5c47614752e536b9a6f96b18539dfc03.ppt
- Количество слайдов: 141

SPECTROSCOPY By Dr. S. R. Mane M. Sc. B. Ed. Ph. D. Associate Prof. and Head, Department of Chemistry, Smt. Kusumtai Rajarambapu Patil Kanya Mahavidyalaya, Islampur. Dist. - Sangli. Email – sambhaji_mane@rediffmail. com

NUCLEAR MAGNETIC RESONANCE (NMR) SPECTROSCOPY
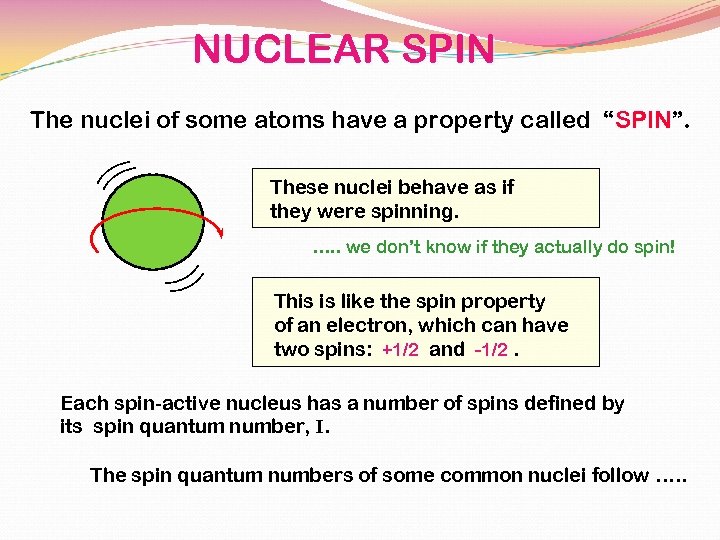
NUCLEAR SPIN The nuclei of some atoms have a property called “SPIN”. These nuclei behave as if they were spinning. …. . we don’t know if they actually do spin! This is like the spin property of an electron, which can have two spins: +1/2 and -1/2. Each spin-active nucleus has a number of spins defined by its spin quantum number, I. The spin quantum numbers of some common nuclei follow …. .
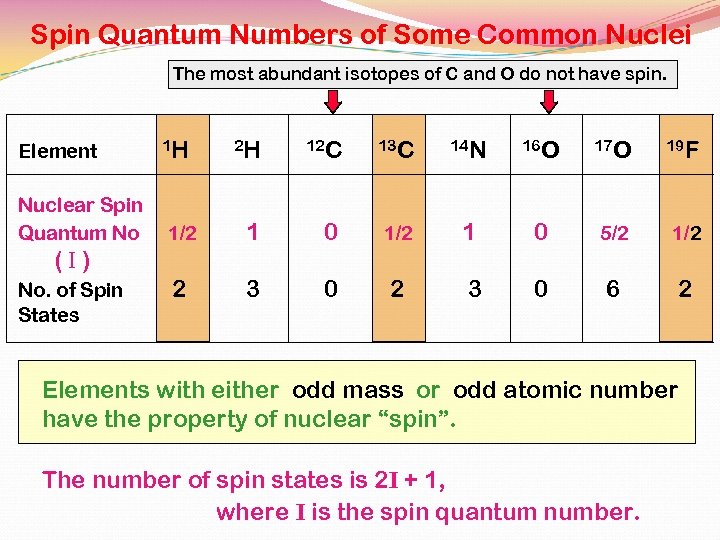
Spin Quantum Numbers of Some Common Nuclei The most abundant isotopes of C and O do not have spin. Element Nuclear Spin Quantum No 1 H 2 H 12 C 13 C 14 N 16 O 17 O 19 F 1/2 1 0 5/2 1/2 2 3 0 6 2 (I) No. of Spin States Elements with either odd mass or odd atomic number have the property of nuclear “spin”. The number of spin states is 2 I + 1, where I is the spin quantum number.
THE PROTON Although interest is increasing in other nuclei, particulary C-13, the hydrogen nucleus (proton) is studied most frequently, and we will devote our attention to it first.
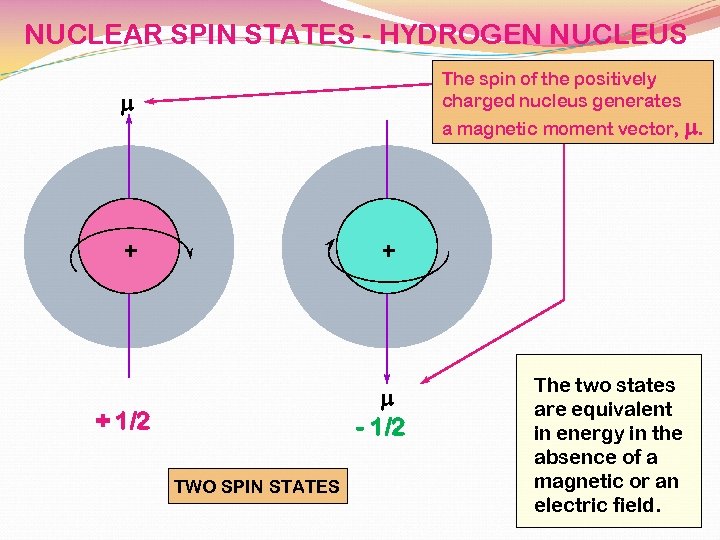
NUCLEAR SPIN STATES - HYDROGEN NUCLEUS The spin of the positively charged nucleus generates m a magnetic moment vector, m. + + m - 1/2 + 1/2 TWO SPIN STATES The two states are equivalent in energy in the absence of a magnetic or an electric field.
THE “RESONANCE” PHENOMENON absorption of energy by the spinning nucleus
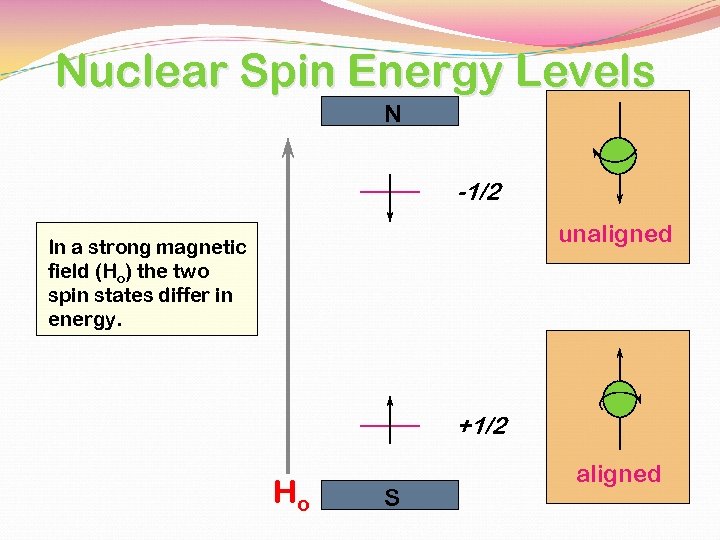
Nuclear Spin Energy Levels N -1/2 unaligned In a strong magnetic field (Ho) the two spin states differ in energy. +1/2 Ho S aligned
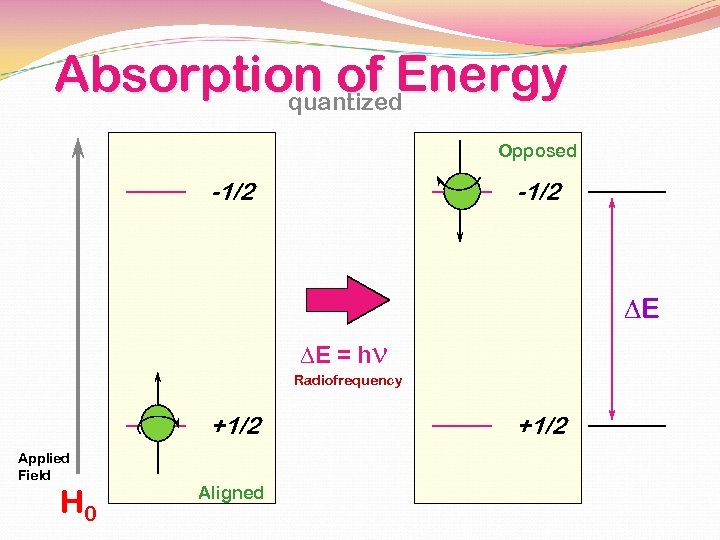
Absorption of Energy quantized Opposed -1/2 DE DE = hn Radiofrequency +1/2 Applied Field H 0 Aligned +1/2
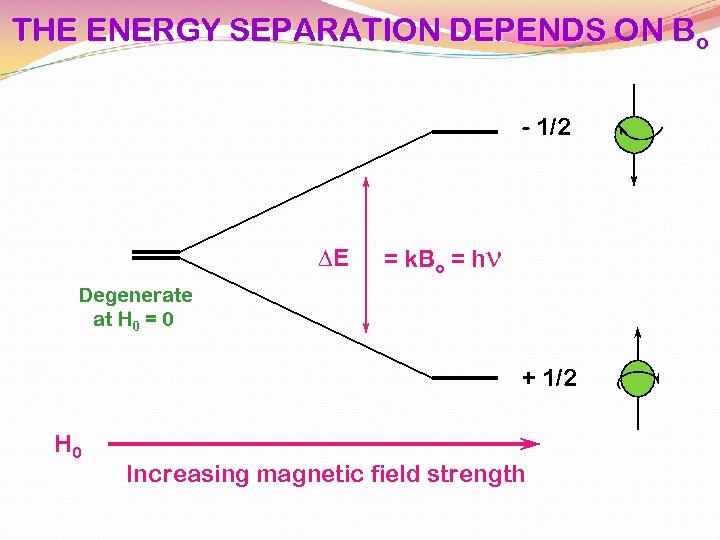
THE ENERGY SEPARATION DEPENDS ON Bo - 1/2 DE = k. Bo = hn Degenerate at H 0 = 0 + 1/2 H 0 Increasing magnetic field strength
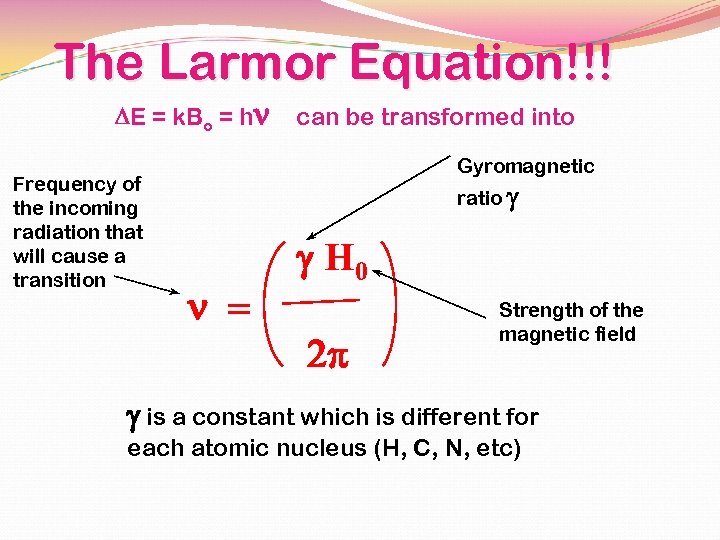
The Larmor Equation!!! DE = k. Bo = hn Frequency of the incoming radiation that will cause a transition can be transformed into Gyromagnetic ratio g n = g H 0 2 p Strength of the magnetic field g is a constant which is different for each atomic nucleus (H, C, N, etc)
A SECOND EFFECT OF A STRONG MAGNETIC FIELD WHEN A SPIN-ACTIVE HYDROGEN ATOM IS PLACED IN A STRONG MAGNETIC FIELD …. . IT BEGINS TO PRECESS OPERATION OF AN NMR SPECTROMETER DEPENDS ON THIS RESULT
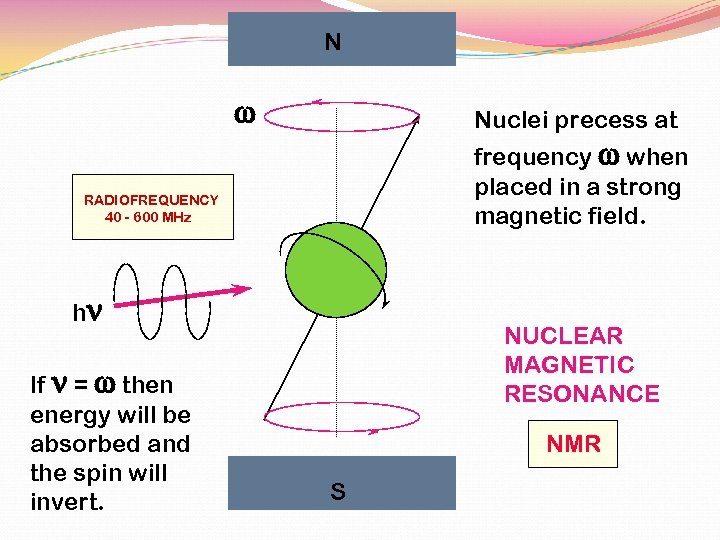
N w Nuclei precess at frequency w when placed in a strong magnetic field. RADIOFREQUENCY 40 - 600 MHz hn If n = w then energy will be absorbed and the spin will invert. NUCLEAR MAGNETIC RESONANCE NMR S
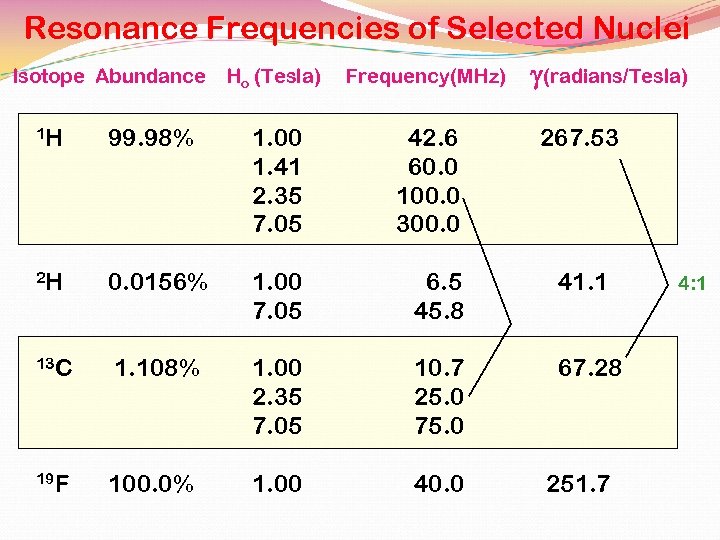
Resonance Frequencies of Selected Nuclei Isotope Abundance Ho (Tesla) Frequency(MHz) g(radians/Tesla) 1 H 99. 98% 1. 00 1. 41 2. 35 7. 05 42. 6 60. 0 100. 0 300. 0 2 H 0. 0156% 1. 00 7. 05 6. 5 45. 8 41. 1 13 C 1. 108% 1. 00 2. 35 7. 05 10. 7 25. 0 75. 0 67. 28 100. 0% 1. 00 40. 0 19 F 267. 53 251. 7 4: 1
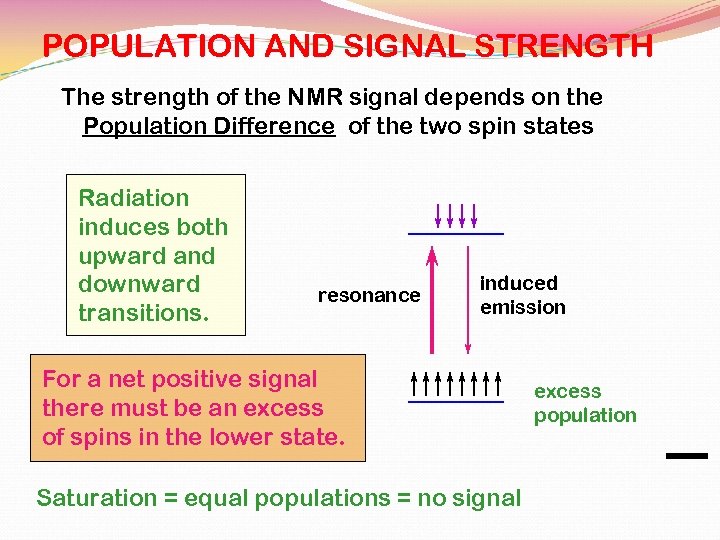
POPULATION AND SIGNAL STRENGTH The strength of the NMR signal depends on the Population Difference of the two spin states Radiation induces both upward and downward transitions. resonance induced emission For a net positive signal there must be an excess of spins in the lower state. Saturation = equal populations = no signal excess population

