a00b3e65b04d732b31906bd1b879edd7.ppt
- Количество слайдов: 34
 Spin transition in ferrous iron in Mg. Si. O 3 perovskite under pressure Koichiro Umemoto Minnesota Supercomputing Institute and Department of Chemical Engineering and Materials Science, University of Minnesota, Minneapolis, MN 55455, USA § Spin transition of Fe 2+ Displacement of low-spin Fe Change of electronic structure § Transition pressure dependence on: Fe concentration Fe configuration § Gradual spin transition of Fe 2+
Spin transition in ferrous iron in Mg. Si. O 3 perovskite under pressure Koichiro Umemoto Minnesota Supercomputing Institute and Department of Chemical Engineering and Materials Science, University of Minnesota, Minneapolis, MN 55455, USA § Spin transition of Fe 2+ Displacement of low-spin Fe Change of electronic structure § Transition pressure dependence on: Fe concentration Fe configuration § Gradual spin transition of Fe 2+
 Collabolators • Renata Wentzcovitch (University of Minnesota) • Yonggang Yu (University of Minnesota) • Ryan Requist (Friedrich Alexandre University, Germany) Acknowledgments • Supported by NSF/EAR-0135533, EAR-0230319, ITR-0426757 (VLab) • Computations were performed at Minnesota Supercomputing Institute and Indiana University‘s Big. Red system
Collabolators • Renata Wentzcovitch (University of Minnesota) • Yonggang Yu (University of Minnesota) • Ryan Requist (Friedrich Alexandre University, Germany) Acknowledgments • Supported by NSF/EAR-0135533, EAR-0230319, ITR-0426757 (VLab) • Computations were performed at Minnesota Supercomputing Institute and Indiana University‘s Big. Red system
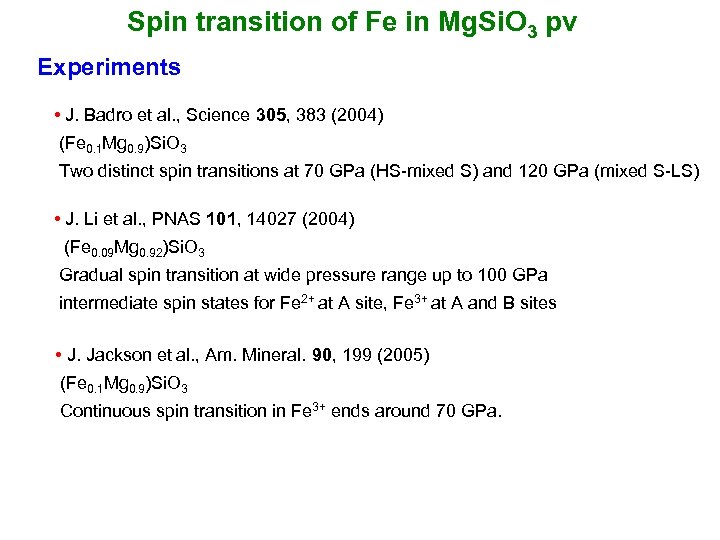 Spin transition of Fe in Mg. Si. O 3 pv Experiments • J. Badro et al. , Science 305, 383 (2004) (Fe 0. 1 Mg 0. 9)Si. O 3 Two distinct spin transitions at 70 GPa (HS-mixed S) and 120 GPa (mixed S-LS) • J. Li et al. , PNAS 101, 14027 (2004) (Fe 0. 09 Mg 0. 92)Si. O 3 Gradual spin transition at wide pressure range up to 100 GPa intermediate spin states for Fe 2+ at A site, Fe 3+ at A and B sites • J. Jackson et al. , Am. Mineral. 90, 199 (2005) (Fe 0. 1 Mg 0. 9)Si. O 3 Continuous spin transition in Fe 3+ ends around 70 GPa.
Spin transition of Fe in Mg. Si. O 3 pv Experiments • J. Badro et al. , Science 305, 383 (2004) (Fe 0. 1 Mg 0. 9)Si. O 3 Two distinct spin transitions at 70 GPa (HS-mixed S) and 120 GPa (mixed S-LS) • J. Li et al. , PNAS 101, 14027 (2004) (Fe 0. 09 Mg 0. 92)Si. O 3 Gradual spin transition at wide pressure range up to 100 GPa intermediate spin states for Fe 2+ at A site, Fe 3+ at A and B sites • J. Jackson et al. , Am. Mineral. 90, 199 (2005) (Fe 0. 1 Mg 0. 9)Si. O 3 Continuous spin transition in Fe 3+ ends around 70 GPa.
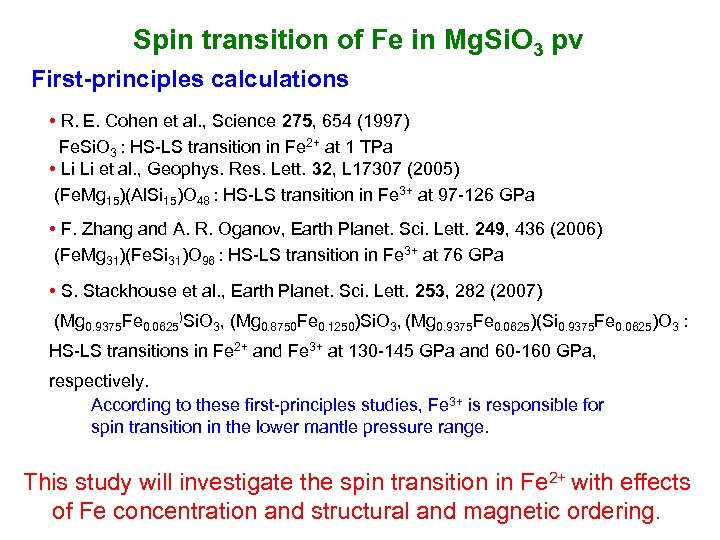 Spin transition of Fe in Mg. Si. O 3 pv First-principles calculations • R. E. Cohen et al. , Science 275, 654 (1997) Fe. Si. O 3 : HS-LS transition in Fe 2+ at 1 TPa • Li Li et al. , Geophys. Res. Lett. 32, L 17307 (2005) (Fe. Mg 15)(Al. Si 15)O 48 : HS-LS transition in Fe 3+ at 97 -126 GPa • F. Zhang and A. R. Oganov, Earth Planet. Sci. Lett. 249, 436 (2006) (Fe. Mg 31)(Fe. Si 31)O 96 : HS-LS transition in Fe 3+ at 76 GPa • S. Stackhouse et al. , Earth Planet. Sci. Lett. 253, 282 (2007) (Mg 0. 9375 Fe 0. 0625)Si. O 3, (Mg 0. 8750 Fe 0. 1250)Si. O 3, (Mg 0. 9375 Fe 0. 0625)(Si 0. 9375 Fe 0. 0625)O 3 : HS-LS transitions in Fe 2+ and Fe 3+ at 130 -145 GPa and 60 -160 GPa, respectively. According to these first-principles studies, Fe 3+ is responsible for spin transition in the lower mantle pressure range. This study will investigate the spin transition in Fe 2+ with effects of Fe concentration and structural and magnetic ordering.
