCT 2009 © 2009 Abbott Laboratories Relative Risk (95% CI) EES better 0. 53 0. 43 0. 30 0. 52 0. 63 0. 44 PES better TLF = cardiac death, target vessel MI, or ischemia-driven TLR; 1 Year = 365 ± 28 days. *Requiring medication
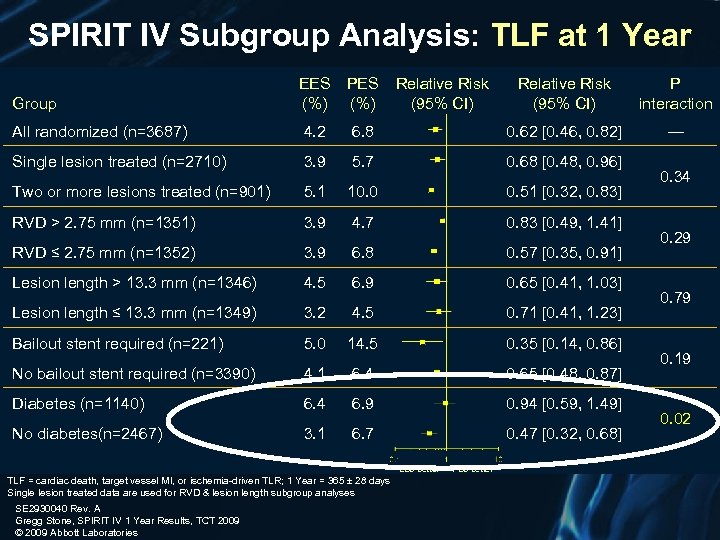
SPIRIT IV Subgroup Analysis: TLF at 1 Year EES (%) PES (%) Relative Risk (95% CI) P interaction All randomized (n=3687) 4. 2 6. 8 0. 62 [0. 46, 0. 82] — Single lesion treated (n=2710) 3. 9 5. 7 0. 68 [0. 48, 0. 96] Two or more lesions treated (n=901) 5. 1 10. 0 0. 51 [0. 32, 0. 83] RVD > 2. 75 mm (n=1351) 3. 9 4. 7 0. 83 [0. 49, 1. 41] RVD ≤ 2. 75 mm (n=1352) 3. 9 6. 8 0. 57 [0. 35, 0. 91] Lesion length > 13. 3 mm (n=1346) 4. 5 6. 9 0. 65 [0. 41, 1. 03] Lesion length ≤ 13. 3 mm (n=1349) 3. 2 4. 5 0. 71 [0. 41, 1. 23] Bailout stent required (n=221) 5. 0 14. 5 0. 35 [0. 14, 0. 86] No bailout stent required (n=3390) 4. 1 6. 4 0. 65 [0. 48, 0. 87] Diabetes (n=1140) 6. 4 6. 9 0. 94 [0. 59, 1. 49] No diabetes(n=2467) 3. 1 6. 7 0. 47 [0. 32, 0. 68] Group Relative Risk (95% CI) EES better TLF = cardiac death, target vessel MI, or ischemia-driven TLR; 1 Year = 365 ± 28 days Single lesion treated data are used for RVD & lesion length subgroup analyses SE 2930040 Rev. A Gregg Stone, SPIRIT IV 1 Year Results, TCT 2009 © 2009 Abbott Laboratories PES better 0. 34 0. 29 0. 79 0. 19 0. 02
![Impact of Diabetes on TLF XIENCE V TAXUS RR [95%CI] = 0. 94 [0. Impact of Diabetes on TLF XIENCE V TAXUS RR [95%CI] = 0. 94 [0.](https://present5.com/presentation/78a44eb300f947784ddd4d7b3a50a18c/image-29.jpg)