CLASSICAL INSTRUMENTATION typical before 1960 field is scanned
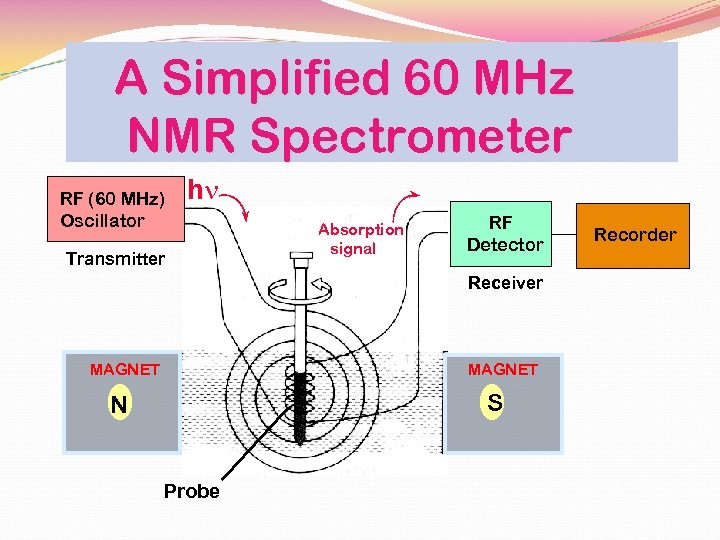
A Simplified 60 MHz NMR Spectrometer RF (60 MHz) Oscillator hn Transmitter Absorption signal RF Detector Receiver MAGNET S N Probe Recorder
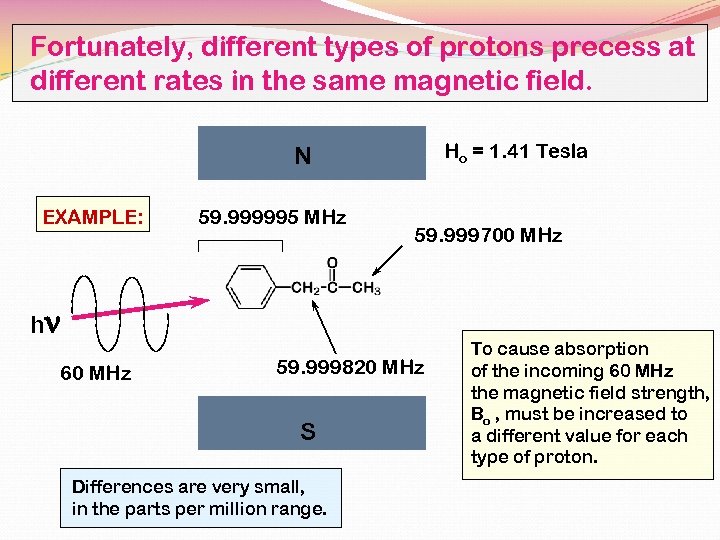
Fortunately, different types of protons precess at different rates in the same magnetic field. Ho = 1. 41 Tesla N EXAMPLE: 59. 999995 MHz 59. 999700 MHz hn 60 MHz 59. 999820 MHz S Differences are very small, in the parts per million range. To cause absorption of the incoming 60 MHz the magnetic field strength, Bo , must be increased to a different value for each type of proton.
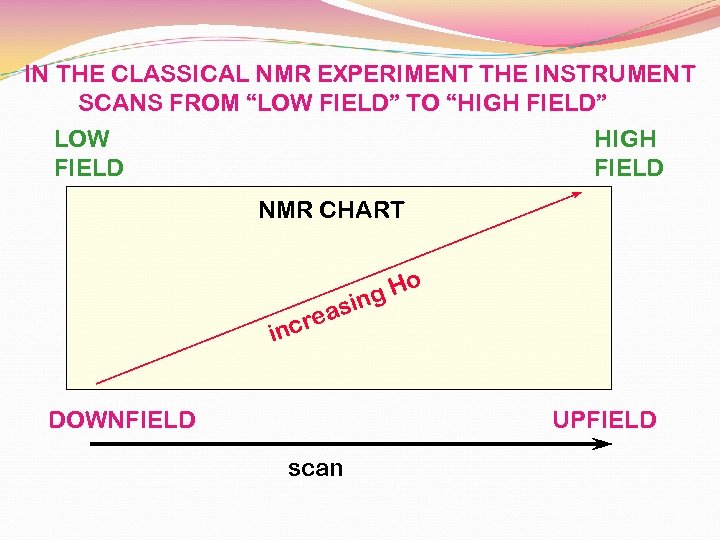
IN THE CLASSICAL NMR EXPERIMENT THE INSTRUMENT SCANS FROM “LOW FIELD” TO “HIGH FIELD” LOW HIGH FIELD NMR CHART o g. H sin a cre in DOWNFIELD UPFIELD scan
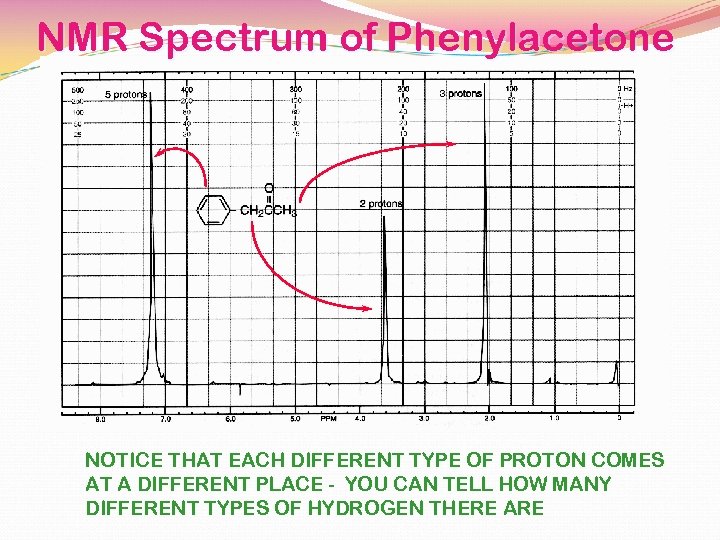
NMR Spectrum of Phenylacetone NOTICE THAT EACH DIFFERENT TYPE OF PROTON COMES AT A DIFFERENT PLACE - YOU CAN TELL HOW MANY DIFFERENT TYPES OF HYDROGEN THERE ARE
MODERN INSTRUMENTATION PULSED FOURIER TRANSFORM TECHNOLOGY FT-NMR requires a computer
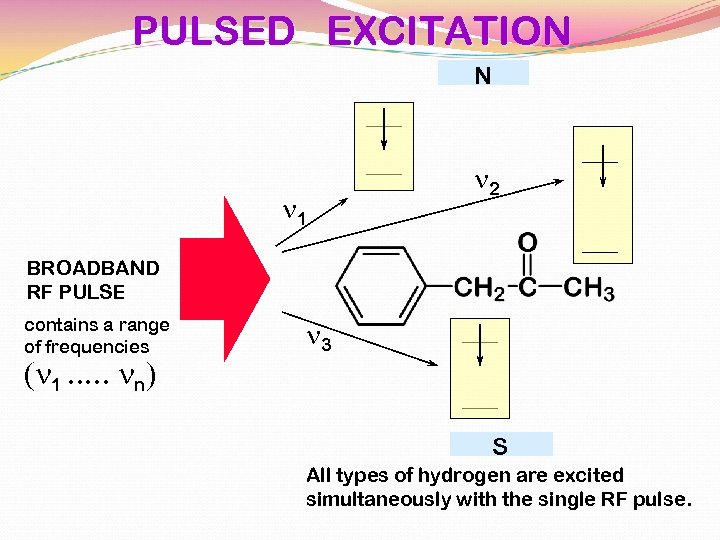
PULSED EXCITATION N n 1 n 2 BROADBAND RF PULSE contains a range of frequencies (n 1. . . nn) n 3 S All types of hydrogen are excited simultaneously with the single RF pulse.
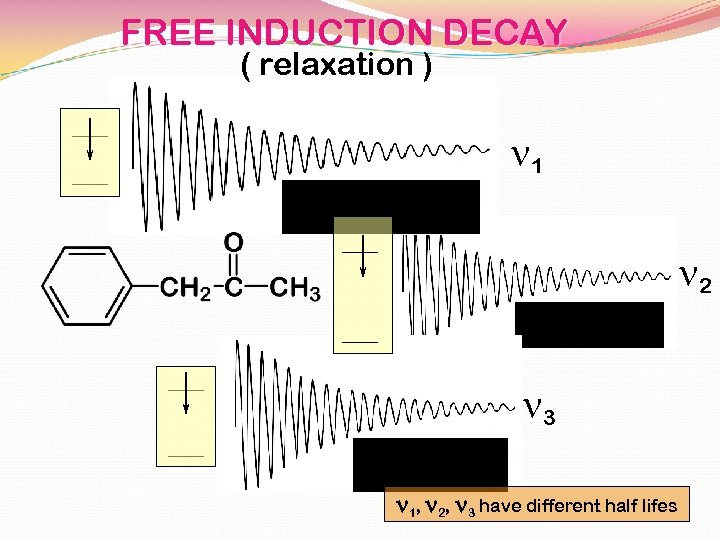
FREE INDUCTION DECAY ( relaxation ) n 1 n 2 n 3 n 1, n 2, n 3 have different half lifes
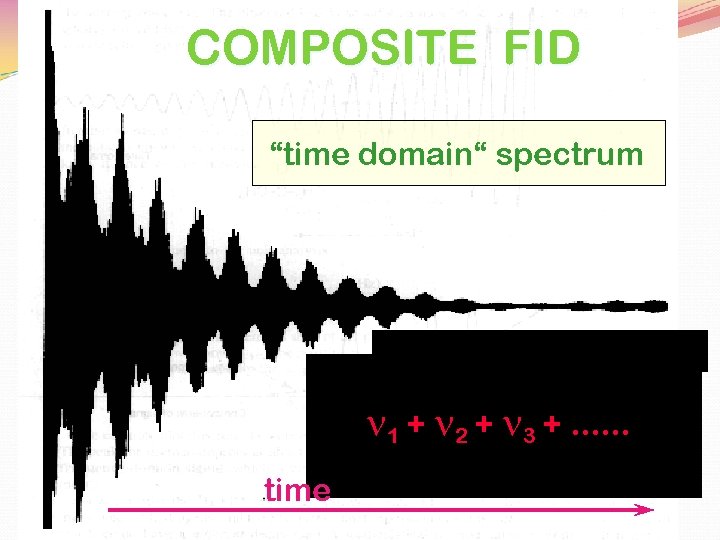
COMPOSITE FID “time domain“ spectrum n 1 + n 2 + n 3 +. . . time
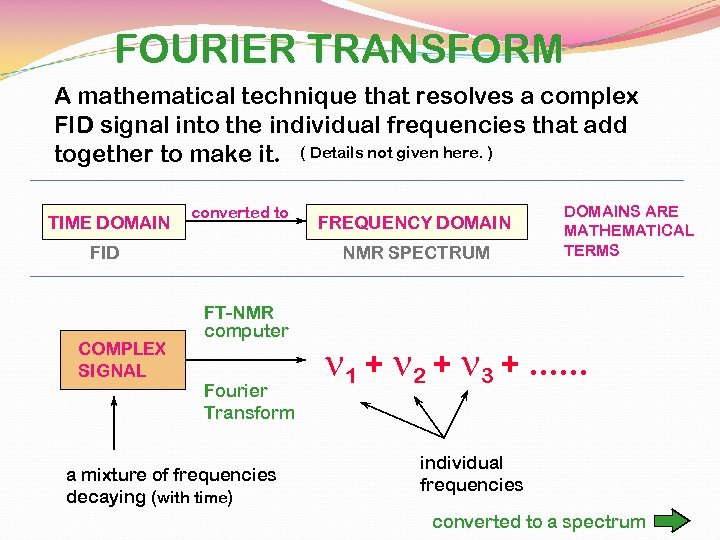
FOURIER TRANSFORM A mathematical technique that resolves a complex FID signal into the individual frequencies that add together to make it. ( Details not given here. ) TIME DOMAIN converted to FID COMPLEX SIGNAL FREQUENCY DOMAIN NMR SPECTRUM FT-NMR computer Fourier Transform a mixture of frequencies decaying (with time) DOMAINS ARE MATHEMATICAL TERMS n 1 + n 2 + n 3 +. . . individual frequencies converted to a spectrum
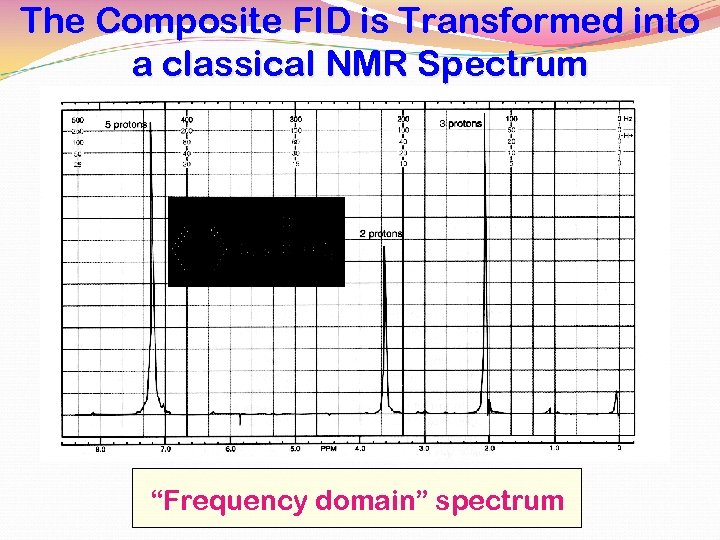
The Composite FID is Transformed into a classical NMR Spectrum “Frequency domain” spectrum
COMPARISON OF CW AND FT TECHNIQUES
CONTINUOUS WAVE (CW) METHOD THE OLDER, CLASSICAL METHOD The magnetic field is “scanned” from a low field strength to a higher field strength while a constant beam of radiofrequency (continuous wave) is supplied at a fixed frequency (say 100 MHz). Using this method, it requires several minutes to plot an NMR spectrum. SLOW, HIGH NOISE LEVEL
PULSED FOURIER TRANSFORM (FT) METHOD FAST THE NEWER COMPUTER-BASED METHOD LOW NOISE Most protons relax (decay) from their excited states very quickly (within a second). The excitation pulse, the data collection (FID), and the computer-driven Fourier Transform (FT) take only a few seconds. The pulse and data collection cycles may be repeated every few seconds. Many repetitions can be performed in a very short time, leading to improved signal …. .
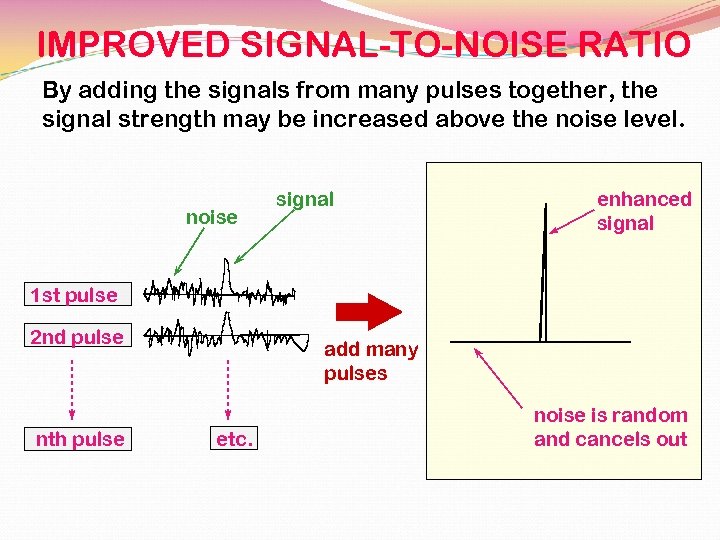
IMPROVED SIGNAL-TO-NOISE RATIO By adding the signals from many pulses together, the signal strength may be increased above the noise level. noise signal enhanced signal 1 st pulse 2 nd pulse nth pulse add many pulses etc. noise is random and cancels out

SPIN-SPIN SPLITTING

SPIN-SPIN SPLITTING Often a group of hydrogens will appear as a multiplet rather than as a single peak. Multiplets are named as follows: Singlet Doublet Triplet Quartet Quintet Septet Octet Nonet This happens because of interaction with neighboring hydrogens and is called SPIN-SPIN SPLITTING.
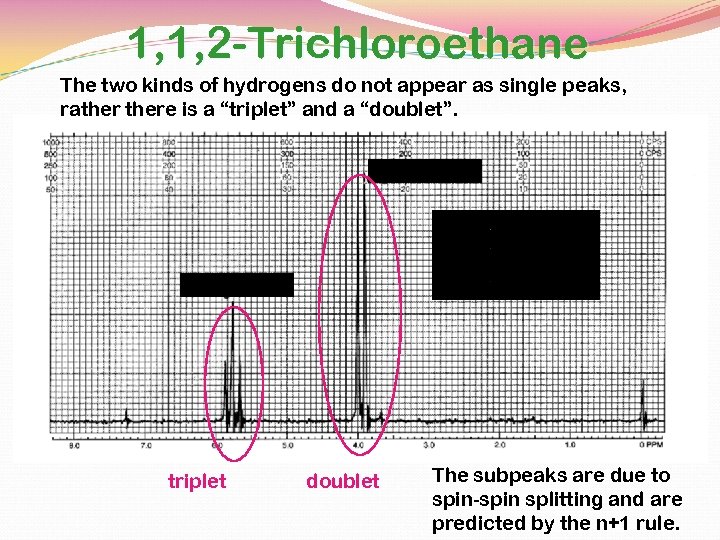
1, 1, 2 -Trichloroethane The two kinds of hydrogens do not appear as single peaks, rathere is a “triplet” and a “doublet”. integral = 2 integral = 1 triplet doublet The subpeaks are due to spin-spin splitting and are predicted by the n+1 rule.
n+1 RULE
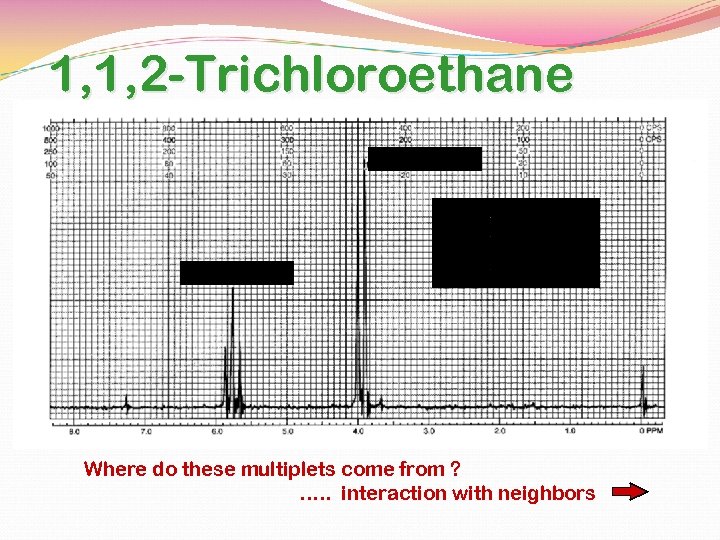
1, 1, 2 -Trichloroethane integral = 2 integral = 1 Where do these multiplets come from ? …. . interaction with neighbors
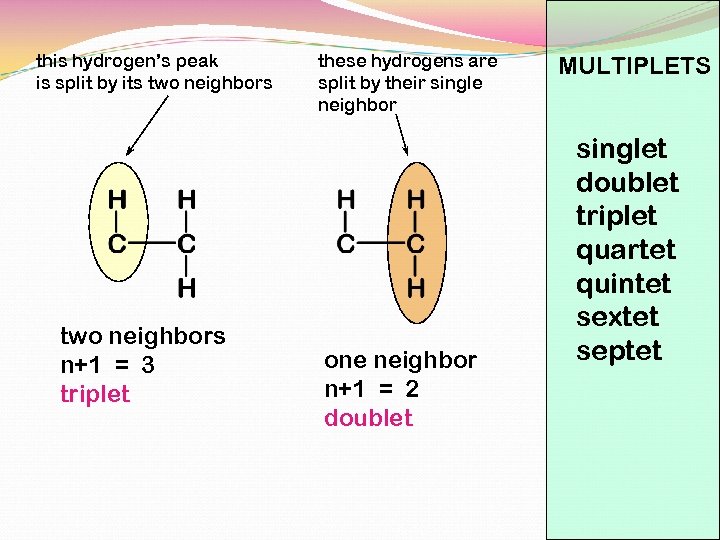
this hydrogen’s peak is split by its two neighbors n+1 = 3 triplet these hydrogens are split by their single neighbor one neighbor n+1 = 2 doublet MULTIPLETS singlet doublet triplet quartet quintet sextet septet
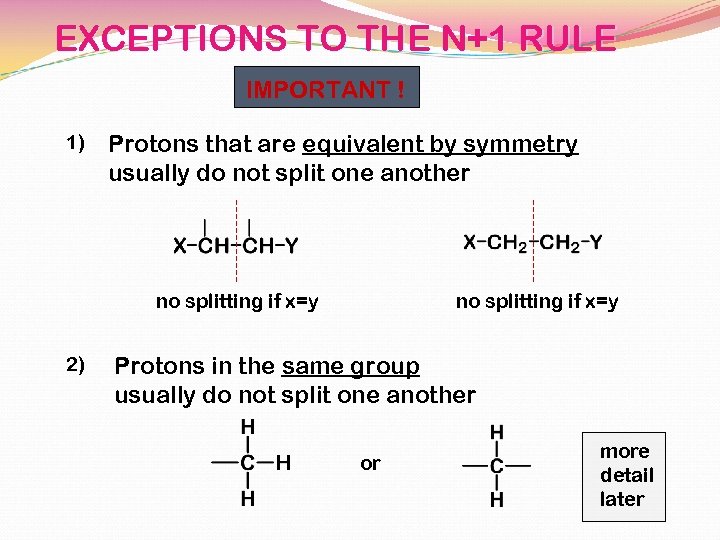
EXCEPTIONS TO THE N+1 RULE IMPORTANT ! 1) Protons that are equivalent by symmetry usually do not split one another no splitting if x=y 2) no splitting if x=y Protons in the same group usually do not split one another or more detail later
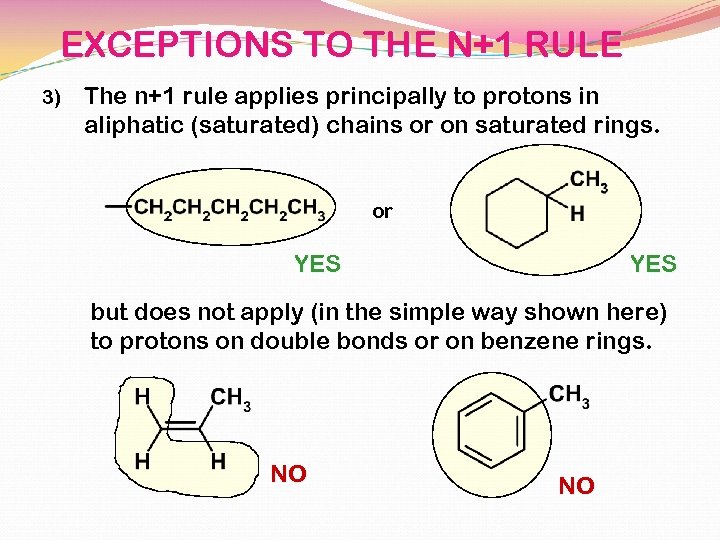
EXCEPTIONS TO THE N+1 RULE 3) The n+1 rule applies principally to protons in aliphatic (saturated) chains or on saturated rings. or YES but does not apply (in the simple way shown here) to protons on double bonds or on benzene rings. NO NO