Spin transition of Fe in Mg. Si. O 3 pv First-principles calculations • R. E. Cohen et al. , Science 275, 654 (1997) Fe. Si. O 3 : HS-LS transition in Fe 2+ at 1 TPa • Li Li et al. , Geophys. Res. Lett. 32, L 17307 (2005) (Fe. Mg 15)(Al. Si 15)O 48 : HS-LS transition in Fe 3+ at 97 -126 GPa • F. Zhang and A. R. Oganov, Earth Planet. Sci. Lett. 249, 436 (2006) (Fe. Mg 31)(Fe. Si 31)O 96 : HS-LS transition in Fe 3+ at 76 GPa • S. Stackhouse et al. , Earth Planet. Sci. Lett. 253, 282 (2007) (Mg 0. 9375 Fe 0. 0625)Si. O 3, (Mg 0. 8750 Fe 0. 1250)Si. O 3, (Mg 0. 9375 Fe 0. 0625)(Si 0. 9375 Fe 0. 0625)O 3 : HS-LS transitions in Fe 2+ and Fe 3+ at 130 -145 GPa and 60 -160 GPa, respectively. According to these first-principles studies, Fe 3+ is responsible for spin transition in the lower mantle pressure range. This study will investigate the spin transition in Fe 2+ with effects of Fe concentration and structural and magnetic ordering.
 Method n LDA (Ceperlay-Alder) and GGA (Perdew-Burke-Ernzerhof) n Vanderbilt ultrasoft pseudopotentials for Fe, Si, and O Von-Barth & Car pseudopotential for Mg n Plane wave cut-off energy : 40 Ry n Variable Cell Shape Molecular Dynamics for structural search n Supercell (up to 160 atoms) n Quantum-ESPRESSO package (www. pwscf. org)
Method n LDA (Ceperlay-Alder) and GGA (Perdew-Burke-Ernzerhof) n Vanderbilt ultrasoft pseudopotentials for Fe, Si, and O Von-Barth & Car pseudopotential for Mg n Plane wave cut-off energy : 40 Ry n Variable Cell Shape Molecular Dynamics for structural search n Supercell (up to 160 atoms) n Quantum-ESPRESSO package (www. pwscf. org)