Impact of Diabetes on TLF XIENCE V TAXUS RR [95%CI] = 0. 94 [0. 59, 1. 49] p=0. 80 TLF (%) RR [95%CI] = 0. 47 [0. 32, 0. 68] p<0. 0001 52/1652 TLF = cardiac death, target vessel MI, or ischemia-driven TLR SE 2930040 Rev. A Gregg Stone, SPIRIT IV 1 Year Results, TCT 2009 © 2009 Abbott Laboratories 55/815 49/761 Pinteraction = 0. 02 26/379
![Impact of Diabetes Type on TLF XIENCE V TAXUS RR [95%CI] = 1. 16 Impact of Diabetes Type on TLF XIENCE V TAXUS RR [95%CI] = 1. 16](https://present5.com/presentation/78a44eb300f947784ddd4d7b3a50a18c/image-30.jpg)
Impact of Diabetes Type on TLF XIENCE V TAXUS RR [95%CI] = 1. 16 [0. 51, 2. 62] p=0. 83 TLF (%) RR [95%CI] = 0. 86 [0. 49, 1. 50] p=0. 64 33/562 18/264 16/199 Diabetes not requiring insulin TLF = cardiac death, target vessel MI, or ischemia-driven TLR SE 2930040 Rev. A Gregg Stone, SPIRIT IV 1 Year Results, TCT 2009 © 2009 Abbott Laboratories 8/115 Diabetes requiring insulin Pinteraction = 0. 56
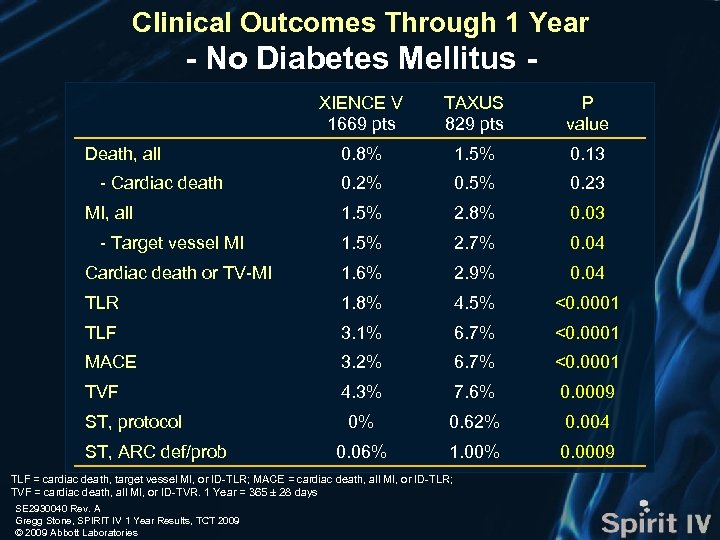
Clinical Outcomes Through 1 Year - No Diabetes Mellitus XIENCE V 1669 pts TAXUS 829 pts P value 0. 8% 1. 5% 0. 13 0. 2% 0. 5% 0. 23 1. 5% 2. 8% 0. 03 1. 5% 2. 7% 0. 04 Cardiac death or TV-MI 1. 6% 2. 9% 0. 04 TLR 1. 8% 4. 5% <0. 0001 TLF 3. 1% 6. 7% <0. 0001 MACE 3. 2% 6. 7% <0. 0001 TVF 4. 3% 7. 6% 0. 0009 0% 0. 62% 0. 004 0. 06% 1. 00% 0. 