SOME COMMON PATTERNS
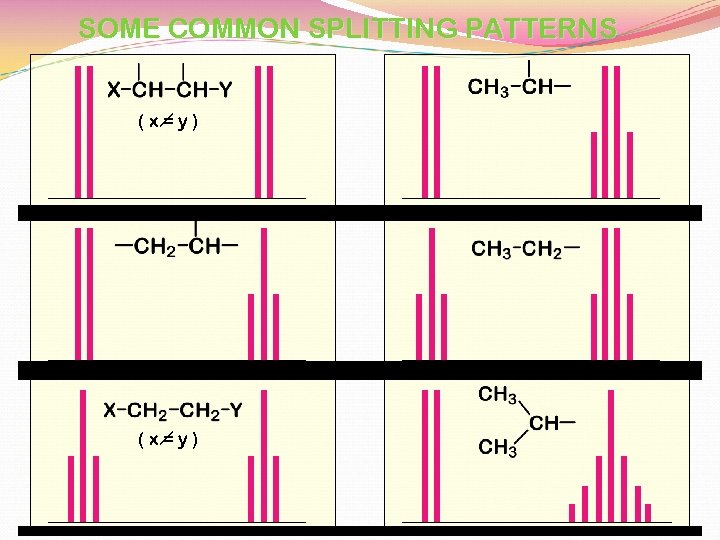
SOME COMMON SPLITTING PATTERNS (x=y)

SOME EXAMPLE SPECTRA WITH SPLITTING
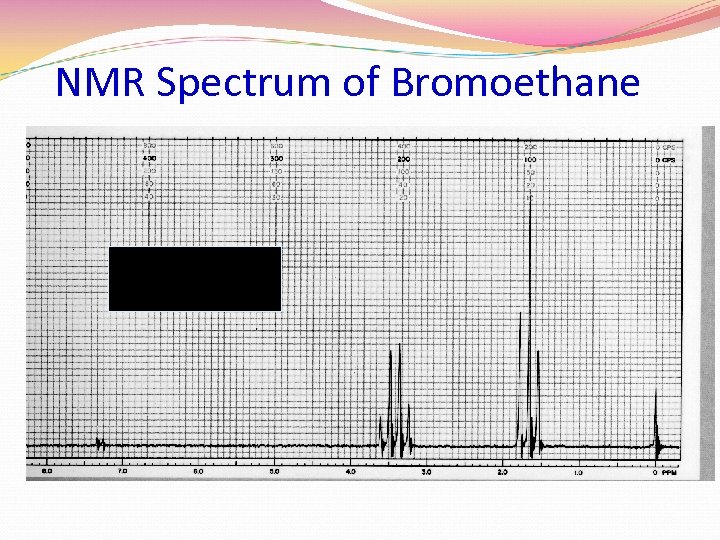
NMR Spectrum of Bromoethane
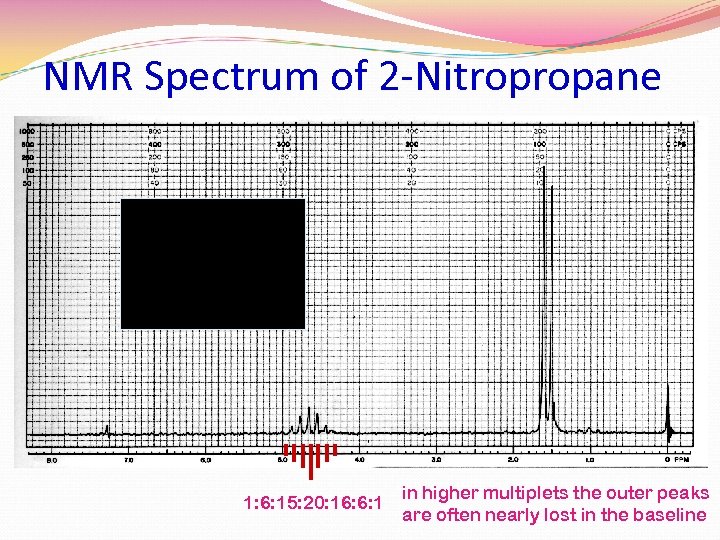
NMR Spectrum of 2 -Nitropropane 1: 6: 15: 20: 16: 6: 1 in higher multiplets the outer peaks are often nearly lost in the baseline
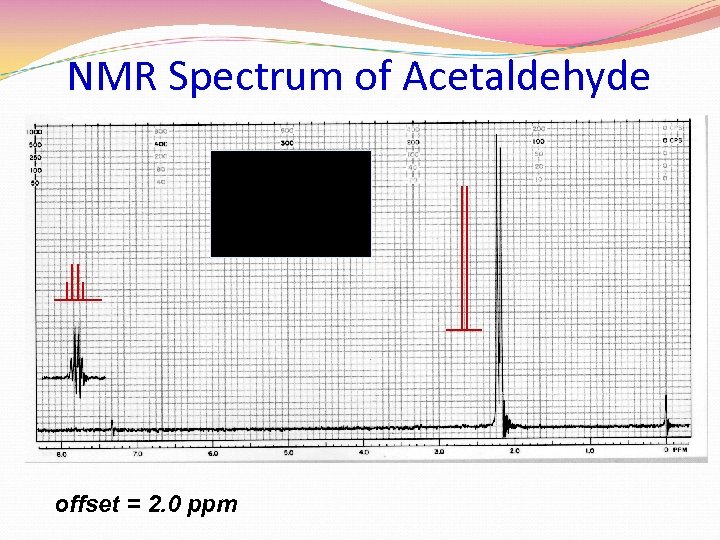
NMR Spectrum of Acetaldehyde offset = 2. 0 ppm
INTENSITIES OF MULTIPLET PEAKS PASCAL’S TRIANGLE
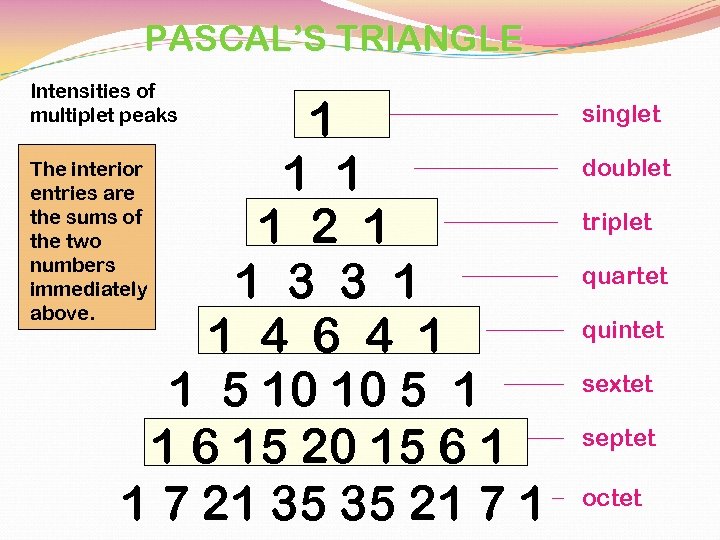
PASCAL’S TRIANGLE Intensities of multiplet peaks 1 The interior 1 1 entries are the sums of 1 2 1 the two numbers immediately 1 3 3 1 above. 1 4 6 4 1 1 5 10 10 5 1 1 6 15 20 15 6 1 1 7 21 35 35 21 7 1 singlet doublet triplet quartet quintet sextet septet octet

THE ORIGIN OF SPIN-SPIN SPLITTING HOW IT HAPPENS
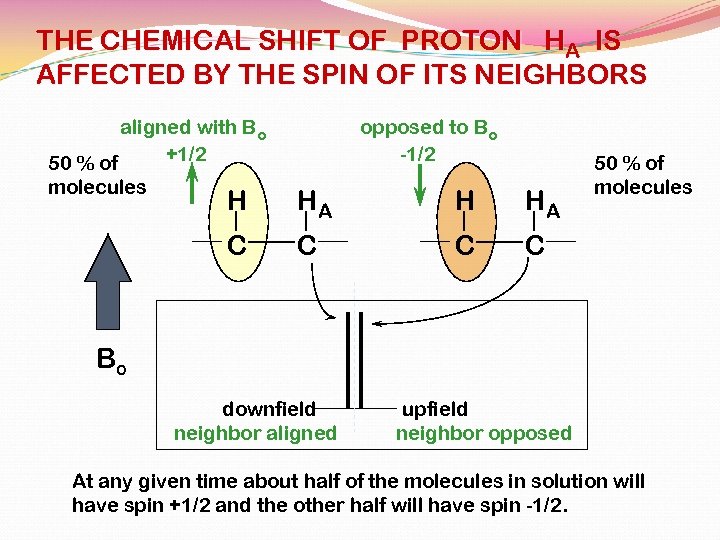
THE CHEMICAL SHIFT OF PROTON HA IS AFFECTED BY THE SPIN OF ITS NEIGHBORS aligned with Bo +1/2 50 % of molecules opposed to Bo -1/2 H HA C C C 50 % of molecules C Bo downfield neighbor aligned upfield neighbor opposed At any given time about half of the molecules in solution will have spin +1/2 and the other half will have spin -1/2.
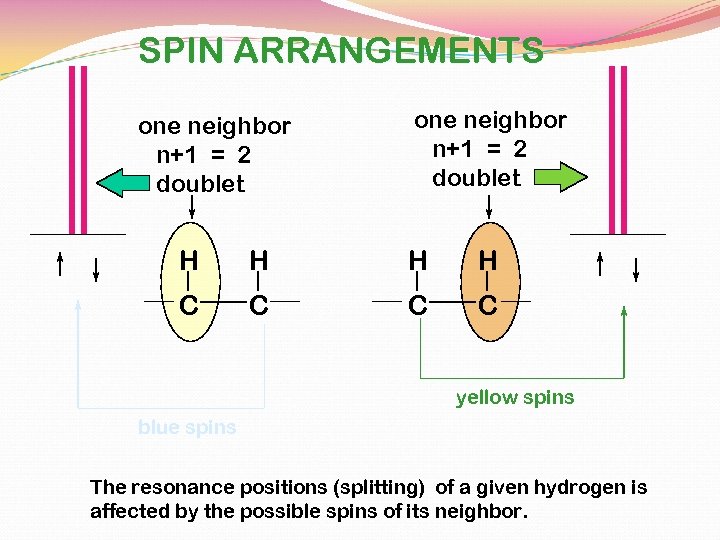
SPIN ARRANGEMENTS one neighbor n+1 = 2 doublet H H C C yellow spins blue spins The resonance positions (splitting) of a given hydrogen is affected by the possible spins of its neighbor.
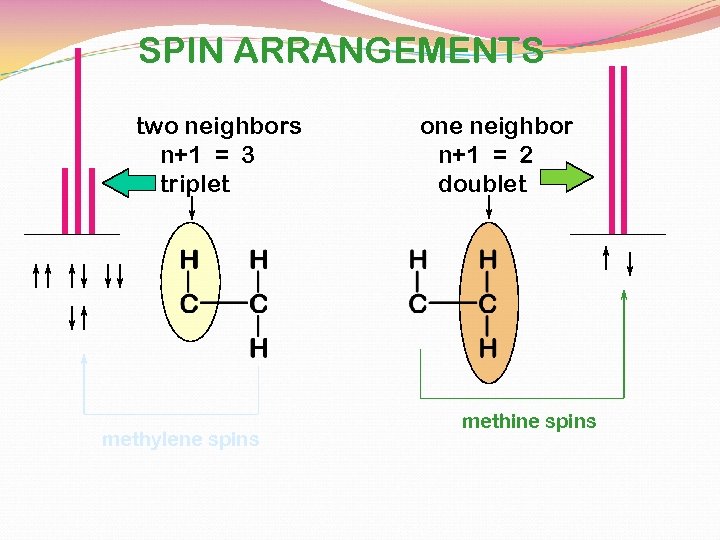
SPIN ARRANGEMENTS two neighbors n+1 = 3 triplet methylene spins one neighbor n+1 = 2 doublet methine spins
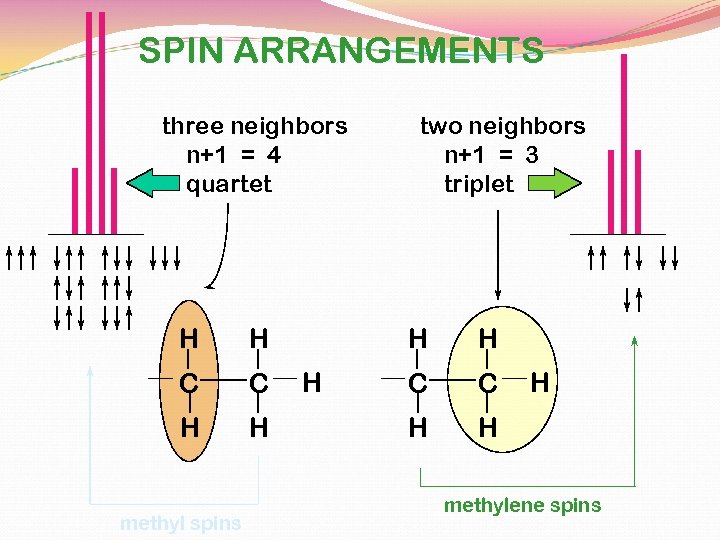
SPIN ARRANGEMENTS three neighbors n+1 = 4 quartet H H C C H H methyl spins two neighbors n+1 = 3 triplet H H H C C H H H methylene spins