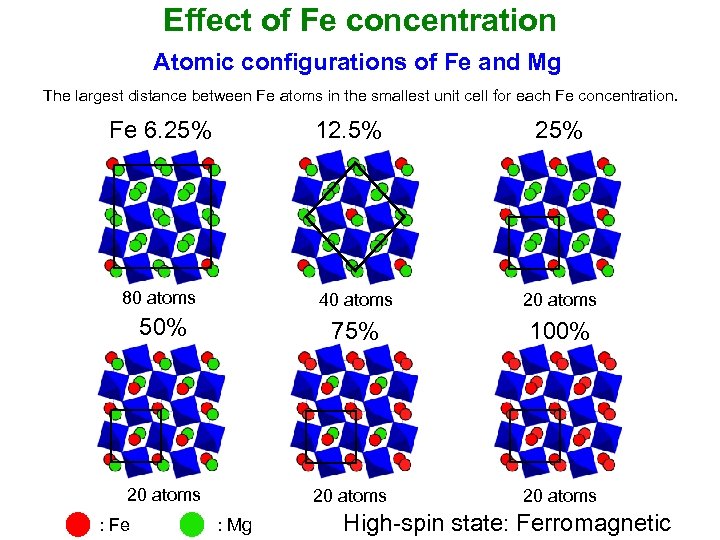 Effect of Fe concentration Atomic configurations of Fe and Mg The largest distance between Fe atoms in the smallest unit cell for each Fe concentration. Fe 6. 25% 12. 5% 25% 80 atoms 40 atoms 20 atoms 50% 75% 100% 20 atoms : Fe : Mg High-spin state: Ferromagnetic
Effect of Fe concentration Atomic configurations of Fe and Mg The largest distance between Fe atoms in the smallest unit cell for each Fe concentration. Fe 6. 25% 12. 5% 25% 80 atoms 40 atoms 20 atoms 50% 75% 100% 20 atoms : Fe : Mg High-spin state: Ferromagnetic
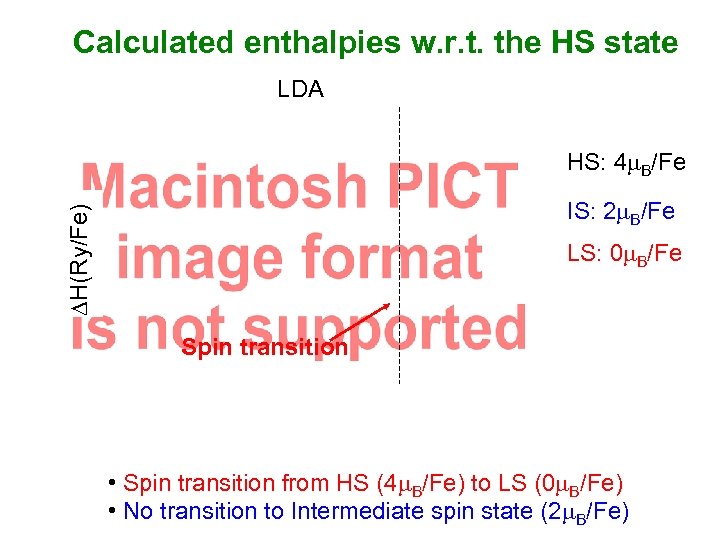 Calculated enthalpies w. r. t. the HS state LDA HS: 4 m. B/Fe DH(Ry/Fe) IS: 2 m. B/Fe LS: 0 m. B/Fe Spin transition • Spin transition from HS (4 m. B/Fe) to LS (0 m. B/Fe) • No transition to Intermediate spin state (2 m. B/Fe)
Calculated enthalpies w. r. t. the HS state LDA HS: 4 m. B/Fe DH(Ry/Fe) IS: 2 m. B/Fe LS: 0 m. B/Fe Spin transition • Spin transition from HS (4 m. B/Fe) to LS (0 m. B/Fe) • No transition to Intermediate spin state (2 m. B/Fe)
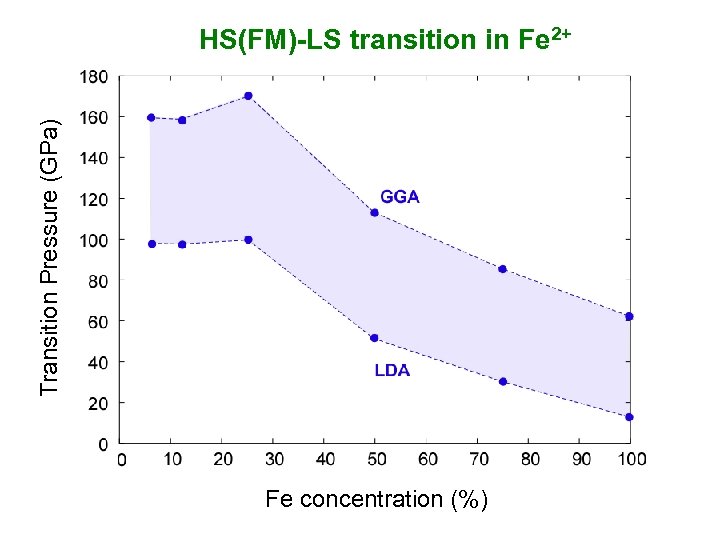 Transition Pressure (GPa) HS(FM)-LS transition in Fe 2+ Fe concentration (%)
Transition Pressure (GPa) HS(FM)-LS transition in Fe 2+ Fe concentration (%)