0009 Death, all - Cardiac death MI, all - Target vessel MI ST, protocol ST, ARC def/prob TLF = cardiac death, target vessel MI, or ID-TLR; MACE = cardiac death, all MI, or ID-TLR; TVF = cardiac death, all MI, or ID-TVR. 1 Year = 365 ± 28 days SE 2930040 Rev. A Gregg Stone, SPIRIT IV 1 Year Results, TCT 2009 © 2009 Abbott Laboratories
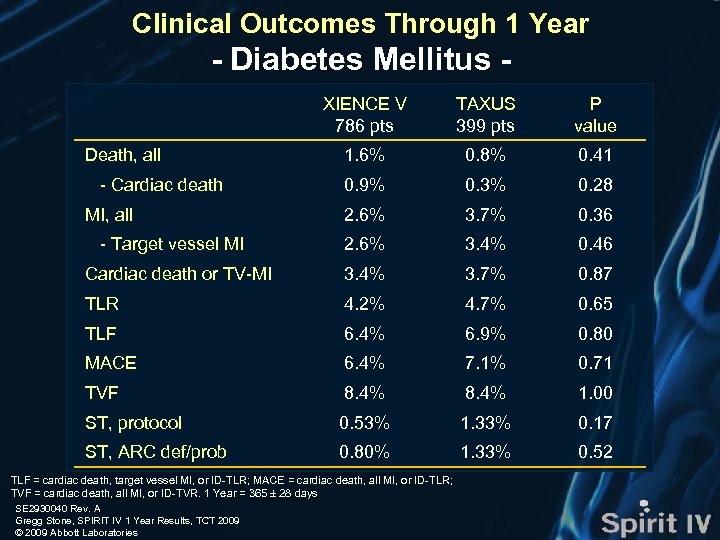
Clinical Outcomes Through 1 Year - Diabetes Mellitus XIENCE V 786 pts TAXUS 399 pts P value 1. 6% 0. 8% 0. 41 0. 9% 0. 3% 0. 28 2. 6% 3. 7% 0. 36 2. 6% 3. 4% 0. 46 Cardiac death or TV-MI 3. 4% 3. 7% 0. 87 TLR 4. 2% 4. 7% 0. 65 TLF 6. 4% 6. 9% 0. 80 MACE 6. 4% 7. 1% 0. 71 TVF 8. 4% 1. 00 ST, protocol 0. 53% 1. 33% 0. 17 ST, ARC def/prob 0. 80% 1. 33% 0. 52 Death, all - Cardiac death MI, all - Target vessel MI TLF = cardiac death, target vessel MI, or ID-TLR; MACE = cardiac death, all MI, or ID-TLR; TVF = cardiac death, all MI, or ID-TVR. 1 Year = 365 ± 28 days SE 2930040 Rev. A Gregg Stone, SPIRIT IV 1 Year Results, TCT 2009 © 2009 Abbott Laboratories
![XIENCE V: Stent thrombosis results [name of file], 35 XIENCE V: Stent thrombosis results [name of file], 35](https://present5.com/presentation/78a44eb300f947784ddd4d7b3a50a18c/image-33.jpg)
XIENCE V: Stent thrombosis results [name of file], 35
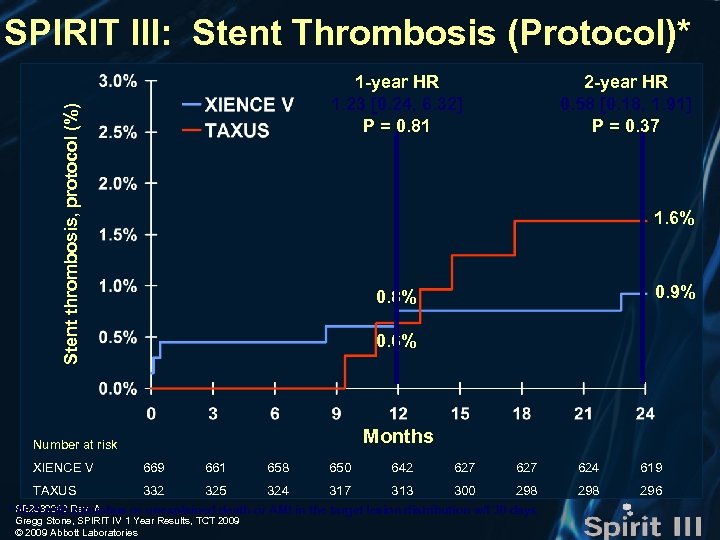
SPIRIT III: Stent Thrombosis (Protocol)* Stent thrombosis, protocol (%) 1 -year HR 1. 23 [0. 24, 6. 32] P = 0. 81 2 -year HR 0. 58 [0. 18, 1. 91] P = 0. 37 1. 6% 0. 9% 0. 8% 0. 6% Months Number at risk XIENCE V 669 661 658 650 642 627 624 619 TAXUS 332 325 324 317 313 300 298 296 * SE 2930040 Rev. A ACS with thrombus or unexplained death or AMI in the target lesion distribution w/I 30 days Gregg Stone, SPIRIT IV 1 Year Results, TCT 2009 © 2009 Abbott Laboratories