THE COUPLING CONSTANT
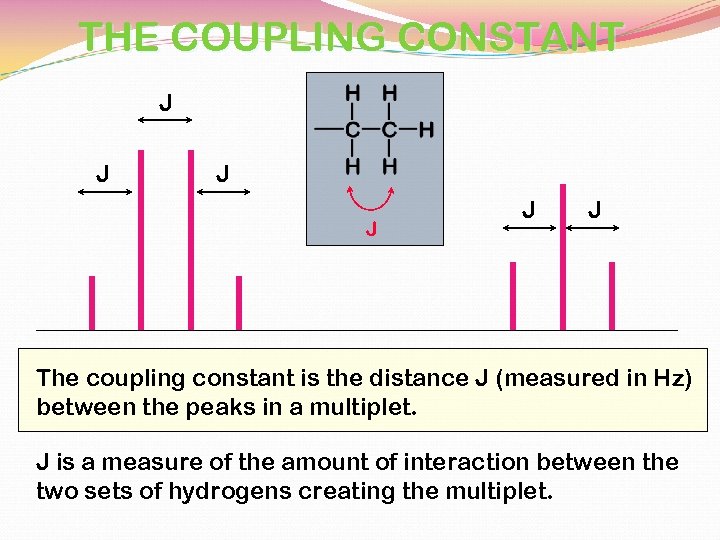
THE COUPLING CONSTANT J J J The coupling constant is the distance J (measured in Hz) between the peaks in a multiplet. J is a measure of the amount of interaction between the two sets of hydrogens creating the multiplet.
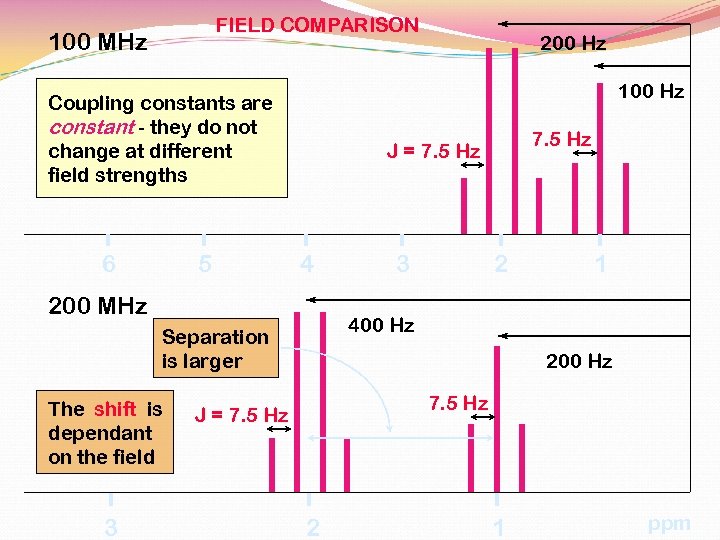
FIELD COMPARISON 100 MHz 100 Hz Coupling constants are constant - they do not change at different field strengths 6 5 4 3 2 1 400 Hz Separation is larger 3 7. 5 Hz J = 7. 5 Hz 200 MHz The shift is dependant on the field 200 Hz 7. 5 Hz J = 7. 5 Hz 2 1 ppm
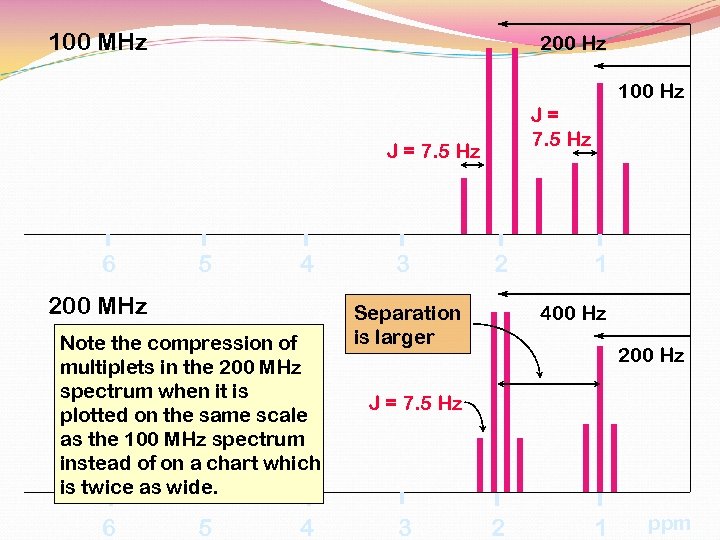
100 MHz 200 Hz 100 Hz J= 7. 5 Hz J = 7. 5 Hz 6 5 4 200 MHz Note the compression of multiplets in the 200 MHz spectrum when it is plotted on the same scale as the 100 MHz spectrum instead of on a chart which is twice as wide. 6 5 4 3 2 Separation is larger 1 400 Hz 200 Hz J = 7. 5 Hz 3 2 1 ppm
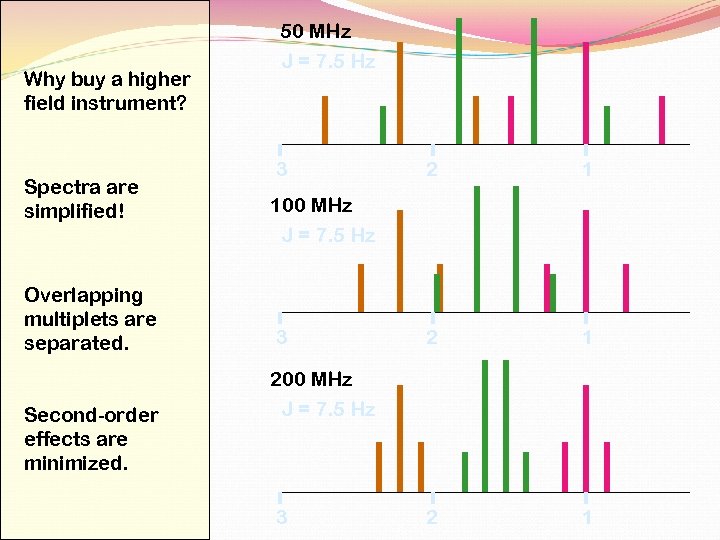
Why buy a higher field instrument? Spectra are simplified! Overlapping multiplets are separated. Second-order effects are minimized. 50 MHz J = 7. 5 Hz 3 2 1 2 1 100 MHz J = 7. 5 Hz 3 200 MHz J = 7. 5 Hz 3
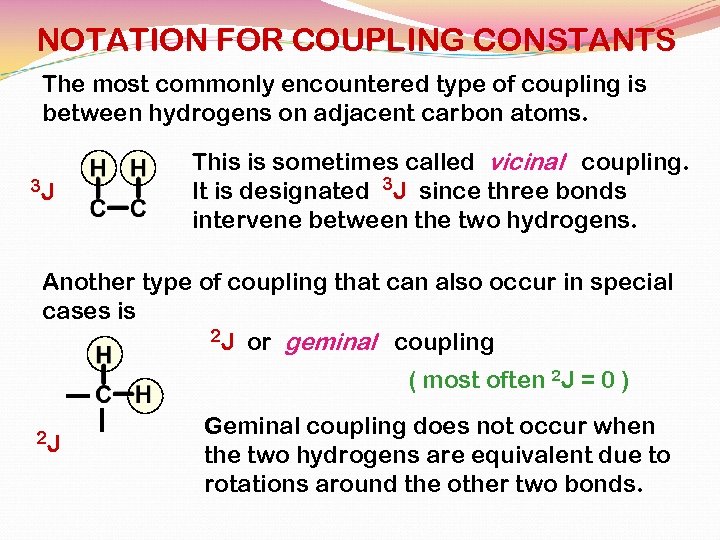
NOTATION FOR COUPLING CONSTANTS The most commonly encountered type of coupling is between hydrogens on adjacent carbon atoms. 3 J This is sometimes called vicinal coupling. It is designated 3 J since three bonds intervene between the two hydrogens. Another type of coupling that can also occur in special cases is 2 J or geminal coupling ( most often 2 J = 0 ) 2 J Geminal coupling does not occur when the two hydrogens are equivalent due to rotations around the other two bonds.
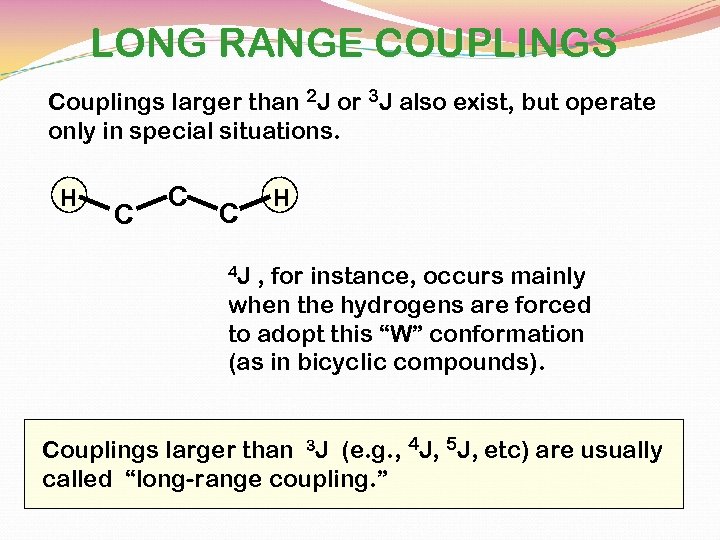
LONG RANGE COUPLINGS Couplings larger than 2 J or 3 J also exist, but operate only in special situations. H C C C H 4 J , for instance, occurs mainly when the hydrogens are forced to adopt this “W” conformation (as in bicyclic compounds). Couplings larger than 3 J (e. g. , 4 J, 5 J, etc) are usually called “long-range coupling. ”
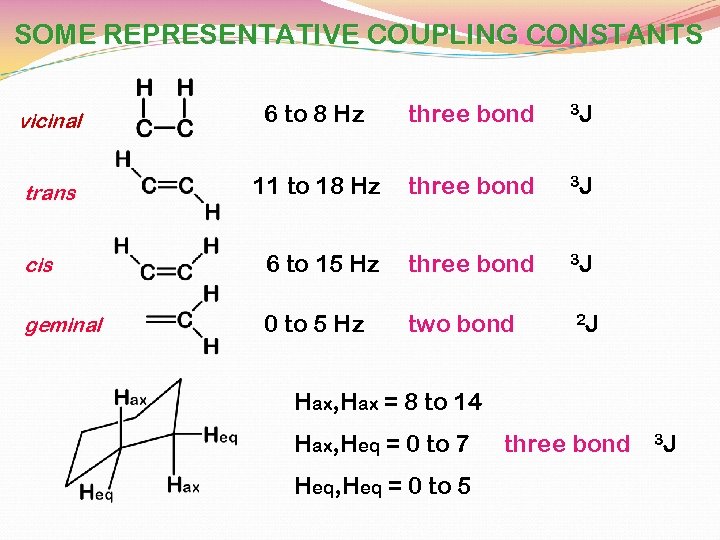
SOME REPRESENTATIVE COUPLING CONSTANTS vicinal 6 to 8 Hz three bond 3 J trans 11 to 18 Hz three bond 3 J cis 6 to 15 Hz three bond 3 J geminal 0 to 5 Hz two bond 2 J Hax, Hax = 8 to 14 Hax, Heq = 0 to 7 Heq, Heq = 0 to 5 three bond 3 J
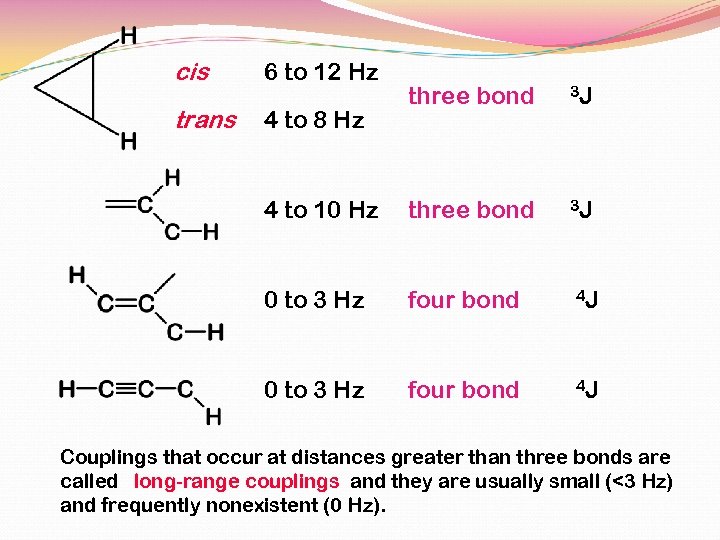
cis 6 to 12 Hz trans 4 to 8 Hz three bond 3 J 4 to 10 Hz three bond 3 J 0 to 3 Hz four bond 4 J Couplings that occur at distances greater than three bonds are called long-range couplings and they are usually small (<3 Hz) and frequently nonexistent (0 Hz).

OVERVIEW
TYPES OF INFORMATION FROM THE NMR SPECTRUM 1. Each different type of hydrogen gives a peak or group of peaks (multiplet). 2. The chemical shift (d, in ppm) gives a clue as to the type of hydrogen generating the peak (alkane, alkene, benzene, aldehyde, etc. ) 3. The integral gives the relative numbers of each type of hydrogen. 4. Spin-spin splitting gives the number of hydrogens on adjacent carbons. 5. The coupling constant J also gives information about the arrangement of the atoms involved.
SPECTROSCOPY IS A POWERFUL TOOL Generally, with only three pieces of data 1) empirical formula (or % composition) 2) infrared spectrum 3) NMR spectrum a chemist can often figure out the complete structure of an unknown molecule.
EACH TECHNIQUE YIELDS VALUABLE DATA FORMULA Gives the relative numbers of C and H and other atoms INFRARED SPECTRUM Reveals the types of bonds that are present. NMR SPECTRUM Reveals the enviroment of each hydrogen and the relative numbers of each type.

INTEGRATION
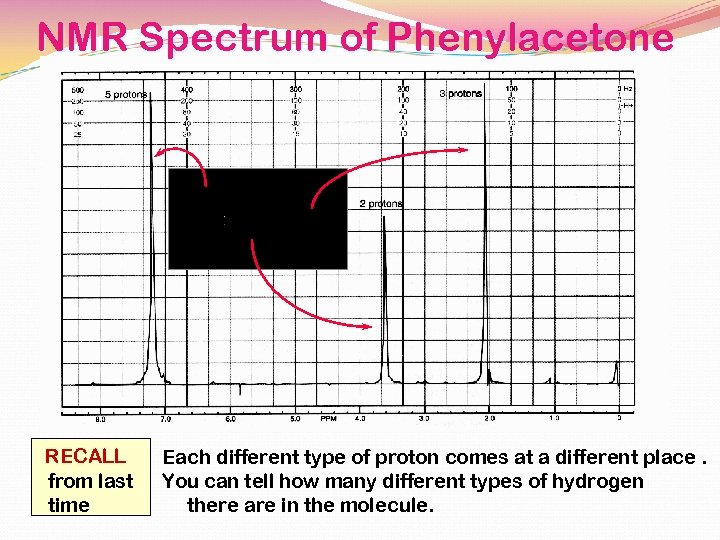
NMR Spectrum of Phenylacetone RECALL from last time Each different type of proton comes at a different place. You can tell how many different types of hydrogen there are in the molecule.

INTEGRATION OF A PEAK Not only does each different type of hydrogen give a distinct peak in the NMR spectrum, but we can also tell the relative numbers of each type of hydrogen by a process called integration. Integration = determination of the area under a peak The area under a peak is proportional to the number of hydrogens that generate the peak.
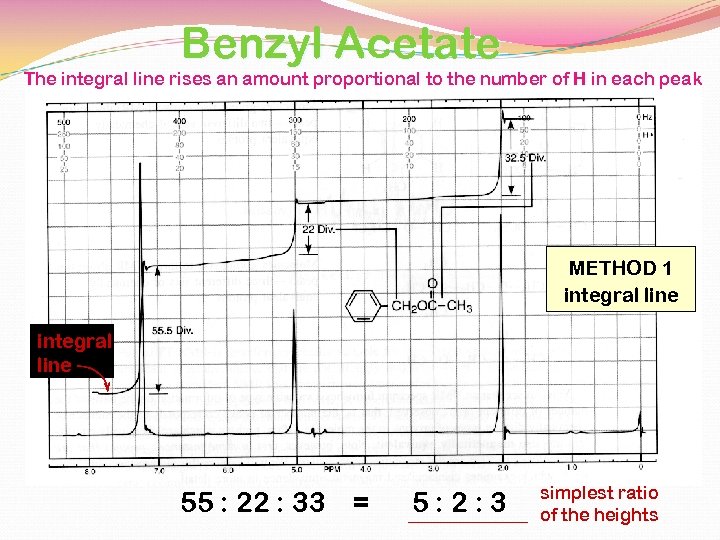
Benzyl Acetate The integral line rises an amount proportional to the number of H in each peak METHOD 1 integral line 55 : 22 : 33 = 5: 2: 3 simplest ratio of the heights
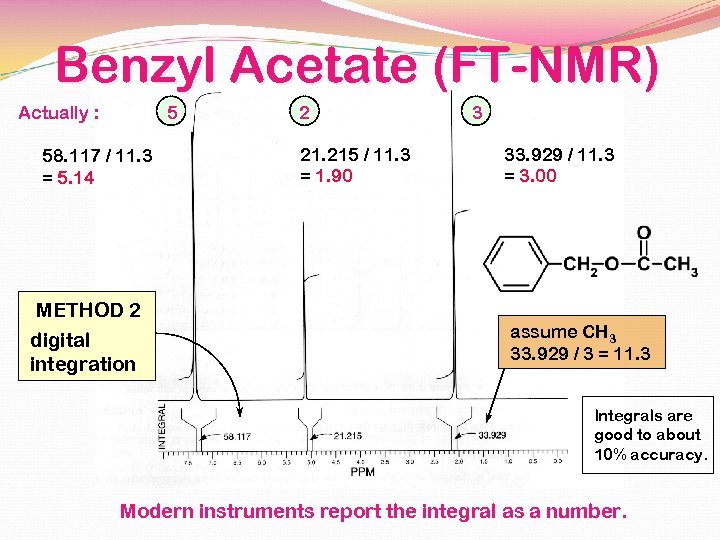
Benzyl Acetate (FT-NMR) Actually : 5 58. 117 / 11. 3 = 5. 14 METHOD 2 digital integration 2 21. 215 / 11. 3 = 1. 90 3 33. 929 / 11. 3 = 3. 00 assume CH 3 33. 929 / 3 = 11. 3 Integrals are good to about 10% accuracy. Modern instruments report the integral as a number.