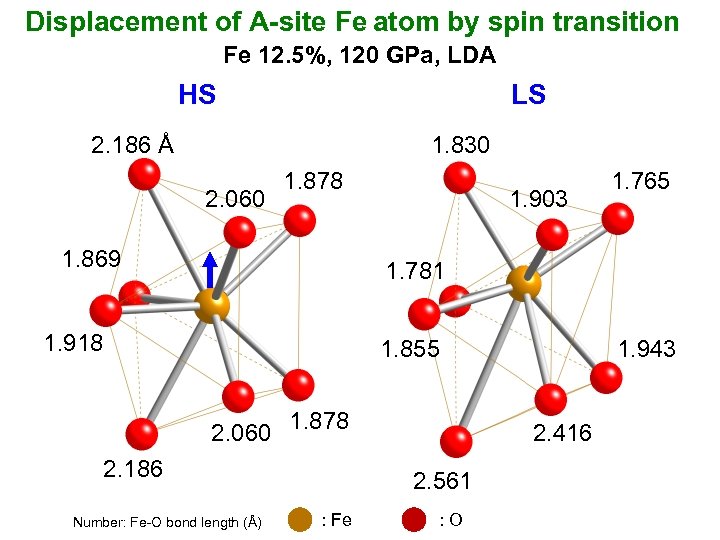 Displacement of A-site Fe atom by spin transition Fe 12. 5%, 120 GPa, LDA HS LS 2. 186 Å 1. 830 2. 060 1. 878 1. 869 1. 903 1. 765 1. 781 1. 918 1. 855 2. 060 1. 878 2. 186 Number: Fe-O bond length (Å) 2. 416 2. 561 : Fe 1. 943 : O
Displacement of A-site Fe atom by spin transition Fe 12. 5%, 120 GPa, LDA HS LS 2. 186 Å 1. 830 2. 060 1. 878 1. 869 1. 903 1. 765 1. 781 1. 918 1. 855 2. 060 1. 878 2. 186 Number: Fe-O bond length (Å) 2. 416 2. 561 : Fe 1. 943 : O
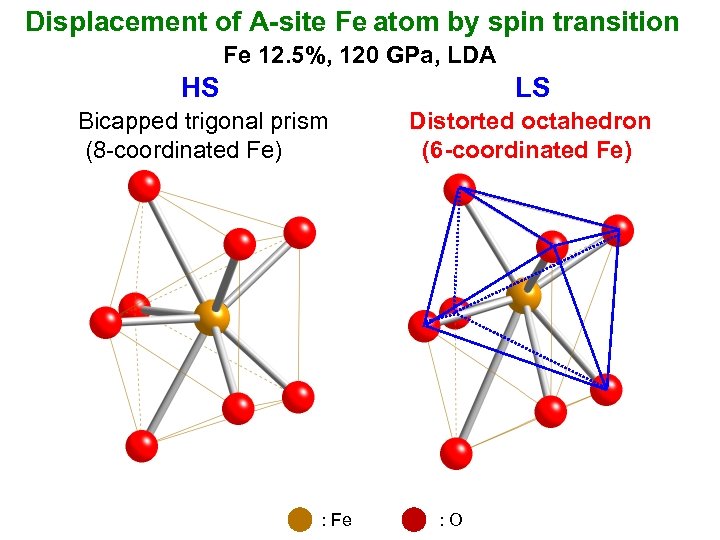 Displacement of A-site Fe atom by spin transition Fe 12. 5%, 120 GPa, LDA HS LS Bicapped trigonal prism (8 -coordinated Fe) Distorted octahedron (6 -coordinated Fe) : Fe : O
Displacement of A-site Fe atom by spin transition Fe 12. 5%, 120 GPa, LDA HS LS Bicapped trigonal prism (8 -coordinated Fe) Distorted octahedron (6 -coordinated Fe) : Fe : O
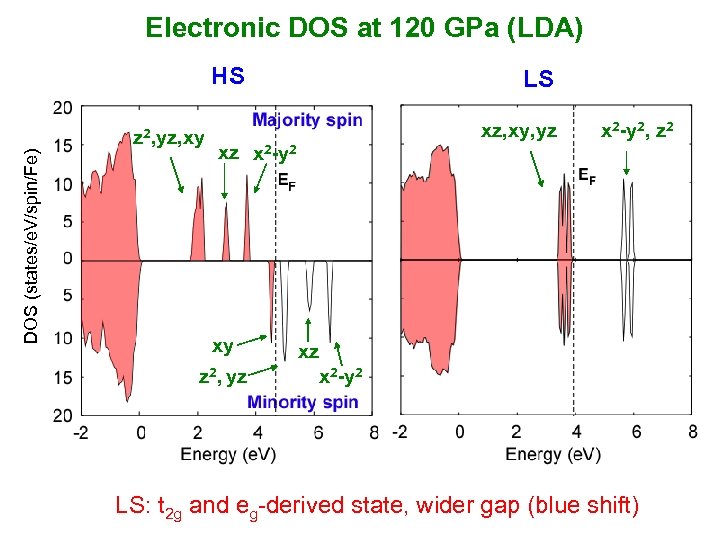 Electronic DOS at 120 GPa (LDA) HS DOS (states/e. V/spin/Fe) z 2, yz, xy LS xz, xy, yz xz x 2 -y 2 xy z 2, yz x 2 -y 2, z 2 xz x 2 -y 2 LS: t 2 g and eg-derived state, wider gap (blue shift)
Electronic DOS at 120 GPa (LDA) HS DOS (states/e. V/spin/Fe) z 2, yz, xy LS xz, xy, yz xz x 2 -y 2 xy z 2, yz x 2 -y 2, z 2 xz x 2 -y 2 LS: t 2 g and eg-derived state, wider gap (blue shift)
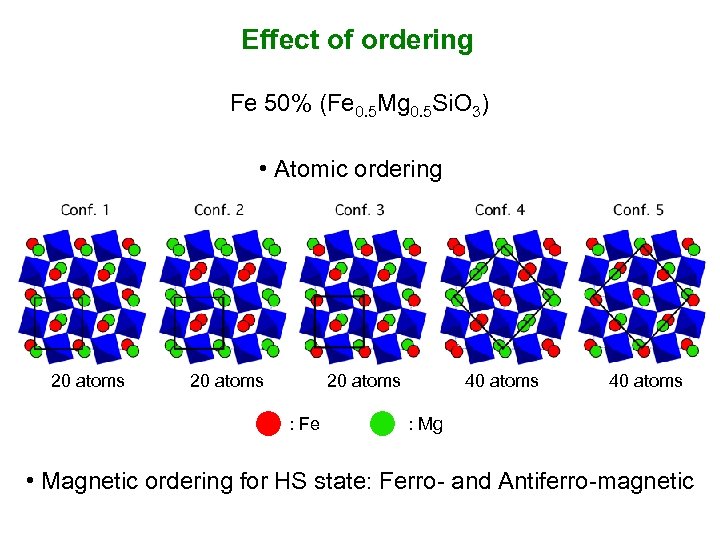 Effect of ordering Fe 50% (Fe 0. 5 Mg 0. 5 Si. O 3) • Atomic ordering 20 atoms : Fe 40 atoms : Mg • Magnetic ordering for HS state: Ferro- and Antiferro-magnetic