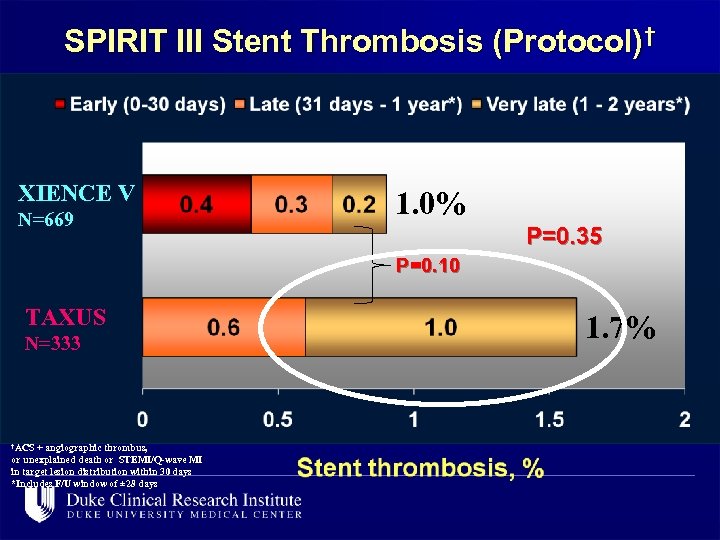
SPIRIT III Stent Thrombosis (Protocol)† XIENCE V N=669 1. 0% P=0. 35 P=0. 10 TAXUS N=333 †ACS + angiographic thrombus, or unexplained death or STEMI/Q-wave MI in target lesion distribution within 30 days *Includes F/U window of ± 28 days 1. 7%
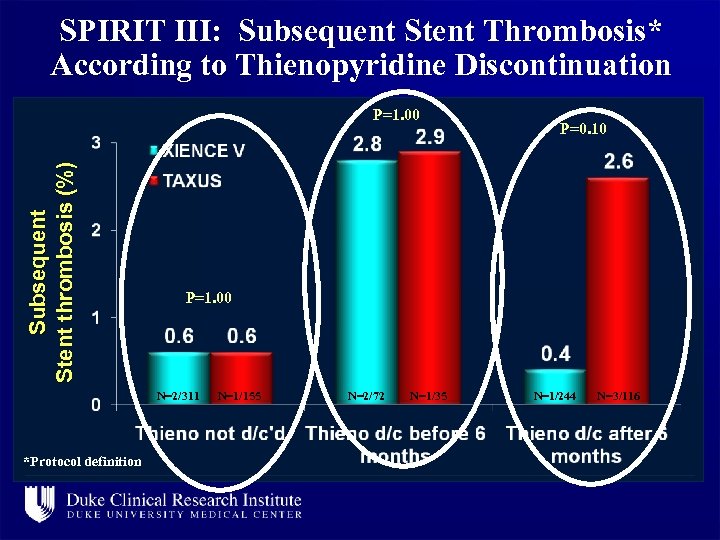
SPIRIT III: Subsequent Stent Thrombosis* According to Thienopyridine Discontinuation Subsequent Stent thrombosis (%) P=1. 00 N=2/311 *Protocol definition P=0. 10 N=1/155 N=2/72 N=1/35 N=1/244 N=3/116
![SPIRIT IV: Stent Thrombosis (Protocol Definition)* Stent thrombosis (%) XIENCE V TAXUS HR [95%CI] SPIRIT IV: Stent Thrombosis (Protocol Definition)* Stent thrombosis (%) XIENCE V TAXUS HR [95%CI]](https://present5.com/presentation/78a44eb300f947784ddd4d7b3a50a18c/image-37.jpg)
SPIRIT IV: Stent Thrombosis (Protocol Definition)* Stent thrombosis (%) XIENCE V TAXUS HR [95%CI] = 0. 20 [0. 06, 0. 63] p=0. 002 0. 82% Δ 0. 66% 0. 16% Months Number at risk XIENCE V 2458 2427 2413 2389 2377 TAXUS 1229 1198 1187 1177 1169 *ACS + angiographic thrombus, or unexplained death or STEMI/Q-wave MI in TL distribution within 30 days SE 2930040 Rev. A Gregg Stone, SPIRIT IV 1 Year Results, TCT 2009 © 2009 Abbott Laboratories FOR IMPORTANT SAFETY INFORMATION SEE FINAL SLIDE.