DIAMAGNETIC ANISOTROPY SHIELDING BY VALENCE ELECTRONS
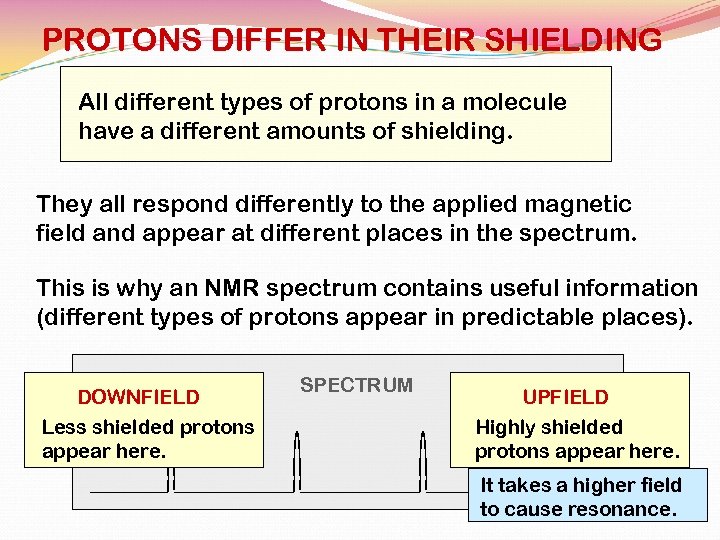
PROTONS DIFFER IN THEIR SHIELDING All different types of protons in a molecule have a different amounts of shielding. They all respond differently to the applied magnetic field and appear at different places in the spectrum. This is why an NMR spectrum contains useful information (different types of protons appear in predictable places). DOWNFIELD Less shielded protons appear here. SPECTRUM UPFIELD Highly shielded protons appear here. It takes a higher field to cause resonance.
CHEMICAL SHIFT
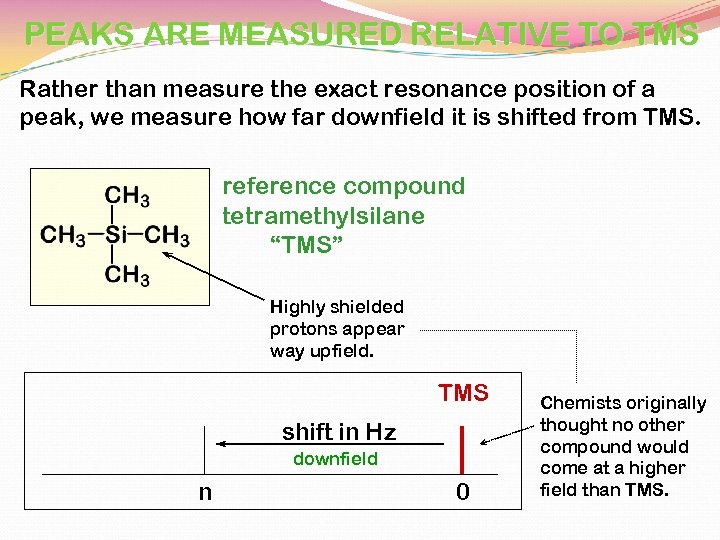
PEAKS ARE MEASURED RELATIVE TO TMS Rather than measure the exact resonance position of a peak, we measure how far downfield it is shifted from TMS. reference compound tetramethylsilane “TMS” Highly shielded protons appear way upfield. TMS shift in Hz downfield n 0 Chemists originally thought no other compound would come at a higher field than TMS.
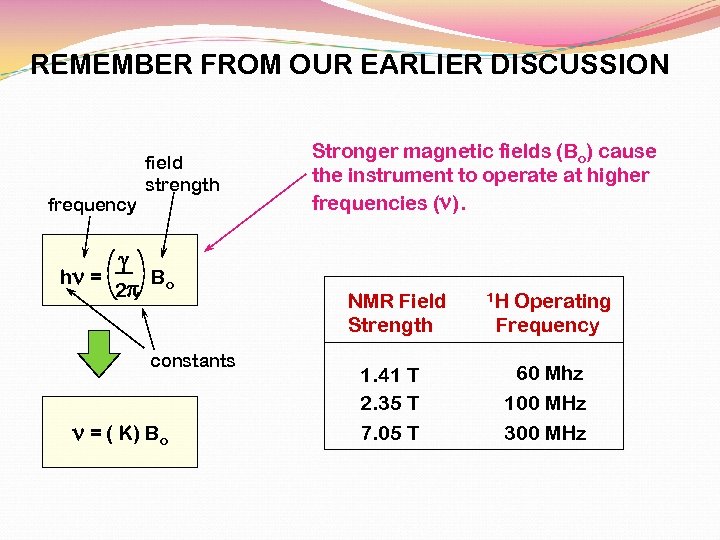
REMEMBER FROM OUR EARLIER DISCUSSION field strength frequency g hn = B 2 p o constants n = ( K) Bo Stronger magnetic fields (Bo) cause the instrument to operate at higher frequencies (n). NMR Field Strength 1. 41 T 2. 35 T 7. 05 T 1 H Operating Frequency 60 Mhz 100 MHz 300 MHz
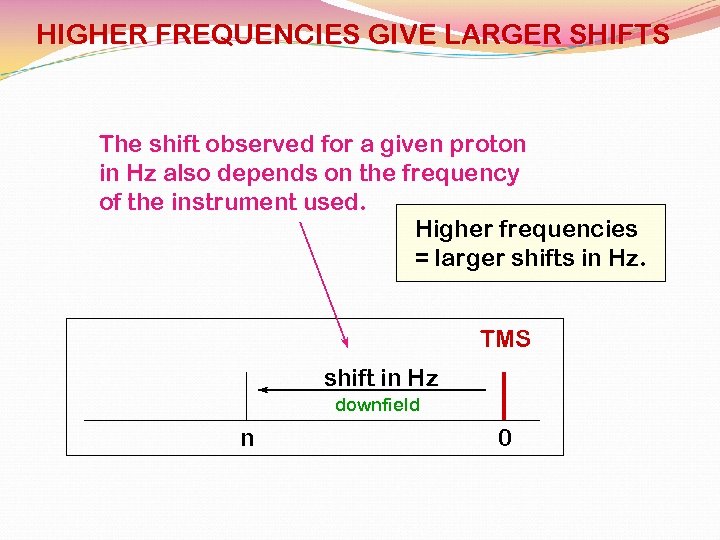
HIGHER FREQUENCIES GIVE LARGER SHIFTS The shift observed for a given proton in Hz also depends on the frequency of the instrument used. Higher frequencies = larger shifts in Hz. TMS shift in Hz downfield n 0
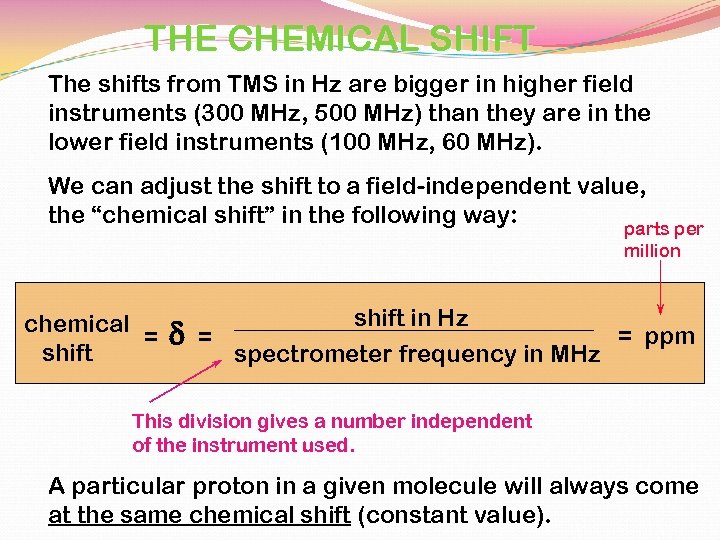
THE CHEMICAL SHIFT The shifts from TMS in Hz are bigger in higher field instruments (300 MHz, 500 MHz) than they are in the lower field instruments (100 MHz, 60 MHz). We can adjust the shift to a field-independent value, the “chemical shift” in the following way: parts per million chemical = shift d shift in Hz = ppm = spectrometer frequency in MHz This division gives a number independent of the instrument used. A particular proton in a given molecule will always come at the same chemical shift (constant value).
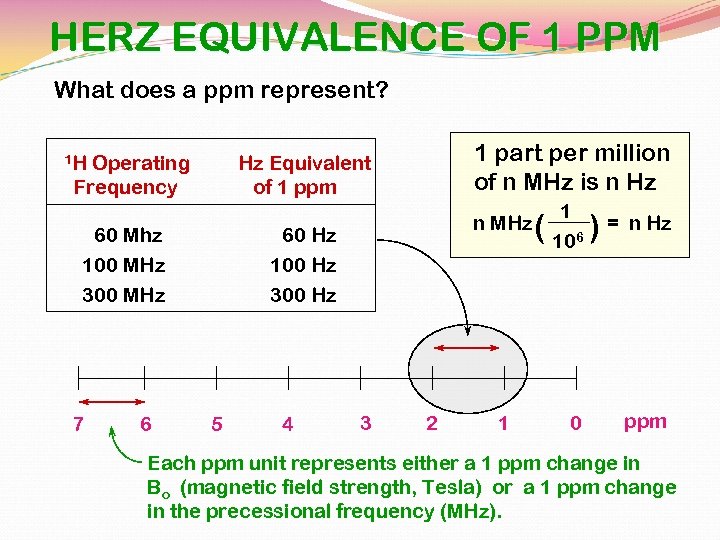
HERZ EQUIVALENCE OF 1 PPM What does a ppm represent? 1 H Operating Frequency 60 Mhz 100 MHz 300 MHz 7 6 1 part per million of n MHz is n Hz Hz Equivalent of 1 ppm n MHz 60 Hz 100 Hz 300 Hz 5 4 3 2 1 ( 1 106 0 )= n Hz ppm Each ppm unit represents either a 1 ppm change in Bo (magnetic field strength, Tesla) or a 1 ppm change in the precessional frequency (MHz).
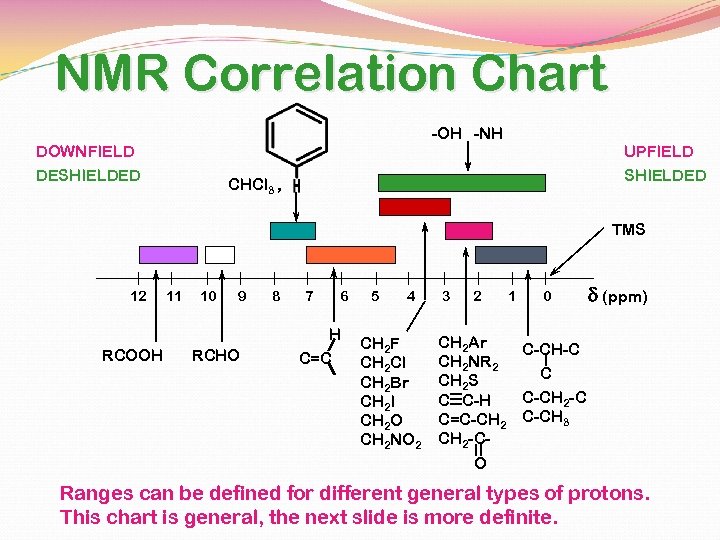
NMR Correlation Chart -OH -NH DOWNFIELD DESHIELDED UPFIELD SHIELDED CHCl 3 , TMS 12 RCOOH 11 10 9 RCHO 8 7 6 H C=C 5 4 CH 2 F CH 2 Cl CH 2 Br CH 2 I CH 2 O CH 2 NO 2 3 2 1 0 d (ppm) CH 2 Ar C-CH-C CH 2 NR 2 C CH 2 S C-CH 2 -C C C-H C=C-CH 2 C-CH 3 CH 2 -CO Ranges can be defined for different general types of protons. This chart is general, the next slide is more definite.
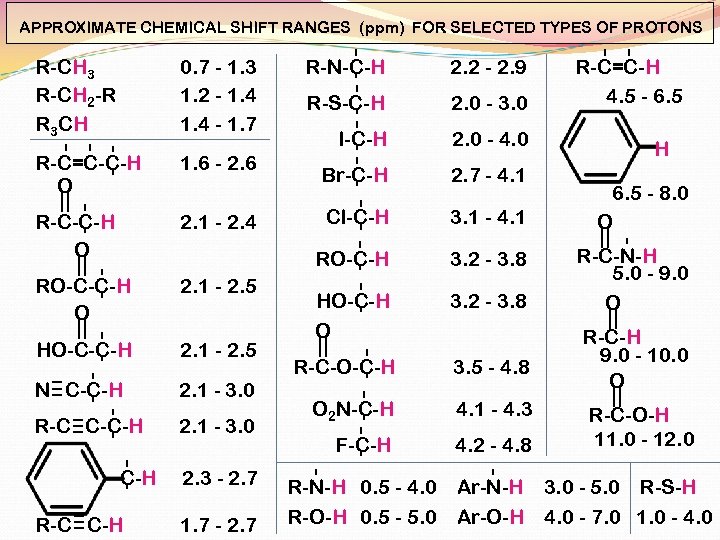
APPROXIMATE CHEMICAL SHIFT RANGES (ppm) FOR SELECTED TYPES OF PROTONS R-CH 3 R-CH 2 -R R 3 CH 0. 7 - 1. 3 1. 2 - 1. 4 - 1. 7 R-C=C-C-H O 1. 6 - 2. 6 R-C-C-H O 2. 1 - 2. 4 RO-C-C-H O 2. 1 - 2. 5 N C-C-H 2. 1 - 3. 0 R-C C-C-H 2. 1 - 3. 0 C-H R-C C-H 2. 3 - 2. 7 1. 7 - 2. 7 2. 2 - 2. 9 R-S-C-H 2. 0 - 3. 0 I-C-H 2. 0 - 4. 0 Br-C-H 2. 7 - 4. 1 Cl-C-H 3. 1 - 4. 1 RO-C-H HO-C-C-H R-N-C-H 3. 2 - 3. 8 HO-C-H O 3. 2 - 3. 8 R-C-O-C-H 3. 5 - 4. 8 O 2 N-C-H 4. 1 - 4. 3 F-C-H 4. 2 - 4. 8 R-N-H 0. 5 - 4. 0 R-O-H 0. 5 - 5. 0 R-C=C-H 4. 5 - 6. 5 H 6. 5 - 8. 0 O R-C-N-H 5. 0 - 9. 0 O R-C-H 9. 0 - 10. 0 O R-C-O-H 11. 0 - 12. 0 Ar-N-H 3. 0 - 5. 0 R-S-H Ar-O-H 4. 0 - 7. 0 1. 0 - 4. 0
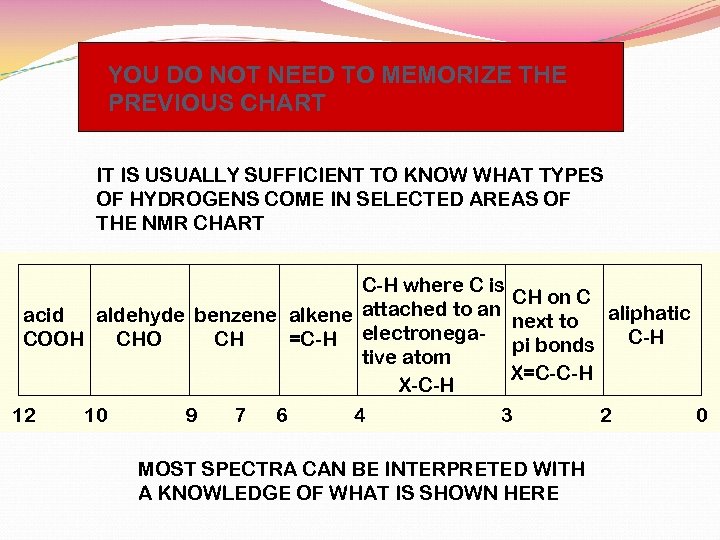
YOU DO NOT NEED TO MEMORIZE THE PREVIOUS CHART IT IS USUALLY SUFFICIENT TO KNOW WHAT TYPES OF HYDROGENS COME IN SELECTED AREAS OF THE NMR CHART C-H where C is CH on C attached to an aliphatic acid aldehyde benzene alkene next to C-H COOH CHO CH =C-H electronega- pi bonds tive atom X=C-C-H X-C-H 12 10 9 7 6 4 3 2 0 MOST SPECTRA CAN BE INTERPRETED WITH A KNOWLEDGE OF WHAT IS SHOWN HERE

DESHIELDING AND ANISOTROPY Three major factors account for the resonance positions (on the ppm scale) of most protons. 1. Deshielding by electronegative elements. 2. Anisotropic fields usually due to pi-bonded electrons in the molecule. 3. Deshielding due to hydrogen bonding. We will discuss these factors in the sections that follow.

DESHIELDING BY ELECTRONEGATIVE ELEMENTS
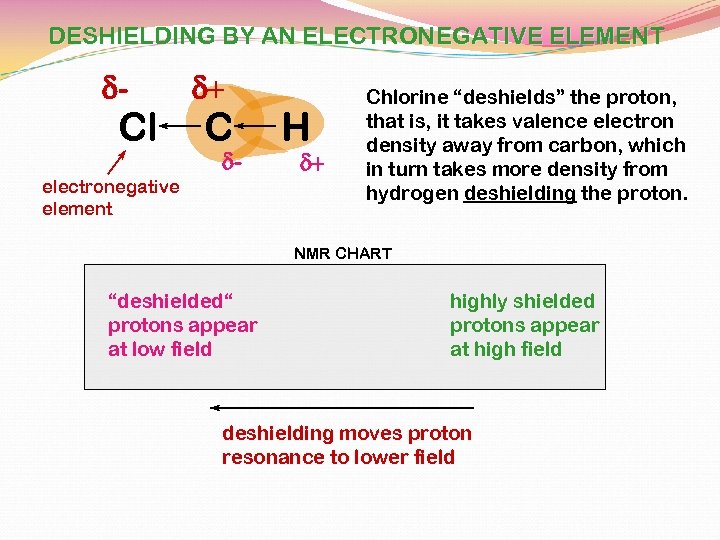
DESHIELDING BY AN ELECTRONEGATIVE ELEMENT d- Cl d+ C d- electronegative element H d+ Chlorine “deshields” the proton, that is, it takes valence electron density away from carbon, which in turn takes more density from hydrogen deshielding the proton. NMR CHART “deshielded“ protons appear at low field highly shielded protons appear at high field deshielding moves proton resonance to lower field
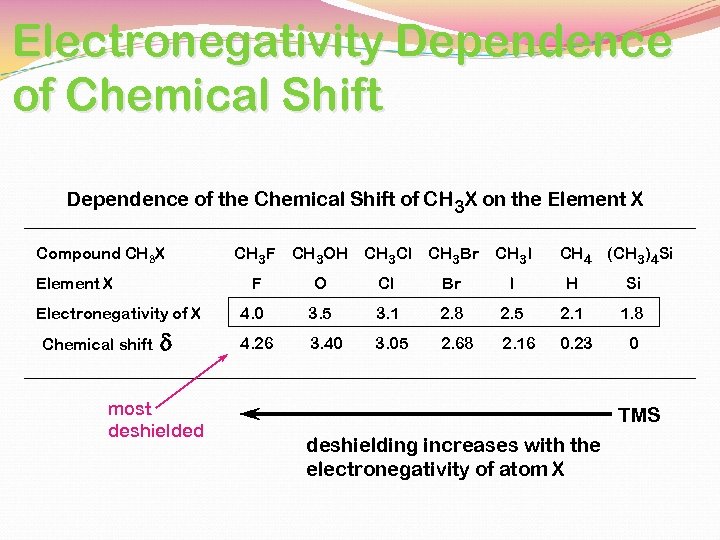
Electronegativity Dependence of Chemical Shift Dependence of the Chemical Shift of CH 3 X on the Element X Compound CH 3 X Electronegativity of X Chemical shift d most deshielded CH 3 OH CH 3 Cl F Element X CH 3 F CH 3 Br CH 3 I CH 4 (CH 3)4 Si O Cl Br I H Si 4. 0 3. 5 3. 1 2. 8 2. 5 2. 1 1. 8 4. 26 3. 40 3. 05 2. 68 2. 16 0. 23 0 TMS deshielding increases with the electronegativity of atom X
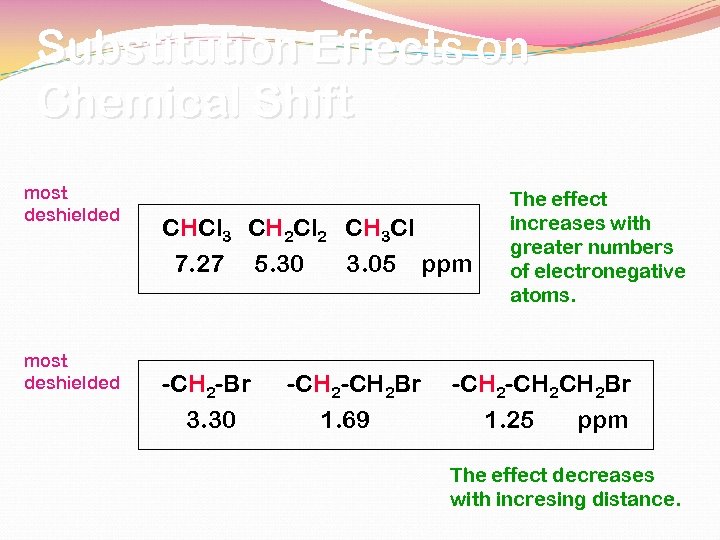
Substitution Effects on Chemical Shift most deshielded CHCl 3 CH 2 Cl 2 CH 3 Cl 7. 27 5. 30 3. 05 ppm -CH 2 -Br 3. 30 -CH 2 Br 1. 69 The effect increases with greater numbers of electronegative atoms. -CH 2 CH 2 Br 1. 25 ppm The effect decreases with incresing distance.