Effect of ordering Fe 50% (Fe 0. 5 Mg 0. 5 Si. O 3) • Atomic ordering 20 atoms : Fe 40 atoms : Mg • Magnetic ordering for HS state: Ferro- and Antiferro-magnetic
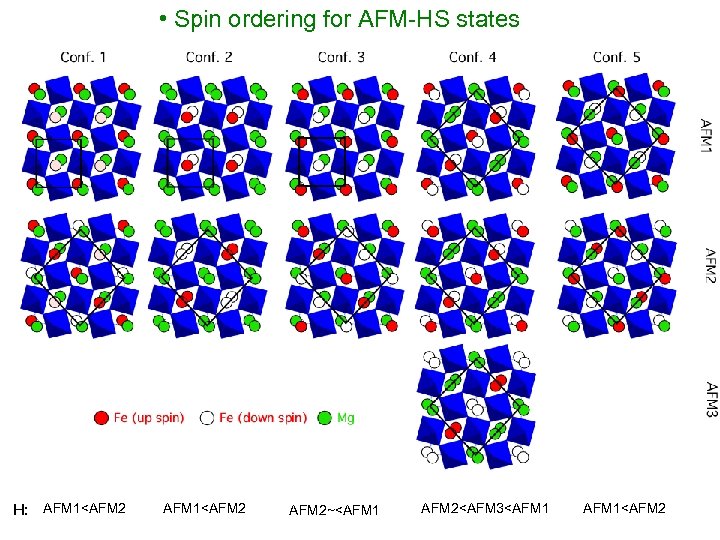 • Spin ordering for AFM-HS states H: AFM 1
• Spin ordering for AFM-HS states H: AFM 1
 DH (Ry/Fe 0. 5 Mg 0. 5 Si. O 3) FM Fe 0. 5 Mg 0. 5 Si. O 3 (LDA)
DH (Ry/Fe 0. 5 Mg 0. 5 Si. O 3) FM Fe 0. 5 Mg 0. 5 Si. O 3 (LDA)
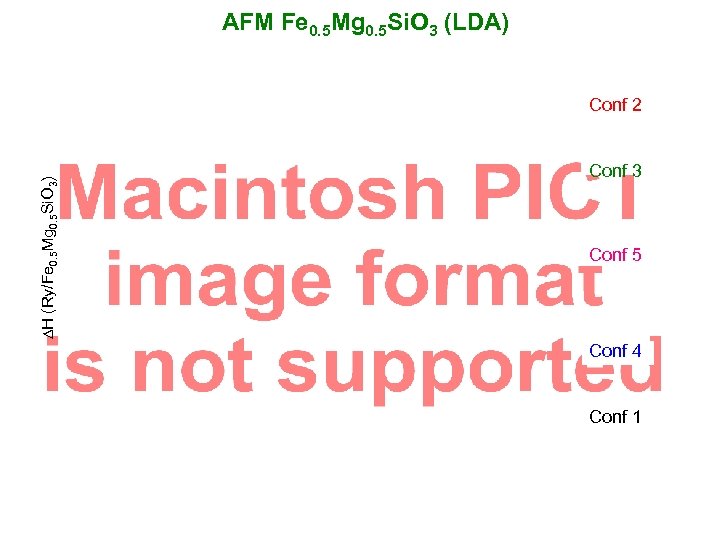 AFM Fe 0. 5 Mg 0. 5 Si. O 3 (LDA) DH (Ry/Fe 0. 5 Mg 0. 5 Si. O 3) Conf 2 Conf 3 Conf 5 Conf 4 Conf 1
AFM Fe 0. 5 Mg 0. 5 Si. O 3 (LDA) DH (Ry/Fe 0. 5 Mg 0. 5 Si. O 3) Conf 2 Conf 3 Conf 5 Conf 4 Conf 1
 DH (Ry/Fe 0. 5 Mg 0. 5 Si. O 3) LS Fe 0. 5 Mg 0. 5 Si. O 3 (LDA)
DH (Ry/Fe 0. 5 Mg 0. 5 Si. O 3) LS Fe 0. 5 Mg 0. 5 Si. O 3 (LDA)
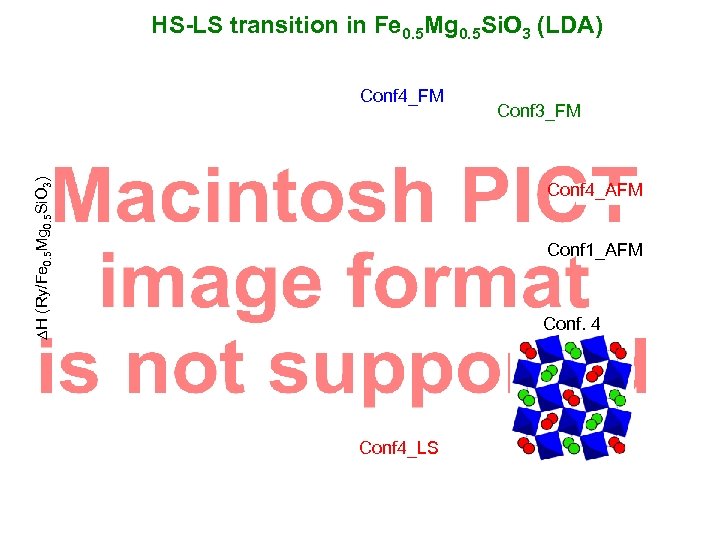 HS-LS transition in Fe 0. 5 Mg 0. 5 Si. O 3 (LDA) DH (Ry/Fe 0. 5 Mg 0. 5 Si. O 3) Conf 4_FM Conf 3_FM Conf 4_AFM Conf 1_AFM Conf. 4 Conf 4_LS
HS-LS transition in Fe 0. 5 Mg 0. 5 Si. O 3 (LDA) DH (Ry/Fe 0. 5 Mg 0. 5 Si. O 3) Conf 4_FM Conf 3_FM Conf 4_AFM Conf 1_AFM Conf. 4 Conf 4_LS
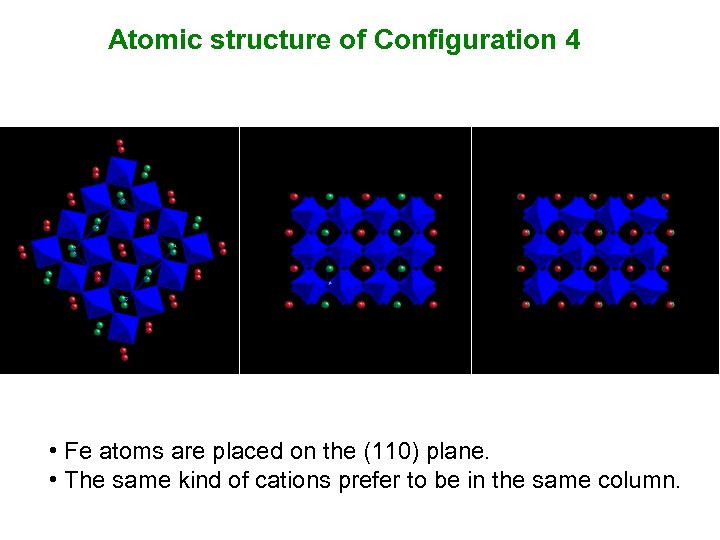 Atomic structure of Configuration 4 • Fe atoms are placed on the (110) plane. • The same kind of cations prefer to be in the same column.
Atomic structure of Configuration 4 • Fe atoms are placed on the (110) plane. • The same kind of cations prefer to be in the same column.