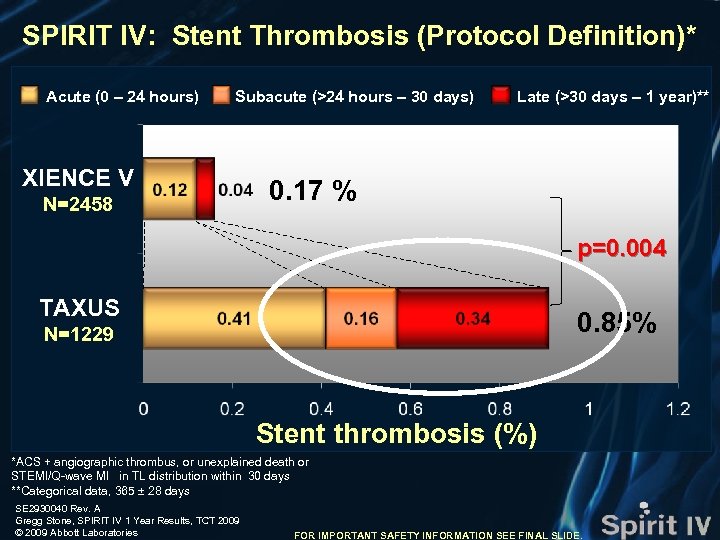
SPIRIT IV: Stent Thrombosis (Protocol Definition)* Acute (0 – 24 hours) Subacute (>24 hours – 30 days) XIENCE V N=2458 Late (>30 days – 1 year)** 0. 17 % p=0. 004 TAXUS 0. 85% N=1229 Stent thrombosis (%) *ACS + angiographic thrombus, or unexplained death or STEMI/Q-wave MI in TL distribution within 30 days **Categorical data, 365 ± 28 days SE 2930040 Rev. A Gregg Stone, SPIRIT IV 1 Year Results, TCT 2009 © 2009 Abbott Laboratories FOR IMPORTANT SAFETY INFORMATION SEE FINAL SLIDE.
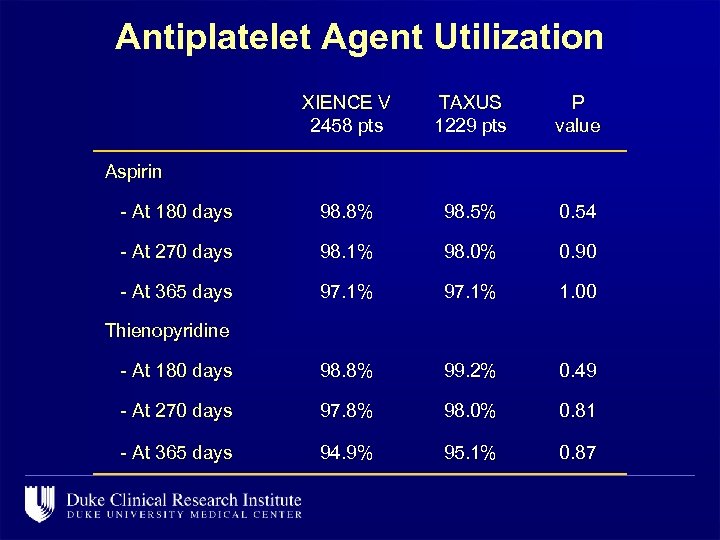
Antiplatelet Agent Utilization XIENCE V 2458 pts TAXUS 1229 pts P value - At 180 days 98. 8% 98. 5% 0. 54 - At 270 days 98. 1% 98. 0% 0. 90 - At 365 days 97. 1% 1. 00 - At 180 days 98. 8% 99. 2% 0. 49 - At 270 days 97. 8% 98. 0% 0. 81 - At 365 days 94. 9% 95. 1% 0. 87 Aspirin Thienopyridine

SPIRIT IV Subgroup Data: Conclusions n SPIRIT IV, the SPIRIT “family” of trials and COMPARE make Xience V the most extensively studied medical device in history: l 4 indpendent prospective, trials l All randomized Gen I vs. Gen II DES “head to head” l With and without protocol catheterization l Progressively complex patient populations l Ongoing follow up now out to 3 years
SPIRIT IV Subgroup Data: Conclusions n ALL available data consistent: l Lower TVR l Lower TVF l Lower MACE n Both safety and efficacy margins consistently widen from year 1 to year 3 n Better results in ALL subgroups except diabetics, where results are same compared to first generation DES n Low LAST, esp when DAPT <1 year

SPIRIT IV: Insight Into Subgroups Data Mitchell W. Krucoff, MD, FACC Professor, Medicine/Cardiology Duke University Medical Center Director, Cardiovascular Devices Unit Duke Clinical Research Institute [name of file], 44
78a44eb300f947784ddd4d7b3a50a18c.ppt