ANISOTROPIC FIELDS DUE TO THE PRESENCE OF PI BONDS The presence of a nearby pi bond or pi system greatly affects the chemical shift. Benzene rings have the greatest effect.

fields add together
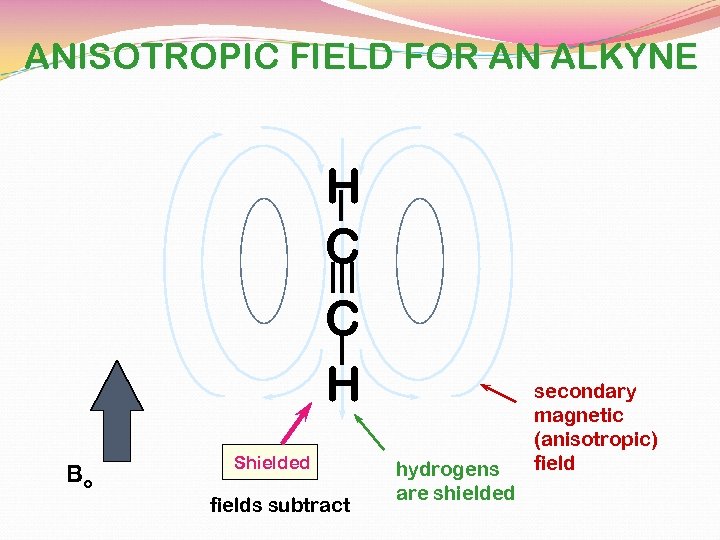
ANISOTROPIC FIELD FOR AN ALKYNE H C C H Bo Shielded fields subtract hydrogens are shielded secondary magnetic (anisotropic) field

HYDROGEN BONDING
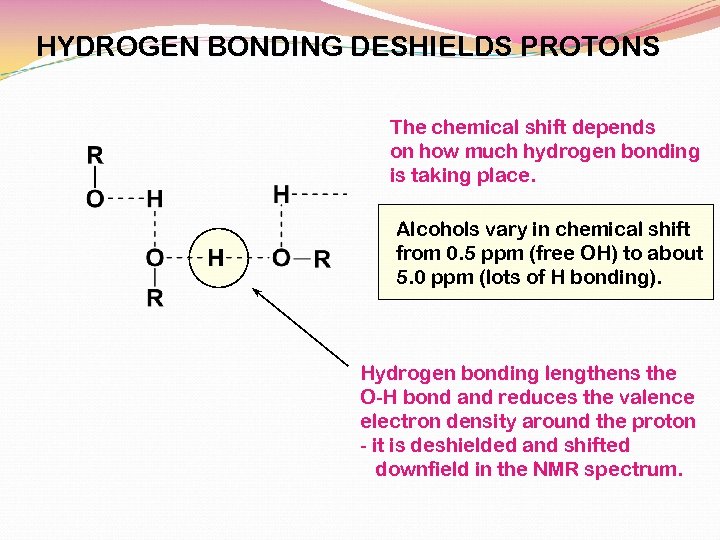
HYDROGEN BONDING DESHIELDS PROTONS The chemical shift depends on how much hydrogen bonding is taking place. Alcohols vary in chemical shift from 0. 5 ppm (free OH) to about 5. 0 ppm (lots of H bonding). Hydrogen bonding lengthens the O-H bond and reduces the valence electron density around the proton - it is deshielded and shifted downfield in the NMR spectrum.
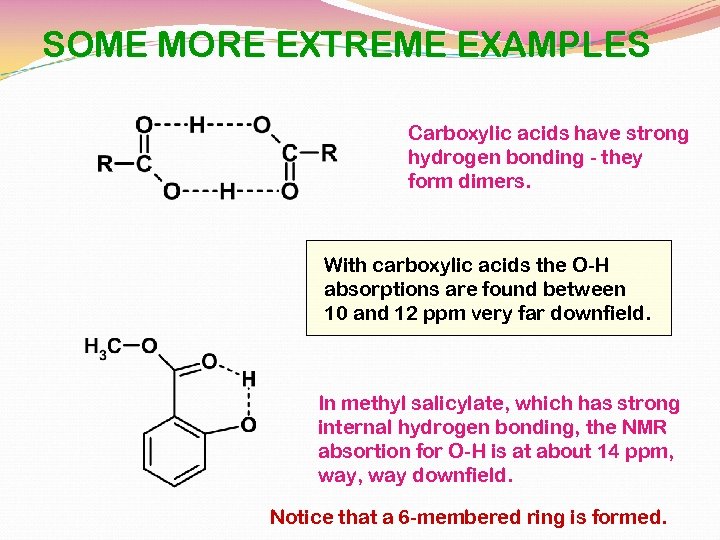
SOME MORE EXTREME EXAMPLES Carboxylic acids have strong hydrogen bonding - they form dimers. With carboxylic acids the O-H absorptions are found between 10 and 12 ppm very far downfield. In methyl salicylate, which has strong internal hydrogen bonding, the NMR absortion for O-H is at about 14 ppm, way downfield. Notice that a 6 -membered ring is formed.
UNEQUAL COUPLING TREE DIAGRAMS SPLITTING DIAGRAMS aka “TREE” DIAGRAMS
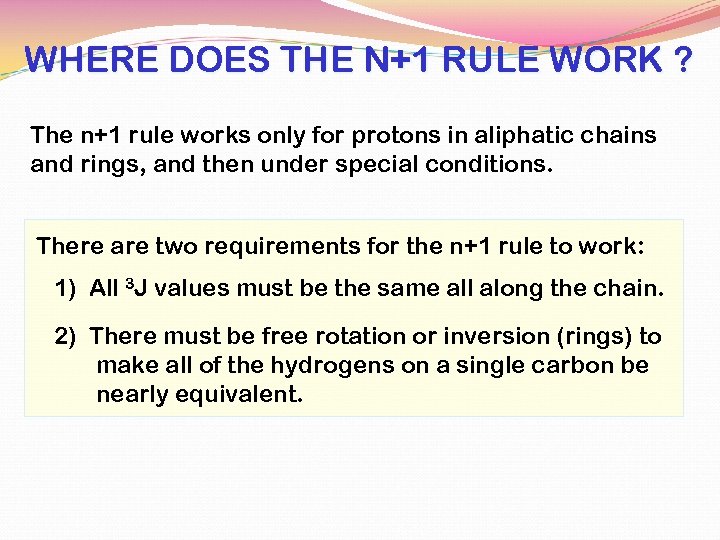
WHERE DOES THE N+1 RULE WORK ? The n+1 rule works only for protons in aliphatic chains and rings, and then under special conditions. There are two requirements for the n+1 rule to work: 1) All 3 J values must be the same all along the chain. 2) There must be free rotation or inversion (rings) to make all of the hydrogens on a single carbon be nearly equivalent.
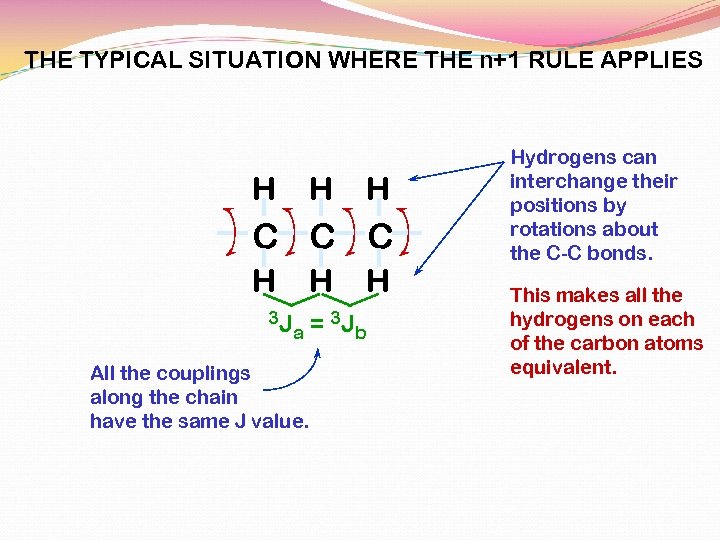
THE TYPICAL SITUATION WHERE THE n+1 RULE APPLIES H H H C C C H 3 J H a All the couplings along the chain have the same J value. H = 3 J b Hydrogens can interchange their positions by rotations about the C-C bonds. This makes all the hydrogens on each of the carbon atoms equivalent.
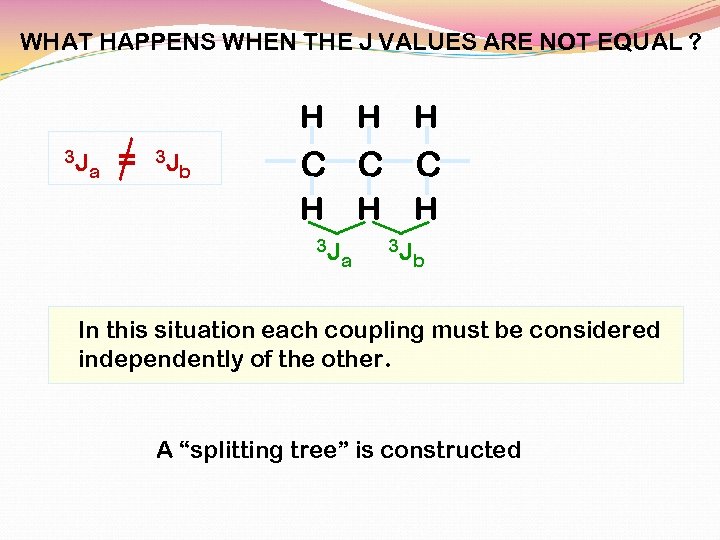
WHAT HAPPENS WHEN THE J VALUES ARE NOT EQUAL ? H 3 J a = 3 J b H H C C C H 3 J H a H 3 J b In this situation each coupling must be considered independently of the other. A “splitting tree” is constructed
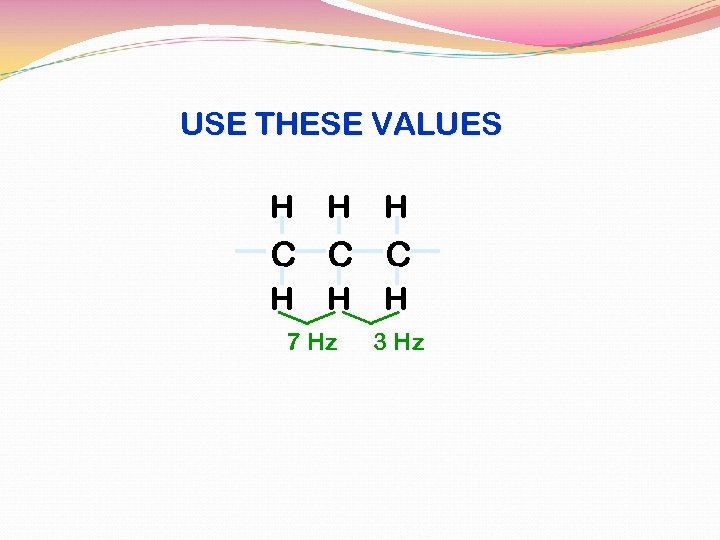
USE THESE VALUES H H H C C C H H 7 Hz H 3 Hz
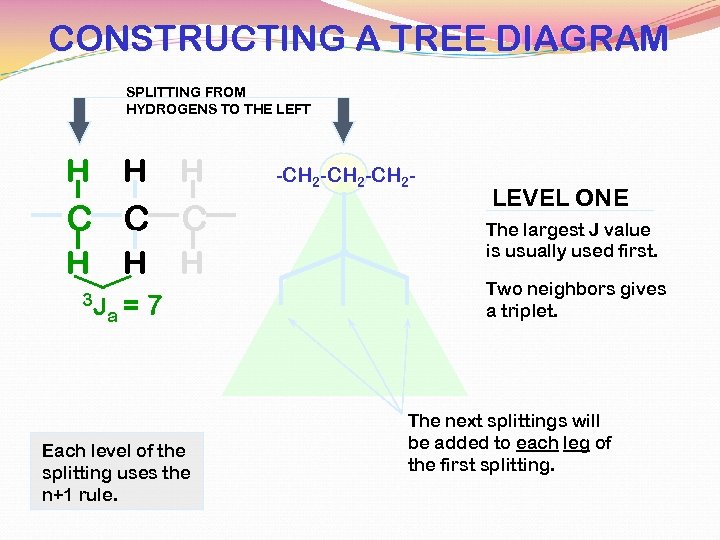
CONSTRUCTING A TREE DIAGRAM SPLITTING FROM HYDROGENS TO THE LEFT H H H C C C H 3 J H a= H 7 Each level of the splitting uses the n+1 rule. -CH 2 -CH 2 - LEVEL ONE The largest J value is usually used first. Two neighbors gives a triplet. The next splittings will be added to each leg of the first splitting.
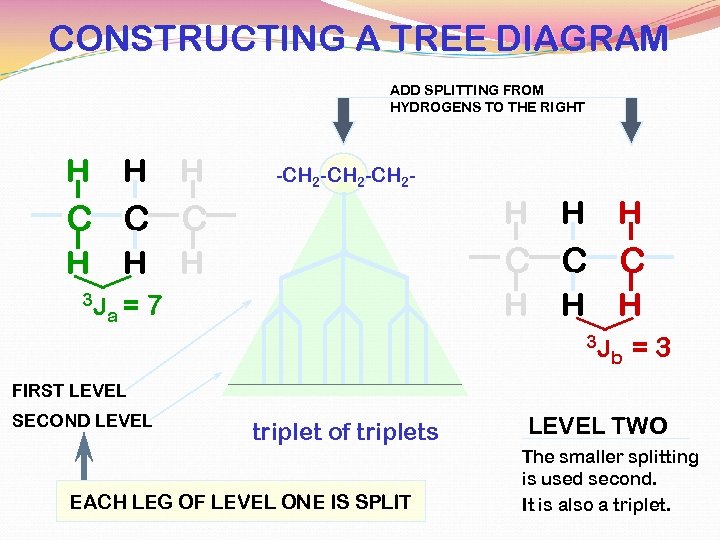
CONSTRUCTING A TREE DIAGRAM ADD SPLITTING FROM HYDROGENS TO THE RIGHT H H H -CH 2 -CH 2 - C C C H H C C C 3 J H a= H H 7 H H 3 J b =3 FIRST LEVEL SECOND LEVEL triplet of triplets EACH LEG OF LEVEL ONE IS SPLIT LEVEL TWO The smaller splitting is used second. It is also a triplet.
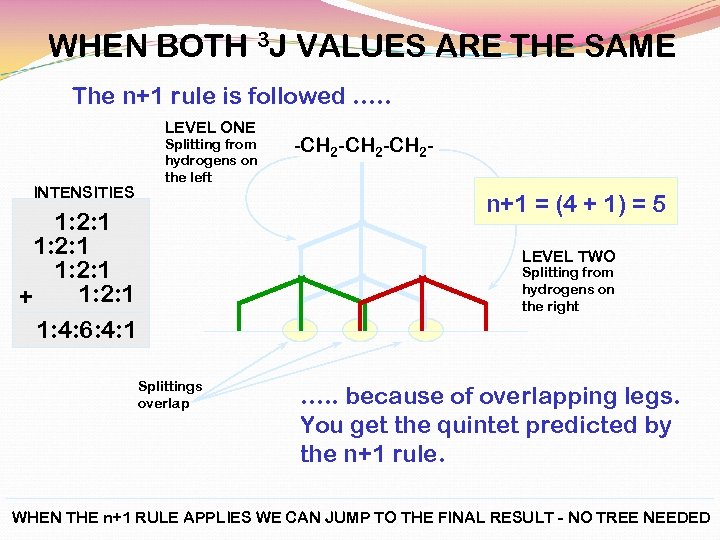
WHEN BOTH 3 J VALUES ARE THE SAME The n+1 rule is followed …. . LEVEL ONE INTENSITIES Splitting from hydrogens on the left -CH 2 -CH 2 - n+1 = (4 + 1) = 5 1: 2: 1 + 1: 4: 6: 4: 1 LEVEL TWO Splitting from hydrogens on the right Splittings overlap …. . because of overlapping legs. You get the quintet predicted by the n+1 rule. WHEN THE n+1 RULE APPLIES WE CAN JUMP TO THE FINAL RESULT - NO TREE NEEDED

2 -PHENYLPROPANAL A case where there are unequal J values.