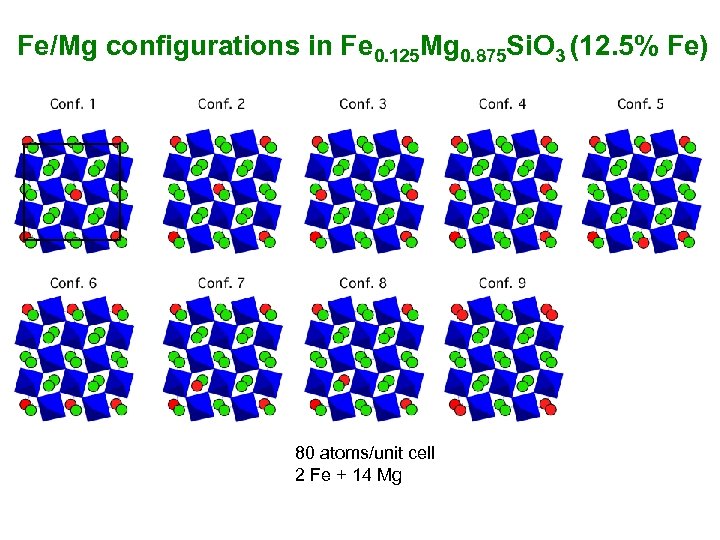 Fe/Mg configurations in Fe 0. 125 Mg 0. 875 Si. O 3 (12. 5% Fe) 80 atoms/unit cell 2 Fe + 14 Mg
Fe/Mg configurations in Fe 0. 125 Mg 0. 875 Si. O 3 (12. 5% Fe) 80 atoms/unit cell 2 Fe + 14 Mg
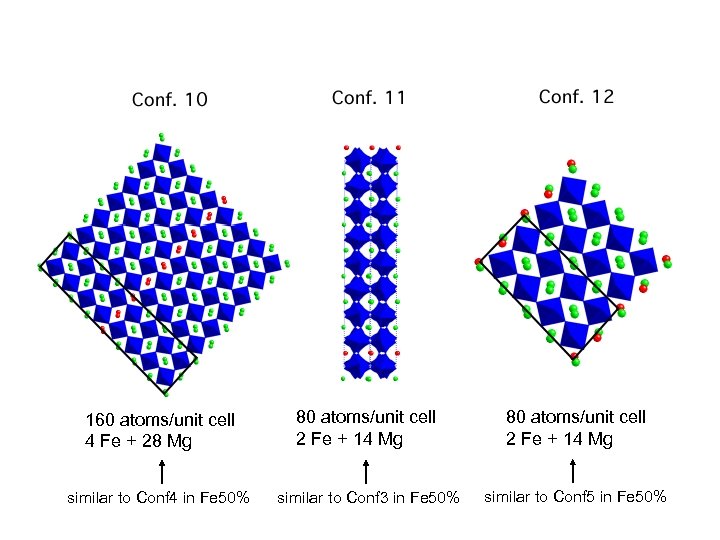 160 atoms/unit cell 4 Fe + 28 Mg 80 atoms/unit cell 2 Fe + 14 Mg similar to Conf 4 in Fe 50% similar to Conf 3 in Fe 50% similar to Conf 5 in Fe 50%
160 atoms/unit cell 4 Fe + 28 Mg 80 atoms/unit cell 2 Fe + 14 Mg similar to Conf 4 in Fe 50% similar to Conf 3 in Fe 50% similar to Conf 5 in Fe 50%
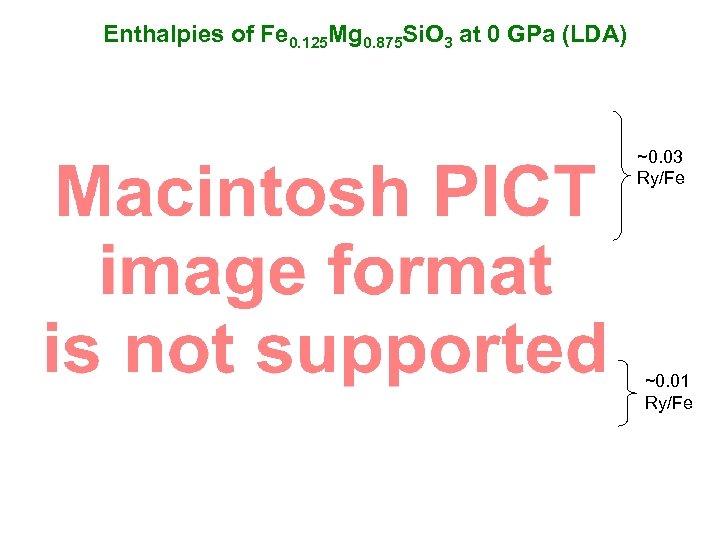 Enthalpies of Fe 0. 125 Mg 0. 875 Si. O 3 at 0 GPa (LDA) ~0. 03 Ry/Fe ~0. 01 Ry/Fe
Enthalpies of Fe 0. 125 Mg 0. 875 Si. O 3 at 0 GPa (LDA) ~0. 03 Ry/Fe ~0. 01 Ry/Fe
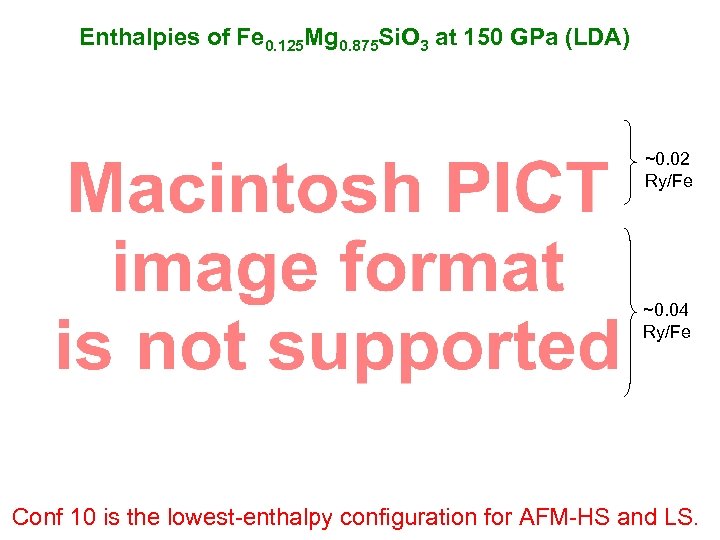 Enthalpies of Fe 0. 125 Mg 0. 875 Si. O 3 at 150 GPa (LDA) ~0. 02 Ry/Fe ~0. 04 Ry/Fe Conf 10 is the lowest-enthalpy configuration for AFM-HS and LS.