PURE ETHANOL
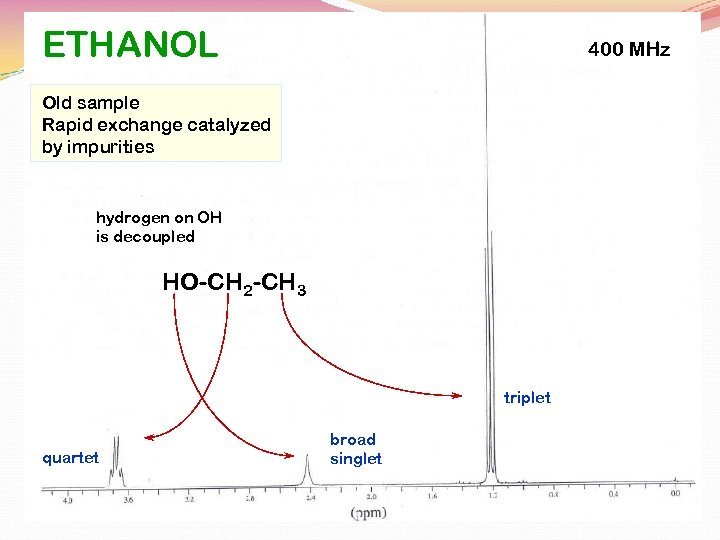
ETHANOL 400 MHz Old sample Rapid exchange catalyzed by impurities hydrogen on OH is decoupled HO-CH 2 -CH 3 triplet quartet broad singlet
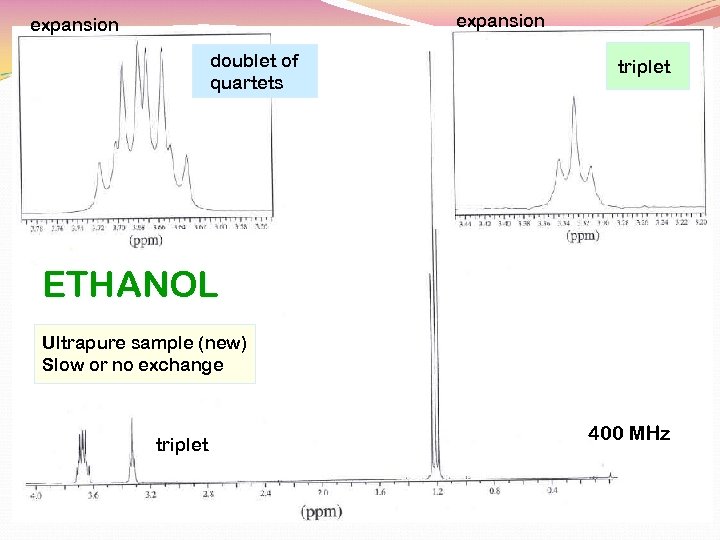
expansion doublet of quartets triplet ETHANOL Ultrapure sample (new) Slow or no exchange triplet 400 MHz
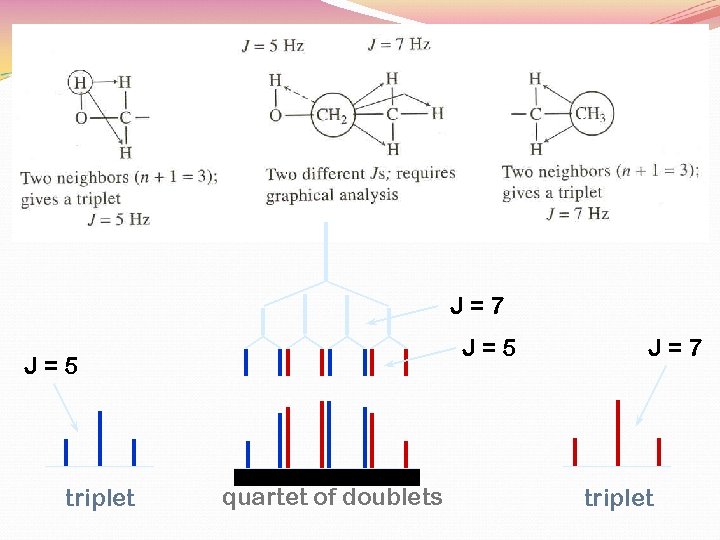
J=7 J=5 triplet quartet of doublets J=7 triplet

VINYL ACETATE ALKENE HYDROGENS
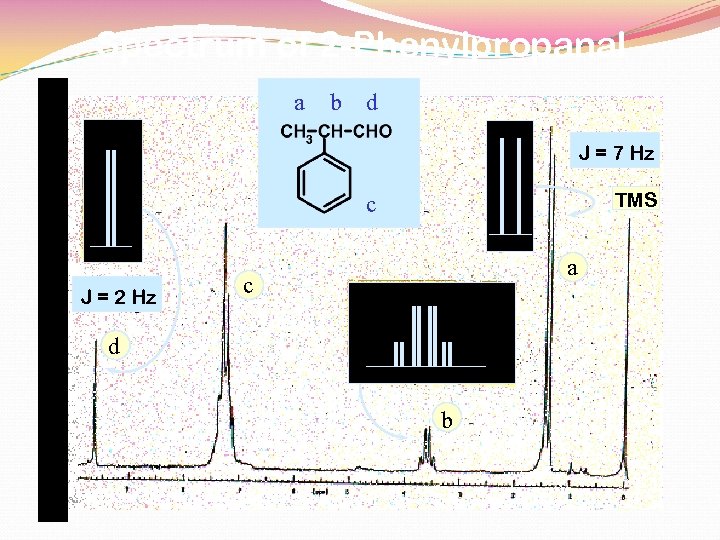
Spectrum of 2 -Phenylpropanal a b d J = 7 Hz TMS c J = 2 Hz a c d b
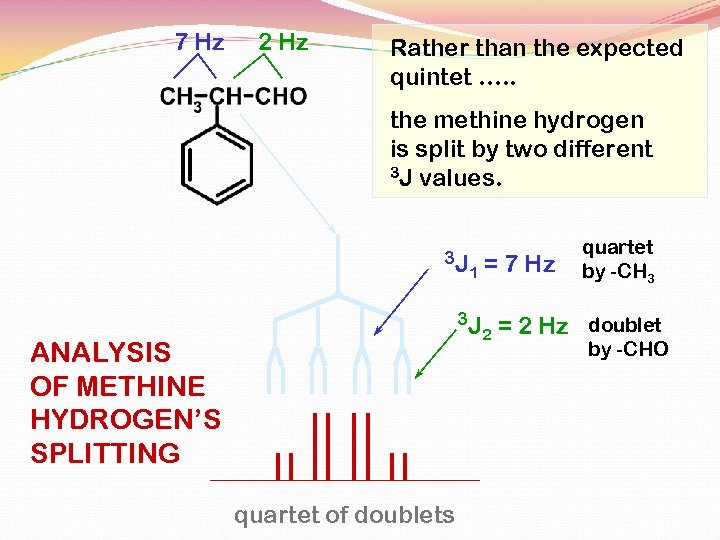
7 Hz 2 Hz Rather than the expected quintet …. . the methine hydrogen is split by two different 3 J values. 3 J 1 3 J ANALYSIS OF METHINE HYDROGEN’S SPLITTING quartet of doublets = 7 Hz 2 quartet by -CH 3 = 2 Hz doublet by -CHO

PURE ETHANOL
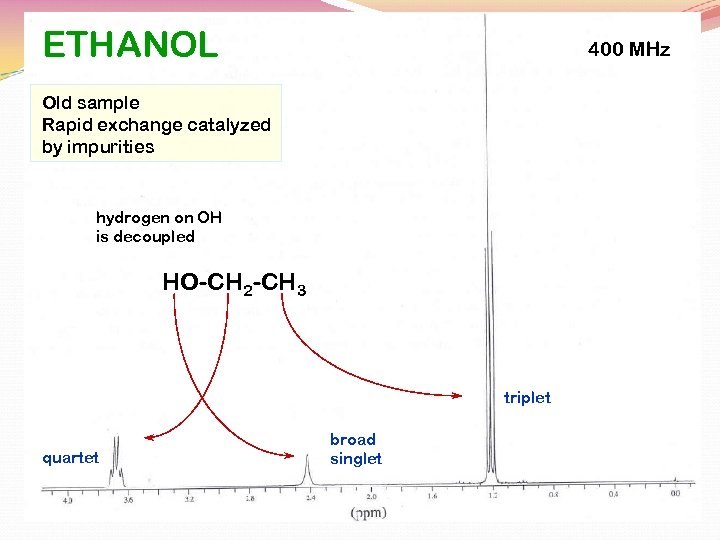
ETHANOL 400 MHz Old sample Rapid exchange catalyzed by impurities hydrogen on OH is decoupled HO-CH 2 -CH 3 triplet quartet broad singlet
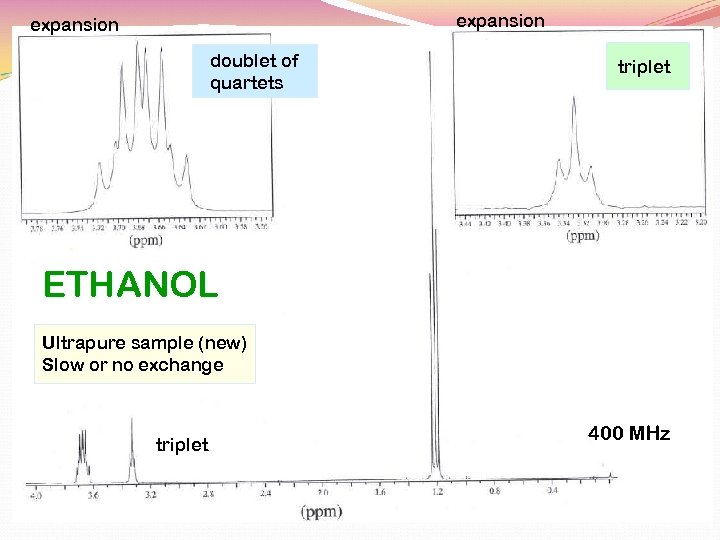
expansion doublet of quartets triplet ETHANOL Ultrapure sample (new) Slow or no exchange triplet 400 MHz
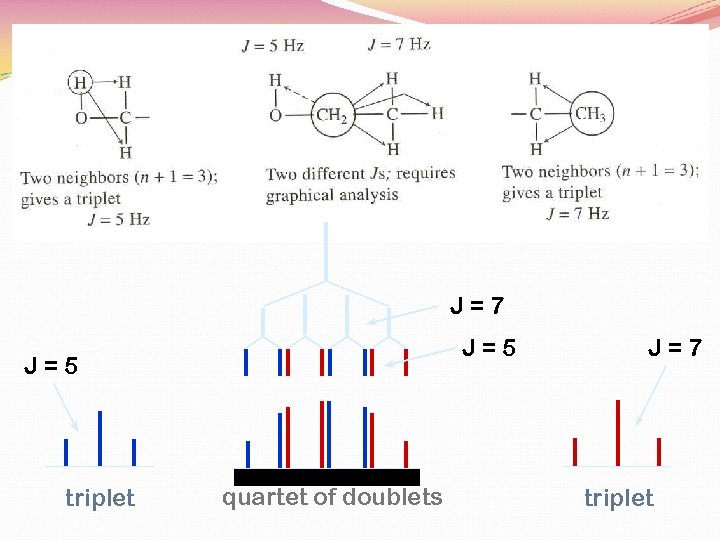
J=7 J=5 triplet quartet of doublets J=7 triplet

VINYL ACETATE ALKENE HYDROGENS
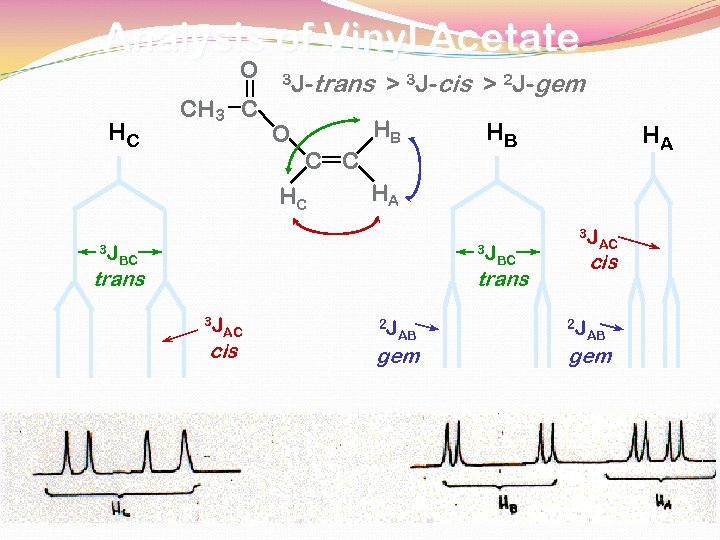
Analysis of Vinyl Acetate O HC CH 3 C 3 J- O HB C C HC 3 J trans > 3 J-cis > 2 J-gem HB HA HA 3 J BC trans 3 J cis BC trans 3 J AC cis 2 J AB gem AC 2 J AB gem
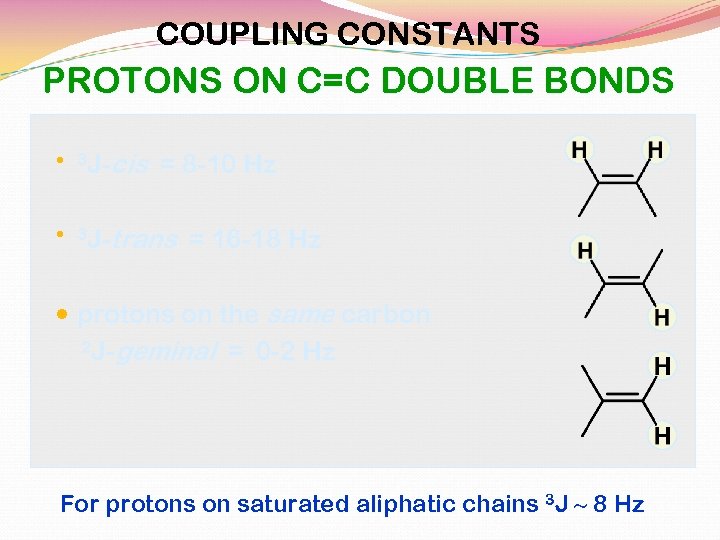
COUPLING CONSTANTS PROTONS ON C=C DOUBLE BONDS 3 J- cis = 8 -10 Hz 3 J- trans = 16 -18 Hz protons on the same carbon 2 J-geminal = 0 -2 Hz For protons on saturated aliphatic chains 3 J ~ 8 Hz
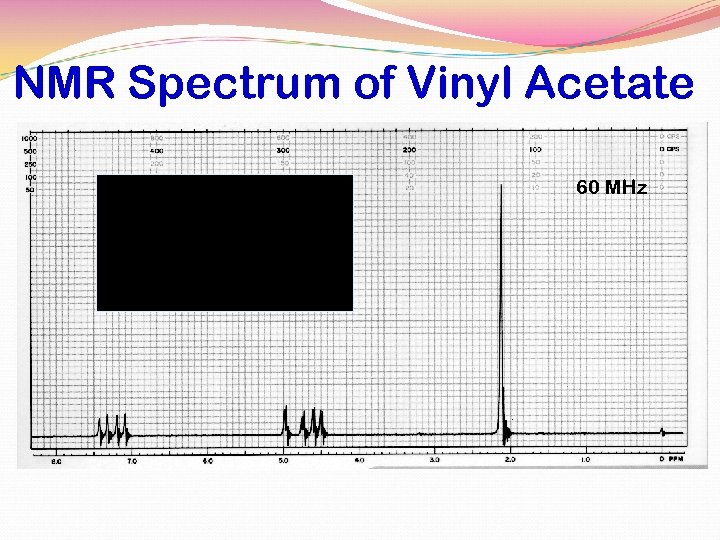
NMR Spectrum of Vinyl Acetate 60 MHz
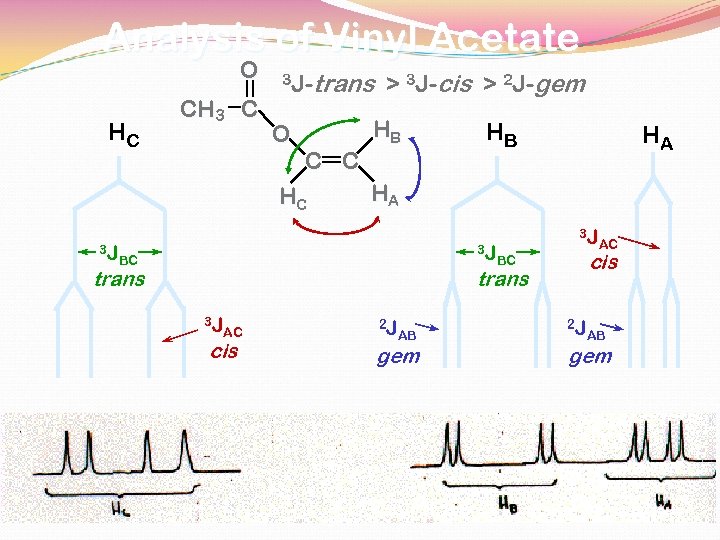
Analysis of Vinyl Acetate O HC CH 3 C 3 J- O HB C C HC 3 J trans > 3 J-cis > 2 J-gem HB HA HA 3 J BC trans 3 J cis BC trans 3 J AC cis 2 J AB gem AC 2 J AB gem

2, 4 -DINITROANISOLE BENZENE HYDROGENS
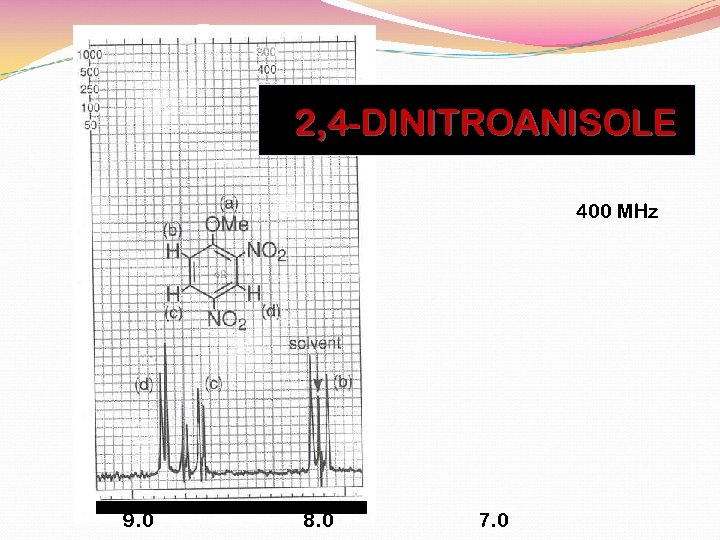
2, 4 -DINITROANISOLE 400 MHz 9. 0 8. 0 7. 0
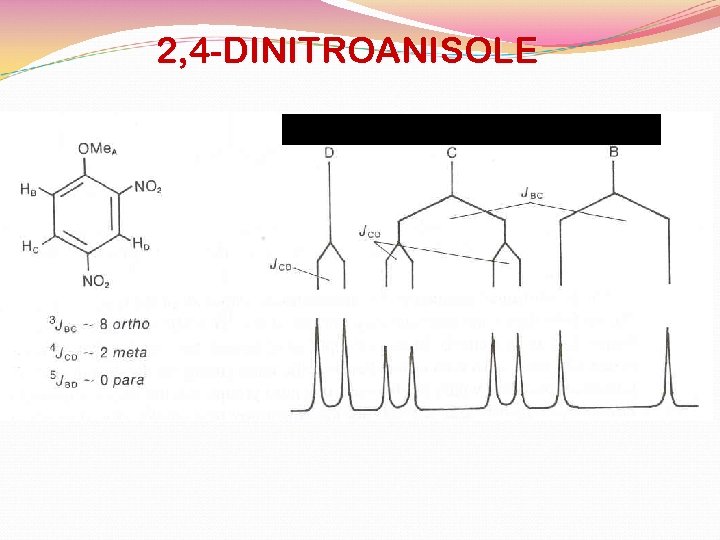
2, 4 -DINITROANISOLE 8. 72 ppm 8. 43 ppm

AROMATIC RINGS

BENZENE RING HYDROGENS Ring current causes protons attached to the ring to appear in the range of 7 to 8 ppm. An anisotropy diagram (next slide) shows the origin of the effect. Protons in a methyl or methylene group attached to the ring appear in the range of 2 to 2. 5 ppm.


MONOSUBSTITUTED RINGS
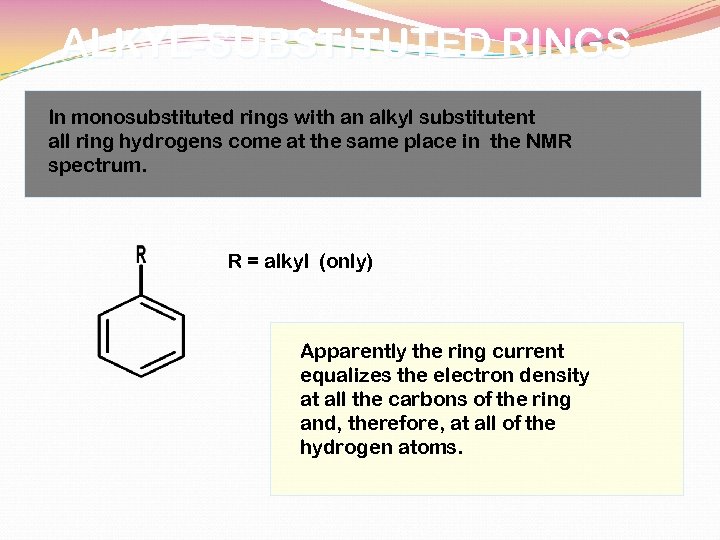
ALKYL-SUBSTITUTED RINGS In monosubstituted rings with an alkyl substitutent all ring hydrogens come at the same place in the NMR spectrum. R = alkyl (only) Apparently the ring current equalizes the electron density at all the carbons of the ring and, therefore, at all of the hydrogen atoms.
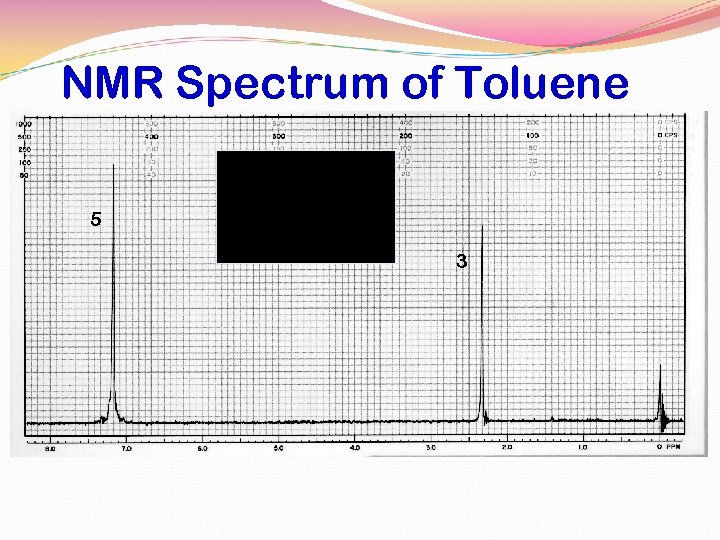
NMR Spectrum of Toluene 5 3
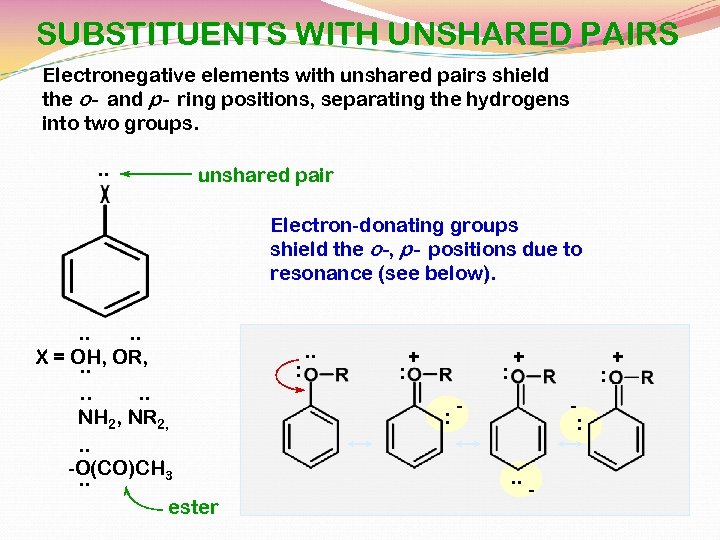
SUBSTITUENTS WITH UNSHARED PAIRS Electronegative elements with unshared pairs shield the o- and p- ring positions, separating the hydrogens into two groups. . . unshared pair Electron-donating groups shield the o-, p- positions due to resonance (see below). . . X = OH, OR, . . . NH 2, NR 2, . . -O(CO)CH 3. . ester : . . : + : - : . . - +
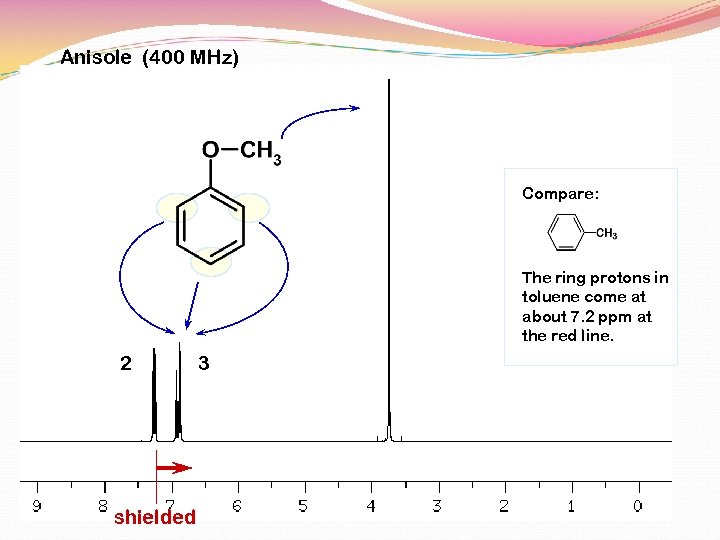
Anisole (400 MHz) Compare: The ring protons in toluene come at about 7. 2 ppm at the red line. 2 shielded 3
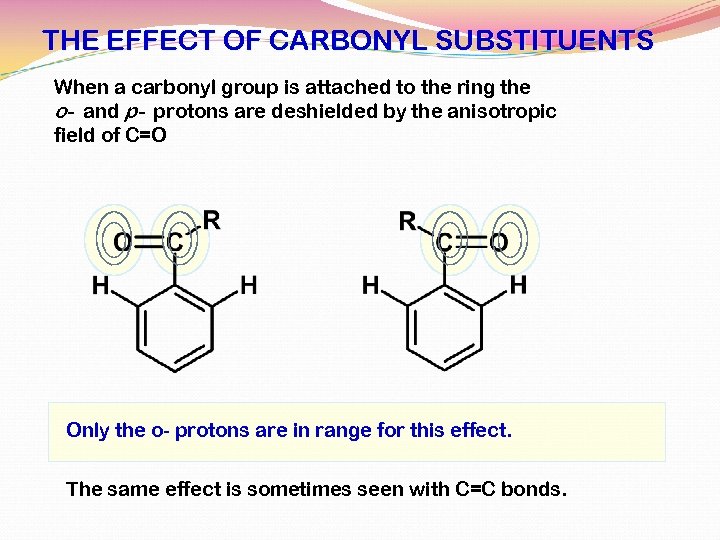
THE EFFECT OF CARBONYL SUBSTITUENTS When a carbonyl group is attached to the ring the o- and p- protons are deshielded by the anisotropic field of C=O Only the o- protons are in range for this effect. The same effect is sometimes seen with C=C bonds.
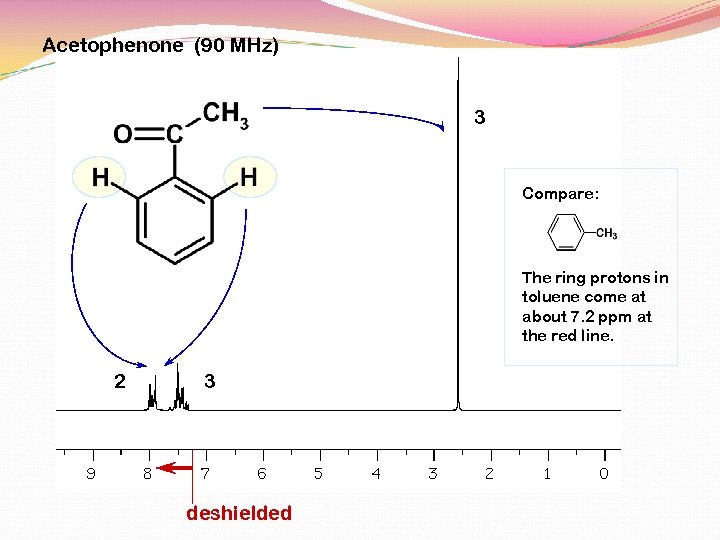
Acetophenone (90 MHz) 3 Compare: The ring protons in toluene come at about 7. 2 ppm at the red line. 2 3 deshielded

para -DISUBSTITUTED RINGS

para-Disubstitution 1, 4 -Disubstituted benzene rings will show a pair of doublets, when the two groups on the ring are very different an example: 1 -iodo-4 -methoxybenzene
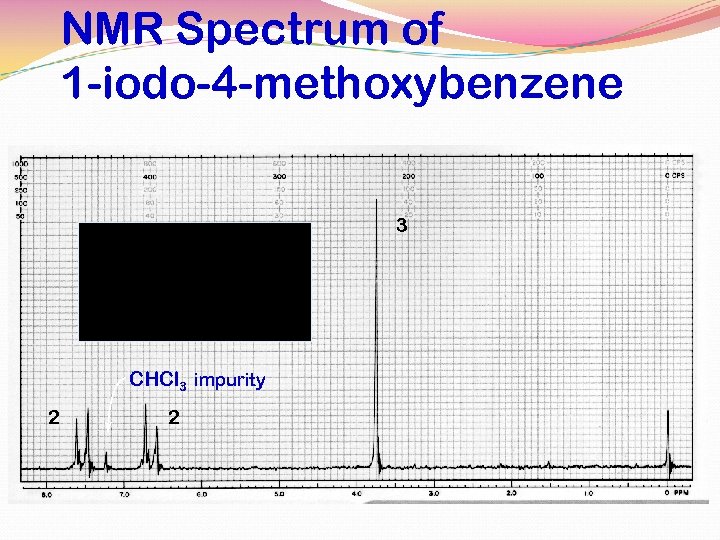
NMR Spectrum of 1 -iodo-4 -methoxybenzene 3 CHCl 3 impurity 2 2
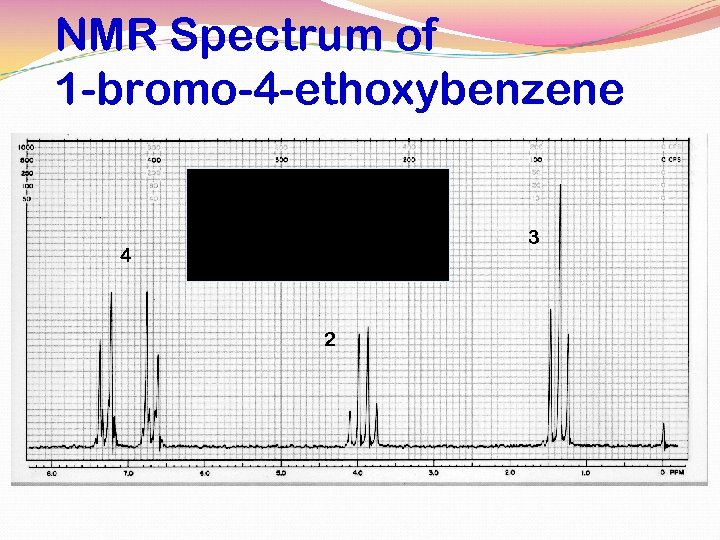
NMR Spectrum of 1 -bromo-4 -ethoxybenzene 3 4 2
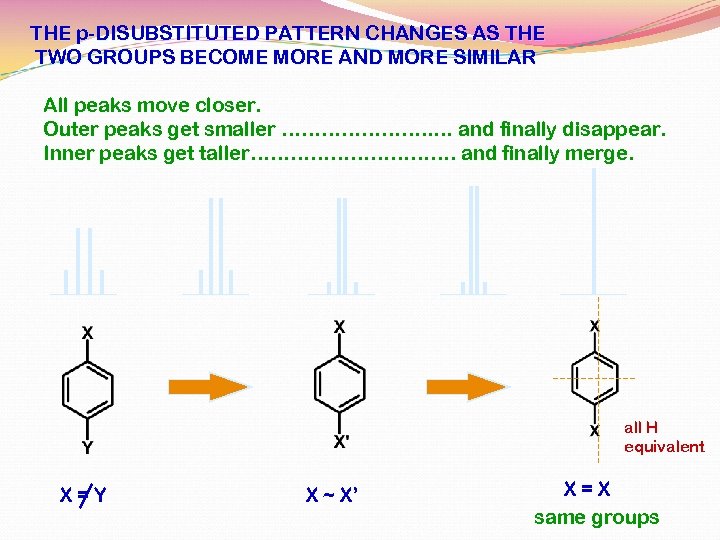
THE p-DISUBSTITUTED PATTERN CHANGES AS THE TWO GROUPS BECOME MORE AND MORE SIMILAR All peaks move closer. Outer peaks get smaller …………………. . … and finally disappear. Inner peaks get taller……………. and finally merge. all H equivalent X=Y X ~ X’ X=X same groups
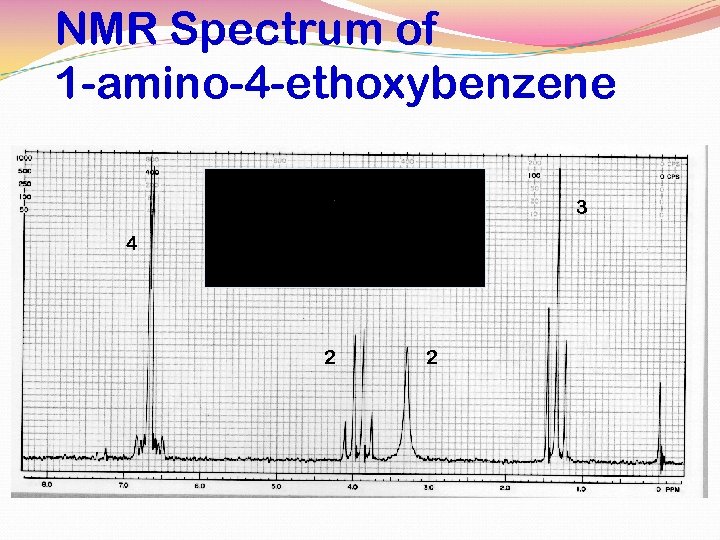
NMR Spectrum of 1 -amino-4 -ethoxybenzene 3 4 2 2
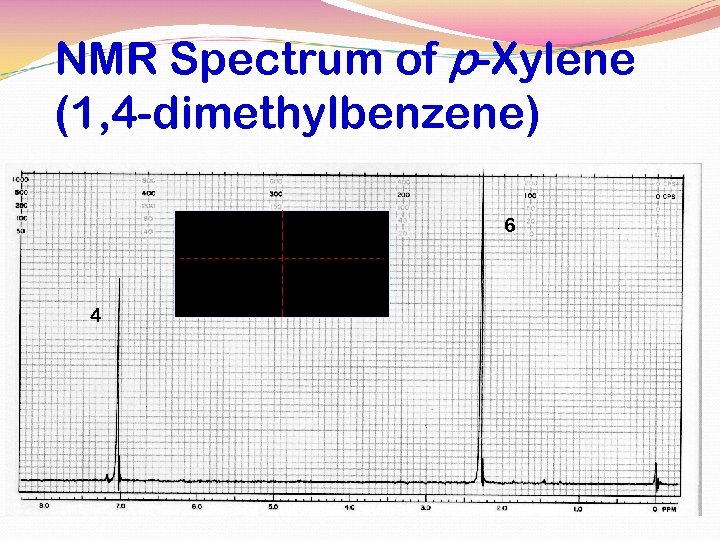
NMR Spectrum of p-Xylene (1, 4 -dimethylbenzene) 6 4

HYDROXYL AND AMINO PROTONS
Hydroxyl and Amino Protons Hydroxyl and amino protons can appear almost anywhere in the spectrum (H-bonding). These absorptions are usually broader than other proton peaks and can often be identified because of this fact. Carboxylic acid protons generally appear far downfield near 11 to 12 ppm.
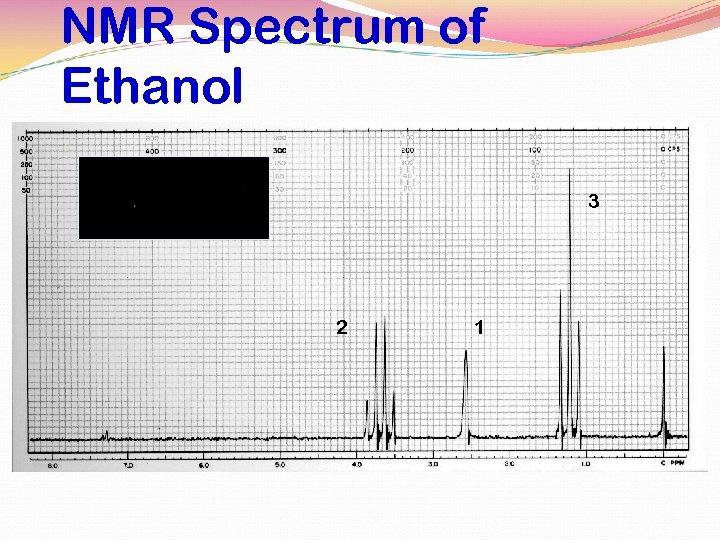
NMR Spectrum of Ethanol 3 2 1
SPIN-SPIN DECOUPLING BY EXCHANGE In alcohols coupling between the O-H hydrogen and those on adjacent carbon atoms is usually not seen. C O This is due to rapid exchange of OH hydrogens between the various alcohol molecules in the solution. H H In ultrapure alcohols, however, coupling will sometimes be seen. R-O-Ha + R’-O-Hb R-O-Hb + R’-O-Ha The exchange happens so quickly that the C-H group sees many different hydrogens on the O-H during the time the spectrum is being determined (average spin = 0)
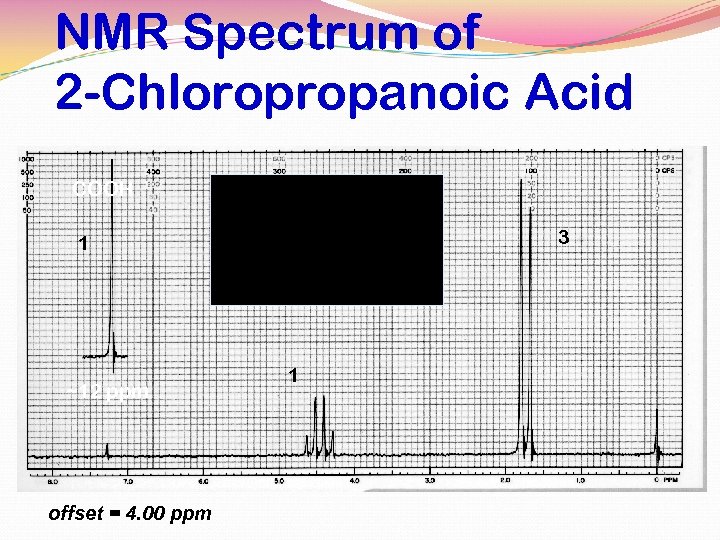
NMR Spectrum of 2 -Chloropropanoic Acid COOH 3 1 ~12 ppm offset = 4. 00 ppm 1
5c47614752e536b9a6f96b18539dfc03.ppt