Enthalpies of Fe 0. 125 Mg 0. 875 Si. O 3 at 150 GPa (LDA) ~0. 02 Ry/Fe ~0. 04 Ry/Fe Conf 10 is the lowest-enthalpy configuration for AFM-HS and LS.
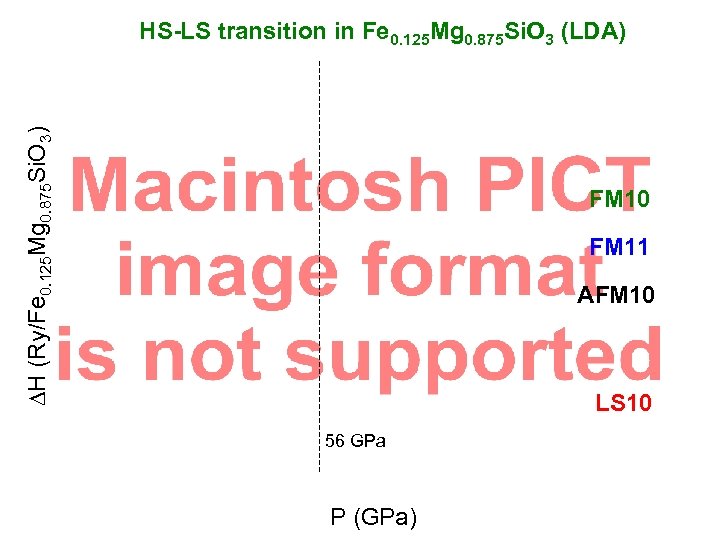 DH (Ry/Fe 0. 125 Mg 0. 875 Si. O 3) HS-LS transition in Fe 0. 125 Mg 0. 875 Si. O 3 (LDA) FM 10 FM 11 AFM 10 LS 10 56 GPa P (GPa)
DH (Ry/Fe 0. 125 Mg 0. 875 Si. O 3) HS-LS transition in Fe 0. 125 Mg 0. 875 Si. O 3 (LDA) FM 10 FM 11 AFM 10 LS 10 56 GPa P (GPa)
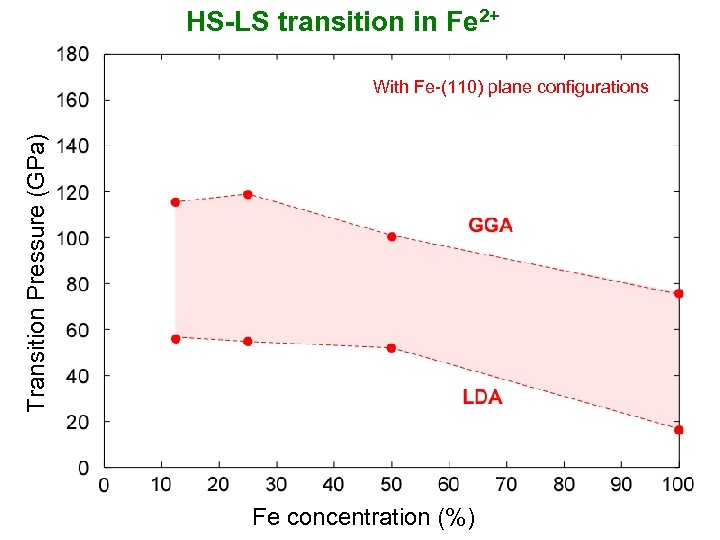 HS-LS transition in Fe 2+ Transition Pressure (GPa) With Fe-(110) plane configurations Fe concentration (%)
HS-LS transition in Fe 2+ Transition Pressure (GPa) With Fe-(110) plane configurations Fe concentration (%)
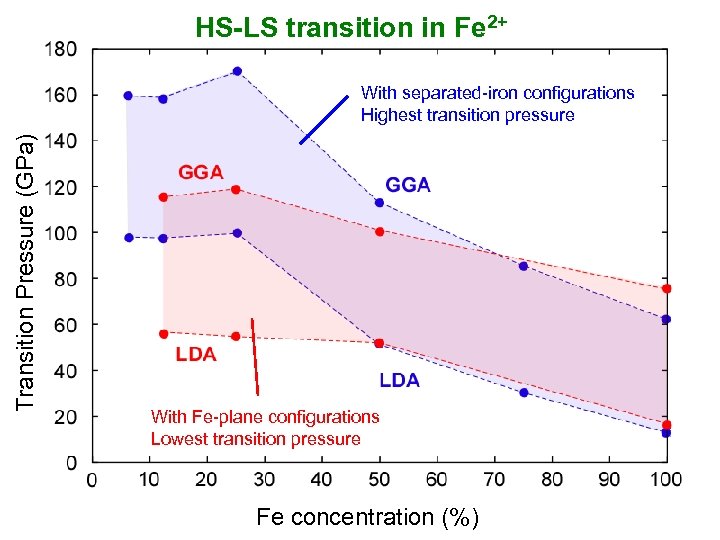 HS-LS transition in Fe 2+ Transition Pressure (GPa) With separated-iron configurations Highest transition pressure With Fe-plane configurations Lowest transition pressure Fe concentration (%)
HS-LS transition in Fe 2+ Transition Pressure (GPa) With separated-iron configurations Highest transition pressure With Fe-plane configurations Lowest transition pressure Fe concentration (%)
 Configuration vs HS-LS transition pressure in Fe 0. 125 Mg 0. 875 Si. O 3 (LDA) With separated-iron configurations With Fe-plane configurations
Configuration vs HS-LS transition pressure in Fe 0. 125 Mg 0. 875 Si. O 3 (LDA) With separated-iron configurations With Fe-plane configurations
 At high temperature… • All configurations with different transition pressures should appear locally. • Fe planes (conf. 10) with different sizes should exist locally. The larger (smaller) size of Fe plane gives the lower (higher) spin transition pressure. Possibility of gradual spin transition in Fe 2+ at the A site
At high temperature… • All configurations with different transition pressures should appear locally. • Fe planes (conf. 10) with different sizes should exist locally. The larger (smaller) size of Fe plane gives the lower (higher) spin transition pressure. Possibility of gradual spin transition in Fe 2+ at the A site
 Electronic DOS of Fe 12. 5% at 150 GPa (LDA) With separated-iron configuration With Fe-plane configuration FM AFM LS LS
Electronic DOS of Fe 12. 5% at 150 GPa (LDA) With separated-iron configuration With Fe-plane configuration FM AFM LS LS
 Summary • Fex. Mg 1 -x. Si. O 3 shows tendency to display atomic and magnetic (AFM) order at 0 K. This tendency decreases with temperature. • Spin transition in Fe 2+ occurs at 0 K in the pressure range found experimentally, i. e. , at lower mantle pressures. At high temperature, this transition should be broad and pass through mixed spins states. • In highly-ordered structures, where Fe‘s are close to each other and they are on the (110) plane, the spin transition pressure is the lowest. This is consistent with the transition pressure dependence on Fe concentration. • In the LS state, electronic structure of Fe at the A site becomes similar to that of Fe at the octahedron (t 2 g and eg-derived states) with a large gap.
Summary • Fex. Mg 1 -x. Si. O 3 shows tendency to display atomic and magnetic (AFM) order at 0 K. This tendency decreases with temperature. • Spin transition in Fe 2+ occurs at 0 K in the pressure range found experimentally, i. e. , at lower mantle pressures. At high temperature, this transition should be broad and pass through mixed spins states. • In highly-ordered structures, where Fe‘s are close to each other and they are on the (110) plane, the spin transition pressure is the lowest. This is consistent with the transition pressure dependence on Fe concentration. • In the LS state, electronic structure of Fe at the A site becomes similar to that of Fe at the octahedron (t 2 g and eg-derived states) with a large gap.



 Spin transition of Fe in Mg. Si. O 3 pv Experiments • J. Badro et al. , Science 305, 383 (2004) (Fe 0. 1 Mg 0. 9)Si. O 3 Two distinct spin transitions at 70 GPa (HS-mixed S) and 120 GPa (mixed S-LS) • J. Li et al. , PNAS 101, 14027 (2004) (Fe 0. 09 Mg 0. 92)Si. O 3 Spin transition at wide pressure range up to 100 GPa intermediate spin states for Fe 2+ at A site, Fe 3+ at A and B sites • J. Jackson et al. , Am. Mineral. 90, 199 (2005) (Fe 0. 1 Mg 0. 9)Si. O 3 Continuous spin transition in Fe 3+ ends around 70 GPa. Calculations • R. E. Cohen et al. , Science 275, 654 (1997) Fe. Si. O 3 : HS-LS transition in Fe 2+ at 1 TPa • Li Li et al. , Geophys. Res. Lett. 32, L 17307 (2005) (Fe. Mg 15)(Al. Si 15)O 48 : HS-LS transition in Fe 3+ at 97 -126 GPa • F. Zhang and A. R. Oganov, Earth Planet. Sci. Lett. 249, 436 (2006) (Fe. Mg 31)(Fe. Si 31)O 96 : HS-LS transition in Fe 3+ at 76 GPa • S. Stackhouse et al. , Earth Planet. Sci. Lett. 253, 282 (2007) This study will investigate the spin transition in Fe 2+ with effects (Mg 0. 9375 Fe 0. 0625)Si. O 3, (Mg 0. 8750 Fe 0. 1250)Si. O 3, (Mg 0. 9375 Fe 0. 0625)(Si 0. 9375 Fe 0. 0625)O 3 : HS-L of Fe concentration and structural and 60 -160 GPa, respectively. transitions in Fe 2+ and Fe 3+ at 130 -145 GPaand magnetic ordering.
Spin transition of Fe in Mg. Si. O 3 pv Experiments • J. Badro et al. , Science 305, 383 (2004) (Fe 0. 1 Mg 0. 9)Si. O 3 Two distinct spin transitions at 70 GPa (HS-mixed S) and 120 GPa (mixed S-LS) • J. Li et al. , PNAS 101, 14027 (2004) (Fe 0. 09 Mg 0. 92)Si. O 3 Spin transition at wide pressure range up to 100 GPa intermediate spin states for Fe 2+ at A site, Fe 3+ at A and B sites • J. Jackson et al. , Am. Mineral. 90, 199 (2005) (Fe 0. 1 Mg 0. 9)Si. O 3 Continuous spin transition in Fe 3+ ends around 70 GPa. Calculations • R. E. Cohen et al. , Science 275, 654 (1997) Fe. Si. O 3 : HS-LS transition in Fe 2+ at 1 TPa • Li Li et al. , Geophys. Res. Lett. 32, L 17307 (2005) (Fe. Mg 15)(Al. Si 15)O 48 : HS-LS transition in Fe 3+ at 97 -126 GPa • F. Zhang and A. R. Oganov, Earth Planet. Sci. Lett. 249, 436 (2006) (Fe. Mg 31)(Fe. Si 31)O 96 : HS-LS transition in Fe 3+ at 76 GPa • S. Stackhouse et al. , Earth Planet. Sci. Lett. 253, 282 (2007) This study will investigate the spin transition in Fe 2+ with effects (Mg 0. 9375 Fe 0. 0625)Si. O 3, (Mg 0. 8750 Fe 0. 1250)Si. O 3, (Mg 0. 9375 Fe 0. 0625)(Si 0. 9375 Fe 0. 0625)O 3 : HS-L of Fe concentration and structural and 60 -160 GPa, respectively. transitions in Fe 2+ and Fe 3+ at 130 -145 GPaand magnetic ordering.
 DH (Ry/f. u. ) P (GPa) LDA-CA, 3 s&3 p: valence
DH (Ry/f. u. ) P (GPa) LDA-CA, 3 s&3 p: valence


